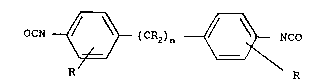Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ - ' - 1338690
Water-dilutable binders and their use for coating mater-
ials, in particular in automotive construction
The invention relates to aqueous binders which
are particularly suitable for the preparation of cathodic
coating materials. Coatings produced using the water-
dilutable binder~ according to the invention have an
excellent corrosion protection action and improved stone-
chip resistance.
It i~ ~n~n that, after salt formation, variou~
organic binder systems can be dispersed in an aqueous
medium and deposited by an electrophoretic method, with
application of an electric current, on a conductive
metallic article ;mmersed in the aqueous bath. The
electrophoretic finishes or electrocoating finishes which
are predominantly used in practice and have good throwing
power and very good corrosion protection on metallic sur-
faces, for example metal sheets, are cathodic binder~.
The thermal crosslinking of the deposited films is
effected, for example, by incorporating partially blocked
polyisocyanates or by admixing fully blocked polyiso-
cyanates, as described in, for example, D~-A-20 57 799,
DE-A-21 31 060, DE-A-25 31 960 and DE-A-26 03 666. In
these patent descriptions, general lists of the suitable
blocked polyisocyanates are compiled or novel blocking
agents are described. There i~ no indication of mixtures
of special polyisocyanates which improve the stone-chip
re~istance.
DE-C 35 45 205 describes electrocoating materials
which, in addit1on to the binder, contain crosslinking
mixtures of a reactive thinner A and a compound B which
contains at least three blocked isocyanate groups and has
a number average molecular weight of 750-10,000. The
component A used as a thinner may be a compound which has
two blocked isocyanate groups and may have a low number
average molecular weight of less than 715 and a low amine
number of less than 30. The solvent character of the
reactive ~hi~er A results in large film thicknesses. No
improved stone-chip protectlon i~ de~cribed.
- 2 - 1 338 690
_ In DE-A-30 04 538, a polyisocyanate mixture
consisting of partially and completély blocked aromatic
polyisocyanates and a non-blocked bifunctional polyiso-
cyanate is used so that the cathodic electrocoating primer
in the two-coat system does not cause any deterioration in
the quality of the top coat as a result of coloured cleavage
products during baking. In DE-A-34 32 233 and EP-A-249 884,
blocked polyisocyanate mixtures having different reactivi-
ties, that is to say having different blocking groups, are
used in order to avoid substrate defects in the baked film.
In EP-A-201 444, in addition to a blocked polyisocyanate as
a reactive crosslinking agent, a blocked polyisocyanate
having a low glass transition temperature (Tgc22C) is added
as a reactive diluent, in order to reduce the content of
coalescence agents and obtain an electrocoating film having
good levelling properties. None of these examples of mix-
tures gives any indication of an improvement in the stone-
chip resistance as a result of a specific choice of
polyisocyanate mixtures.
The market requirements with regard to the proper-
ties of coating materials which can be deposited at the
cathode are increasing constantly because of the reduction
in the solvent content and in the baking temperature while
maintaining or improving the throwing properties and
corrosion protection properties. Today, particular value is
placed on increased stone-chip resistance.
The object of the invention is to prepare binder
systems for aqueous cathodic electrocoating materials, which
meet the above requirements and have improved stone-chip
resistance compared with the known coating materials.
It has now been found, surprisingly, that this
object can be achieved by a specific choice of the blocked
polyisocyanates used.
The invention relates to water-dilutable binders,
contalnlng
A) 40 to 90~ by weight of water-dilutable base resins
containing hydroxyl groups corresponding to a hydroxy
number of 30 to 500 and amino groups corresponding to an
amine number of 30 to 150 and
.~
_ - 3 ~ 1338690
having a number averaqe molecular weight Mn of 500
to 20,000 and
B) 10 to 60% by weight of a completely blocked polyiso-
cyanate mixture consisting of
1. one or more terminal blocked diisocyanates which
contain urethane group~ and optionally urea
groups, are free of OH groups and are reaction
products of H-reactive bifunctional compounds,
which optionally contain tertiary amino groups,
with diisocyanates, having a number average mole-
cular weight Nn of 750 to 8,000 and an amine num-
ber of 30 to 150, and
2. aromatic and/or aliphatic blocked polyisocyanates
which have more than two blocked isocyanate
groups and a number average molecular weight Mn
of 500 to 1,500,
the components Bl and B2 being present in a weight
ratio of 1 : 1 to 1 : 10.
The base resins present as component A in the
water-dilutable binder according to the invention contain
hydroxyl ylOu~ primary and/or secondary amino groups as
H-reactive groups which can react with blocked-i~ocyanate
groups. Tertiary amino gto~p~, quaternary ~mino glo~s,
quaternary phosphonium groups and/or ternary sulphonium
groups are used for achieving the water-dispersibility or
water-solubility. The base resins may be present a~ mix-
tures, and each component of the mixtures may carry one
or ~ore of the stated functional groups. It is also
possible to admix components without such functional
y~ou~s~ provided that the water-dilutability i~ not
adversely affected.
The water-dilutable base re~in (component A) can
be a polyadduct, polyconden~ate or polymer having a
number average molecular weight Mn of 500 to 20,000, in
particular 1,000 to 10,000 (measured by gel permeation
chromatography, calibrated with polystyrene fractions).
Its viscosity i~ preferably 0.05 to 10 Pa.s, in par-
ticular 0.1 to 5 Pa.s, measured in 50% strength solution
in monoglycol ethers (in particular butoxyethanol) at
~~` ~ 4 - 1338690
`_
25C. Its glass transition temperature is, in particular,
-50 to +150C, preferably -20 to +50C. The suitable mean
molecular weights or viscosities can also be obtained by
admixing resins having higher or lower molecular weight
or viscosity.
The chemical composition can be selected from a
very wide range of classes of compounds. For the solu-
bility, it is important that at least one group capable
of salt formation, such ~8, for example, primary, secon-
dary or tertiary amino groups, is present per molecule.
However, quaternary ammonium or phosphonium salt groups,
as well as ternary sulphonium groups, may also be present
(optionally as a proportion of the total) in the mole-
cule. For the crosslinking capability, it is important
that they possess on average at least two H-reactive
sites, for example two hydroxyl ylo~ps and/or primary
and/or secondary amino groups. If the component (A)
contains a sufficient amount of amino groups 80 that it
becomes water-soluble or water-dispersible after protona-
tion with acids, it is possible, by emulsification with
- blocked polyisocyanates (component B), to prepare water-
dispersible binders for baking finishes, from which bin-
ders it is possible, after electrophoretic deposition and
thermal crosslinking, to produce particularly good anti-
corrosion primer coats or one-coat finishes.
The water-dilutable base resin (component A)
preferably has an amine number of 30 to 150 (mg of ROH
per g of solid resin) or 0.5 to 2.7 milliequivalents of
cationic groups per g of solid resin and a hydroxyl
number of 30 to 500 or 0.9 to 8.9 milliequivalents of
hydroxyl groups per g of solid resin. The upper limit of
the amine number is preferably 120, particularly pre-
ferably 100, and the lower limit of the amine number is
preferably 45, particularly preferably 70. If the amine
number is too low, the solubility is too low, or an
excessively high degree of neutralization results in
excessively acidic pH values in the electrophoretic
baths. If the amine number is too high, the deposited
film exhibits poor adhesion or an uneven surface having
-_- ~ 5 ~ 1338690
~ different film thicknesses i~ formed. The hydroxyl
groups present in the molecule are important for the
crosslinking reactions which take place during baking.
The number of these hydroxyl groups i8 at least 2,
preferably at least 3 and particularly preferably at
least 4 hydroxyl groups per molecule. The upper limit of
the hydroxyl number is preferably 400 and particularly
preferably 300. The lower limit of the hydroxyl num~er
i8 preferably S0, particularly preferably 100 If the
hydroxyl number is too low, films which still undergo
surface swelling in organic solvents, such as acetone,
methyl ethyl ketone are formed during crosslin~i~g. If,
on the other hand, the hydroxyl number is too high, the
film becomes too brittle and may also remain too hydro-
philic. Resins having quaternary ammonium or phosphoniumgroup~ or ternary sulphonium 9~0~pB are generally admixed
for the preparation of suitable pigment paste binders.
40 to 60% by weight of the water-dilutable base
resin (component A) are mixed with 10 to 60% by weight of
the blocked polyisocyanate mixture (component B), in par-
ticular 50 to 80% by welght of component A with 20 to 50%
by weight of component B, particularly preferably SS to
70~ by weight of component A with 30 to 45% by weight of
component B. The ratio of the number of equivalents of
primary or secondary amino groups and hydroxyl groups of
component A to the blocked isocyanate groups of component
B is in the range from 1 : 0.1 to 1 : l.S, preferably 1
: 0.7 to 1 : 1.2 and particularly preferably 1 : 1. The
components (A) and (B) can be mixed in the cold or hot
state and may also be precondensed, in particular at
ele~ated temperatures. During this procedure, the com-
ponents (A) and (B) react with one another to a certain
extent without the mixture losing it~ heat-curability and
the ability to be rendered water-~oluble by protonation
with acids.
The preparation of basic polyamine resins which
are used according to the invention as component (A) is
known and is described in many patent publications, for
example
- 6 - 133869U
1. adducts of modified polyepoxides and ketimine~,
which are prepared from polyamines having a secon-
dary and at least one primary amino group ( DE-A-27
01 002 and DE-A-20 57 799).
2. adducts of modified polyepoxides and polyamines (DE-
A-37 12 805) or ammonia (DE-A-36 24 313).
3. modified epoxy resins whi;h are obtained by reaction
with unsaturated or basic monoisocyanates ( DE-A-27
07 405 or DE-A-27 07 482).
4. Nannich bases, obtAine~ from modified phenols by
reaction with formaldehyde and secondary amines or
amino alcohols (DE-A-27 51 499, DE-A-27 S9 428 and
EP-A-209 857).
5. copolymers which contain at least one basic acryloyl
or vinyl monomer (DE-A-15 46 854, DE-A-20 57 799 and
DE-A-34 36 346).
.
- 6. reaction products of maleated or epoxidized poly-
butadiene oils with amines (DE-A-27 28 470 and DE-
A-27 32 736).
7. aminopolyurethAne~ ContAining OR groups (DE-A-34 65
329).
8. polycondensates of polycarboxylic acids and a poly-
amine which contains at least two basic ~mino 9LOU~
(US-A-2 450 940).
9. adducts of polycarboxylic acid resins and alkylene-
imine (US-A-3 403 088).
10. basic resins which contain quaternary ammonium or
phosphonium salt groups or ternary sulphonium salt
y~O~p3 (DE-A-25 31 960).
_ - 7 - 1 3 3 8 6 9 0
- 11. basic resins which, in addition to hydroxyl and
amino groups, also contain blocked isocyanate
groups.
The chemical composition of a preferred amino-
epoxy resin and its properties can be varied within awide range, for example by
- the choice of the epoxy resins and of the amines,
~ - the number of amino and hydroxyl groups,
- the molecular weight and the molar ratio of base
resins and crosslinking agents and
- the ratio of hard and soft molecular segments.
Resins contA i ni ng epoxy groups and preferably
having terminal 1,2-epoxy groups are polyglycidyl ethers,
polyglycidylamines or polyhydrocarbons contAining epoxy
groups, having a mean molecular weight of 140-4,000 and
an epoxide equivalent weight of about 70 to 2,600. Exam-
ples of suitable epoxy resins are compounds of the
general formula
. , ~
(1) CH2 ~ ~ 2
n
in which
A represents the radical of a polyhydric, preferably di-
hydric, alcohol, phenol, amine or corresponding hetero-
cyclic compounds and n is a number from 1.5 to 6, prefer-
ably 1.8-4, preferably 2, and which may also be used as
mixtures.
Particularly preferably used are polyglycidyl
ethers which contain about two 1,2-epoxy groups per
molecule, having a mean molecular weight of about 300 to
1,500 and an epoxide equi~alent weight of about 170 to
1,000, in particular 180 to 500,
in which
A represents the radical of a polyhydric, preferably di-
hydric, alcohol, phenol, amine or corresponding hetero-
cyclic compounds and n is a number from 1.5 to 6, prefer-
ably 1.8-4, preferably 2, and which may also be used as
_ - 8 - - 1 3 3 8 69 U
mixtures.
They are prepared, for example, by reaction of
epihalohydrins or methylepihalohydrins, preferably
epichlorohydrins, with dihydric phenols, and the mole-
cular weight can be ad~usted by selecting the molarratios and adding suitable basic catalysts, such as
ammonium or phosphonium salts. The formula (1) includes,
for example, resins of the following formulas
(2) ~2~ 2~-R - ~ 2 ~ 2 - -~2~a ~ C~2
m
wherein m is 0 to 5, preferably 0 to 2, and R is prefer-
ably the radical of a bisphenol of the following struc-
ture:
(3) ~ y ~
- wherein Y is -CH2-, -C(CH3~ 2- ~ -CO- ~ -S- ~ - -SO- ~ -S2- or
- -C(CCl3~2- and preferably -C(CH3)2-. The aromatic rings
can optionally be substituted by halogens or alkyl
groups.
Typical dihydric phenols are hydroquinone,
resorcinol, 1,5-dihydroxynaphthalene, p,p'-dihydroxy-
diphenylpropane, p,p'-dihydroxybenzophenone, p,p'-di-
hydroxydiphenylmethane, p,p'-dihydroxydiphenylethane,
p,p'-dihydroxydi-tert-butylphenylpropane or bis-(2-
hydroxynaphthyl)-methane. Industrial mixtures, such as
dihydroxydiphenylpropane, in particular the 4,4'-isomers
cont A i ning small amounts of 2,2~-or 4,2~-isomer~, are
preferred. The epoxy resins described can also be
completely or partially hydrogenated, such as, for
example, 1,4-bis-(2,3-epoxypropoxy)-cyclohex~e or can be
used as mixtures of compounds having different structures
and molecular weights.
These polyepoxy resins which have been described
can optionally be partly replaced by more resilient
- 9 - 1338690
modifications. They are formed from dihydric phenols, in
particular bisphenols, by reaction of the polyepoxy
resins with polyalcohols, preferably long-chain dialk-
anols (HO-E-OH), such as butane-1,4-diol or heY~ne-1,6-
S diol in the presence of suitable catalysts, with forma-
tion ofs
(4) ~--CH~20-R--~-CH2-~H-CH2-o---E--0 CN2 ~ 2 2 ~ 2
or by reaction of polyhydric phenols with alkylene ox-
ides, such as ethylene oxide, propylene oxide, butylene
oxide or stearyl oxide, and subsequent reaction with epi-
chlorohydrin, with formation of:
(5) ~2 / CH2 0 ~H-CH2-0-R--O-CH2-~H--O-CH2- ~ 2
wherein R' denotes hydrogen or a lower alkyl radical,
preferably -CH3 or -C2HS. Also suitable are polyglycidyl
ethers of polyhydric slcohols, which sre characterized by
the following genersl formuls and are embraced by the
above genersl formula (1):-
(6) \2 ~ -CH2~-(CHR )p O-CH2 ~ ~ 2
wherein R'' = hydrogen or a lower, optionally substituted
alkyl radical, preferably -CH3 or -C2H~ and p is 2 to 15.
Typical examples of these are the reaction products of
epichlorohydrin and ethylene glycol, 1,2- and 1,3-propyl-
ene glycol, 1,2- and 1,4-butAne~iol, 1,5-pentAn~iol and
2-ethylheYAne-1,6-diol, as well as compounds such as
1,2,6-heYAnetriol or bis-(4-hyd oAy~yclohexyl)-2~2-
propane. However, suitable polyglycidyl ethers may also
correspond to the formula:
(7) C\2~-C~ O-(CHR~)r~ -cH2-~ ~ 2
which is likewise embraced by the general formula (1),
wherein R'' has the same meaning as above and r denotes
- lo - 1 3 38 6 9 ~
2 to 6 and q denotes 1 to 20. Typical examples of these
are the reaction products of epichlorohydrin and the
polyethers obtained from ethylene glycol, 1,2-propylene
glycol or 1,2-butylene glycol, such as polyethylene gly-
5 c018, polypropylene glycols or polybutylene glycols hav-
ing various molecular weights.
The formula (1) also embraces heterocyclic
polyepoxy compounds, which can likewise be used, such as
1,3-diglycidyl-5,5-dimethylhydantoin or trigl~_idyl
isocyanurate. Another suitable class of polyepoxides
comprises polyglycidyl ethers of phenolic novolak resins.
They are formed by condensation of formaldehyde with
phenols in a molar ratio of 1 : 0.5 to 1 : 0.8 under
acidic conditions and then reacted with epichlorohydrin.
They have an epoxide equivalent weight of 150 to 300,
preferably 170 to 210 and contain about 2 to 4 glycidyl
groups per molecule. In the case of this resin system,
it must be borne in mind that it generally has a higher
mean molecular weight, for example be~ el. 474 and 3,000.
The resins can be defunctionalized by reaction with
monoalkylphenols, such as nonylphenol.
Amino y~o~ps are advantageously introduced by
sub~ecting the epoxy groups to an addition reaction with
NH-reactive compounds. The reaction is therefore carried
out using an approximately equimolar ratio, optionally a
small excess of epoxy groups, in order to compensate for
the consumption of epoxy groups for side reactions, or to
ensure complete incorporation of the amines. Primary
amines react with two epoxy groups and thus result in
chain extension. In general, the epoxy resins are
dissolved in organic solvents, such as aliphatic alco-
hols, monoalkyl ethers of ethylene glycol or propylene
glycol, or the corresponding dialkyl ethers. The reac-
tion of the amines begins at as low as room temperature
and is generally exothermic. By increasing the reaction
temperature to about 50-150C, preferably 60-85C, for
stability reasons it must be ensured that, after the end
of the reaction, epoxy groups are no longer present. All
amines in the mixture may be reacted simultaneously with
~ 13386g0
~ the epoxy groups or a stepwise procedure can be adopted,
that is to say one or more basic intermediates containing
epoxy groups can be prepared in different sequences.
For the reaction with the epoxy resins, amines
from the following ylo~ps are advantageously selected:
1. Mono- or dihydroxyalkylamines of the general formula
(8) H-N--R'~H (9) H-N-(R' H)2
wherein
-R is -H or an alkyl r~icA 1 having 1 to 8 C atoms,
preferably methyl or ethyl, and
- 10 -R'- is an alkylene radical having 2 to 8 C atom~,
preferably ethylene or propylene.
Because of their hydroxyl y-o~ps, which are preferably
primary ones, amines of this type improve the reactivity
of the ba~e resin. Typical example~ of these are amino-
ethanol, N-methylaminoethanol, N-ethylaminoethanol, di-
ethanolamine,aminoisopropanol,N-methylaminoisopropanol,
N-methylamino-n-propanol, N-ethylaminoisopropanol and
diisopropanolamine.
2. N,N-Dialkylami~oA1~ylamine~ of the general formula
~ ~ ~R~
( 10) H-l'll-R '~ ( 11) HH- (R ' -1~
R R~ R~ 2
wherein
-R denotes H or -R'',
-R'- denotes an alkylene radical having 2 to 8 C
atom~, preferably ethylene or propylene, and
-R'' denote~ an alkyl radical having 1 to 8 C atoms,
preferably methyl or ethyl.
Because of the dialkylamino g O~p3, amines of
this type improve the basicity and hence the solubility
of the base resin. Suitable examples for this purpose
are N-dimethylaminoethylamine, N-diethyl-N'-methylamino-
p,o~lamine,diethylaminoethylamine,dimethylaminop o~amine, diethylaminopL6p~1amine and dimethylaminoneo-
- - 12 ~ 1338 690
pentylamine.
3. Long-chain secondary diamines which are used with
chain extension for elastification and are of the
general formula
(121 H-~-R' ~ H
R R
wherein
-R denotes an alkyl group or hydroYyalkyl group
having 1 to 8 C atoms and
-R' denotes an alkylene group which optionally con-
tains one or more oxygen atoms in the chain and has
2 to 12 C atoms, preferably 4 to 8 C stoms.
The secondary diamine can also be prepared by reacting
the corresponding primary alkylene~i~mine with glycidyl
ethers or glycidyl esters. Typical examples are N,N'-
~1 A lkyldiamino~lkAne~ such as N,N~-dimethyldiamino-
h~YAn~ bis-N,N'-cyanoethylalkylene~iAmines or preferably
the reaction product of h~YA~e~i~mine with 2 moles of
~~~ ~ Cardura E, the glycidyl ester of versatic acid. Exten-
- sion of the elastifyin7~chain can be achieved, with for-
mation of urea groups, by reacting 2 molecules of the
secondary diamines described above with 1 mole of diiso-
cyanate. The secondary diamine may also have an asym-
metric structure if the two substituents are different.
For example, the diamine may be a reaction product of N-
h~L~eLhylethyle~e~iAmine or N-dimethylaminoethyl-
propyl~n~i~mine with Cardura E.
For further modification of the epoxy resins, itis also possible to use primary monoalkylamines and/or,
preferably, secondary dialkylamines, such as diethyl-
amine, n-octylamine, N-methyl-N-ethylhexylamine, di-
dodecylamine or metho~yp o~ylamine.
Primary amino group~ are preferably incorporatedin the resin base structure by reacting resins cont~;ning
at least one, preferably at lea~t two, epoxy y 0~8 per
molecule with an amino- and/or hydroxyl-contAi n ing
ketimine and/or aldimine. The preferred ketimines are
~- - 13 - 1 ~ 3 8 6 9 0
reaction products of ketones and alkylamines contAining
secondary amino groups, such as methyl isobutyl ketone
and diethylenetriamine.
The ketimines are prepared by known methods by
eliminating water from the corresponding polyamines of
the general structure R-NH-R'-NH2 or from the corre~pond-
ing aminoalcohols of the general structure HO-R-NH2 and
the suitable aliphatic ketones, ~uch as diethyl ketone,
methyl isobt~.yl ketone, ethyl n-propyl ketone, or, for
example, cyclopentanone, cycloheYA~one or acetophenone.
The reaction conditions (reaction temperature, choice of
solvent) must be such that no substances, such as water,
which break the ketimine bond remain in the reaction
product.
The ketimine protects the primary amino group in
such a way (cf. US-A-3 523 925) that this group can be
reacted without difficulties with the epoxy base resin
via a further functional group, for example a hydroxyl
group or, preferably, a secondary amino group. Through
the choice of the molar ratios of the components used, it
must be ensured that no unconverted low molec~lar weight
amine remains~b~hind in the mixture. The reaction of the
secondary amino group of the polyaminoketimine with the
epoxy group begins at as low as room temperature and is
generally exothermic. In order to achieve complete con-
version, it is necessary, as a rule, to increase the tem-
perature temporarily to 50-120C.
Another group of water-dilutable base resins
(component A) comprises polymer resins which contain pri-
mary, secondary and/or tertiary amino groups and option-
ally onium salt ylOup~. They can be prepared according
to the prior art, as described in, for example, DE-A-15
46 854, DE-A-20 57 799, DE-A-23 25 177 or DE-A-23 57 152.
Ethylenically unsaturated monomers employed are virtually
all monomers capable of undergoing free radical polymer-
ization, the usual restrictions for copolymerizations
which are prescribed by the Q and e scheme according to
Alfrey and Price or by the copolymerization parameters
being applicable (cf. Brandrup and Immergut, Polymer
_ ~ 14 ~ 1 33869 0
.
Handbuch (Polymer Manual), 2nd edition, John Wiley &
Sons, New York 1975). Since component A) is a poly-
(meth)acrylate re~in having amino groups, the resin is
dilutable with water after neutralization with organic
acids. Such an amino- and hydroxyl-contA;ning copolymer
is preferably obtAin~ by polymerization in solution.
For the preparation of this component A), it is poss-
ible to use mixtures of different unsaturated monomers
which contain b~sic nitrogen atoms or into which such a
basic nitrogen atom csn be introduced by chemical react-
ions. Thus, the component A) is based on, for example,
a) 6 to 40 parts by weight of monomers contAining amino
groups,
b) 4 to 50 parts by weight of monomers contAini~g
hydroxyl groups,
each of these monomers being capable of free rA~i
polymerization, and
c) 10 to 90 parts by weight of further monomers which
are capable of undergoing free radical polymeriza-
tion and, apart from an unsaturated double bond,
contain no further reactive groups,
optionally up to 10 parts by weight of component c) being
replaced by polyunsaturated monomers capable of under-
going free radical polymerization.
The monomers contA i n i ng amino groups and monomers
contAining hydroxyl y~OU~8 ~ each of which is capable of
undergoing free radical polymerization, need not be used
in the form of a mixture. It i8 also possible to use
monomer types which contain both amino g o~ps and hy-
droxyl y~o~ps at the same time. In this case, 8 to 60
parts by weight of the monomers contAi~;ng amino ~o~0
and hydroxyl y~o~ and 10 to 90 parts by weight of the
monomers capable of undergoing free radical polymeriz-
ation and contAining no further reactive g Ou~3 are
employed, optionally up to 10 parts by weight of the
latter being polyunsaturated monomers capable of under-
going free radical polymerization.
For example, monomers of the following general
formula:
_ - 15 ~ 13 3869 0
(l3) R-CH=CR'-X-~-N(R' )2
wherein R denotes -R' or -X-C~H2tl,
R' denotes -H or -C~H~1,
R'' denotes -R', -CDH~OH and/or -CnH~NR2,
X denotes -COO-, -CONH-, -CH20- or -O-,
A denotes -CnH~- or -CnH2n-~-CH2- and
OH
n denotes 1 to 8, preferably l to 3,
are used as monom rs which can be sub~ected to free radi-
cal polymerization and contain amino groups or N ylou~s.
Examples of un~aturated monomers contAining N
groups are N-dialkyl- or N-monoalkylaminoalkyl (meth)-
acrylates, such as, for example, N-diethylaminoethyl
methacrylate or N-tert-butylaminoethyl acrylate, or the
corresponding N-alkanol compounds, N-dialkyl- or N-
lS monoalkyl amino~lkyl(meth)acrylamide, ~uch as, for exam-
ple, N-dimethylaminoethylacrylamide, or the corres~on~;~g
N-AlkAnol compounds and/or vinyl contA i n i ng heterocyclic
compounds having one or more basic nitrogen atoms, such
as, for example, N-vinylimidazole.
Monomers which can be sub~ected to free radical
- polymerization and contain hydroxyl ylo~ps are understood
as being those which, in addition to a polymerizable
ethylenically unsaturated group, also contain at least
one hydroxyl group on a C2 to C20 linear, branched or
cyclic carbon skeleton.
These are mainly unsaturated esterification pro-
ducts of the general formula
(14) R-CH=CR'-X-B
wherein R, R' and X are as defined above and
B is a linear or branched Cl6 alkyl group having
1-3 OH groups.
Hydroxyalkyl (meth)acrylates, such as, for exam-
ple, 2-hydroxyethyl acrylate, 2-hydroxypropyl methacryl-
ate, butane-1,4-diol monoacrylate, 2,3-dihydroxypropyl
methacrylate, pentaerythritol monomethacrylate or poly-
propylene glycol monoacrylate, or else dihydroxyalkyl
fumarates are particularly suitable. However, it is also
possible to u~e N-hydroxyalkyl(meth)acrylamides or N-
~_ - 16 - 133869 0
-
- hydroxyalkylfumaric acid mono- or diamides, -~uch as, for
example, N-hydroxyethylacrylamide or N-(2-hydroxypropyl)-
methacrylamide. Particularly elastic properties can be
obtained when a reaction product of hydroxyalkyl (meth)-
S acrylate with ~-caprolactam is used. Other compounds
contAi~ng hydroxyl g ou~8 are allyl alcohol, monovinyl
ethers of polyalcohols, in particular diols, such a~ the
monovinyl ether of ethylene glycol or of butAnediol, and
allyl ethers or est~s contAining hydroxyl groups, such
as 2,3-dihydroxypropyl monoallyl ether, trimethylol-
propane monoallyl ether or allyl 2,3-dihydroxypropionate.
Hydroxyethyl, hydroxypropyl and/or butane-1,4-diol mono-
(meth)acrylate are particularly suitable.
The choice of the monomers which can be sub~ected
to free radical polymerization and contain no further
reactive g o~ is based on the mechAnicAl properties of
the film and on the compatibility of the resin combinat-
ion used. Alkyl acrylate~, alkyl methacrylates, dialkyl
maleates and/or dialkyl fumarates are used, the alkyl
radicals consisting of 1 to 20 carbon atoms and being
-^ arranged in a lineAr or branched aliphatic chain and/or
as a cycloaliphatic and/or (alkyl)aromatic radical.
~Hard~ monomers having a high glass transition temper-
ature as polymers are, for example, monomers of the
vinylaromatic type, such as styrene, ~-substituted
styrenes, such as ~-methylstyrene, o-, m- and p-alkyl-
~tyrenes, such as vinyltoluene or p-tert-butylstyrene,
halogenated vinylbenzenes, such as o- or p-chlorostyrene,
methacrylates having a short chain, such as methyl meth-
acrylate, ethyl methacrylate, propyl methacrylate, butylmethacrylate, cyclohexyl methacrylate, isobornyl meth-
acrylate, dihydrodicyclopentadienyl methacrylate, (meth)-
acrylamide and/or else (meth)acrylonitrile. "Soft~
monomers, on the other hand, are acrylates having a long
alcohol chain, such as n-butyl acrylate, isobutyl acryl-
ate, tert-butyl acrylate, 2-ethylhexyl acrylate and/or
lauryl acrylate. It is also possible to use unsaturated
ethers, such as ethoxyethyl methacrylate or tetrahydro-
furfuryl acrylate. Monomers of the vinyl ester type,
~ 7 _ 13~8690
preferably vinyl esters of ~-branched monocarboxylic
acids, in particular vinyl versatate, can also be incor-
porated as polymerized units if suitable reaction con-
ditions and reaction comonomers are chosen. Ethylenic-
ally polyunsaturated monomers are understood as meaning
compounds having at least 2 double bonds capable of
undergoing free radical polymerization, of the general
formula
(15) R-CH=CR'-D-(-CR' CH-R)~
where m is 1 to 3, preferably m i8 1,
and, in addition to the abovementioned meAnlngs, D is the
general basic supporting chemical skeleton for the react-
ive double bond. Examples of D are the o-, m- or p-
phenyl radical and radicals of the formula -X-alkyl-X'-,
wherein alkyl preferably has 2 to 18 C atoms, X and X'
are identical or different bonding y~oups~ for example
-O-, -CONH-, -COO-, -NHCOO- or -NH-CO-NH-. D may be, for
example, a benzene ring, as in divinylhç~7sne, which may
also be optionally substituted, such as p-methyldivinyl-
benzene or o-nonyldivinylbenzene. Other examples of
suitable polyunsaturated monomers are reaction products
- - - of polyalcohols, in particular dialcohols, with ~,B-
unsaturated carboxylic acids, as already defined. Exam-
ples of these are e~Ane~iol diacrylate, glycol dimeth-
acrylate, 1,4-butAne~;ol diacrylate, 1,6-heYAne~iol di-
acrylate, neopentylglycol dimethacrylate, triethylene
glycol dimethacrylate, polyglycol 400 diacrylate, gly-
cerol dimethacrylate, trimethylolpropane triacrylate
and/or pentaerythritol diacrylate.
The use of bifunctional unsaturated monomers,
such as butAneAiol diacrylate or heyA~e~iol diacrylate,
is preferred. When glycidyl methacrylate and methacrylic
acid are used, the corre~po~ g compound glycerol di-
methacrylate is formed automatically in the polymerizat-
ion. The type and amount of polyunsaturated monomers
must be carefully tailored to the reaction conditions
(catalysts, reaction temperature, solvent) in order to
obtain the desired viscosity without gelling.
The copolymerization is carried out in a known
_ - 18 - 1338 69 0
~ manner by solution polymerization with the addition of
free radical initiators and optionally molecular weight
regulators, at temperatures from 50 to 160C. It is
carried out in a liquid in which monomers and polymers
dissolve together. The content of monomers or polymers
after the polymerization is about 50 to 90% by weight.
Solution polymerization in organic solvents which are
dilutable with water is preferred. Such solvents are,
for example, ethylen~ glycol, ethoxyethanol, butoxy-
ethanol, diethylene glycol, triethylene glycol, diethy-
lene glycol dimethyl ether, propylene glycol, methoxypro-
panol, ethoxypropanol, dipropylene glycol monomethyl
ether, dipropylene glycol dimethyl ether, diacetone-
alcohol, ethanol, isopropanol, sec-butanol, tert-butanol,
acetone, methoxypropanone, dioxane, tetrahydrofuran, N-
methylpyrrolidone or mixture~ of these. In general, the
solvent or solvent mixture i8 heated to the reaction
temperature and the monomer mixture is then allowed to
run in over several hours. In order to be able to carry
out the procedure at the reflux temperature, the in-
itiator i~ matched -to the boiling point of the solvent
~ - mixture. It usually decomposes with a half life of-30
minutes to 10 hours. The initiator is either dissolved
in the monomer mixture at room temperature or, for safety
reasons, i~ metered in separately during the monomer
feed. 0.1 to 5% by weight, preferably 0.5 to 3% by
weight, based on the amount of monomers used, of per-
oxides and/or azo compounds are added as initiators which
are soluble in organic solvents. Examples of peroxides
used are benzoyl peroxide or di-tert-butyl peroxide,
hydroperoxides, ~uch as tert-butyl hydroperoxide or
cumene hydroperoxide, and pere~ter~, such as tert-butyl
peroctoate or tert-butyl perhen7oate. Azo compounds
which undergo thermal decomposition are, for example,
2,2'-azobis-(2-cyanopropane) or l~l~-azobiscyclohey~n~-
carbonitrile. Compounds of the ~ih~n7yl type which form
free radicals, such a~ 1,2-bis-(4-methylphenyl)-1,2-
dicarbethoxy-1,2-dicyanoethane, can also be used as
initiators. By using regulators, the molecular weight
~ - '9 - 1338 690
can be reduced in a known manner. Mercaptans, halogen-
contA~ni~g compounds and other substances which transfer
free radicals are preferably used for this purpose. n-
or tert-dodecyl mercaptan, tetrakis mercaptoacetylpenta-
erythritol, tert-butyl-o-thiocresol, thiosalicylic acid,
buten-1-ol or dimeric ~-methylstyrene are particularly
preferred.
Amino-(meth)acrylate resins can also be prepared
by polymer-analogous reaction. Thus, for example, a co-
polymer contA~ng acrylamido groups can be reacted withformaldehyde and a secondary amine and/or aminoalcohol.
A particularly preferred process is described in DE-A-
34 36 346. Here, monoethylenically unsaturated monomers
contAin~ng epoxy groups are first incorporated in the co-
polymer as copolymerized units. Thereafter, a reactionwith excess _mmonia, primary and/or secondary monoamines
and/or monoaminoalcohols i8 carried out and the excess
amine is then distilled off. A similar reaction can, for
example, preferably be carried out, using equivalent
amount~, with ketimines of polyamines which contain a
secondary amino group and one or more primary amino
- ~ 9l0U~, such as, for example, the monoketimine of methyl
isobutyl ketone and methylaminopropylamine or the di-
ketimine of methyl isobutyl ketone and diethylenetri-
amine.
Resins contA~n~ng onium salt g~o~ are those
which contain, for example, quaternary ammonium salt
y~ou~s~ quaternary phosphonium salt groups and/or ternary
sulphonium salt groups. They can be prepared, for exam-
ple, by reacting epoxy resins with tertiary ~m~ne salts,sulphide/acid mixtures or phosphine/acid mixtures. The
reaction temperature i8 not particularly critical and is
chosen as a function of the starting materials and their
reaction rates. Frequently, the reaction takes place
sufficiently rapidly at room temperature or at elevated
temperatures of up to 70C. In some case~, it is advis-
able to use higher temperatures of about 110C. A solvent
is not generally necessary, although it is often used for
better control of the reaction. Examples of suitable
_ - 20 - 1 3 3 8 69 0
solvents are aromatic hydrocarbons, monoalkyl ether~ of
ethylene glycol or propylene glycol and aliphatic alco-
hols. The acids used are in general those which form
corresponding quaternary ammonium salts, sulphonium salt~
or phosphonium salts. Organic acids having a dissocia-
tion con~tant greater than about 10-5 are preferred.
Examples of suitable acids are formic acid, acetic acid,
propionic acid and lactic acid, as well as boric acid or
phosphoric acid. Substituted or unsubs~ tuted amines
which are suitable for the preparation of quaternary am-
monium salts are those which do not interfere with the
reaction of the amine salt with the polyepoxide and do
not lead to gelling. Preferred amines are tertiary tri-
alkylamines, such as trimethylamine, triethylamine, tri-
isopropylamine, methyldibutylamine, diethylbutylamine orelse dimethylaminoethanol or N-methyldiisopropanolamine.
For the preparation of resins which contain ter-
tiary sulphonium bases, it is possible to use any sul-
phides which react with epoxy groups and do not contain
any groups which interfere with the reaction. The
sulphide may be an aliphatic, mixed aliphatic-aromatic,
- - aralkylic or cyclic sulphidQ. Examples-of such sulphides
are dialkyl sulphides, such as diethyl sulphide, dipropyl
~ulphide, dibutyl sulphide or dihexyl sulphide, or alkyl
phenyl sulphides, such as diphenyl sulphide, or ethyl
phenyl sulphide, alicyclic sulphides, such as tetra-
methylene sulphide or pentamethylene sulphide, hydroxy-
alkyl sulphides, such as, for example, diethanol, dipro-
panol or dibutanol thioether. The polyepoxide can also
be reacted with mercaptans, and the ternary sulphonium
~alt then be formed by reaction with 1,2-epoxides in the
presence of an acid.
Resins having quaternary phosphonium ~alt groups
are prepared using any phosphine~ which do not contain
any groups which interfere. Examples of ~uch phosphines
are aliphatic, aromatic or alicyclic phosphine~, the
following phosphines being mentioned as specific exam-
ples: lower trialkylphosphines, such as trimethylphos-
phine, methyldiethylphosphine, triethylphosphine,
~ 21 - 1338690
tripropylphosphine or tributylphosphine, mixed lower
alkylphenylphosphines, such as phenyldimethylphosphine,
phenyldiethylphosphine, phenyldipropylphosphine, di-
phenylmethylphosphine, diphenylethylphosphine, diphenyl-
propylphosphine or triphenylphosphine, and alicyclic
phosphines, such as tetramethylenemethylphosphine.
The ratio of tertiary amine, sulphide or phos-
phine to acid is not particularly critical. At least one
equivalent of acid is preferably used ~r each desired
mole for conversion into an onium salt. The ratio of
amine acid salt, sulphonium salt or phosphonium salt to
the epoxy compound may vary. The optimum amounts depend
on the specific starting materials. In general, about
one to about SO parts by weight of salt can be used per
lS about lOO parts of polyepoxide. In some cases, less than
100% of the nitrogen is in the form of quaternary ammon-
ium salt g o~p8. This is the case, for example, if
primary and secondary amines are used for the preparation
of the resins having quaternary ammonium salt ~lO~B.
Polymer resins having onium salt y oups can be
prepared, for example, by-copolymerization of unsaturated
- monomers which can be sub~ected to free radical polymer-
ization and, in addition to the reactive unsaturated
double bond, also contain a quaternary ammonium group, a
tertiary sulphonium group or a quaternary phosphonium
salt group, such as, for example, the reaction product of
glycidyl (meth)acrylate and trialkylamines, in the pres-
ence of water or acids.
A selected mixture of two completely bloc~ed
polyisocyanates having different compositions is used as
the cross1 i~king agent (component B):
Component Bl is a modified diisocyanate which is free of
OH ~ oups~ contains urethane groups and optionally urea
groups and has blocked terminal -NCO yro~ps and a mean
molecular weight of 750 to 8,000. The lower limit is
preferably l,OOO, particularly preferably l,500; the
upper limit of the molecular weight is preferably 6,000,
particularly preferably 4,000. For improving the solu-
bility properties, the component Bl can contain tertiary
. - 22 ~ 1 3 3 869 0
amino groups, the amine number being 30 to 150. The
upper limit of the amine number is preferably 100, par-
ticularly preferably 60.
Component B1 is prepared by reacting bifunctional
compounds contAininq active hydrogen, at reaction temper-
atures from room temperature to 100C, with diisocyanates
in the presence of solvents which. are inert to NC0
groups, or at 110 to 180C with blocked diisocyanates.
The H-reactive bifunctional compounds used are di~co-
hols, diamines, aminoalcohols and/or polyesterdiols.
Examples of suitable dihydroxy compounds are the
various isomers of linear, branched and cyclic hydro-
carbon compounds which have 2 to 20 carbon atoms and two
secondary and/or primary hydroxyl y~Oup8 but may also
contain tertiary amino y oups in the C chain or as a side
chain. Typical examples of these are ethylene glycol,
propylene 1,2-glycol, propylene 1,3-glycol, butane-1,4-
diol, neopentylglycol, pentane-1,5-diol, heYAne-1,6-diol,
hexylene glycol, trimethylheYAne-1,6-diol, decane-l,10-
diol, bis-(hydroxymethylene)-cycloh~YA~e, bisethoxylated
~ or bispropoxylated- bisphenol A or the corresponding
- hydrogenation products, N-methyldiethanolamine, N-ethyl-
diethanolamine, N-methyldiisopropanolamine, N-methyl-
ethanolamineor2-dimethylamino-2-methylpropane-1,3-diol.
Relati~ely high molecular weight dihydroxy com-
pounds are, for example, polyesterdiols, polycaprolac-
tonediols, polycaprolactamdiols or polyetherdiols.
Polyesterdiols are prepared, for example, by reacting the
abovementioned diols with aliphatic, cycloaliphstic or
aromatic dicarboxylic acids, such as succinic acid,
glutaric acid, adipic acid, pimelic acid, suberic acid,
azelaic acid, sebacic acid or tetradecane-1,4-dicarbox-
ylic acid and the isomers of cyclohe~Ane~icArboxylic acid
or phthalic acid. Dicarboxylic acids which, as a result
3S of neighbouring group effects following esterification,
do not undergo accelerated hydrolysis are preferred. In-
stead of the dialcohols, or else, instead of a proportion
of these, it is possible to use long-chain primary and
secondary diamines, such as 1,6-heY~n~ mine~ adducts of
- 23 ~ 1 3 3 8 69 0
-
2 moles of glycidyl ethers or glycidyl esters with
heYAne~iamine, bis-N,N'-cyanoethylethylenediamine or bi~-
N,N'-cyanoethylpolyoxypropylenediamine. Aliphatic poly-
ester diols, such as neopentylglycol hydroxypivalate, or
reaction products of, for example, adipic acids and
butane-1,4-diol, sebacic acid and neopentylglycol,
azelaic acid and heyAne-1,6-diol, optionally with the
addition of a proportion of N-methyldiethanolamine, are
particularly -referred. The particularly preferred ali-
phatic polyester polyols are linear and have no branches,are reacted in a molar ratio of (n + 1) moles of di-
hydroxy compounds to n moles of dicarboxylic acid, in the
melt or in the presence of inert solvents, such as
xylene, and have a number avera~e molecular weight of 250
to 3,000; the lower limit is preferably 400, particularly
preferably 600. The upper limit is preferably 2,000,
particularly preferably 1,500. If the molecular weight
is too small, the water resistance of the resulting film
may suffer; if, on the other hand, it is too high, the
adhesion to other layers may be reduced.
Diisocyanates which are suitable for the prep-
aration of component B1 correspond to the general- for-
mulae 16, 17 and 18:
(16) 0=C=N-R-N=C=0
in which
R represents an aromatic hydrocarbon radical which is
optionally substituted by one or more alkyl groups or has
methylene bridges and possesses a total of 6 to 15 carbon
atoms, a branched or linear aliphatic hydrocarbon radical
having 2 to 18, preferably 6 to 10, carbon atoms, a
cyclic hydrocarbon radical having 6 to 15 carbon atoms or
a heterocyclic ring.
All isomers or isomer mixtures of organic diiso-
cyanates can be used. Suitable aromatic diisocyanates
3S are, for example, phenylene diisocyanate, toluylene di-
isocyanate, xylylene diisocyanate, biphenylene diiso-
cyanate and naphthylene diisocyanate.
Aliphatic-aromatic diisocyanates of the formula
- 24 - 1338690
OCN _~(CR2)n~NC~
wherein the radicals R are identical or different and
denote hydrogen or an alkyl radical having 1 to 8 C
atom~, preferably 1 or 2 C atom~, especially -CH3, and n
is an integer from 1 to 10, preferably 1 to 3, are pre-
ferably u-ed. Typical examples of this type of dii~o-
cyanate~ are diphenylmethane -2,4'- and/or -4,4'-diiso-
cyanate, 3,2'-dicyanato-4-methyldiphenylmethane and di-
phenylpropane d$isocyanate.
Another group of preferably used diisocyanates
10- are those whose NC0 group is bonded directly to a linear,
branched or cycloaliphatic radical. Such diisocyanates
are compounds of the formulas
(18) osc=N ( CR2 ) rN-Cd
wherein r is an integer from 2 to 2~, in particular 6 to
8, and the radicals R, which may be-identical or differ-
ent, represent hydrogen or an alkyl radical having 1 to
8 C atoms, preferably 1 or 2 C atoms.
These include, for example, propylene diisocyan-
ate, ethylethylene dii~ocyanate, dimethylethylene diiso-
cyanate, methyltrimethylene diisocyanate, trimethylheYAne
diisocyanate, cyclopentylene diisocyanate and isophorone
diisocyanate. The diisocyanates which are defined by
this formula and whose -NC0 groups are ho~d~ via -CH2-
y Gup8 to a linear, branched or cycloaliphatic, especial-
ly non-branched linear aliphatic, radical are particu-
larly preferred. Typical examples of these are tri~eth-
ylene 1,3-diisocyanate, tetramethylene 1,4-dii~ocyanate,
pentamethylene 1,5-diisocyanate, hexamethylene 1,6-diiso-
cyanate, ~oA~cAne 1,12-diisocyanate and octadecane 1,18-
diisocyanate.
The polyisocyanates used as component B2 differ
from component B1 in that, on average, they
~ - 25 1 ~ 3 8690
advantageously provide, for crosslinking, more than two
reactive isocyanate groups per molecule, which are
blocked by protective groups. Trivalent and polyvalent,
for example trivalent to pentavalent, particularly
S preferably trivalent, aromatic and/or aliphatic blocked
polyisocyanates having a number average molecular weight
Mn of SOO to 1,SOO are preferably used for this purpose.
Polyisocyanates which have ~ oved particularly suitable
are the so-called ~coating polyisocyanates~, which are
prepared from the diisocyanates already described. They
can be prepared, for example, by oligomerization, by
reaction of diisocyanates with water or by reaction of
diisocyanates with low molecular weight trivalent or
polyvalent compounds having functional hydrogen, ~uch as
lS polyalcohols, polyamines and aminoalcohols. Triisocyan-
ates which have proved satisfactory are products formed
by trimerization of diisocyanates or by reaction of
diisocyanates with trifunctional compounds contAining OH
or NH groups. Thus, tris-(6-isocyanatohexyl)-biuret is
formed from, for example, hexAne diisocyanate and water.
Trimerization of the heYA~e diisocyanate gives, for
example, tris-(6-isocyanatohexyl) isocyanurate,-possibly
as a mixture with its higher homologs. Further examples
are isocyanurates obtAine~ from isophorone diisocyanate,
diisocyanatotoluene or mixtures of diisocyanatotoluene
and hexamethylene diisocyanate. Other very useful
polyisocyanates are the polyisocyanates which have
urethane groups and are obtained, for example, by react-
ing Qxcess amounts of 2,4-diisocyanatotoluene with
simple, polyhydric alcohols having a molecular weight of
63 to 300, such as trimethylolpropane, trimethylolethane
or glycerol, if necessary removing the unconverted excess
diisocyanate by distillation. Another group of poly-
functional isocyanates comprises oYA~iA7inetrione alkyl
diisocyanates, which can be sub~ected to an addition
reaction with trimethylolpropane. Polyisocyanates having
a higher functionality can also be prepared by reacting
2 moles of triisocyanates with bifunctional compounds
having active hydrogen, such as dialcohols, diamines or
- 26 ~ 1 338690
aminoalcohols, such as ethanolamines or N-methyldiethano-
lamines.
Free isocyanate groups (in the components Bl and
B2) are blocked together or individually 80 that they are
protected at room temperature from reactions with water
or with the active hydrogen atoms of the base resin
(hydroxyl or amine hydrogen yLo~ps). Suitable blocking
agents are monofunctional compounds contAi~i~g acidic
hydrogen and only a sin~1e amine, amide, imide, lactam,
thio or hydroxyl group. In general, volatile compounds
cont~ n ~ ng active hydrogen and having low molecular
weights, preferably not more than 300, more preferably
not more than 200, are used. They are advantageously
reacted with the isocyanate groups at temperatures above
50C, preferably between 80 and 120C. The blocking a~ent
is used in amounts such that one equivalent of blocking
agent is present per one equivalent of NC0, and, if
required, conventional catalysts, such as basic catal-
ysts, for example tertiary amines, or small amounts of
tin salts, such as tin(II) octoate or dibutyltin dilaur-
ate, may be concomitantly used. Examples of ~uitable
blocking agents are secondary or tertiary,~ aliphatic or
cycloaliphatic alcohols, such as isopropanol, tert-
butanol, 2-ethylheYAnol, furfurol, cycloheYAnol or
hydroxyalkyl esters, dialkylaminoalcohols, such as
dimethylaminoethanol, oximes, such as formaldehyde oxime,
acetaldehyde oxime, methylethyl ketoxime, cycloheYA~one
oxime, trimethylcycloheY~one oxime, 2,2,6,6-tetramethyl-
piperid-4-one oxime, acetophenone oxime, benzophenone
oxime or diethylglyoxime, lactams, such as ~-caprolactam,
~-valerolactam, ~-butyrolactam, pyrrolid-2-one, hydrox-
amic acids and their esters, such as acethydroxamic acid
or benzhydroxamic acid, phenols, such as phenol, cresol,
tert-butylphenol or dimethylaminophenol, N-alkylamides,
such as methylacetamide, imidazoles, such as 2-methylimi-
dazole, imides, such as phthalimide or N-hydroxymale-
imide, and compounds which undergo enolisation, such as
malonic esters, acetlc esters or enamines having NH
function ~ OU~3.
~ ~ - 27 - 1 3 ~ 8 6 9 0
- However, ~-hydroxyglycol~ or -glycol ether~ and
glycolamides are also recommended. Oximes and lactones
are of particular intere~t a~ blocking agent~ since the
polyisocyanates blocked with these react at relatively
S low temperatures. It is al~o possible to u~e more than
one type of protective group for blocking, preferably
protective groups having different reactivities. Thus,
it is possible, for example, to use a mixture of two or
more polyisocyanates blocked with different pro~:~ctive
groups, or to use one polyisocyanate which is blocked
with two or more different protective y~O~p8.
- Particularly preferred blocking agents in the
process according to the invention are compounds of the
formula
(19) X - H,
in which X represents
/~
C112)n n ~ 3 - 1
~ ~R ~ R :~H~3t~CgHlg -
2 0 -O-N=C~ Rl -~, -CnH2n ~ 1
R2 R2 = Rl (n-2 -5)
- O- N ~ )
Preferred example~ are ~-caprolactam, methyl
ethyl ketoxime and butoxyethanol. To carry out the
blocking reaction, in general the isocyanate component is
initially taken and the reactant added. The reaction can
be carried out in the Ah~encs of a solvent or in the
presence of suitable (inert) solvents.
The blocked polyisocyanates are stirred into the
base resin either in succession in any order or as a mix-
ture. The mixing ratios of components Bl to B2 are 1 :
~ 28 - 1 338690
- 1 to 1 s 10, preferably 1 : 2 to 1 : 5. The optimum is
obtAi~e~ by means of experiments where the crosslinking
density and re~ults from the stone-chip impact test are
adapted to one another. A~ the level of component B1 in-
creases, the hAke~ film becomes more resistant to stone-
chips; as the level of component B2 increases, the film
becomes more resistant to solvents. If the coated
article is heated to a temperature which is sufficient to
eliminate the blocking of ~he isocyanate, the coating is
0 crossl ink,e~ or cured to give 8 protective, insoluble
film. The protective group is eliminated at hA~lng
temperatures of less than 210C, preferably lesff than
190C, in particular below 180C, and on the other hand
above 110C, preferably above 140C, particularly pre-
lS ferably above 150C, 80 that the isocyanate group liber-
ated can react with the base resin.
The crosslinking of the water-dilutable base
resin with blocked polyisocyanates can, if required, be
accelerated by adding 0.01 to 2% by weight, especially
O.S to 1% by weight, based on solid resin, of catalysts,
such as strongly basic tertiary amines and/or^ active
metal compounds. ~ particular effect, which is sometimes
synergistic, is achieved by the combination of the basic
medium of the deposited resins and the metal salts of
bismuth, lead, cobalt, iron, antimony and/or tin(II) and
tin(IV) compounds. Catalysts such as iron(III) acetyl-
acetonate, zinc acetylacetonate, dibutyltin dilaurate,
di-n-butyltin oxide, dibutyltin dioctylmaleate, tin octo-
ate, tin oleate, tetrabutyl titanate and/or cobalt 2-
ethylheY~no~te, are particularly preferred. Preferredcatalysts are those which are soluble only to a res-
tricted extent in the electrocoating bath and are elec-
trophoretically deposited in finely divided form with the
coating and can be uniformly distributed in the film
without giving rise to levelling problems during hAki~g.
If unsaturated double bonds are present in the resin, the
conventional metal drying agents, optionally in emulsion
form, can also be added to improve the curing properties.
By protonation with acids, the cationic binder
- 29 ~ 133 869 0
mixed with the crosslinking agent is rendered water-
dilutable in a manner known per se. Examples of acids
are formic acid, lactic acid, acetic acid, propionic
acid, citric acid, malonic acid, acrylic acid, phosphoric
acid and alkylphosphoric acid. Low molecular weight
monobasic organic carboxylic acids are preferred. It is
necessary to add at least sufficient acid to form a
stable emulsion of the cationic binder and the crosslink-
ing agent. An excess of acid, that i~ to say a degree of
neutralization of more than 100%, is advantageously
avoided. The NEQ value (milliequivalents of acid per 100
g of solid resin) is in general 20 to 80. A~ low an MEQ
value as possible is desirable, in order to obtain as
high a deposition equivalent as possible.
15The content of organic solvents in the coating
material, for example the electrocoating bath, should be
less than 10~, in particular less than 3%. The solvents
used are alcohols, glycol ethers and ketoalcohols as well
as Al~rhAtic and/or aromatic hydlorArhons having va~rious
chain lengths. In making the choice, it must be borne in
mind that the crosslinking agent is not water-soluble and
~ proportions of suitable solvents may facilitate and
stabilize the dispersing process. As the solvent content
increases, the throwing power deteriorates, the deposited
film thickness increases and overcoating may occur.
Water-insoluble solvents have a greater effect in this
respect than water-soluble ones. To improve levelling
and to reduce the film resistance, i8 iS also possible to
add a proportion of a water-insoluble, high-boiling sol-
vent, such as hexylene glycol, phenoxyethanol, phenQxy-
propanol, ethylh~YAnol, iso~e~Anol or 2,2,4-trimethyl-
pentane-1,3-diol monoisobutyrate.
To prepare coating materials or finishes from the
binder dispersions prepared according to the invention,
it is possible to incorporate by dispersion pigments,
fillers, anticrater agents, corrosion inhibitors and/or
conventional coating auxiliaries in a conventional manner
at a suitable point in the production process. To permit
the preparation of dispersions having a low solvent
- 30 - 1338690
content, the solvent is distilled off before or after the
preparation of the aqueous dispersion. If water-dilut-
able solvents having a lower boiling point than water,
such as, for example, ethanol, are used, the solvent can
be distilled off under mild conditions, in vacuo at
temperatures of 40 to 50C. If solvents which are not
water-dilutable and form an azeotropic mixture are used,
the solvent is distilled off with the circulating water,
via a reparator. ~~
The solids content of the coating material (for
example the electrocoating bath) according to the inven-
tion is advantageously 5 to 60% by weight after dilution
with water. When the finish is ad~usted to a higher
solids content of 25 to 50% by weight, preferably 30 to
45% by weight, water-dilutable hA~ing finishes, which can
be applied by immersion, spraying, roller-coating, etc.,
to the article to be coated, are obtA;~e~. If, on the
other hand, dilution is carried out to a solids content
of 5 to 30% by weight, preferably 10 to 20% by weight,
the finish is suitable for electrophoretic deposition.
The bath is stirred constantly in order to maintain a
uniform temperature at the cathode surface and toUprevent
the insoluble constituents of the dispersion, for example
the pigments, from settling out. The pH of the coating
material (for example the electrocoating bath) is in
general between 4.0 and 8.0, preferably between 5.5 and
7.5. If the pH i8 too low, it is likely that the acid
will attack the iron of tanks, pipelines and pumps. The
electrophoretic deposition is advantageously carried out
no earlier than 24 hours after preparation of the bath.
During this time, it is advantageous to carry out con-
tinuous stirring in order to obtain uniform distribution.
The anodes used are electrically conductive, non-corrod-
ing electrodes, for example of stainless steel or gra-
phite. The article to be coated at the cathode, and theanode, are immersed in the aqueous bath in the manner
familiar for electrophoretic deposition. All metallic-
ally conductive workpieces can be coated, such as copper,
aluminium, tin, zinc, iron and alloys of these metals.
- 31 - 1338690
- During the deposition, the bath is kept at temperature~
of, advantageously, about 15 to 3SC. The solids content,
deposition temperature and deposition time and the
voltage are chosen 80 that the de~ired film thickness is
S obtAineA after washing off with ultrafiltrate and/or
water and ba~ing at temperatures of 130 to 230C. Thus,
for example, the film thickness increases with increasing
coating time and deposition ~oltage. On application of
an electric cu~-ent with a voltage of, advantageously, SO
to 500 volt be~ en the metallically conductive workpiece
and a counter-electrode, the water-dilutable base resin
coagulates at the cathode. It thus transports the water-
insoluble crosslink~ng agent, pigments, catalysts, etc.
with it. During this procedure, the ratio of pigment to
synthetic resin binder in the deposited film may change
in favour of the pigment. At the same time, water and
the acid used for neutralization accumulate in the bath.
Replenishing must therefore be carried out using concen-
trated finishes which compensate for this change by means
of altered ratios of amounts. This correction can also
- be made by means of suitable apparatuses, for example
- - electrodia1ysis~methods or ultrafiltration. ~ _ - -
According to the invention, it is also possible
to prepare a concentrated paste binder which is to be
diluted with water and contains, for example, onium salts
and has, for example, a solids content of about 85 to 50%
by weight; this may be pigmented in a conventional manner
in a ball mill, three-roll mill or a pearl mill. For
thi~ purpose, conventional pigments, as de~cribed in, for
example, DIN 55,944, fillers, corrosion inhibitors and
coating auxiliaries, such as anticrater agentR, levelling
agents or antifoam~, can be added. Of course, the sub-
stances which are chosen are tho~e which do not undergo
troublesome reactions with water in an acidic to neutral
medium, do not entrain any water-soluble foreign ions
and, during ageing, are not precipitated in a form such
that they cannot be resuspenAe~ by stirring. The finish-
es are particularly suitable for the electrocoating of
metals and, after hA~i ng for, preferably, lS to 45
_ - 32`- 1338690
- minutes at 140 to 180C, give smooth prime coats having
improved flexibility and stone-chip resistance. The pig-
ment/binder ratio is dependent on the dispersibility and
viscosity of the binder and is in general between 0.1 :
1 and 1.5 : 1.
Water-dilutable base resin Al
Intermediate: In a reaction flask equipped with
a stirrer, a dropping funnel and a reflux condenser, S82
g of Yylene, 1,504 g of an epoxy resin b~ ed on bisphenol
A and having an epoxide equ~valent weight of 188 and 824
g of n-octylphenol were heated to 100C under an inert
gas. After the addition of 0.8 g of a 50% strength
aqueous solution of tetrabutylammonium chloride, the mix-
ture was heated to 150C and kept at this temperature
until the epoxide equivalent weight was 640. Thereafter,
the mixture was cooled to 50C and a mixture of 720 g of
xylene and 720 g of ethylene~i~minQ was added, the tem-
perature of the exothermic reaction increasing to about
105C. This temperature was maintA;~e~ for 3 hours, a
vacuum was applied and the excess ethylene~i~mine was
distilled--off. The amine residues were distilled off
- with steam until the distillate passing over had an~amine
number of less than O.3.
Solids content: 93.5% by weight (after heating
for 30 minutes at 180C)
Am~ne numbers 177 (mg of KOH per g of solid
resin)
1,000 g of the intermediate were heated with 580
g of methyl isobutyl ketone until water was separated off
with the ~olvent. After 26 g of water had been separated
off, the mixture was cooled to 40-45C, and a solution of
244 g of heY~ne diisocyanate in 488 g of dry methyl i80-
butyl ketone was slowly added dropwise in the course of
two hours. Thereafter, the temperature was increased to
80C and this temperature was maintAi~e~ until the NCO
number was O. The solvent was distilled off in vacuo and
the residue was diluted with 150 g of ethoAy~o~anol.
Solids content: 84.7% by weight (after heating
for 30 minutes at 150C)
---- - 33 - 1338690
Amine numbers 68
Viscositys 590 mPa.s (after dilution to 50%
by weight with ethoxypropanol at
25C).
S Cro881i nki ~g agent Bl
Polyesters In a three-necked fla~k e~uipped with
a stirrer, a thermometer and a Vigreux column, 2,044 g of
adipic acid and 1,890 g of butane-1,4-diol were carefully
melted. Thereafter, the m'xture was heated to 230C with
elimination of water of reaction, but the top temperature
of the column was kept at 98 to 100C. The reaction was
terminated after an acid number of less than 2 was
obtained.
Polyurethane resin: In a three-necked flask
equipped with a stirrer and a thermometer, and under a
dry stream of inert gas, 980 g of polyester, 238 g of N-
methylethanolamine and 238 g of N-methylpyrrolidone were
mixed while heating at 35C. Thereafter, a mixture of
1,100 g of isophorone diisocyanate and 476 g of N-methyl-
pyrrolidone was allowed to run in over 1 hour, the react-
ion temperature increasing to 80C. At an NCO number of--
- 4.2, the mixture was cooled to 50C~and 206 g of methyl
ethyl ketoxime were added. As a result of the exothermic
reaction, the temperature increased. The mixture was
kept at 80C until the NCO number was less than 0.1
Solids contents 81.8% by weight (after heating
for 30 minutes at 150C in a
through-circulation oven)
Amine number: 42.8 (mg of ROH per g of solid
resin)
Viscosity: 1.9 Pa.s (after dilution to 609~
by weight with N-methylpyrrol-
idone).
Cros~linking agent B2
875 g of Desmodur L, a reaction product of 1 mole
of trimethylolpropane with 3 moles of toluylene diisocy-
anate, which had been dissolved in ethyl acetate to give
a 75% strength solution, were heated to 90~C in the
absence of moi~ture and while passing dry inert gas over
~ 34 - 13~8690
the solution and stirring thoroughly at 90C. 342 g of
~-caprolactam were added ~lowly in the course of 3 hours
80 that a reaction temperature of 100C was not exceeded.
This temperature was maint~ine~ until the NCO number had
fallen to below 0.1%. The solvent was substantially dis-
tilled off in vacuo, and the residue was then diluted to
80% by weight with butoxyethanol.
Comparative Experiment
208 g of titanium dioxide and 2 g o-' carbon black
were ground in 248 g of water-dilutable synthetic resin
binder Al with the addition of 162 g of ethoxypropanol,
at about 50C for 30 minutes in a dissolver (3,000 revolu-
tion~ per minute). Thereafter, 49.6 g of water-dilutable
Al, 210 g of crosslinking agent B2, 5 g of dibutyltin
dilaurate and 6.4 g of formic acid were admixed and the
mixture was then diluted slowly with 2,609 g of deminer-
alised water.
Example
As for the comparative experiment, using the
following amountss
248 g of water-dilutable base resin Al
162 g of ethoxypropanol
208 g of titanium dioxide
2 g of carbon black
49.6 g of water-dilutable base resin Al
157.5 g of crossli~ g agent B2
51.3 g of crossli nki ng agent Bl
5 g of dibutyltin dilaurate
6.1 g of formic acid
2,610 g of demineralised water.
Using the above baths, electrophoretic deposition
was carried out on phosphatized steel sheet under the
following conditions and with the following results:
~ ~ 35 ~ 1 3 ~ 8 6 9 0
~ Comparative
Baths Example Example
5.6 5.7
pH
Bath conductivity (~Scm~l) 584 600
Solids content 17% by wt. 17% by wt
NEQ value 23.5 19
Voltage/film th~c~neRs 2! 35 260 V 2' 30 21W
18 ~m 18 ~m
De?osition equivalent (C/g) 22 1;
Coating film resistance
(n x 105) 4300 3150
Pendulum hardness 138 ~ 130~
Impact test 20 inch pound 35 inch
pound
Crosshatch test 1-2 (n-8-) 0 (8-)
Solvent resistance 8. 8.
Salt spray test 720 h/
Bo 132 U = 0.5 mm U = 0.5 _
Stone-chip impact test: Chip area:
Mono-stone-chip: + 20C 6 mm2 3 mm2
--20C 9 mm2 3 mm2
~ Multi-~tone-chip - n.s. 8.
8. = satisfactory
n.s. = not sstisfactory