Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
-1- 13393 1~
5H-IMIDAZOPYRROLOPYRIDINE, QUINO1INE, THIENO- AND
FUROPYRIDINE, DIHYDROTHIENO- AND FUROPYRIDINE 2-5-DIONES
This application is a divisional application of
application Serial No. 460,029 filed on July 31st, 1984.
The invention of the parent application relates to
novel herbicidally effective, dihydroimidazopyrrolopyridines
and derivatives thereof which are useful for the control of
undesirable monocotyledonous and dicotyledonous plant
species. The invention of the parent application also relates
to a process for the preparation of said herbicidally
effective compounds.
More particularly, the invention of the parent
application relates to novel,herbicidally effective,
dihydroimidazopyrrolopyridines, quinolines, thieno- and
furo[2,3-b]pyridines, dihydrothieno- and furo[2,3-b]pyridines,
thieno- and furo[3,2-b]pyridines and dihydrothieno- and
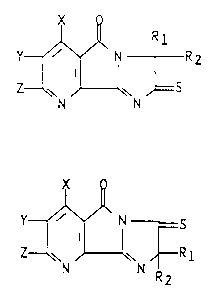
furo[3,2-b]pyridines depicted by formula (I) below:
1339~1~
R l
Y~' ~ R 2
Z w
N N
wherein Rl and R2 each represent Cl-C3 alkyl or cyclo-
propyl, with the proviso that the sum of
the number of carbon atoms in Rl and R2 is
2 to 5; and when Rl and R2 are taken together
with the carbon to which they are attached,
they may form a C3-C6 cycloalkyl ring
optionally substituted with methyl;
W is oxygen or sulfur;
A is hydrogen, hydroxyl, C3-C6 alkenyloxy, C3-C6 alkynyl-
oxy, Cl-C6 alkylthio, NR13R14 or Cl-C6
alkoxy optionally substituted with phenyl,
halophenyl, Cl-C3 alkylphenyl, Cl-C3 alkoxy-
phenyl or di-Cl-C3 alkylaminophenyl;
R13 is hydrogen, Cl-C4 alkyl optionally substituted
with phenyl, halophenyl, Cl-C3 alkylphenyl
or Cl-C3 alkoxyphenyl;
R14 is hydrogen or Cl-C4 alkyl;
X is hydrogen, halogen or methyl;
133~31~
Y and Z are each hydrogen, halogen, Cl-C6 alkyl, Cl-C4
hydroxyalkyl, Cl-C6 alkoxy, Cl-C4 alkyl-
thio, phenoxy, Cl-C4 haloalkyl, OCF2CHF2,
OCF3, OCHF2, nitro, cyano, NR4Rs, C3-Cg
straight or branched alkenyloxy optionally
substituted with one to three halogens,
C3-Cg straight or branched alkynyloxy option-
ally substituted with one to three halogens,
or phenyl optionally substituted with one
Cl-C4 alkyl, Cl-C4 alkoxy or halogen;
R4 is hydrogen or Cl-C4 alkyl;
Rs is Cl-C4 alkyl;
And, when taken together, Y and Z may form a ring in
which YZ is represented by
(1) the structure: -(CH2)n-, where n is an
integer of 2, 3 or 4, provided that X is
hydrogen; or
(2) by the structure: -C~=IC-lC-lC-
where, L, M, R7 and R8 each represent hydro-
gen, halogen, Cl-C4 alkyl, Cl-C4 alkoxy,
Cl-C4 alkylthio, Cl-C4 alkylsulfonyl, Cl-C4
haloalkyl, NO2, CN, phenyl, phenoxy, amino,
OCF3, OCHF2, OCF2CHF2, Cl-C4 alkylamino,
dialkyl(Cl-C4)amino, chlorophenyl, methyl-
phenyl, C3-Cg straight or branched alkenyl-
oxy optionally su.bstituted with one to
three halogens, c3-cg straight or branched
alkynyloxy optionally substituted with one
3~ to three halogens, or phenoxy substituted
with one Cl, CF3, NO2 or CH3 group, with
the proviso that only one of L, M, R7 or R8
may represent a substituent other than
hydrogen, halogen, Cl-C4 alkyl or Cl-C4
alkoxy; or
133931S
--4--
(3) by the structures:
-C=IC-B-, -B-C=IC- , -CH-ICH-B-, or -B-CH-CH-;
RloRg ~gRlo R12~11 RllR12
where B is oxygen or sulfur; Rg and Rlo
each represent hydrogen, halogen, phenyl,
or Cl-C4 alkyl; Rll and R12 each represent
hydrogen, Cl-C4 alkyl or phenyl;
and when Rl and R2 are not the same, the cis- or trans-
isomers or mixtures thereof or the optical isomers
(cis- or trans- or mixtures thereof).
As used in the present specification and
claims, the term "halogen" means F, Cl, Br or I, unless
otherwise specified.
It should, of course, be understood that when
Rl and R2 are not the same, the formula (I) compounds
can exist as both the cis- and trans-isomers. These
can be illustrated as follows:
~N~--'1ll R2
Z W
N N
A H
(Ia) cis, and
X
Y ~ N - I
~I R 2
Z~\ J~ W
N - N
A H
(Ib) tran 5 .
1339315
--5--
Especially preferred compounds of the present
invention are more precisely illustrated by formulas
II, III, IV, V, VI, VII and VIII shown below.
Preferred dihydroimidazopyrrolopyridine com-
pounds of this invention are depicted by formula (II),
hereinafter illustrated:
R I
Y~ N R2
Z J ~ W
N ' N
( I I )
wherein A, Rl, R2, W and X are as defined in reference
to formula I above; Y and Z each, independently, rep-
resent hydrogen, halogen, Cl-C6 alkyl, Cl-C6 alkoxy,
CN, N02, OCF3, OCHF2, OCF2CHF2, phenoxy, Cl-C4 halo-
alkyl, Cl-C4 alkylthio, Cl-C4 hydroxyalkyl, NR4Rs,
C3-Cg straight or branched alkenyloxy optionally sub-
stituted with one to three halogens, C3-Cg straight or
branched alkynyloxy optionally substituted with one to
three halogens, or phenyl optionally substituted with
one Cl-C4 alkyl, Cl-C4 alkoxy or halogen; R4 is hydrogen
or Cl-C4 alkyl; Rs is Cl-C4 alkyl; and when taken toge-
ther Y and Z may form a ring in which YZ are represented
by the structure: -(CH2)n-, where n is an integer of 2,
3 or 4; and when Rl and R2 are not the same, the cis-
or trans-isomers or mixtures thereof or the optical
isomers (cis- or trans- or mixtures thereof).
1~39~1~
--6--
Preferred dihydroimidazopyrroloquinolines are
illustrated by formula (III):
R ~ J : w
N N
A H
R8
(III)
wherein A, Rl, R2, W and X, are as defined above in
reference to formula I, and L, M, R7 and R8 represent
lS hydrogen, halogen, Cl-C4 alkyl, Cl-C4 alkoxy, Cl-C4
alkylthio, Cl-C4 alkylsulfonyl, Cl-C4 haloalkyl, N02,
CN, phenyl, phenoxy, amino, CF3, OCHF2, OCF2CHF2, Cl-C4
alkylamino, dialkyl(Cl-C4)amino, chlorophenyl, methyl-
phenyl, C3-Cg straight or branched alkenyloxy option-
20 ally substituted with one to three halogens, C3-Cg
straight or branched alkynyloxy optionally substituted
with one to three halogens, or phenoxy substituted with
one Cl, CF3, NO2 or CH3 group, with the proviso that
only one of L, M, R7 or Rg, may represent a substituent
25 other than hydrogen, halogen, Cl-C4 alkyl or Cl-C4
alkoxy; and when Rl and R2 are not the same, the cis-
or trans-isomers or mixtures thereof or the optical
isomers (cis- or trans- or mixtures thereof).
133931S
--7--
Preferred dihydroimidazopyrrolothieno- and
furo[3,2-b]pyridines and dihydroimidazopyrrolodihydro-
thieno- and furo[3,2-b]pyridines are, respectively,
5 illustrated by formulas (V) and (VI), shown below:
,1~ R 1
N R2
R 10~ /)~\ W
N N
A H
(V) and,
R
Rll ~ ~ W
A H
( V I )
wherein A, Rl, R2, W and B, are as defined above in
reference to formula I; Rg and Rlo each represent
hydrogen, halogen, Cl-C4 alkyl or phenyl; and Rll and
R12 each represent hydrogen, Cl-C4 alkyl or phenyl; and
25 when Rl and R2 are not the same, the cis- or trans-isomers
or mixtures thereof or the optical isomers (cis- or
trans- or mixtures thereof).
The preferred dihydroimidazopyrrolothieno-
and furo[2,3-b]pyridines and the dihydroimidazopyrrolo-
30 dihydrothieno- and furo[2,3-b]pyridines are, respecti-
vely, depicted by formulas (VII) and (VIII) illustrated
as follows:
133931~
--8--
~ Rl
R10.~ ~ R2
Rg W
B N - N
A H
(VII ) and,
R12~ ~ Rl R2
Rll W
B N N
A H
( V I I I )
wherein A, Rl, R2, W and B, are as defined above in
20 reference to formula I, Rg and Rlo each represent
hydrogen! halogen, Cl-C4 alkyl or phenyl; Rll and R12
each represent hydrogen, Cl-C4 alkyl or phenyl; and
when Rl and R2 are not the same, the cis- or trans-
isomers or mixtures thereof or the optical isomers
25 (cis- or trans- or mixtures thereof).
It is obvious that in the case of formula
(VI) and (VIII) that when either Rll or R12 is other
than hydrogen, additional optical and cis- and trans-
isomers are possible, and all of these are considered
to be within the scope of the invention.
133931~
g
Although dihydroimidazoisoindolediones are
described as herbicidal agents in United States Patent
4,041,045 issued August 9, 1977, said publication does
not render the compounds of this invention obvious
since it does not disclose or imply the disclosure of
herbicidal compounds containing a pyridine or substituted
pyridine ring. Moreover, it is surprising to find that
the formula I compounds of this invention are frequently
found to be highly selective herbicidal agents effective
for the preemergence and postemergence control of a
wide variety of undesirable broadleaf weeds and grasses
in the presence of graminaceous crops such as corn,
rice and wheat; leguminous crops such as soybeans and
in the presence of a variety of other crops including
cotton.
In accordance with the process of the present
invention, the novel formula (I) dihydroimidazopyr~olo-
pyridines, quinolines and the like can be prepared by
reaction of the appropriately substituted or unsubsti-
tuted formula (XII) 2-(2-imidazolidinyl)nicotinic acid;
2-(2-imidazolidinyl)quinoline-3-carboxylic acid; 2-(2-
imidazolidinyl)thieno- or furo[3,2-b]pyridine-6-carbo-
xylic acid; 2-(2-imidazolidinyl)dihydrothieno- or furo-
[3,2-b]pyridine-6-carboxylic acid; 2-(2-imidazolidinyl)-
thieno- or furo[2,3-b]pyridine-5-carboxylic acid or
2-(2-imidazolidinyl)dihydrothieno or furo[2,3-b]pyridine-
5-carboxylic acid, with at least one molar equivalent
and preferably a slight excess of acetic anhydride in
the presence of pyridine and acetonitrile.
~ 1~3931~
- 1 o -
In practice, it is generally desirable to warm the
reaction mixture to about 40 to 60~C until an essen-
tially clear solution is obtained. Cooling of the
thus-prepared solution to about ambient temperature or
below then yields the desired formula (I) dihydroimidazo-
pyrrolopyridine, quinoline, thieno or furo-pyridlne or
dihydrothieno or furopyridine, corresponding to the
isomeric mixture of the substituted or unsubstituted
acid employed as starting material for the reaction.
The reaction may be graphically illustrated
as follows:
l_ y ~ OOH
N N R2 + ~ +
HN - W
(XII)
R 1
(CH3C0)2o l I
Z~\, ~ ~=W
N , N
(I)
wherein X, Y, Z, Rl, R2 and W are as described for
3~ formula (I) above.
133931S
Similarly, in an alternate procedure it has
also been found that the substituted or unsubstituted
2-(2-imidazolidinyl)nicotinic acid; 2-(2-imidazoli-
dinyl)quinoline-3-carboxylic acid; 2-(2-imidazolidinyl)-
thieno- or furo[3,2-b]pyridine-6-carboxylic acid; 2-(2-
imidazolidinyl)dihydrothieno- or furo[3,2-b]pyridine-6-
carboxylic acid; 2-(2-imidazolidinyl)thieno- or furo-
[2,3-b]pyridine-5-carboxylic acid or 2-(2-imidazo-
lidinyl)dihydrothieno- or furo[2,3-b]pyridine-5-carbo-
xylic acid, which are themselves pre- and postemergence
herbicides, can be converted to the corresponding
formula (I) dihydroimidazopyrrolopyridine, quinoline,
thieno- or furo[3,2-b]pyridine, dihydrothieno- or furo-
[3,2-b]pyridine, thieno- or furo[2,3-b]pyridine or the
dihydrothieno- or furo[2,3-b]pyridine, by reaction
thereof with at least an equimolar amount of N,N'-
dicyclohexylcarbodiimide in the presence of a chlori-
nated hydrocarbon solvent such as methylene chloride,
dichloroethane, chloroform or the like. The reaction
may be conducted at ambient temperature and yields the
desired formula (I) compound on evaporation or separa-
tion of the solvent from the reaction mixture. The
reaction may be graphically illustrated as follows:
133931~
Y~COOH
N ~ N--R2 + ~N=C=~3
HN--W
(X~ I I )
~ R 1
Z W
N . N
~ H
( I )
wherein R1, R2, X, Y, Z and W are as described with
respect to formula (I) above. It should be understood
that if a cis-, trans- or mixture of cis- and trans-
imidazolidinone is employed as starting m~terial in the
above reactions, then the cis-, trans- or mixture of
cis- and trans- formula (I) compounds is obtained.
These formula (I) dihydroimidazopyrrolopyridines, quino-
lines, thieno- and furopyridines and dihydrothieno- and
furopyridines, are unexpectedly substantially more
selective than their 2-(2-imidazolidinyl)acid precursors.
~ 1339315
-13-
Many of the formula (I) dihydroimidazopyrrolo-
pyridine, quincline, thieno- and furopyridine and
dihydrothieno- and furopyridine, compounds of the present
invention may also be prepared by reduction of the corres-
ponding formula (XIV) 5H-imidazopyrrolopyridine, quinoline,
thieno- or furopyridine, or dihydrothieno- or furopyridine,
2,5-dione. This reduction can be achieved by reaction of
the formula (XIV) 2,5-dione with at least an equimolar
amount of a reducing agent such as sodium or lithium
borohydride in alcohol or aqueous alcohol or tetrahydrofuran
or aqueous tetrahydrofuran, at a temperature between about
-10 and +5~C. Acidification of the reaction mixture to a
pH of about 3, using a strong mineral acid such as
concentrated sulfuric acid, then yields the desired formula
(I) product. The reaction may be graphically illustrated as
follows:
b~ R l
y ~ / ~ N R2 + NaBH4 (XIV)
Z~\ J~, W
N N
' R 1
11 . (I)
Z--N - N
H H
The compounds of formula (XIV) and their isomers in which R
and R2 have exchanged positions with W form the subject of
this divisional application.
133931~
-14-
wherein X, Y, Z, W, Rl and R2 are as described with
respect to formula (I) above.
-This procedure usually forms a mixture of the
cis and trans-isomers of the formula (I) compounds.
The preparation of the formula XIV 5H-imidazo-
pyrrolopyridines and imidazopyrroloquinolines in which
W is oxygen is described in the European Patent Appli-
cation 0,041,623, published December 16, 1981.
In all the cases described above, the products
10 from the reaction are those in which A is hydrogen. In
order to prepare these compounds in which A is a group
other than hydrogen, advantage is taken of the ability
of the /C=N- function in the imidazopyraolopyridines,
quinolines, thieno- and furopyridines to add a variety
of nucleophiles such as alcohols, amines and thiols.
The reaction may be graphically illustrated as follows:
X R
Y ~ Rl
l I + AH
Z--~ / ~\\ W
N N
Z~\~
W
N A HN
-15- 133931~
wherein AH is, for example, CH30H, CH3SH, CH3NH2. This
reaction may be catalyzed by acids such as ~-toluene-
sulfonic acid or bases such as tertiary amines.
In practice it has been found that many sub-
stituted and unsubstituted aromatic and heteroaromatic
imidazolidinone and imidazolidinethione compounds
utilized as starting materials for the above described
reactions by formula (I) can be prepared by reduction
of the corresponding formula (XV) imidazolinone or
1 imidazolinethione with, for example, at least about an
equimolar amount of sodium cyanoborohydride in the
presence of a solvent such as Cl-C4 aliphatic alcohol,
aqueous alcoholic mixture or ether, followed by acidi-
fication to a pH between about 2.5 and 5 and preferably
between 3 and 4, with a strong mineral acid such as
hydrochloric acid, or an organic acid such as acetic or
the like. This reduction is generally conducted at a
temperature between 0 and 40~C and is particularly
effective for treatment of 2-(2-imidazolinyl)nicotinic
acids and esters, but preferably the methyl esters. It
is likewise effective for reduction of the imidazolinyl
function 2-(2-imidazolinyl)thieno and furo[3,2-b]-
pyridine-6-carboxylic acid esters and 2-(2-imidazolinyl)-
thieno and furo[2,3-b]pyridine-5-carboxylic acid esters.
The above described reduction may be graphic-
ally illustrated as follows:
-16- 13393 ~5
X X
Y~\ :OOR Y~\~ COOR
I R NaCNBH3 ll H R Cis
5z~\ ~ \~/ N R2 Z--~N ~/ N ~ ll
HN =W HN ',~/
(XV) +
X
Y~CO O R
Z~ ?~ H R 1 Tran 5
H N~',~l
wherein ~ may be hydrogen, but preferably a methyl
group and Rl, R2, W, X, 'f and Z, are 2s defined in
reference to sai.d formula I. When R is a methyl group
then this can advantageously be removed by reaction of
the methyl ester in a C1-C4 aliph21ic alcohol, prefer-
ably absolute methanol, and admixing therewith at least
one equivalent of strong base.
As shown above, the imidazolidinones and
25 imidazolidinethiones are obtained as a mixture of cis-
and trans-isomers when Rl and R2 are not the same.
These isomers are obtained in vari2ble amounts. The
mixtures are useful as such, but can frequently be
- separ2ted chromatographic211y to give the pure cis- and
trans-isomers, both of which are effective herbicidal
agents.
133~31~
-l7-
Since the above-described reduction is not a
universal method for the prep2ration of all substituted
and unsubstituted aromatic and heteroaromatic imidazo-
lidinones and imidazolidinethiones, a variety of
synthetic routes have been explored in order to provide
effective procedures for the manufacture of the imid2zo-
lidinones and imidazolidinethiones employed as starting
materials for the preparation of the formula (I)
compounds of this invention.
Accordingly, it has now been determined that
both the oxo and thioxo derivatives of 2-(2-imidazoll-
dinyl)nicotinates and 2-(2-imidazolidinyl)quinoline-3-
carboxylates can be synthesized by heating to refluxing
temperature, a mixture of a formula IX aminoamide or
aminothioamide with about an equimolar amount of an
appropriate formula X substituted or unsubstituted
lower alkyl, 2-formylpyridine-3-carboxylate or 2-formyl-
quinoline-3-carboxylate, in the presence of an inert
organic solvent such as benzene, toluene, or the like,
and a strong organic acid, such as p-toluenesulfonic
acid, under a blanket of nitrogen. The thus-formed
ester may then be converted to the corresponding formula
(XII), acid, used as starting materials in the syntnesis
of the formula (I) compounds of the present inventicn,
by dissolving or dispersing the imidazolidinone ester
or imidazolidinethione ester in a C--C4 aliphatic alcohol,
preferably absolute methanol and admixing therewith at
least one equivalent of strong base.
133931~
-18-
In practice, the base is generally dissolved in water
and the mixture heated to between about 20 and 50~C.
The mixture is then cooled and adjusted to pH 6.5 to
7.5, and preferably about pH 7, with a strong mineral
acid such as hydrochloric acid to yield the imidazo-
lidinone or imidazolidinethione acid wherein Rl, R2,
- R3, W, X, 'f and Z are as defined above. The reaction
is illustrated in Flow Diagram (I).
3o
-19- 133~31S
F L OW D I AGRAM
Y ~:O O R ~1 ~
I + NH2--C, ~--NH2
Z--\\N /--CHO R2
(X) ( IX)
X X pTSA
Y~--COOR + Y~COOR
z~\\ ~ H Rl N ~ N ~
H N ~ H N ~,~/
Tran s Ci 5
( L I I a ) ( L I I b )
1. CH30H/NaOH
2. Acid
Y~COOH
N ~ N--R 2
H N =W
3o
( X I I )
133~315
-20-
wherein R is Cl-C4 alkyl; and Rl, R2, X, Y, Z and W are
as described for formula (I) above.
- The reaction of an aldehyde of formula (X)
with an ~-aminoamide or thioamide of formula (IX) under
acid catalysis gives the corresponding Schiff's base as
the initial product. Whether the Schiff's base of
general formula
X
Y~ ~C O O R ~1 W
Z~, ~: H=N~ ~--N H 2
R2
is isolated as such or cyclizes under the reaction
conditions to the desired imidazolidinone depends on
some unknown subtle factors. Nevertheless, if the
Schiff's base is isolated it can, in a separate reaction,
be cyclized with trifluoroacetic acid to the imidazo-
lidinone.
Substituted Cl-C4 alkyl 2-formylnicotinates
which are useful in the preparation of 2-(2-imidazoli-
dinyl)nicotinatic acids and esters by the aldehyde
route described above and illustrated in Flow Diagram
I, for synthesis of formula LIIa and LIIb substituted
5-oxo-2-imidazolidinyl nicotinates and the formula
(XII) acids, can be prepared from substituted Cl-C12
alkyl 2-methylnicotinates. For convenience and clarity,
the following synthesis is described using substituted
methyl 2-methylnicotinates as illustrative of this
. class of reactions.
133931S
- -21-
In accordance with the process, equivalent
amounts of a substituted methyl 2-methylnicotinate,
represented by formula LIII and m-chloroperbenzoic acid
are admixed in the presence of a chlorinated hydrocarbon
such as methylene chloride, chloroform or the like. The
reaction mixture is heated to refluxing temperature,
then cooled to ambient temperature and excess peracid
destroyed by addition of excess l-hexene. Thereafter
the solution is washed with sodium bicarbonate solution,
dried and concentrated to give the corresponding sub-
stituted methyl methylnicotinate l-oxide of formula LIV.
The formula LIV l-oxide is then heated to about 70 to
95~C with an excess of acetic anhydride to yield the
formula LV substituted methyl 2-acetoxymethylnicotinate.
A cosolvent such as pyridine or pyridine/dimethoxyethane
may also be used in the reaction, but is not essential.
Oxidation of the formula LV acetoxymethylnicotinate
with hydrogen peroxide in acetic acid yields the methyl
2-acetoxymethylnicotina~e l-oxide represented by formula
LVI. This l-oxide is then readily converted to the
formula LVII methyl 2-diacetoxymethylnicotinate by
reaction with an excess of acetic anhydride at a tem-
perature between about 70 and 95~C, with or without a
cosolvent such as pyridine or pyridine/dimethoxyethane.
Treatment of the formula LVII methyl diacetoxymethyl-
nicotinate with an alkali metal alkoxide such as sodiummethoxide, sodium ethoxide, potassium butoxide, or the
like, in the presence of a Cl-C4 aliphatic alcohol then
yields the substituted alkyl formylnicotinate such as
methyl 2-formylnicotinate of formula LVIII.
- 13393l~
Alternatively, it has also been found that
the reaction of a substituted Cl-C12 alkyl 2-methyl-
nicotinate, depicted by formula LIII, with benzaldehyde
at an elevated temperature, yields the formula LIX
methyl 2-styrylnicotinate which, when ozonized gives
the formula LVIII substituted alkyl formylnicotinate.
Additionally, it has been found that treat-
ment of the formula LV substituted methyl 2-acetoxy-
methylnicotinate with an alkali metal alkoxide such as
sodium methoxide, in the presence of a lower aliphatic
alcohol at an elevated temperature, yields the cor-
responding substituted methyl 2-hydroxymethylnicotinate
of formula LX. The substituted methyl 2-hydroxymethyl-
nicotinate is then converted to the formula LVIII
substituted methyl formylnicotinate by oxidation with
selenium dioxide or lead tetraacetate.
The above reactions are graphically illus-
trated in Flow Diagram II.
1333315
-23-
FLOW DIAGRAM II
Y ~ OOCH3 ~ CHO
Z H3
(LIII)
Y ~ OOCH3
~ Z H = CHC6H5
Y ~ COOCH3 N
Z ~ ~ ~ CH3 (LIX)
+~
-O (LIV)
o3
Y ~ OOCH3
Acetic anhydride
z_ N {HO
tLVIII)
X ~ X
Y ~ COOCH3 Y ~ OOCH3
I NaOMe/MeO H ll
Z--N--CH20COCH3 Z-- ~ ~--CH20H
(LV) .(LX)
H2o2 SeO2 or
Pb(OAc)4
-24- 133~31S
FLOW DIAGRAM II (Continued)
Y ~ OOCH3 Y ~ OOCH3
Z H20COCH3 HO
~ (LVI) (LVIII)
Acetic anhydride
X
Y ~ OOCH3
H(OCOCH3)2
N
(LVII)
CH30H, CH30Na
X
y ~ OOCH3
N
(LVIII)
3o
133931S
-25-
The reduction of quinolinic acid diesters
with diisobutylaluminum hydride is also an effective
route to alkyl 3-formylnicotinates. The synthesis of
these quinolinic acid diesters is described in European
Patent Application 81103638.3, Publication Number
0 041 623.
The aldehyde route to the preparation of the
formula LIIa and LIIb substituted (5-oxo(and thioxo)-2-
imidazolidinyl)nicotinates is likewise effective for
the preparation of the substituted and unsubstituted
(5-oxo-2-imidazolidinyl)quinoline-3-carboxylates from
the substituted 2-formylquinoline-3-carboxylates.
The process for the preparation of these
substituted 2-formylquinoline-3-carboxylate interme-
diates involves the reaction of an appropriatelysubstituted aniline, depicted by formula LXI:
NH2
~R8
L~R7
M
( LXI )
wherein L, M, R7 and R8 are as defined in reference to
formula III quinolines; with approximately an equimolar
amount of a keto-ester depicted by formula LXII and
having the structure:
R '--C0--CH2COOR "
(LXII)
13393l~
-26-
wherein R' is CH3 or COOR" and R" is C1-C4 alkyl. This
reaction is optionally conducted in the presence of an
organic sulfonic acid such as p-toluenesulfonic acid
hydrate, camphorsulfonic acid, or aniline hydrochloride,
in the presence of an organic solvent such as cyclo-
hexane, toluene, benzene, xylene, monochlorobenzene,
orthodichlorobenzene and mixtures thereof, or the like
at a temperature from about 20 to 110~C. It is pre-
ferred to continuously remove the water which is formed
during the reaction by distillation either at atmospheric
or under reduced pressures of as low as 50 mm of Hg
while mzintaining the reaction temperature in a range
of 75 to 80~C. The reaction yields the B-anilino-~,B-
unsaturated ester of formula LXIII i.e.,
H~ { 00 R "
~ /C R
R7 H
(LXIII)wherein L, M, Q, R7, R'j and R" are as described above.
The thus-formed B-anilino-~,B-unsaturated
Z5 ester of formula LXIII is then reacted with an approxi-
mately equimolar amount of ar; immonium salt having the
structure:
e e
Cl { H N ( R "' )2 Cl ,
3o
(LXIV)
-27- ~ 1 339 31 5
wherein R~ is C1-C6 alkyl or
~ ~ e
Cl - CH N (CH2)n Cl
(LXIVa)
wherein n is 4 or 5, and referred to respectively as
formula LXIV or LXIIa. The reaction is conducted in
the presence of a hydrocarbon solvent such as toluene
or a chlorinated hydrocarbon solvent such as methylene
chloride, dichloroethane, orthodichlorobenzene, chloro-
benzene, or mixtures thereof, at a temperature between
about 40 and 110~C, for a period of time sufficient to
essentially complete the reaction and yield the formula
LXV alkyl ester of 2-methyl-3-quinolinecarboxylic acid,
if R' is CH3 in the formula LXVII B-anilino-~.B-
unsaturated ester or the quinoline-2,3-dicarboxylate if
R' is COOR" in the formula LXVIII B-anilino-~,B-unsatu-
rated ester.
Alternatively, the formula LXI substituted
20 aniline, wherein L, M, R7 and R8 are as described above,
can be reacted with about an equimolar amount of a
formula LXVI acetylene dicarboxylate having the structure: -
R"OOC { _C { OOR",
where R" is C1-C4 alkyl. This-reaction is generally
carried out in the presence of a solvent such as
dichloroethane or a C1-C4 alcohol such as methanol, at
a temperature between O and 100~C to yield a B-anilino-
3 ~,B-unsaturated ester as formula LXIII. The B-anilino-
~,B-unsaturated ester of formula LXIII is then reacted
with an immonium salt depicted by formula LXIV having
the structure:
1339315
-28 -
e e
Cl~H--N--( R " ' ) 2 ~ Cl
wherein R"' is C1-C6 alkyl or LXIVa having the structure:
e~ e
Cl{H N ( CH 2 ) n ~ Cl
where n is 4 or 5. While the anion in formulas LXIV or
LXlVa is shown as Cl , it should be recognized that
when POCl3 is used to prepare the Vilsmeier rea2ent,
the anion is P02Cl2. This reaction is generally con-
ducted in the presence of a solvent such as methylene
chloride, dichloroethane, monochlorobenzene, ortho-
dichlorobenzene, or toluene at a tempera~ure between 40and 110~C for a period of time sufficient to complete
the reaction and yield the quinoline-2,3-dicarboxylate
shown as formula LXVa having the structure:
OOR"
OOR"
' N
R7
(LXVo)
wherein L, M, Q, R7 and R" are as described above.
The immonium salt formula LXIV or LXIVa
utilized in the above cyclization reactions may, here-
after, be referred to as the Vilsmeier reagent. This
reagent may be generated from a (N,N-dialkyl or N-
alkyl,N-phenyl) formamide reaction with POC13, COCl2,
ClCO-COCl or SOCl2 in a hydrocarbon or chlorinated
hydrocarbon solvent.
133~3 1 5
-29-
Conversion of the 2-methyl-3-quinoline-
carboxylate shown as formula LXV in which R'=CH3 to the
corresponding aldehyde of formula LXVII can be achieved
in a manner similar to that described above for the
conversion of the substituted 2-methylnicotinate of
formula LIII to the corresponding 2-formylnicotinate of
LVIII.
Conversion of the quinoline-2,3-dicarboxylate,
shown as formulas LXV and LXVa, to the corresponding
aldehyde shown as formula LXVII having the structure:
~ OOR"
R7 ~ \ ~ HO
~ N
R8
(LXVII)
where L, M, R7, R8 and R" are as defined above, can be
achieved by reaction of the formula LXV quinoline-2,3-
dicarboxylate with diisobutylaluminum hydride. The
reaction is preferably conducted in the presence of a
non-protic solvent such as tetrahydrofuran under a
blanket of inert gas.
These reactions are graphically illustrated
in Flow Diagram III below.
.
133g31~
-30-
FLOW DIAGRAM III
~H2
~R8
. 5 L ~ R7 +
M
(LXI)
R' -CO - CH2COOR" or R"02C - C_C { OOR"
(LXII) (LXVI)
Solvent Solvent
-H20
~ HfiCOOR" ~ HfiCOOR"
20 R7 ~ N /C R' R7 ~ C - COOR"
H H
R8 R8
(LXIII) (LXIII)
25Cl-CH=N-(R"')2 Cl Cl-CH=N-(R"')2 Cl
(LXIV) (LXIV)
or or
Ci-CH=N~_~(CH2)n Cl ClCH=N~_~(CH2)n Cl
(LXIVa) (LXIVa)
-31- 133931~
FLOW DIAGRAM III (Continued)
(LXIVa) (LXIVa)
L ,L
~/~O O R " 1~--CO O R "
R7 ~ N R' R7 ~ OOR"
R8 R8
(LXV) (LXVa)
(iBU)2AlH (iBu)2AlH
when R '= COOR "
- ~ OO R "
R7 ~ N HO
R8
(LXVII)
3o
13~931~
-32-
The 2-t2-imidazolidinyl)thieno- and furo-
[3,2-b]pyridine-6-carb~xylates; 2-(2-imidazolidinyl)-
2,3-dihydrothieno- and furo[3,2-b]pyridine-6-carbo-
xylates; 2-(2-imidazolidinyl)thieno- and furo[2,3-b]-
pyridine-5-carboxylates and 2-(2-imidazolidinyl)-2,3-
dihydrothieno- and furo[2,3-b]pyridine-5-carboxylates,
useful as intermediates in the preparation of the
formula (I) compounds of this invention can be obtained,
by reduction of the corresponding (2-imidazolin-2-yl)-
thieno- and furo[2,3-b] and [3,2-b]pyridines with
sodium cyanoborohydride. These 2-(2-imidazolin-2-yl)-
thieno- and furo[2,3-b] and t3,2-b]pyridine inter-
mediates, necessary for the preparation of the formula
V, VI, VII and VIII, thieno- and furopyridines, of the
present invention are described in the copending
Canadlan patent application of Marinus
Los, David William Ladner and Barrington Cross, Serial
455,718~2 , flled ~June 1, 1984.
The 2-(2-imidazolin-2-yl)thieno and furo-
[2,3-b] and t3,2-b]pyridine intermediates, ~sed in the
synthesis of the 2-(2-imidazolidinyl)thieno- and fu-~-
[2,3-b] and [3,2-b]pyridines starting materials for the
compounds of the present invention are depicted by
formulas Va, VIa, VIIa and VIIIa, illustrated below.
~ ~ OOR Rll ~ ~ OOR
Rl o N ~Y N--R 2 Rl 2 N /~ N--R 2
H~--W HN~W
(Va ) ( VIa )
1~39315
-33 -
Rlo~ ~OOR R12 J~COOR
R9 B N~/ N--R2 11~ B N ~/ --R2
HN =W HN--W
(VIIa) (VIIIa)
wherein R is hydro~en or Cl-C4 alkyl and R1, R2, Rg,
1o Rlo, R11, R12, B and W are as described above in
reference to compounds of formula V, VI, VII and VIII.
While for convenience, the imidazolinone and
imidazolinethione intermediates referred to throughout
are illustrated by single structures, it should be
15 recognized that the imidazolinyl function in these
compounds may exist in either tautomeric for~, i.e:
H Rl Rl
_~ N--R 2 1~ N--R 2
N~--W HN--W
The formula Va, VIa, VIIa and VIIIa, inter-
mediates for the compounds of the present invention may
be prepared from the appropriately substituted thieno-
and furot2,3-b] and [3,2-b]pyridinedicarboxylic acids
25 and esters of formulas LXXI znd LXXIa illustrated below.
Since Rg and R10 represent substituents
selected from hydrogen, halogen, Cl-C4 alkyl and phenyl,
and R11 and R12 represent hydrogen, Cl-C4 alkyl and
- -phenyl; for the purposes of the following discussion,
3~ which relates to the preparation of the formula Va,
VIa, VIIa and VIIIa, 2-(2-imidazolin-2-yl)thieno and
furo[2,3-b] and [3,2-b]pyridines, compound structures
involved in the synthesis under discussion will be
illustrated with Rg and R10.
1339~1~
34
Rlo I ~CCC)2R~ ~ ~CO2;R~
Rg ~ B ~ N CO~R~ and Rlo N C02R
~ ~)
whereln Rg, Rlo and B are as prevlously descrlbed and R" ls
methyl or ethyl.
Methods sultable for preparing formula Va, VIa, VIIa
and VIIIa unsaturated compounds whereln - - ls a double bond
from the formula (LXXI) and (LXXIa) pyrldlnedlcarboxyllc acld
esters are lllustrated ln Flow Dlagram IV below.
Thus formula (LXXI) and (LXXIa) dlesters may be
hydrolyzed to the correspondlng thleno- and furo-2,3-pyrldlne-
dlcarboxylic aclds of formula (LXXII) and (LXXIIa) by reactlon
thereof wlth a strong base such as potasslum hydroxlde or
sodlum hydroxlde. Acld anhydrldes of formula (LXXIII) and
(LXXIIIa) may then be prepared by treatment of the formula
(LXXII) and (LXXIIa) pyrldinedlcarboxyllc aclds wlth, for ex-
ample, acetlc anhydrlde. Reactlon of formula (LXXIII) and
(LXXIIIa) anhydrldes wlth an approprlately substltuted amino-
carboxamlde or amlnothlocarboxamlde depicted by formula (IX)
ylelds carbamoyl nlcotlnlc aclds of formula (LXXIV) and
(LXXIVa). Treatment of the thus-formed formula (LXXIV) and
(LXXIVa) carbamoyl nlcotlnlc aclds wlth about 2 to 10 molar
equlvalents of aqueous or aqueous alcohollc sodlum or potas-
slum hydroxlde, preferably under a blanket of lnert gas such
as nltrogen, coollng and acldlfylng to pH 2 to 4 wlth a strong
mlneral acld such as hydrochlorlc acld or sulfurlc acld glves
herblcldally effectlve 6-(4,4-dlsubstltuted-5-oxo-(orthlono)-
2-lmldazolln-2-yl)thleno- and furo[2,3-_]pyrldlne-5-carboxyllc
aclds, and 5-(4,4-dlsubstltuted-5-oxo(or thlono)-2-lmldazolln-
2-yl)thleno- and furo[3,2-_]pyrldlne-6-carboxyllc aclds
encompassed by formulas (Va) and (VIIa).
61109-7307D
,~
.
~ 133~1s
Formula (Va) and (VIIa) 5-(2-imidazolin-2-
yl)thieno- and furopyridine esters, wherein R represents
a substituent other than hydrogen or a salt-forming
cation, and R1, R2, Rg, Rlo and B are as described
above can be prepared by reacting a novel thieno- or
furoimidazopyrrolopyridinedione, represented by formulas
- (LXXV) and (LXXVa), hereinbelow, in Flow Diagram (V),
with an appropriate alcohol and correspondin~ alkali
metal alkoxide at a temperature ranging between about
20 and about 50~C.
Formula (LXXV) and (LXXVa) thieno- and furo-
imidazopyrrolopyridinediones may conveniently be pre-
pared from formula (VIIa) and (Va) acids, where R is H
by treatment with one equivalent of dicyclohexylcarbo-
diimide in an inert solvent such as methylene chlorideas illustrated in Flow Diagram (V) below.
3o
-36- 1339~15
FLOW DIAGRAM (IV)
5 Rlo ~ ~ ~ OOR" ~ ~ OOR"
Rg B N OOR" Rlo OOR"
(LXXI) (LXXIa)
l. Aqueous ethanolic NaOH
. 2. HCl
Rlo ~ ~ OOH R9 ~ B ~ OOH
Rg OOH Rlo OOH
B N N
20 (LXXII) (LXXIIa)
Ac20
R9~ B ~ , Rlo~
(LXXIII) (LXXIIIa)
133931~
-37-
FLOW DIAGRAM ( IV) (Continued )
(LXXIII) (LXXIIIa)
Rll
NH2~--CW--NH2
R2
( I X )
Rl 0~ ~OOH ~1 ~ ~OOH ~1
9 B N ONH~W--NH2 Rl o N ONH~W--NH2
R2 R2
( LXX IV ) ( LXX IVa )
NaOH
R~ B ~/ N--R2 R~ I ~/ --R2
N~ W
H
1339315
-38-
FLOW DIAGRAh~ (V)
R~4~N ~ Rlo~ ~OOH R
H N N '~
DCC
R 1 ~ ~ N--=0 1~~N --O
R9 Rlo
B N N R2 N r~ R2
Rl R
( LXXV ) ( LXXVa )
R O-M +
Rl~ ~COOR3 Rl : R91~ B ~{c~R3 Rl
~1~ ~</r~ R 2 R 1 o ~ N ~/L< N--W
3o
-39- I 339 3I~
where Ml is an alkali metal, and X, Y, Z, Rl, R2 are as
above defined and R3 is C1-C4 alkyl.
Many formula (LXXI) thieno[2,3-b]pyridinedi-
carboxylic acids and tLXXIa) thieno[3,2-b]pyridinedi-
carboxylic acids may conveniently be prepared by reactingthe appropriately substituted 2 or 3-aminothiophene of
formula (LXXXIV) or (LXXXIVa) with a C1-C4 alkyl ester
of acetylenedicarboxylic acid of formula (IX) as
described by Bleckert et al. Chem. Ber. 1978, 106, 368.
The thus-formed ~-aminothieno-~,~-unsaturated ester of
formula (LXXXV) or (LXXXVa) is then reacted with an
immonium salt depicted by the formula Cl-CH=N-(R''')2
e ~-_ e
Cl wherein R''' is Cl-C6 alkyl or Cl-CH=N (CH2)n' Cl
where n' is 4 or 5, in the presence of a low boiling
chlorinated hydrocarbon solvent such as methylene
chloride or dichloroethane at a temperature between
about 40 and 90~C, for a period of time sufficient to
essentially complete the reaction and yield the formula
(LXXI) [2,3-b]thieno- or (LXXIz) [3,2-b]thieno-2,3-
pyridinedicarboxylic acid as the dialkyl ester asillustrated in Flow Diagr2m (VI) below.
The furo[3,2-b]pyridinedicarboxylic acids may
be prepared by reacting 3-amino-2-formylfur2n of formula
(LXXVI) prepared by the method of S. Gronowitz et al.,
Acta Chemica Scand B29 224(1975) with ethyl oxalacetate
to give the furopyridine compounds directly, as illus-
trated in Flow Diagram (VII) below while the furo[2,3-b]-
pyridine compounds where Rg and Rlo are H are obtained
by bromination of the reaction product (LXXVII) of
3o aceto2cet2mide with the diethyl ester of ethoxymethylene-
oxal2cetic acid followed by treatment with sodium boro-
hydride and para-toluene sulfonic acid in refluxing
xylene as illustrated in Flow Diagram (VIII) below.
133~31S
-40-
FLOW DIAGRAM (VI)
Rlo Rlo NH2
I I or ll I
Rg - S -NH2 Rg ~ /
(LXXXIV) (LXXXIVa)
R"02C-C-C-COOR"
(IX)
Rlo I I Rlo ~--C - COOR"
{ OOR" or R9 ~ S
HC--COOR "
(LXXXV) (LXXXVa)
e
Cl{H=N - ( R " ' ) 2 .Cle
or
Cl-- CH=N ( CH2 ) n ~ . Cl
Rlo ~ OOR" R9 ~ ~OOR "
3~ Rg /JL-COOR" Rlo OOR "
S N N
(LXXI) (LXXIa)
13393l~
-41 -
FLOW DIAGRAM (VI I )
~ R9~0~CHO
Rl o ' NH2
(LXXVI)
C2H502C--lCI--CH2--C~2C2H5
R91~~02C2H5
Rlo02C2H5
3o
-42- ' 133931~
FLOW DIAGRAM (VI I I )
8 8 CO2C2 H5
CH3 { -CH2 { -NH2 + C2HsO ~-C-CO-C02C2H5 C2HsOH/NaOAc
H
CH3 - C ~ 02C2H5 48PHBr/Br2
O CO2C2Hs
H
(LXXVII)
BrCH2{~C02C2H5 NaBH4
~ ~ ~ ~2C2H5
H
O,H
BrCH2--CH--~co2c2H5
l 11 (C2H5)3N
20~ ~ N ~ C~2C2H5
H
OH
25~ 02C2H5 p-Toluenesulfonic acid
02C2H5
~02C2H5
~ 2C2H5
O N
3o
1339315
Substituents represented by Rg and Rlo in
formula (Va), (VIIa), (LXXV) and (LXXVa) compounds of
the present invention may be prepared either by using
the appropriately substituted starting material for the
preparation of formula (LXXI) and (LXXIa) thieno- and
furopyridine-5,6-dicarboxylic acid esters or by electro-
philic substitution (halo~enation, nitr2tion, sulfon2-
tion, etc.) directly upon formula (LXXI) or (LXXI2)
diesters or Formula (Va) or (VIIa) final products,
wherein at least one of Y or Z is hydro~en. These
substituted formula (LXXI), (LXXIa), (Va) and (VIIa)
compounds then may be used as startin~ m2teri21s for
additional Rg and Rlo substitution by dlsplacement,
reduction, oxidation, etc. Representative substituted
(LXXI) and (LXXIa) compounds which m2y be prep2red by
these procedures are as illustrated below.
Rlo~ ~C 02R R91~ ~~2R
Rg B - N o2R Rlo 02R
(LXXI) (LXXIa)
_ Rg Rlo R
S H H CH3
S H Br CH3
- S CH3 H CH3
S H Cl CH3
S Cl Cl CH3
S H I CH3
S H No2 CH3
S Br Br CH3
13~931~
-44-
B Rg R10 R
S CH3 Cl CH3
S H CH3 CH3
S Cl H CH3
S CH3 CH3 CH3
S H CN CH3
S H OCH3 CH3
S H N(CH3)2 CH3
S H SCH3 CH3
S H OCr2H CH3
O H H C2H5
O H Br CH3
O H Cl CH3
o CH3 H CH3
~ CH3 H C2H5
O H CH3 CH3
O C2H5 H CH3
O H C2H5 CH3
o CH3 CH3 CH3
S -(CH2)3- CH3
S -tCH2)4- CH3
S -(CH)4- CH3
S C6H5 H CH3
O C6H5 H CH3
~CH3
S H CH3
S H OC6H5 CH3
O H OC6H5 CH3
~ CF3 H CH3
'~ 1339~1~
Additionally, novel herbicidal 2,3-dihydro-
thieno[2,3-b] and [3,2-b]pyridine compounds may be
obtained by starting the sequence in Flow Diagram (VI)
above with a dihydrothiophenimine hydrochloride. Novel
herbicidal 2,3-dihydro furo[2,3-b] and [3,2-b]pyridines
may be prepared by catalytic reduction of the formula
(Va) or (VIIa) (2-imidazolin-2-yl) product, or (LXXI)
- and (LXXIa) furo[2,3-b] and [3,2-b]pyridine-5,6-diesters
as for example with hydrogen and palladium on carbon,
provided that Rg and Rlo are substituents which are not
reduced by such a procedure. This then provides novel
2,3-dihydro herbicidal compounds illustrated below.
R9 ~ <N R2 Rl~ ~ \/N R2
B N N~W N N--W
H H
wherein Rg, Rlo, B, W, Rl, R2 and RB are as described
for (Va) and (VIIA).
The formula I dihydroimidazopyrrolopyridines
and derivatives of the present invention are highly
effective preemergence and postemergence herbicidal
agents, useful for the control of a wide variety of
undesirable monocotyledonous and dicotyledonous plant
species. Surprisingly, it has also been found that
these formula I compounds are very active against a
wide variety of weed species but well tolerated by a
- number of crops including: graminaceous crops such as
sunflower, corn, rice, turf, and wheat; leguminous
crops such as soybeans and other crops including cotton.
133931~
-46-
While herbicidal selectivity of the formula I compounds
of this invention may vary with compound structure from
crop to crop, the presence of the dihydroimidazopyrrolo-
pyridine function, which is unique to all of the formula
I compounds of this invention, appears to impart signi-
ficant herbicidal selectivity to said compounds. This
selectivity thus permits application of the active
compounds to newly planted fields or to maturing crops
for control of undesirable grasses and broadleaf weeds
0 in the presence of said crops.
It is also surprising to find that the com-
pounds of this invention, frequently exhibit plant
growth regulating activity when employed at non-herbicidal
rates of application.
In practice, the formula I dihydroimidazo-
pyrrolopyridines and derivatives thereof may be applied
to the foliage of undesirable monocotyledonous or
dicotyledonous plants or to soil containing seeds or
other propagating organs of said plants such as tubers,
rhizomes or stolons, at ranges generally between about
0.032 and 4.0 kg/ha, and preferably between about 0.063
and 2.0 kg/ha, although rates as high as 8.0 kg/ha may
be used if desired.
Effective plant growth regulating activity
such as dwarfing, antilodging, increased branching,
increased tillering and the like, is generally obtained
when the above-said formula I compounds are applied to
crops at rates below herbicidal rates. Obviously, this
rate will vary from compound to compound.
The formula I compounds of the present inven-
tion may be applied to the foliage of plants or to soil
containing seeds or other propagating organs thereof,
in the form of a liquid spray, as a ULV concentrate or
as a solid formulat-on.
1339~1~
Formula I compounds may also be prepared as
wettable powders, flowable concentrates, emulsifiable
concentrates, granular formulations or the like.
A typical emulsifiable concentrate can be
- 5 prepared by dissolving about 5 to 25% by weight of the
active ingredient in about 65 to 90p by weight of
N-methylpyrrolidone, isophorone, butyl cellosolve,
methylacetate or the like and dispersing therein about
5 to 1 Od by weight of a nonionic surfactant such as an
alkylphenoxy polyethoxy alcohol. This concentrate is
dispersed in water for application as a liquid spray or
it may be applied directly as an ultra low volume con-
centrate in the form of discrete droplets havin& a mass
median diameter between about 17 and 150 microns
particle size.
Wettable powders can be prepared by grindin&
together about 20 to 45p by weight of a finely divided
carrier such as kaolin, bentonite, diatomaceous earth,
attapulgite, or the like, 45 to 80p by weight of the
active compound, 2 to 5p by weight of a dispersing
agent such as sodium lignosulfonate, and 2 to 5% by
weight of a nonionic surfactant, such~ as octylphenoxy
polyethoxy ethanol, nonylphenoxy polyethoxy ethanol or
the like.
A typical flowable liquid can be prepared by
admixing about 40% by weight of the active 1ngredient
with about 2% by weight of a gelling agent such as
bentonite, 3~ by weight of a dispersing agent such as
sodium lignosulfonate, 1% by weight of polyethylene
3~ glycol and 54~ by weight of water.
-' 1339315
-48-
When the compounds of the invention are to be
used as herbicides where soil treatments are involved,
the compounds may be prepared and applied as granular
products. Preparation of the granular product can be
achieved by dissolving the active compound in a solvent
such as methylene chloride, N-methylpyrrolidone or the
like and spraying the thus-prepared solution on a
granular carrier such as corncob grits, sand, attapul-
gite, kaolin or the like.
The granular product thus-prepared generally
comprises about 3 to 20% by weight of the active ingre-
dient and about 97 to 80% by weight of the granulzr
carrier.
In order to facilitate a further under-
standing of the invention, the following examples are
presented primarily for the purpose of illustrating
certain more specific details thereof. The invention
is not to be deemed limited thereby except as defined
in the claims. Unless otherwise noted, all parts are
by weight.
49 133931~
EXAMPLE 1
Preparation of 7-ethyl-1,9b~(and ~)-dihydro-3~-isopro-
pyl-3-methyl-5~-imidazo~1',2':1,2]pyrrolo~3,4-b~pyridine-
2(3H)~5-dione
H5 C2~\~~ O H
~, N---CH(CH3 )2 lN
HN--O
HsC2 ~ N CH(CH3)2
(CH3C0)2o ¦ ~1
O
N ' N
A suspension of 1 g of cis- and trans-5-ethyl-
2-(4-isopropyl-4-methyl-5-oxo-2-imidazolidinyl)-nicotinic
acid and 0.4 mL pyridine and 0.5 mL acetic anhydride in
10 mL acetonitrile is warmed to about 50~C until a
clear solution is obtained. Upon cooling the solution
to room temperature, a solid crystallizes. The solid
is collected by filtration and recrystallized from
ethyl acetate to give analytically pure 7-ethyl-1,9b
~(and ~)-dihydro-3~-isopropyl-3-methyl-5H-imidazo[1',-
2':1,2]pyrrolo[3,4-b]pyridine-2(3H),5-dione, mp 170-177~C.
3o
-50- ~' 13393~5
EXAMPLE 2
Preparation of 7-dimethyl-1,9bB-dihydro-3~-isopropyl-
3,5H-imidazo[1',2':1,2]pyrrolo[3,4-~]pyridine-2(3H),-
5-dione
CH3 ~ 00H
~JIIII H NH CH3 + ~N=C N~
CH(CH3)2
HN ----~
CH3 ~ 1I CH(CH3)2
~cO
N . N
~ H
A mixture containing 1.08 g of cis-2-(4-iso-
propyl-4-methyl-5-oxo-2-imidazolidinyl)-5-methyl-nicotinic
acid and 0.89 g of N,N'-dicyclohexylcarbodiimide in
25 mL methylene chloride is stirred at room temperature
for 18 hours. The mixture is filtered and concentrated
in vacuo. The crystalline residue is recrystallized
from methylene chloride to give analytically pure 7-
dimethyl-1,9bB-dihydro-3~--sopropyl-3,5H-imidazo[1',2':
1,2]pyrrolo[3,4-b]pyridine-2(3H),5-dione, mp 1~1-193~C.
3o
133931~
EXAMPLE 3
Preparation of 1,9b~(and ~)-dihydro-3~-isopropyl-3-
methyl-5H-imidazo[1',2':1,2]pyrrolo[3,4-b]pyridine-2-
(3H),5-dione
CH(CH3)2 + NaBH4
~=0
N N
/~ N CH ( CH 3 ) 2
1 5 ,L O
N ' N
~ H
To a suspension of 1.7 g sodium borohydride
in 120 mL absolute ethanol cooled to 0~C is added drop-
wise a solution of 10.8 g 3-isopropyl-3-methyl-5H-i.~idazo-
[1',2':1,2]pyrrolo[3,4-b]pyridine-2(3H),5-dione in
120 mL dry tetrahydrofur2n, maintaining the temperature
between 0 and 5~C. The mixture is stirred at room
temperature for two hours and added to ice water. The
aqueous mixture is acidified to pH 3 with concentrated
sulfuric acid and extracted with methylene chloride.
Extracts are dried and concentrated in vacuo to give a
solid. The solid is washed with anhydrous ether and
air dried to give 1,9b~(and ~)-dihydro-3~-isopropyl-3-
3~ methyl-5H-imidazo[1',2':1,2]pyrrolo[3,4-b]pyridine-2(3H),-
5-dione, mp 170~C (dec).
133931~
EXAMPLE 4
Preparation of formula (I) dihydroimidazopyrrolo?yridines
or derivatives thereof
Following one of the procedures described in
Examples 1, 2 or 3, the compounds of formula (I),
reported in Table I below, were prepared.
3o
o. o. o. u~ o.
~) O u a~ o C~,
6~ , ~1,,,,
3 0 0 0 0 0 0
C~ O ~ O ~D C~
~ ~ o o o
--ZI
/111 I G
X~ ~Z U ~ C~ ~ ~ ~ ~
r~
U~
cn
C 3 1 0 0 ~ ~ ~ ~
-
cn
,_, U
r ._
r
~L ZI
x~\ z
u ~f
G
U
~a
._
O
L~
1339315
-54-
oooooooooooo
o C~ ~ ~ ~ C
~ 1 ~ , , , , , , , , , , ,
EiIoooooooooooo
U~ ~ o ~ ~ o o ~ ~ ~ ~ '~
~ o o C~ ~ ~ o
C~1
C ~ ,,~,
u~l
._, I ~ o ~ ~ ~ ~ ~ ~
31 0 0 0 o o o o u~ o o
0~
. ~ ~ T C ~n~ T
.C C~l ~
C C~ T
X ¦ T T T T T 5~ T C T T' ~ ~
~ T
~ v ~, t~ e~ ~ ~ ~ ~ ~ ~ c~ ~
CY: I
~1
13~31~
o o o o o o U~ o o o o
.
oooooo~o o o o
c~J o o c~ c~ ~r ~ ~-- ~ o
o ~ ~ ~ ~) o a~ ~ ~ ~ u~
cn
C ~ ~D
J
Cl~ I
- 31 o U~ o o o o o c~ o o o
C~ I ~ ~ ~ T ,C T = ~ ~ ~3 Z
-- ~ -- C~ ~ C T
T
X ¦ ~ T T -- ~ T T T
T ~ ~ r T
C~l ¦ ~ T ~
~l
~_ ~ ~ ~
1339315
-56-
EXAMPLE 4-A
Preparation of3-isopropyl-3-methyl-5H-imidazo[1',2':1,2]-
pyrrolo[3,4-b]pyridine-2(3~),5-dione
~COOH
N/~ CH(CH3)2
H~ --O
AcOH
.AC20
~ ~ CH(CH3)2
N N
A stirred mixture of 123.2 g acid in 300 mL
acetic acid and 89 mL acetic anhydride is heated at
reflux for four hours. The mixture is concentrated
in vacuo and the residue dissolved in toluene and again
concentrated. The residue is slurried in hexane, fil-
tered and washed several times with hexane to give a
90% of product, mp 105-112~C. NMR and Gc analysis
indicztes this material to consist of 80-87% of the
desired 2,5-dione and the balance of the material being
the corresponding 3,5-dione.
~57~ ~ 1 3~9 3
EXAMPLE 4-B
Preparation of 1,9b-dihydro-3~-isopropyl-3-methyl-9b~-
propoxy-5H-imidazo[1',2':1,2]pyrrolo[3,4-b]pyridine-2-
(3H),5-dione
CH3
N N
~ ~ ~
N - N
n-C3H70 H
The solution containing 54 g dione in 200 mL
n-propanol is heated at reflux for one hour. The solu-
tion is slowly cooled to 0~C and after one hour, the
white crystals removed by filtration. The solids are
washed with hexane and dried to give 41.6 of the 9b~-
25 propoxy derivative mp 129-132~C. This material can be
recrystallized from ether-hexane to give analytically
pure material mp 135-137.5~C. This product can also be
obtained by running the reaction at room temperature.
Using essentially the same conditions but
30 utilizing the appropriate nucliophile and the appro-
priate 2,5-dione, the following dihydro derivatives are
prepared.
1339~1~
-58-
CCCCC
~)
o
o U~
N ¦ T T S T T
~ ¦ T -r T X ~ T
X~Z ~ C 111
1, . ~ o o o ~
31 0 0 0 0 0 0
~C~ ~ C~
.. ~C~
1339~15
-59-
~ ~ CC C C
s~~_1 Ll LLl
O
N
C~ ~
X IT' ~C~ ~ ~ -- T' --
I
) v v ~ o o
I
3 1 0 0 0 0 0 U~ O C~
V V ~ V V V C~
V ~
V ~
133~15
EXAMPLE 5
Preparatlon of methyl 2-(4-lsopropyl-4-methyl-5-oxo-2-
lmldazolln-yl)nlcotlnate
Thls method lnvolves the formatlon of trycycllc com-
pounds, wlthout lsolatlon, dlrectly formlng the nlcotlnlc acld
esters:
o
~ N-C-~30NH2 DBU > ~ R2
N ~ CH~CH3~ N N
COOCH3
CH3 MdDH
HN~ ==O
~H3
N CH~CH
~ N
COO~I3
A mlxture of 25 g amlde and 1 mL 1,5-dlazablcyclo-
[5.4.0]undec-5-ene(DBU) ln 500 mL xylene ls heated under re-
flux for one hour under a Dean-Stark water separator. The
mlxture ls cooled somewhat, the water separator removed, 100
mL anhydrous methanol added and the mixture heated under re-
flux for one hour. The solvents are then removed ln vacuo and
the product lsolated by chromatography as to glve 13.65 g pro-
duct mp 120-122~C. Other esters as descrlbed ln Example 28 in
the same manner uslng the approprlately substltuted amlde
startlng materlal. Thls procedure ls also descrlbed ln the
Canadlan Patent 1,187,498 whlch lssued on May 21, 1987 on
Marlnus Los.
-~r
61 1339~1S
EXAMPLE 6
Preparatlon of methyl 2-(4-isopropyl-4-methyl-5-oxo-2-
lmidazolin-2-yl)nicotlnate
CCKX~H3lH3 ~ CCXX~CHH
N CONH - I-CONH2 ~N ~ - CH(CH
CH(CH3~ HN - ~
Method A
A mlxture of 13.65 g of the nlcotlnate and 9.69 g
phosphorus pentachloride in 110 mL dry toluene is heated with
stlrrlng to 80~C. After one and one-half hours, the thlck
mlxture ls cooled, filtered and the solid washed with ether
and dried. This is the hydrochlorlde salt of the deslred
product.
This salt is dissolved in 60 mL water; neutralized
with sodium bicarbonate, the resulting preclpitate removed by
flltration, washed wlth water and air-drled to glve the
product.
Method B
A mlxture of 5.0 g nlcotlnate and 7.1 g phosphorus
pentachlorlde ln 40 mL phosphorus oxychlorlde is stirred at
room temperature overnlght. THe phosphorous oxychloride is
removed ln vacuo, the residue suspended in 40 mL toluene and
again concentrated. This is repeated. Water (40 mL) is added
to the residue and the mlxture heated to reflux and held there
for one hour. After cooling, the mixture is extracted wlth
methylene chlorlde, the extract dried and concentrated to glve
1.05 g of the deslred product. The pH of the aqueous phase
from the methylene chlorlde extraction is ad~usted to 5-6 wlth
sodlum blcarbonate solutlon and the mlxture extracted agaln
wlth methylene chlorlde. The drled extract was concentrated
and the residue crystallized to give a further 2.65 g of the
desired product.
1339315
-62-
The following nicotinic acid esters are
prepared by one or more of the methods described above:
Y~OOR
N ~/ N--R 2
Hr~ - O
1339~15
-63-
u~ o ~ o ~n o o o o o
O ~ ~ o _ o o
lr~ ~ In 3 ~
0~
m o o o Ln o -o o o o
~ o m ~ 3
C~ ¦ T ~ T ~ T
~ ¦ T ,1_ T T -- T ~ C
X I ~ _ T -- _ T ~ X T
-
-
_ T -- -- t~l
~ 3
T T T
~ 3 T T
._ ¦ ~ T ~ ~ _
T U~
T T T T T T -- I .~.
133~
-64-
o o o
o o Lr~ ~ o o
.. ~ .Ln ~ ~ o
~ L~ ~ ~ ~ ~I t~) 3 0
O ~ ~ a~ o 0 ~
E O O
O oo ~ o O o O O
U~~ O 0 3 t~J O '- 0
t~J ¦ T =:C T :C T ~ T -- T ~
~-4 ¦ T = _~ T ~ = ~ T
X ¦ _ = = T . S T = -- _ ~ --
-- _ T ~ T ~ 2 c~
_ _ -- ~ -- -- _ _
--I ~ ~ C ~ ~ ~ T 'r ~ S'
CC C.) -- ~)
L
~r
~ ~r o ~
~ O -- /t~l ~ 111 0 C_) 11 C) t~
-65-
1339315
o o ,- o o o o
o o ~ U~ o o
o ~ ~ C~ ~ ~ L~
C ~
O O O ~) Ln O O O Lt~ O O O
t~¦ -- T X C T -- T T T S T -- O
¦ _ ~ ~ T 3 = ~ ~ T ~ T
X I -- T _ _~. T ~ ,T T T ~ T
~ ~J ~ N ~J~ T ~ ~
T -- ~ ~ T -- T C_)
T-- T T T T 3 ~ y --
~__ T T ~ T~ _3~ ~ ~ ~ ~ ~
T I~_ I~ :C ~' T ~
,~ rt~l r--T ~ C ) T
T
y y ~ T y ~ t_ C ~ T
' ~ 1339315
-66-
o o o o o U~ o o L~
~ ~ ~ 0 ~ o
OQ ~I ~ ~ I I I ~ I I I I I
F O O O bl~
O O L~ O Lr~ O O L~ O O Lt~ O
3 0 L~ J 3 ~ 3 3
3 r~ D ~) O ~
¦ T ~ -- T -- X ~ ~ T T ~ ~ , I ~ =
~ ¦-- T T a T ~ T ~ X ~ T
X ¦ , = _ _ T ~ = ~ ~ ~ _ T -- -- --
~ 2 T ~ ~ ~1
(~I I 3 ~ -- :C T ~J T -- ~ T
T T
.--1 ~ T T T ~ T (~J T ~ ~ ~ ~ ~--
~ I ~ (D
C-- ~ T o~ ~
T In ~_) 2 t~J T T
T T T T T T _~ _ T ~
133931~
-67-
o o o o o o o
~~ CO ~ ~8
~ ~ ~ ~ o ~ ~
o ~ ._._
E O OO
~n o o u~ o o
O ~ ~
c T T _ ~ ~ ~S' C
T ~ I T I = _ T~: I
X¦ I T I I I T -- = =
-- -- I ~ _T
T ~
N~ I T _ -- ~ ~I = I
--I - ~ I T X I I IT T
(~ ~ ~ o o _ 8
T ~? C b~ ~~ -
T ~ ~ -- C TT C_)
-68-
13393l 5
o o o o
O
~ o ~ ~ ,~,
o
Q l l l l O O
O O O O O
~) ~ O ~i t~i
O O
T ~, ~ T
-r T
X I-L. 3 C_) ~ _ =~r
~_1 ~ 2 I: T-- T ~
¢
-69- 133931~
o o Lr~
3 Ln 3
O _ _ _ _ _
E~ O O O O O
O O O
T T T T ~ =
~1 ¦ ~ I ~ T T ~ T T
X ¦ T T :C -- T , _ _
T -- _ _
-- T _ T ~ I I r
3 ~ 3
T -- T
~ 8~, ~, T
T '~O ~ --' ~ \~ 111
U C ) C~
T ~ ~_~ T
- - 13393~
- 7 0 -
Ln o o o o 0 o CO o ~ U~ U~ o
~ o ~ ~~ ~ ~ ~ o ~
O O ~ ~ O ~o
O ~ ~ ~~ ~~
E l l l l l + l+
Lr O O L~ O ~n 11 o It o 11 u~ o o
. a . a. a.
~ ~) O ~~J ~~r~3 ~ _
o a~ ~ o ~5 o ~so
-
C~
T --~ T -- ~ Z _ _
~ ¦ T T T T T
X I = T TT -- _ ~ ~r _ T
_ _ __ _ _ _ _ _ _ _
T --_ -- -- -- T ~ ~ =
~J ¦ T = -- ~r --
-- 1:: =T T T ~r ~-- --
U~
T ~.0
'
' I
~: o~ 5 ~ T T ,_~
-71- 133~31~
o o o o o o U~
~ U~ ~ ~ ~ o
~ ~) O ~ ~ N 3
~ ~ o a~
O ~ C~ ~ .
~IIII I IIIII
o o o ~ o O O O O
cr) ~ O ~OO In 3 U~ ~
Y
~D ~ I C~
~lo~ ~ ~
-
. r
~ --
-
~, I _ _ _ ~ _ _ _ _ ,. _ _
Lr~ ~ Ln
-72- 133931~
~ o U~ o o o o
0 3 0 a' ~ t~i ~i 0
~ ~ ~ U:~ ~ . - ~ 3
O '
L~ O O O Ln O O O
~o o cr~ O
o ~-- ~ ~ a~
-
T
-
t~
~ ¦ T ~ _) ~ -- T T
X I -- T X ~ T -- --
_
T ~ -- ~ -- --. T
_
~ ¦ = T T -- -- -- ~
.
T T
C~ ~ C,)
T ~ ~ ~
133931~
-73-
EXAMPLE 7
Preparation of cis- and trans-methyl 6-(allyloxy)-2-
(4-isopropyl-4-methyl-5-oxo-2-imidazolidinyl)nicotinate
OOCH3
I NaCNBH3
CH2=CHCH20 N ~/ N - CH(CH3)2
HN---~~=O
~ OOCH3
CH2=CHCH20 ~ \ ~ ~ H CH3 +
HN - = O
~ OOCH3
CH2=CHCH2 O ~ \N ~ N CH(CH3)2
HN - O
A solution containing 7.0 g (22.1 mmol)
methyl 6-(allyloxy)-2-(4-isopropyl-4-methyl-5-oxo-2-
imidazolin-2-yl)nicotinate in 70 mL absolute methanol
is cooled to 0~C and a few drops of methyl orange
indicator added. To the stirred solution is added
1.8 mL (22.1 mmol) concentrated HCl. The red solution
is warmed to room temperature and 1.4 g (22.1 mol)
sodium cyanoborohydride is added. Slowly, the solution
turns to an orange color (pH ~4) and 2N methanolic HCl
is added to the mixture until a red tint is observed
(pH -3). This procedure of pH adjustment is repeated
133931~
-74-
until there is no longer a change. After stirring
overnight at room temperature, the solution is cooled
to 0~C, the pH adjusted to ~0 with concentrated HCl to
decompose residual NaCNBH3. The pH is then adjusted to
5-6 with 5 N NaOH. The methanol is removed in vacuo
and enough water added to the residue to dissolve
inorganic salts. This mixture is thoroughly extracted
with CH2Cl2, the extracts dried and concentrated. The
residue (~7.8 g) is a thick oil which is chromatographed
on 350 g silica gel. Using 1:1 CH2Cl2-hexane followed
by ether as eluants results in the separation of
0.35 g starting material. Further elution with ether
results in the isolation of 1.87 g of the trans-isomer,
and further elution with 10~ methanol in ether gives
5.2 g of the cis-isomer.
The trans-isomer is recrystallized from
CH2Cl2-hexanes to give 1.16 g of analytically pure
trans-methyl 6-(allyloxy)-2-(4-isopropyl-4-methyl-
5-oxo-2-imidazolidinyl)nicotinate, mp 144-142~C.
Similarly the cis-isomer is recrystallized
from CH2Cl2-hexane to give 4.6 g analytically pure
cis-methyl 6-(alloxy)-2-(4-isopropyl-4-methyl-5-oxo-
2-imidazolidinyl)nicotinate, mp 120-122~C.
Using essentially the same procedure but
substituting the appropriate 5-oxo- or 5-thioxo-imidazo-
linyl nicotinate for methyl 6-(allyloxy)-2-(4-isopropyl-
4-methyl-5-oxo-2-imidazolin-2-yl)nicotinate, gives the
following 5-oxo- and 5-thioxoimidazolidinyl nicotinates.
Other compounds that can be prepared by the
above procedure are described in Table III below.
_75_ 133931~
o~
o
, -- ~
~ a~ ~
._, t~
O
C' G E
~t _
C ~1 1
E ._, . o
H C~ ~
tY ~ O ~
~ Z I -'
a ~ c ~- ~
X~\ /Z ~t O E ~ ~
~ ~ E C _
C
¦ = -- T
~'
CC--
H ._
H E ~n
H ._ ~ ~ ¦ ' --
m
c
-
E Xl -- T
C~
o ~ / ~ 31 o O O
c ~o Z
X--<~\ /z ~ I
~ ln 3
--r ~
C~
T
IX t~
lll
-76- ~ 133 9 3 1~
D~
,_~ r
~ ~ C_) E
C ~-- o ~,
~: ~ C
t; C' E
CJ L~
._ ._, ~
H
.~ J
C) ~
O ~ O O O O O
.
--I O ~ ~ O _ O O
~ ~ ~ ~ ~ ~ = ~ J
._ ~
~: C~ I I I I I
C ~-~ O
t' J~ Q o L~ o o o o o
t~ O E
~ 0 ~ 0 ~ 0 0
._ .~ ~- J ~ J =
E '- ~ ~ ~ ~ ~ ~
H
~1 5 5 55555
~;
-
-
g ~1 5 5 5555 _
H
H
H
m xl = 5 55 -555
C
~'
31 0 0 O O O O O
J J
_ ~ ~ ~ ~ = 5
555
5 5
I
55 =
Y~ Y~
555 _
5 ~ 5 ~ ~ 5
5 ~ 55
111 ~O 111 ~O ~O 111
5 5 55555
u~ ~
~ C ~
~ a E c~ ~ 133931~
C~ ~, o
C' .
o ~
.~, ~,
~ c ~nl ~
o o o o U~
. . o
~ o ~. ~o
C 1 ~ ~oo '' ''
C ~
t~ . o
t-~ ~ ~ oo o o o
G ~ . .. . .
O U~ ~J ~ C~
E~ -- cr. ~ 0 c~ ~1
' I
~_ ) I ~ ~ ~ ~~
~: ~ ~\ /
a ~) Y \~j/ c c c '
,_
x I ~ ~ :~
E-
3i0 0 0 0 0 0 0 0
u~ b u~
~ ~ ~ ~ _, ~ ~
-78- 1339315
o In o o o
C~1 ~ _ o t~,
~ -- O 3
r
o o o o
~ ~J -- 2 o ~
. ,~ _ _ . .
~,
C, ~C 3 u~
t~ O E ~ ~ ~ ~J
-- E
H ~ O O~l) O
~ L~\ cr~ o
-- 3 _ ~
O U~ O O O O Lr~ O
- :~ ~ O ~ 0 3 ~i ~ o . ~ri ~
t' ~' O
8 Q Lr O O O U~ O O O O O O
O O O~ O ~ G~
E ~: O ~ t~ ~O a~ ~ a' ~ --
H
t--
C.) _ ~) -- T
O
H '-41 S c_) _ _ = _ _ T
X I S S S S S S S S S S
31 0 0 0 0 0 0 0 0 0 0 0
~ s ~ s ~ ~ s s s
S T S ~ _ S S T S _ _
S S :~ S S S S S S ~ S
. .
-
S
S ~O
S S S S -- ~ ~ S~ ~ S~
-- C~
-79- o ~3393I5
~ ~ .
0
~ ~5 o o o
~~ a,
C ~ Oo o o
.
o
E c ~ ,_
~ 0 l l l
t~ L~ o o
o C~ o CO o ~ o o o o
- ~ o ~ ~~
o ~7 o C~i oo ~ ~ ~ ~ ._ o
C C~ I + I +
C ~ ~U~ 11 o 11 o 11 Lr~ o O o o O O
.~ o ~ O ~ ~ a
E c ,~
C~
.~ Z
a
.C
c ~ ~ ~ s
_,
~
X I C T r ~ ~ ~ ~r T ~ ) T
31 0 0 0 0 0 0 0 0 0 0
T ~ T
~n Ln ~~l ~)
z ~
-80 -
~ 1339~
~, a
~ C~
C . ~o
C' J~ C~
~ O E
'C C)
H
O O U~
3 Lr~ _
~a o o. o.
C, E
E '-
C~ I ~ 3: --
-
-
._
-
H
c xl
C
31 O O O
T 'r ~ ~V
_ ..
~ T
- 8 1 -
~. ~~ 133931~
o ~ _
~ ~ U~
. . ~ .
o o o
C ~ .
. ~
C~ o L~ o
C . o
r ~ c ~
tl O E 3 ~ ~n
E C
H U~
._~ O O O
C~'
~O
3 L
~ .--
O O O O ~ O L~
~O ~ ~ . .
_ ~ ~ ~ O ~ ~
~3 ooa:~ ~ ~ ~ ~ o
._ ~
IIIIII+
C ~-- O
c~ ~ Q ln o o u~ o O I I O O
~ O E ~ ~ ~ . ~ ~ 2
~ ~ o ~ u~
._ ._, o ~a:) ~ o ~ ~ o
E . ~ ~ ~ ~ ., ~ ~
H
~ s = s s I ~ ~
,_ .
._
O ~I s--ss== s~
H
H
H
X I S S S S S S S S
E-
3 1 0 0 0 0 0 o u~ v~
s .~ s s s s s s
C~l S T S S S ~ S
~ s s s s s s s s
L~
~ s
c: s c~ s
c~ 8 ~ 8
S -- S S S S S S
-82- 13~31S
V~
.,
C. ,o
t~ ~ Q
t~ O E
.C .C)
H
.,_, I
O O O
O o ~U~ O
._ _ ~
~ O E ~ ~ ~ O
E t- o Cfr~
H ~-
T T
aJ ,
.~
o ~ T -r = S
H
t~ T
m x I T ~_) -- _ T
31 O O O O O O
~ _ _ _ _ _
T ~ T ~r r ~
Y~
-83-
' 133931~
. ~,
._ ~r
C~
C . o
~ ~ a
t~ C E
._ _
F C o~
~1 -1
C~
C ~- O O OO O O
~ ~ a
tl O E
H
C~l ¦ T T
-
C
-
C >'~ ¦ , T , = _
H
H
~ X I T T = T
C
31 00 0 0 0
N ~ t~ J N
T TT ~ T
_
_ T T
T T -- T T
t~ T
~/> 8 ~, ~, T
T ~ G~
~O ~ _ T
-84- 133931~
I
~ a
C ~- o
O E
_ ._
~ U~ I
cl
~ ~. ~. ~. o o o
0
~ ~ o
C-- .L.~ ~ ~ ~ ~ C~ ~ ~
O ~--O O
C'~ ~ C~ o o U~ o o o Ln
8 E ~ ~D ~ ~ u~ ~ ~
~ ~ o C~
H
C~l ¦ T T T _ T _ T
a
-
-
~ ~1 -- ~ ~ ~ = = ~ m
H
m X ¦ ~ _ T = T
C
31 O O O O O O O O
~ -- T T ~ -- T
_
~ T _ T -- S T
8 ~ ~, u
~ ~ ~ ~ T T T
-85- ' 133931~i
o
-
o
.
C C~
C . ,o
C' ~ CL o
~ O E
._ ._, cr~
E s ~1 ~
C~ O
a~
O o o
~ o ~ O
~ a, ~ ~ cr~ ~ O
r ~ _ ~ _
C ~--O O O OO
C O O O ~ O o
tt O E
._ ._, o ~ ~ ~ O
_~ T T 5 T ~: S -- --
c
._
C
O C~
S ~ ~ ~ T -- T _ ~ O
H
H
H
C~
C X ¦T _ ~ ~ T ~ T ~
31 0 0 0 0 0 0 0 0 0 0
TS T T :C '
-- ~ S _ ~ Ll~ S U~ T
T-- T ~ S ~ S S,
~1 ' -- " '' '-' '-'
:c ~ T
O S~) ~ C~
~ C. I 11 11 t~
S~ I ,_ :~
~)~' ~ ~) C ~
Il. ~\~ _. I ~ 11 S
-- ~ ~ S
S ~ ~ S'
~ 133931~
86
o
o
C
C L O
~ ~ ;
C ~--~ O O
O E 3
E C
H U~ ¦
C)l O
O O o ~ o o ~ ~ O o O
.
r ~ ~ ~ . .
._ ~ E
C ~-~ O bO
~; ~ Q L~-\ O O L~ O O L~ O O O O
~ O E
C C~ 3 ~~ ~ 3 ~ ~ _ u~ 3 0
E ~~ . ~ . _ . ~ ~ . . ~ .
H
C~ I = S S :~ S ~ S = = S
~ ,
.~ S
J~
C ~ I ~ _ S
H
H
H
C X I S T = ~ = T S S C~ -- T
31 O O O O O O O O O O O O
~ S TT ~ S ~
~ S~ T,~/-- T t~J ~ --
I ',
_ S ~J S T ~S C -- ~ T -~
H
S :~
S S
O
Z
I I I C~ S ~ S
T S -- S T ~ S c~
-87- 13393I~
o
U~
~ C
.~ ~.,, .
~, C:)
C)
C . o
~ CL o
O E
._ ._ O
H ra~ I ~
._, o
~ a~
o o o o o
o ~ ~ Ln o o
C ~--O O
t~ v Q o ~ Lr~ o o o Lr~ O o O
t~ O E
E '--
C~l ¦ T ~ = r ~ =
._
T ~ -- ~: T T --~ ~_ I ~ _
H
H
H
C X I T ~:C ~ -- T ~r -- = -- =
E-
31 0 0 0 0 0 0 0 0 0 0 0
T ~ ~ T .,_ _, T
T T ~ -- T T ~ ~7 _
-- ~ T T T -- T
A
C L~
r) ~.D T 7~ 111 ~) C_)
\
C I y c~ ~ T T
-88- 133931~
Ln ~
~ o
C ., o ~ o
O E ~
H C) I o O
- O O ~ ~. Lr ~
C ~-- O O
t~ O E ~ ~ O u~ o o o o
C~ ~ _ ~ o
~ ¦ ~ T ~ T'~
-
O ~ ¦ T 3 ~ = _ _ T --
H
C XI ~ _
31 0 0 0 0 0 0 0 0 0
~ _ _ _ _ _ _
T ~ ~
_ _ _ ~ _ ~ _ _ _
~m 15
C: O
-89-
ooooo
~ 1339315
C~ I,III
C
~ ooooo
C 1~ o
~,
C ~ o o o o U-~
~' -- E _I o ~n o
.~ ~ ~ ~ o o
E c
U~ I I I II I
~I o o o o
c~l co o a~
o oooooo
o ~ ~ 1-- ~ ~ o
C~ a o ~ o ~ ~) o r-
~ C ~) ~,, I I I , , I I
C--o O
~ lc E ~ ~ ~ ~ ~ ~ ~
~ o o ~ a: o ~
E C
a ~ c~
5 ~) O
_~ -O ~
31 0 0 00 0 0 0 0
c'J
~ l c~
'
IY ~ ~
1339~1~
-so-
EXAMPLE 8
Preparation of cls- and tr2~s-5-ethyl-2-(4-isopropyl-
4-methyl-5-oxo-2-imidazolidinyl)nicotinic acid
C2H5 ~COOH C2H5~COOH
CH3 NaCNBH~ ~ I H H CH3
N--~/ N--CH(CH3)2 N--~N--CH(CH3)2
HN~O HN~O
To a stirred slurry of 2.89 g 5-ethyl-2-(4-
isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl)nicotinic
acid in 20 mL methanol and one equivalent of 2N methanolic
HCl is added under nitrogen o.6 g sodium cyanoboro-
hydride. Methanolic HCl is added to maintain a pH of
2-3. After stirring the pH of the mixture is adjusted
to 1 with concentrated HCl and after 15 minutes, agzin
adjusted to 3 with saturated NaHC03 solution. After
filtration, the solution is extracted with ethyl acetate.
The pH of the aqueous phase is again adjusted to 3 and
again extracted with ethyl acetate. A crystalline
precipitate of cis- and trans-5-ethyl-2-(4-isopropyl-
4-methyl-5-oxo-2-imidazolidinyl)nicotinate is formed
which can be recrystallized from ethanol to give the
product as a white crystalline solid mp 208-210~C. This
contains about 66% of the cis- and 34p of the trzns-
iscmer.
3~
'~' 1339~1~
-91-
EXAMPLE 9
Preparation of cis-6-(allyloxy)-2-(4-isopropyl-4-methyl-
5-oxo-2-imidazolidynyl)nicotinic acid
~ OOCH3
CH2=CHCH20 ~ \N ~ N\---cH(cH3)2
HN - O
~ OOH
CH2=CHCH20 ~ \N ~ N~--cH(cH3)2
HN - O
To a solution containing 3.9 g (12.2 mmol)
cis-methyl 6-(allyloxy)-2-(4-isopropyl-4-methyl-5-
oxo-2-imidazolidinyl)nicotinate in a minimum absolute
methanol (~15 mL) is added 12.2 mL 2N NaOH solution. A
precipitate results and the mixture is heated with
stirring to 45~C and maintained at that temperature for
one hour. The solution becomes clear. It is cooled to
0~C and 12.2 mL 2N HCl added. A solid precipitates
which is collected, washed with ether and air dried.
This material (3.2 g) is recrystallized from methylene
chloride-hexane to give 2.3 g analytically pure cis-
6-(allyloxy)-2-(4-isopropyl-4-methyl-5-oxo-2-imidazo-
lidinyl)nicotinic acid, mp 193-194~C.
1339315
-92-
By using essentially the same procedure, but
substituting the appropriate methyl 5-oxo or thioxo-
imidazolinyl nicotinate or quinoline-3-carboxylate for
cis-methyl 6-(allyloxy)-2-(4-isopropyl-4-methyl-5-oxo-
. 5 2-imidazolinyl)nicotinate, the following 5-oxo or
5-thioxoimidazolidinyl nicotinic, or quinoline-3-
carboxylic acids are prepared. The reaction can be
illustrated as follows using nicotinates as represen-
tative of the reaction.
Y~COOR Y~COOH
Z~ N R l 3 . z~ H ~1
HN~--W HN eW
93 ' 133931S
, ~.
C~ l C ~ 01 '~1 C cnl u~ I C
. ~ .~ .~ .~ ~ ~Y I ~~ .~ .~ .~ ~ .~ .
U C~ ~ ~ ~ ~ C~ ~ t~ ~ C~ ~J
~,
~oooo~ooooooooo
o ~ 'J _ ~ ~ o o~ ~ _ ~ C~i ~J
~ o oo r~ a~ o~ o c~ ~ O ~ ~ ~ C~
ooU~ooooooooooo
00 ~ ~ ~ O ~ ~ 1-- ~D ~ O 1~ 0 --
o o oo r~ a~ co O 1~ oo o
~.
~r: _ , I
~) t~)
2 ~ 2
~ I ~ ~ 222222211
X 122222 ~ ~ 2222222
3100000000 ~ 00000
133931S
-94-
~ SJ
0 ~ 0 ~ 0 0 0
01 @ X @ ~ ~1 X @ 01 01 @ ~01 @
x
o~oooo~ooooooo o
~ _ ~ co n ~ ~ o ~ ~ u~ ~ ~
O _' ~D O ~ ~ ~ ~ ~ o ~ c~ ,_
I I I I I
oooo~ooooooo~1oC~l
O O ~ O O CO ~D CO ~ ~D O C~
~ T' 111 111
~ O O O
X ¦ ~ C ~ ~ ~ T ~ T'
3 1 0 o o o V~ o o o o o o o o
_95_ 13393
c c U, u' O~ ~n
~,~ ._, ~,.
~ C~ t~ t~
C~ .
o~ o o o o o
.,.U~ C~i ~ o
Y~o ~ ;~ o
I I I I I
o o o ~ o
~ U~ o
C'l
Z ~ T'
~ C~l
F~
~ I ~ O o
X I ~ ~ S ~ ~ ~
3 1 0 0 0 0 0 0
1339~15
-96-
EXAMPLE 10
Preparation of cis-methyl 2-(4-isopropyl-4-methyl-5-
oxo-2-imidazolidinyl)nicotinate hydrochloride
OOCH3
H H CH3 CH~OH
N ~ N ~--CH(CH3)2 HCl
HN - O
OOCH3
N ~ N ~ --CH(CH3)2
HN _ O
To 2.0 g cis-methyl 2-(4-isopropyl-4-methyl-
5-oxo-2-imidazolidinyl)nicotinate is added 25 mL of 2N
methanolic HCl. The solvent is removed in V2CUO and
the residue is crystallized from ethyl acetate-ether to
give the hydroch-loride salt, mp t89-192~C. Other acid
addition salts may be prepared by the above procedure
using the appropriately substituted formula III 2-(2-
imidazolidinyl)nicotinate.
3o
_97_ 133931~
EXAMPLE 11
Preparation of sodium 5-ethyl-2-(4-isopropyl-4-methyl-
5-oxo-2-imidazolidinyl)nicotinate
C2H5~COOH
H CH3 NcOH
N N ~ --CH(CH3)2 MeOH
HN - O
C2 Hs~{OOe
~ ~ N CH3
HN - O
To 1.0 g 5-ethyl-2-(4-isopropyl-4-methyl-
5-oxo-2-imidazolidinyl)nlcotinic acid is added a
solution of 0.1498 g sodium hydroxide in 20 mL absolute
methanol. The mixture is stirred under nitrogen at
room temperature overnight. The solvent is removed to
give a solid which is dried in a vacuum oven at 60~C
for two days. The thus-formed sodium 5-ethyl-2-(4-
isopropyl-4-methyl-5-oxo-2-imidazolidinyl)nicotinate
darkens at 230~C and decomposes at 247-250~C.
133931S
EXAMPLE 12
Preparation of cis-methyl 2-(4-isopropyl-4-methyl-5-
oxo-2-imidazolidinyl)nicotinate
~OOCH3 + NH2 f - fONH2 pTSA
HO CH(CH3)2
1 O ~COOCH3
~, N fH3
HN --O
A solution containing 1.24 g methyl 2-formyl-
pyridine-3-carboxylate (Bull. Soc. Chem. France, 36,
78-83 (1969)], 1.0 g 2-amino-2,3-dimethylbutyramide and
20 g ~-toluene sulfonic acid is heated under reflux
under nitrogen with a Dean-Stark water sepzrator for
six hours. The solution is filtered while hot and the
filtrate concentrated in vacuo to leave a dark oil.
The oil is extracted into ether, the ether concentrated
to give a yellow solid. This solid is recrystallized
from a mixture of hexane-ether and methylene chloride
to give cis-methyl 2-(4-isopropyl-4-methyl-5-oxo-2-
imidazolidinyl)nicotinate, mp 118.5-120~C, identical to
one of the products obtained from the sodium cyanoboro-
hydride reduction of methyl 2-(4-isopropyl-4-methyl-5-
oxo-imidazolin-2-yl)nicotinate. The presence of the
3~ corresponding trans-isomer is indicated by nmr spectro-
scopy. Following the above procedure and using the
appropriately substituted 2-formylpyridine-3-carboxylate
yields the formula III 2-(2-imidazolidinyl)nicotinic
acids and esters reported in Table IV below.
-99- 1339315
. i . . .. .
C) C~ C~ ~ C)
o o Lr~ o o
o a~
E
L~ o o o O
O ~ o
~ 1~l
~; O ~ --, _ T
.~ ~\
~ X--~_fZ
o
~_
C
~ X¦ ~ T T
-
o
m .~ ~
c E y
t~l _ ~ ~ -- -- T
H ~ -- T
H
-
E
O O
C~ y y ~ I ~) ~ ~
0 /~\
~" X--~Z
.
u~ o
G T ~
~r3: I ~ T
-100-
1339315
., ., ., ., ., _ .~ _
o o oLr~ooooo
- ~ ~ ~ ~ ~ o ~ ~ o ~
V ~ E ~J ~ ~ '~ ~ ~ ~ '~
O O
E ~~ ~
Ln o OOOOOOO
1 1~
~ 5 ~
c~; 5 5 s 5 t~ 5 0 C.) c~ --
-
-r
5 ~ 5 t~ --
5 C )
) -- O -- 5 ~)
~ x I -- 5 5 _ 5 5 -- 5 --
J~
o
-
_ _________
-- ~ -- 5 -- -- 5 -- -- 5
C t~J I S
_ _ T _ T 5 5
Lr
o: 5
~r 5 T 5 T 5 ~ ~j
133931~
-1o1-
EXAMPLE 13
Preparation of cis- and trans-methyl 2-(4-isopropyl-4-
methyl-5-thioxo-2-imidazolidinyl)nicotinate
~ OOCH3 fH3
11 +NH2~--CSNH2
CHO
N CH(CH3)2
COOCH3 ~ COOCH3
N ~ '~_-CH(CH3)2 ~N ~ N\' 3 CH(CH )
HN- - S HN - S
Using essentially the same conditions as
described in Example 35, but substituting 2-amino-2,3-
dimethylthiobutyramide for 2-amino-2,3-dimethylbutyramide
gives a mixture of cis- and trans-methyl 2-(4-isopropyl-
4-methyl-5-thioxo-2-imidazolidinyl)nicotinate from
which essentially pure trans-isomer, mp 127-129~C can
be isolated by chromatography of the crude product on
silica gel. The melting point of the cis-isomer is
142-143.5~C.
3o
13~9315
-102-
EXAMPLE 14
Preparation of cis- and trans-ethyl 2-(4-isopropyl-4-
methyl-5-oxo-2-imidazolidinyl-2-yl)~uinoline-3-carboxylate
~cooc2H5 fH3
W~ ~,LCHO + NH2~0NH2
N CH(CH3)2
~CO OC 2 H5 CO OC 2 H5
W' N J'~ ~--CH ( CH3 ) 2 W' N ~ ~_-CH ( CH3 ) 2
HN--O HN--5
Using essentially the same procedure as
described in Example 35, but substituting ethyl 2-~ormyl-
quinoline-3-carboxylate [Godard etal., Bull. Chem. Soc.
France, 906 (1971)] for the methyl 2-formylnicotinate,
there is formed the cis-ethyl 2-(4-isopropyl-4-methyl-
5-oxo-2-imidazolin-2-yl)quinoline-3-carboxylate, mp
156-164~C and trans-methyl 2-(4-isopropyl-4-methyl-5-
oxo-2-imidazolin-2-yl)quinoline-3-carboxylate, mp
163-164~C. Following the procedure of Example 33 but
substituting a substituted 2-formylcarboxylate and
using an appropriately substituted aminoamide in place
of 2-amino-2 t 3-dimethylbutyramide will give the substi-
tuted formula IV 2-(2-imidazolidinyl)quinoline-3-
carboxylate.
3o
- -103- ' 13~31~
EXAMPLE 14-A
Preparation of ethyl 2-[2-(dimethylamino)vinyl]-5-
nitronicotinate
~2Nf ~OOC2H5
H3
(CH3)2NCH(OCH3)2
02N~XOOC2H5
H=CH-N(CH3)2
A solution containing 20.88 g of ethyl 2-
methyl-5-nitronicotinate in 100 mL l,l-dimethoxytri-
20 ethylamine is heated at reflux for three hours and 15minutes. The mixture is cooled and the solid collected
by filtration, washed with methanol and air dried to
give 26 g of the desired enamine as a dark red solid mp
176-179~C.
-104- 13~3~15
EXAMPLE 14-B
Preparation of ethyl 2-[N-(l-carbamoyl-1,2-dimethyl-
propyl)formimidoyl]-5-nitronicotinate
.
02N ~ OoC2H5
H=CH-N(CH3)2
N
1. 03,(CH3)25
fH3
2. NH2 -C - CONH2
CH(CH3)2
~2 ~ ooc2H5 fH3
CH= N - C - CONH2
N
CH(CH3)2
To a solution containing 18.9 g of the enamine
in 200 mL CH2C12 and 10 mL methanol cooled in an ice
25 bath is added ozone from a Welsback ozone generator
operated at 120v and air at 8 psi. This is continued
until the red color of the enamine is discharged. The
ozone is replaced by nitrogen and then 10 mL dimethyl-
sulfide added. After 15 minutes, 9.8 g of 2-amino-2,3-
30 dimethylbutyramide is added, the reaction mixture trans-
ferred to a 500 mL flask and concentrated. The residue
is dissolved in 300 mL toluene and heated at reflux
under a nitrogen atmosphere under a Dean-Stark water
trap. After one hour, the so~vent is removed and the
35 residue, a black gum, which is mainly the Schiff base
is used without further purification.
133931S
-105-
EXAMPLE 14-C
Preparation of cis- and trans-ethyl 2-(4-isopropyl-4-
methyl-5-oxo-2-imidazolidinyl)-5-nitronicotinate
02N ~ oOC2H5 fH3
CH= N - ,C - CONH2
CH(CH3)2
TFA
~ COOC2H5
/ ~ N C~H3
HN---{=O
02N ~ ~~C2 H5
/ ~ N CH3
HN---'=0
133931~
-106-
The crude Schiff base as described above is
dissolved in 50 mL CH2C12 and treated with 4.6 mL tri-
fluoroacetic acid at room temperature. After one hour
an additional 1 mL acid is added and stirring continued
for one hour. The mixture is cooled, 5.5 g sodium
bicarbonate added. After the slow and careful addition
of water, stirring is continued until CO2 evolution
ceases. The pH is adjusted to 7 with saturated sodium
bicarbonate solution and the CH2Cl2 layer removed. The
aqueous phase is reextracted three times with CH2Cl.
The combined extracts are dried and concentrated to
give the crude product as a dark semi-solid. This
material is chromatographed on silica gel. Using ether
and hexane-ethyl acetate mixtures to develop and elute
the products, trans-ethyl 2-(4-isopropyl-4-methyl-5-oxo-
2-imidazoliinyl)-5-nitronicotinate is eluted first and
is recrystallized from CH2Cl2-hexane to give the pure
trans-isomer mp 116-120~C.
The cis-isomer is eluted later and is recrys-
tallized from CH2C12-hexane to give pure cis-ethyl
2-(4-isopropyl-4-methyl-5-oxo-2-imidazolidinyl)-5-nitro-
nicotinate mp 149-150.5~C.
-107- 1339315
EXAMPLE 14-2
Preparation of methyl 2-(4-isopropyl-4-methyl-5-oxo-2-
imidazolin-2-yl)-5-(methylthio)nicotinate
Br~COOCH3
- N ~ / N\__{H(CH3)2
HN - O
NaSCH3
I HF/DMF
CH3S ~ COOCH3
N ~ {H(CH3)2
HN - _ O
To a stirred solution containing 1.0 g bromo
compound in 5 mL THF and 2 mL DMF is added 210 mg sodium
methyl mercaptide under nitrogen. After two hours at
60~C, the mixture is cooled to room temperature, the pH
adjusted to 4 with acetic acid, poured over ice and
extracted with 2 x 50 mL ether. The extract is dried
and concentrated to give a yellow oil which slowly
solidifies. Recrystallization of the solid from
ether/hexane gives pure methyl 2-(4-isopropyl-4-methyl-
5-oxo-2-imidazolin-2-yl)-5-methylthio)nicotinate, mp
107-108~C.
1339315
-108-
EXAMPLE 15
Preparation of dimethyl thieno[3,2-b]pyridine-5,6-
dicarboxylate
I ~ NHC02Prl ~ ~ NH2 DMAD
S POC13/DMF ~ S ~CO2cH3
ICC02CH3 L ~ /,~Co2cH3
C02CH3 N
H
A mixture of isopropyl-3-thiophenecarbamate
(177 g; 0.975 mol) in methanol (1.2 l) and water (2.8 l)
containing sodium hydroxide (200 g) is heated at reflux
for four hours. Methanol is removed under reduced
2 pressure and the cooled reaction extracted with ether
(5 l), and these extracts are washed with water, aqueous
sodium chloride and dried. Evaporation under reduced
pressure affords 3-aminothiophene as an oil in 57p
crude yield.
3-Aminothiophene is redissolved in methanol
(500 mL) cooled in an ice bath and dimethylacetylene-
dicarboxylate (80 g; 0.50 mol) is added dropwise. The
mixture is stirred at room temperature for 15 hours and
30 minutes, the methanol removed under reduced pressure
3 and 1,2-dichloroethane is added. This solvent is also
evaporated off to give dimethyl 3-thienylaminobutene-
dioate as an oil.
1339315
- 109-
A Vilsmeier reagent is prepared by adding
dropwise, with stirring phosphorus oxychloride (86 g,
0.56 mol) to a cooled (5~C) solution of DMF (41 g,
0.56 mol) in 1,2-dichloroethane (200 mL). This reagent
is stirred at room temperature for one hour and 40
minutesr diluted with 1,2-dichloroethane (lOO mL),
cooled to 5~C and then the above dimethyl ester dis-
solved in 1,2-dichloroethane (400 mL) is added to the
Vilsmeier reagent at 5~C dropwise over a 25 minute~ lO period. The reaction temperature is raised to room
temperature for 15 minutes, then to reflux for a further
two hours and 25 minutes. The cooled reaction mixture
is chromatographed directly on a silica gel column
affording 35.7 g (15~) of dlmethyl thieno[3,2-b]-
pyridine-5,6-dicarboxylate mp 124-125.5~C after crystal-
lization from hexane-ethyl zcet2te. A second crop (10.3 g)
with mp 121-124~C is obtained giving an overall yield
from isopropyl 3-thiophenecarbamate of 19p.
Utilizing the above procedure and substituting
the 2ppropriate substituted aminothiophene for isopropyl
3-aminothiophenecarbamate yields the compounds illus-
trated below.
R9~ S ~COOR "
Rlo COOR"
R9 Rlo R" mp~C
H H CH3 126-127
CH3 H CH3
Cl H CH3
1339~15
- 1 1 o -
EXAMPLE 16
Preparation of dimethyl thieno[3,2-b]pyridine-5,6-
dicarboxylate
S ~ CHO l. H2S~4 ~ S ~ 02CH3
NHCOCH3 3 POC13/DMF ~ / ~ 02CH3
To concentrated sulfuric acid (170 mL), stirred
at room temperature is added in portions 3-acetylamino-
2-formylthiophene (17.5 g, 0.103 mol). The mixture is
heated at 50~C for 30 minutes, cooled and poured into
an ice-water mixture. After neutralizing with an
excess of sodium acetate, the mixture is ether (l x 2
l) extracted. The organic layer was dried over anhydrous
Na2S04 and stripped to a dark red gum consistin~ of
3-amino-2-formylthiophene. Dimethylacetylenedicarbo-
xylate (DMAD) (13 mL) in acetic acid (5 mL), piperidine
(5 mL), methylene chloride (100 mL) and toluene (lOO mL)
is added to the 3-amino-2-formylthiophene and the mix-
ture stirred overnight. Methylene chloride is removed
by distillation and then the mixture heated at reflux
for 24 hours. After cooling an additional 13 mL of
DMAD is added and the reaction heated to reflux again
for seven and one-half hours. After standing for 60
hours at room temperature, the solvents are removed and
the dimethyl thieno[3,2-b]pyridine-5,6-dicarboxylate
product is obtained by chromatography, after eluting
with hexane-ethyl acetate, mp 124-125~C.
l3393ls
- 1 1 1 -
EXAMPLE 17
Preparation of dimethyl 3-chloro[3,2-b]pyridine-5,6-di-
carboxylate and dimethyl 2,3-dichlorothieno[3,2-_]-
pyridine-5,6-dicarboxylate
2CH3 C12
C02CH3
~~É02CH3 ~S~c02cH3
Cl 02CH3 ClN C02CH3
A solution of dlmethyl thieno[3,2-b]pyridine-
5,6-dicarboxylate (15 g 0.0525 mol) in acetic acid
(680 mL) and sodium acetate (86 g, 0.093 mol) is main-
tained at 58~C while chlorine is slowly introduced
during five hours and 45 minutes. After reaction is
complete, the mixture is flushed with nitrogen, ethylacetate (200 mL) is added and solid sodium chloride
filtered off and washed with ethyl acetate. The mother
liquors and washes are combined and the solvents removed
under reduced pressure. The residue is dissolved in
methylene chlor_de and the solution washed with water,
back extracted with methylene chloride and the combined
methylene chloride layers washed with aqueous sodium
bicarbonate, dried and stripped to give 18 g of solid.
Chromatography on silica gel with 15p ethyl acetate-
hexane, then 20p ethyl acetate-hexane gives the 2,3-
dichloro compound, mp 173-178~C, 1.3 g, followed by the
3-chlorothieno compound mp 166-173~C after crystalliza-
tion from ethyl acetate-hexane.
133931S
-112-
EXAMPLE 18
Preparation of dimethyl 3-bromothieno[3,2-b]pyridine-
5,6-dicarboxylate
i~ ~ Br2 ~ C02CH3
C02CH3 Br C02CH3
N N
A solution of bromine (20 g, 0.125 mol) in
acetic acid (50 mL) is added dropwise over three hours
to a solution of dimethyl thieno[3,2-b]pyridine-5,6-di-
carboxylate, (26.3 g, 0.104 mol), containing sodium
acetate (17.2 g, 0.2 mol) in acetic acid (300 mL) at
85~C. Additional sodium acetate (18 g) and bromine
(20 g) in acetic acid (50 mL) is added over an hour and
the mixture stirred at 85~C overnight. Bromine (10 g)
is added in one portion then left at 85~C for four
hours. The mixture is cooled and treated with aqueous
sodium bisulfite, diluted with ethyl acetate and con-
centrated. The reaction product is partitioned between
water and methylene chloride and the organic layer
washed with aqueous sodium chloride and the solvent
removed. The residue is washed with ether to give 25 g
of crude product, mp 165-168~C. Recrystallization from
methanol gave needles of dimethyl 3-bromothieno[3,2-b]-
pyridine-5,6-dicarboxylate, mp 168-169~C.
3o
-113- 133931~
EXAMPLE 19
Preparation of thieno[3,2-b]pyridine-5,6-dicarboxylic
acid
--CO 2 CH 3 Na O H , L,~cco 2 H
02CH3 H20 C02H
N N
Dimethyl thieno[3,2-b]pyridine-5,6-dicarboxy-
late (3.75 g, 0.0149 mol) is added to a solution of
sodium hydroxide (1.8 g, 0.045 mol) in water (20 mL)
and the mixture is warmed at 60~C for 20 hours. The
5 reaction mixture is diluted with water, cooled in an
ice bath, and acidified by the addition of concentrated
hydrochloric acid. A precipitate of thieno[3,2-b~-
pyridine-5,6-dicarboxylic acid is filtered off and
dried overnight to give 3.1 g (93p) mp >380~C.
2 Utilizing the above procedure and substituting
the appropriate substituted thieno[3,2-b]pyridine-5,6-
dicarboxylic acid diester yields the compounds illus-
trated below.
R9 ~ S ~ C02H
Rl o C02H
N
3o
1339315
-114-
Rg Rlo mp~C
H H >380
H Cl None taken
H Br >380
H
H F
H CN
H OCH3
H OH
H NO2
H N(CH3)2
CH3 H
H CH3
CH CH3
H OCHF2-
H SCH3
H SO2N(CH3)2
C6Hs H
-(CH2)3-
_(CH2)4-
-(CH)4-
Cl Cl
H C6H5
C6H5 H
H OC6H5
CF3 H
3o
1339~31~
-115-
EXAMPLE 20
Preparation of 3-chlorothieno[3,2-b]pyridine 5,6-
dicarboxylic acid anhydride
~ S ~ - COOH A O
0
3-Chlorothieno[3,2-b]pyridine-5,6-dicarboxylic
acid (1.45 g) is heated at 85 to 90~C for 30 minutes
then 90 to 102~C for 30 minutes in acetic anhydride
(7 mL). The reaction is cooled, the solids filtered
off and washed with ether to give 1.2 g of 3-chloro-
thieno[3,2-b]pyridine-5,6-dicarboxylic aci~ anhydride.
Utilizing the above procedure and substituting
the appropriate pyridine-5,6-dicarboxylic acid for
3-chlorothieno[3,2-b]pyridine-5,6-dic2rboxylic acid
yields the compounds illustrated below.
R9 ~ S
Rlo N
3o
13~93 1 5
-116-
Rg Rlo mp~C
H H 266-267
H Cl Solid no mp
' obtained
H Br >380
Cl H
Cl Cl
H N02
CH3 H
N(CH3)2
H SCH3
H OCH3
H CH3
H F
H
CH3 CH3
H CN
H OCHF2
C6H5 H
H S02N(CH3)2
-(CH2)3-
-(CH2)4-
-(CH)4-
Cl C1
H C6H5
C6H5 H
H OC6H5
CF3 H
3o
133931~i
-117-
EXAMPLE 21
Preparation of 5-[(1-carbamoyl-1,2-dimethylpropyl)-3-
chlorothieno[3,2-b]pyridine-6-carboxylic acid
Cl ~ ~ ~ + NH2- f { ONH2
N CH(CH3)2
o
! ~ OOH fH3
Cl N ONH ~ { ONH2
CH(CH 3 ) 2
2-Amino-2,3-dimethylbutyramide (0.71 ~) 211 in
one portion is added to a stirred solution of 3-chloro-
thieno[3,2-b]pyridine-5,6-dicarboxylic acid anhydride,
(1.2 g) in THF (1.0 mL). After standing for five
minutes, the ice bath is removed and the reaction st~rred
at room temperature for 28 hours. T~F (5 mL) is added
and the mixture heated at reflux for two hours and then
set aside overnight. The cooled mixture is filtered
and the collected solid washed with ether to gi~e 1.4 g
of the desired 5-[(1-carbamoyl-1,2-dimethylpropyl)-
carbamoyl]3-chlorothieno[3,2-~]pyridine-6-carboxylic
acid.
Utilizing the above procedure and substituting
the appropriate pyridine-5,6-dicarboxylic acid anhydride
for 3-chlorothieno[3,2-b]pyridine-5,6-dicarboxylic acid
anhydride and the appropriate aminoamide yields the
compounds illustrated below.
-118~ 3931~
R9~ CC~2H ~1
Rl o CO NH--Cl ~0 NH 2
R2
Rg R10 R1 R2 mp~C
H H CH3 1_C3H7
H Cl CH3 l-C3H7 not p~re
10 Cl H CH3 1-C3H7
Cl Cl CH3 l-C3H7
H Br CH3 l-C3H7
H Me CH3 l-C3H7
H NO2 CH3 l-C3H7
H N(CH3)2 CH3 l-C3H7
H SCH3 CH3 l-C3H7
H OCH3 CH3 l-C3H7
CH3 H CH3 l-C3H7
H SCH3 CH3 l-C3H7
20 H H CH3 C3H7
H H CH3 C2H5
H OCHF2 CH3 1-C3H7
CH3 CH3 CH3 1-C3H7
H CN CH3 l-C3H7
H F CH3 1-C3H7
H I CH3 l-C3H7
H SG2N(CH3)2 CH3 1-C3H7
C6H5 H CH3 1-C3H7
-(CH2)3- CH3 1-C3H7
-(CH2)4- CH3 1-C3H7
-(CH)4- CH3 1-C3H7
H C6H5 CH3 l-C3H7
C6H5 H CH3 1-C3H7
H OC6H5 CH3 1-C3H7
35 CF3 H CH3 i_C3H7
13~93 1~
- 1 1 9
~ EXAMPLE 22
Preparation of 5-(4-isopropyl-4-methyl-5-oxo-2-imidazo-
lin-2-yl)thieno[3,2-_]pyridine-6-carboxylic acid
~02H
NH2 - f- CONH2 . ~ 02H fH3
CH(CH3)2 CONH ~ - CONH2
CH(CH3)2
1. NcOH
2. H+
~ N CH(CH3)2
N
N 0
Thieno[3,2-b]pyridine-5,6-dicarboxylic acid
(2.5 g, 0.011 mol) is heated slowly to 85~C for one
hour with acetic anhydride (25 mL), then cooled, filtered
and washed with diethyl ether to give the anhydride as
3o a solid, mp 266-267~C. A mixture of the anhydride and
2-amino-2,3-dimethylbutyramide (2.6 g, 0.02 mol) in T~F
(70 mL) is stirred at room temperature for 15 hours.
1339315
-120-
After heating at reflux for two hours, the mixture is
cooled and diluted with THF (50 mL). Solid 5-[(1-car-
bamoyl-1,2-dimethylpropyl)carbamoyl]thieno[3,2-b]-
pyridine-6-carboxylic acid is filtered off, washed with
ether and dried. The above solid is mixed with an
aqueous 60 mL) solution of sodium hydroxide (6 g
0.05 mol) and heated at 85~C for two hours and 30
minutes, then set aside at room temperature overnight.
After cooling in an ice bath, the mixture is acidified
to pH 3 with concentrated hydrochloric acid. A solid
(3 g) is filtered off and dried. Crystallization from
ethyl acetate affords (5-(4-isopropyl-4-methyl-5-oxo-2-
imidazolin-2-yl)thieno[3,2-b]pyridine-6-carboxylic
acid, mp 242-244~C in 46% yield.
Utilizing the above procedure and substi-
tuting the appropriate pyridine-5,6-dicarboxylic acid
for thieno[3,2-b]pyridine-5,6-dicarboxylic acid yields
the compounds illustrated below.
-121- 1~3~3
~ ~02H CH3
Rlo /~/N\ - CH(CH3)2
N - - 0
Rg Rlo mp~C
H H 242- 244
H Cl 238-239
H Br 226-227
Cl H 266-267
2 C H 3
N - - 0
H
~,p 239- 241~C
133~315
-122-
EXAMPLE 23
Preparation of diethyl furo[3,2-~]pyridine-5,6-
dicarboxylate
~c ~=02C2H~
NH 2 ~ 2C2 H5
3-Amino-2-formylfuran, prepared from 3-azido-
2-formylfuran (8.9 g 0.065 mol) is dissolved in ethanol
and to this solution diethyl oxzlacetate (12.23 g,
0.065 mol) and ten drops of piperidine are added. In
addition pulverized 3A~ molecular sieve is added and
the reaction stirred at 65-60~C for three hours, then
additional diethyl oxalacetate (2.2 g) is added. The
reaction is essentially complete after 12 hours at
55-60~C. On cooling the reaction is filtered, and the
filtrate concentrated and then dissolved in ethyl
acetate, water washed, then brine washed, dried over
anhydrous magnesium sulfate and stripped to dryness.
The residue is dissolved in 3:1 hexane:ethyl acetate
and passed through a flash chromatographic column in
two stages. First it is filtered by vacuum through a
four to five inch pad of silica from which the last
three fractions containing the required product are
collected and combined. These combined fractions are
then passed through a six inch column eluting under
pressure with ethyl acetate:hexane 3:1 anc 2.1. Diethyl
furo~3,2-bjpyridine-5~6-dicarboxylate 4.15 g (24~) is
obtained after crystallization from hexane-ether, of mp
60-64~C, and with a mass spectrum m/e of 264.
133931~
-123-
Utilizing the above procedure and substituting
the appropriate furan for 3-amino-2-formylfuran yields
the compounds illustrated below.
R 9~ ~ ~~ 2 R "
Rlo 02R
N
Rg R10 R" mp~C
H H C2H5 60-64
H ClC2H5
CH3 H C2H5
H CH3C2H5
C2H5 H C2H5
H C2H5C2H5
CH3 CH3C2H5
3o
-124- 1 ~3931 5
- EXAMPLE 24
Preparation of furo~3,2-b~pyridine-5,6-dicarboxylic
acid
~ ~2C2H~ ~N
Furo[3,2-b]pyridine-5,6-dicarboxylic acid,
diethyl ester (1.1 g, 0.0042 mol) is dissolved in 95p
ethanol (20 mL) containing lOd aqueous sodium hydroxide
(20 mL) and set aside at 0~C for t~o days. The mixture
is cooled, acidified and the solvent removed under
reduced pressure. Water 5 mL is added and the hydrated
product diacid obtained as a brown solid by filtration,
3.31 g (99%), mp 183~C (dec). Anal calcd. as
CgH5N05.2 1/2 H20 C, 42.86; H,3.99; N,5.55 found:
C,42.63; H, 2.63; N,5.46.
3o
- 1~3~31~
-125-
EXAMPLE 25
Preparation of furo[3,2-b~pyridine-5,6-dicarboxylic
acid anhydride
~ C02H
0
Euro[3,2-b]pyridine-5,6-dicarboxylic acid
(3.3 g, 0.0159 mol) in acetic anhydride (100 mL) is
heated to 70-80~C for six hours. The reaction mixture
is cooled, filtered and the solid is washed with ether
to give 3.0l (100%) of crude furo[3,2-b]pyridine-5,6-
dicarboxylic acid anhydride.
3o
133~3~5- -
-l26-
- EXAMPLE 26
Preparation of 5-[(1-carbamoyl-1,2-dimethylpropyl)-
carbamoyl]furo[3,2-b]pyridine-6-carboxylic acid and
5-(4-isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl)furo-
[3,2-b]pyridine-6-carboxylic acid
C~/~ fH(CH3)2
,1 H 2 N~--C O N H 2
N ~ CH3
o
~2H fH3
ONH - C - CONH2
N
CH(CH3)2
l. NaOH
2. Acid
~ ~ O~H CH(CH3)2
N CH3
N N~ ~
3o
-127- 13~931~
Furo[3,2-b]pyridine-5,6-dicarboxylic acid
anhydride (3.01 g, 0.015 mol) is suspended in THF
(100 mL) to which 2-amino-2,3-dimethylbutyramide
(2.3 g, 0.018 mol) is added. After stirring for 20
hours, the solution is stripped to an oily solid which
dissolves in a water/dilute sodium hydroxide solution.
The alkaline solution is extracted with methylene
chloride, and then acidified and reextracted with
0 methylene chloride but on stirring only minute traces
of material is isolated. The water layer is concentrated
to an oily solid which is dissolved in ethanol, filtered
and concentrated to a purple gum which is predominantly
the crude product, 5-[(1-carbamoyl-1,2-dimethylpropyl)-
carbamoyl]furo[3,2-b]pyridine-6-carboxylic acid and is
used without further purification to prepare the final
2-imidazolin-2-yl product by dissolving it in 10%
sodium hydroxide solution (40 mL) and warming at 80~C
for three hours. On cooling the reaction is acidified
and a small amount of solid precipitated out and was
filtered off. Concentration of mother liquors gives a
second crop, which is collected and combined with the
first crop. Purification is effected by taking half of
the material and separating on silica gel preparative
glass plates as bands. The slower running band using
methylene chloride:ethyl acetate:chloroform: methanol
1:1:1:1 as eluant, affords the desired 2-imidazolin-
2-yl product, mp 214-223~C(dec), Esters may then be
prepared by the procedures described in Example 20.
13~9315
-128-
Utilizing the above procedure and substituting
the appropriate furo[3,2-b]pyridine-5,6-dicarboxylic
anhydride yields the compounds illustrated below.
~ ~~ 2 H CH 3
Rlo /~--// N--CH(CH3)2
N
N - ~
Rg Rlo mp~C
H H 214-223 (dec)
H Cl
CH3 H
H CH3
C2H5 H
H C2H5
CH3 CH3
1339~ 1 ~
-129-
EXAMPLE 27
Preparation of dimethyl thieno[2,3-b]pyridine-5,6-
dicarboxylate
C02CH3 DMF ~rC02CH3
C02CH3 POC13 ~ 1 /~C02cH3
A Vilsmeier reagent is prepared by adding
dropwise, with stirring, phosphorus oxychloride
(40.29 g, 0.26 mol) to a cooled (10~C) solution of DMF
(l9.0 g, 0.26 mol) in 1,2-dichloroethane (40 mL) in an
N2 atmosphere. This reagent is stirred at room
temperature for one hour and 45 minutes. Dimethyl-2-
thienylaminobutenedioate (63.4 g, 0.26 mol) dissolved
in 1,2-dichloroethane (300 mL) is added dropwise to the
Vilsmeier reagent at 7-10~C. The reaction temperature
is raised to room temperature for 15 minutes, then to
reflux for 12 hours. The cooled reaction mixture is
concentrated and the residue chromatographed on a
silic2 gel column with ethyl acetate-hexane, affording
dimethylthieno[2,3-b]pyridine-5,6-dicarboxylate (29 g,
45Z) as a solid.
3o
133931S
-130-
Utilizing~the above procedure and substituting
the appropriate dimethyl-2-thienylaminobutenedioate
yields the compounds illustrated below.
R5 ~ C02CH3
Rlo~ ~ ~) {02CH3
Rg R1o mp~C
CH3 H 80-82
H H solid
H CH3
CH~ CH3
H C6H5
C6H5 H
CF3 H
3o
-131- 133~31~
EXAMPLE 28
Prepzration of dimethyl 3-bromothieno[2,3-b]pyridine-
5,6-dicarboxylate
C02CH3 Br2 Br ~ ~ 02CH3
C02CH3 HOAc 02CH3
S N NaOAc S N
Bromine (0.33, 0.00206 mol) in acetic acid
(8 mL) is added to a stirred solution of dimethyl-
thieno[2,3-b]pyridine-5,6-dicarboxylate (0.5 g,
0.00187 mol) in acetic acid containing sodium acetate
(0.31 g, 0.00377 mol) at 40~C. The reaction mixture is
heated at 75~C for 18 hours. Evaluation of the mixture
by tlc (silica gel) indicated incomplete reaction.
Additional bromine (0.33 g) in acetic acid and sodium
acetate (0.31 g) is added and heating at 75~C continued
for six hours. The reaction mixture is diluted with
water and extracted into ethyl acetate. The separated
organic layer is dried over anhydrous MgS04, filtered,
and the filtrate concentrated to an oil which solidifies
on standing. Crystallization of the crude product from
ethyl acetate-hexanes yields the dimethyl 3-bromo-
thieno~2,3-b]pyridine-5,6-dicarboxylate as white needles
mp 86-87.5~C.
- This compound may readily be converted to a
variety of substituted-thieno[2,3-b]pyridine compounds
as illustrated below, while electrophilic substitution
such as nitration or halogenation yields the additional
compounds also listed be~ow.
1339315
-132-
Rl o~ 0 2 CH 3
R~ 02CH3
S N
Rc, Rlo mp~C
H H
H Cl 104-110
H Br 86-87.5
H
H - F
H CN
H SCH3
H OCH3
H N(CH3)2
H OCHF2
H NO2
H CHO
H CH2Cl
CH3 H 80-82
H CH3
Cl H
Cl Cl 84-89
CH3 CH3
H SO2N(CH3)2
-(CH2)4-
3~ -(CH)4-
-(CH2)3-
C6Hs H
H C6H5
H OC6H5
CF3 H
-133- 133~315
- EXAMPLE 29
Preparation of thieno[2,3-b]pyridine-5,6-dicarboxylic
acid
~2CH3 KOH ~ 02H
02CH3 C02H
S N S N
A solution containing dimethyl thieno[2,3-b]-
pyridine-5,6-dicarboxylate (27.75 g, 0.11 mol) and
potassium hydroxide (30.98 g, 0.55 mol) in methanol
(200 mL) under a N2 atmosphere is heated at reflux for
two hours. The reaction mixture is cooled and suffi-
cient water added to dissolve any solids present before
evaporating the mixture to dryness. The resulting
solid is dissolved in a minimum volume of water, cooled
in an ice bath and acidified with concentrated H2S04 to
pH~1. Thieno[2,3-b]pyridine-5,6-dicarboxylic acid is
filtered off and dried overnight to give 23.36 g mp
272-275~C.
Utilizing the above procedure and substitu-
ting the appropriate substituted dialkylthieno[2,3-b]-
pyridine-5,6-dicarboxylate yields the compounds
illustrated below.
3o
1339315
-134-
R 1 0~ ~CC~ 2 H
Rg C02H
S N
Rg Rlo mp~C
H H 272-275
H Cl >300
H Br >315
H
H F
H CN
H SCH3
H OCH3-
H N(CH3)2
H OCHF2
H NO2
H CHO
H CH2Cl
H CH3 180-183 (dec)
CH3 H
Cl H
Cl Cl
CH3 CH3
C6Hs H
H SO2N(CH3)2
-(CH2)3-
_(CH2)4-
-(CH)4-
H OC6H5
H C6H5
CF3 H
- 133~31~
-135-
EXAMPLE 30Preparation of thieno[2,3-b]pyridine-5,6-dicarboxylic
anhydride
~C02H Ac 2~ ~\
~ "ko 2 H P y r, DM E
S N S N
0
Acetic anhydride (37.4 g, 0.366 mol) is added
to a stirred suspension of thieno[2,3-b]pyridine-5,6-
dicarboxylic acid (21.52 g, 0.096 mol) in dimethoxy-
ethane (175 mL) in an inert N2 atmosphere. Upon addition
of pyridine (16.78 g, 0.21 mol) at room temperature an
exotherm to 45~C is observed and a homogeneous so~ution
results. The reaction mixture is then stirred at room
temperature and the resulting solid filtered off, washed
with ether and air dried to give 14.8 g (75p) of thieno-
[2,3-b]pyridine-5,6-dicarboxylic acid anhydride.
Utilizing the above procedure and substituting
the appropriate substituted thieno[2,3-b~pyridine-5,6- -
dicarboxylic acid yields the compounds illustrated
below.
3o
13333IS
-136-
R9 ~ 5 ~
Rg R10 mp~C
CH3 H 176-180
H Br 228.5-231
H Cl 230-300
(slow dec)
H ~,
H
H
H F
H CN
H SCH3
N(CH3)2
H N02
H CH0
H CH2Cl
H CH3
CH3 H
Cl
.Cl Cl
CH3 CH3
C6H5 H
H S02N(CH3)2
-(CH2)3-
-(CH2)4-
_(CH)4-
. H C6H5
H 0c6Hs
CF3 H
133931~i
-l37-
EXAMPLE 31
Preparation of 6-[(1-carbamoyl-1,2-dimethylpropyl)-
carbamoyl]thieno~2,3-b]pyridine-5-carboxylic acid
~\ fH3
1 ~ +H2N~ONH2
S N ~ CH(CH3)2
0
C02H fH3
CONH~{ONH2
S N
CH(CH3)2
2-Amino-2,3-dimethylbutyramide (9.84 g,
0.076 mol) is added to a stirred suspension of thieno-
[2,3-b]pyridine-5,6-dicarboxylic acid anhydride
(14.~ g, 0.072 mol) in TH~ under an inert atmosphere of
N2 at room temperature. The dark solution is stirred at
room temperature overnight and the resulting solid
filtered off, washed with TH~ and air dried to give
17.35 g (72p) of 6-[(1-carbamoyl-1,2-dimethylpropyl)-
carbamoyl]thieno[2,3-b]pyridine-5-carboxylic acid.
Utilizing the above procedure and substituting
the appropriate substituted thieno[2,3-b]pyridine-5,6-
dicarboxylic acid anhydride ylelds the compounds
illustrated below.
1339~15
-138-
Rlo~ ~02H fH3
Rg CH(CH3)2
Rg Rlo mp~C
CH3 H 207-208
H Br 176-178
Cl 156-158
H H
H
H F
H CN
H SCH3
H OCH3
N(CH3)2
H N02
H CHO
H CH2Cl
H CH3
Cl H
Cl Cl
CH3 CH3
C6H5 H
H S02N(CH3)2
-(CH2)3-
-(CH2)4-
_(CH)4-
H C6H5
H OC6Hs
CF3 H
-l39-
EXAMPLE 32 13 3 ~ 31~
Preparation of 6-(4-isopropyl-4-methyl-5-oxo-2-imidazo-
lin-2-yl)thieno[2,3-b]pyridine-5-carboxylic acid
02H fH3
S N CONH - IC { ONH2
CH(CH3)2
02H CH3
N CH(CH3)2
S N N - O
6-[(1-Carbamoyl-1,2-dimethylpropyl)carbamoyl]-
thieno[2,3-b]pyridine-5-carboxylic acid (17.35 g,
0.052 mol) is added to water (225 mL) containing sodium
hydroxide (10.35 g, 0.26 mol). The resulting basic
solution is heated at 80~C for two hours and 45 minutes,
cooled in an ice-water bath and acidified with 6N H2S04.
The product 6-(4-isopropyl-4-methyl-5-oxo-2-imidazolin-
2-yl)thieno[2,3-b]pyridine-5-carboxylic acid is filtered
off, washed with water and air dried yielding 1.54 g,
70.3%, mp 221-223~C.
133931~
-l40-
EXAMPLE 33
Preparation of 2-isopropyl-2-methyl-5H-Imidazo[1',2':1,2]-
pyrrolo[3,4-_}thieno[3,2-_]pyridine-3(2~),5-dione
c=0
N CH(CH3)2
CH3
DCC
N~ \~ CH(CH3)2
CH3
Dicyclohexylcarbodiimide (1.07 g, 0.005 mol)
2 in methylene chloride (20 mL) is added dropwise to a
stirred methylene chloride (30 mL) suspension of 6-
(4-isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl)thieno-
[2,3-b]-5-carboxylic acid (1.5 g, 0.0047 mol) under an
N2 atmosphere. After stirring the reaction mixture for
16 hours, it was clarified by filtration, concentrated
to dryness and the resulting material purified by column
chromatography on silica gel eluting with acetonitrile/
methylene chloride (1/2). The solid product was crystal-
lized from toluene to give the pure 3,5-dione as white
3~ crystals mp 214.5-216.5~C.
1~3931~
-141-
EXAMPLE 34
Preparation of 2-propynyl 6-(4-isopropyl-4-methyl-5-
oxo-2-imidazolin-2-yl)thieno[2,3-b]pyridine-5-carbo-
xylate
< H O H 2 CC _ CH
S N N CH(CH3)2
CH3
~ 02CH2C-CH
/~ H
S N ~N~o
N CH(CH3)2
CH3
Sodium hydride (2.4 g, 60p, 0.126 mol) is
added to the 3,5-dione (0.9 g, 0.003 mol) in propargyl
alcohol (25 mL) at 10~C under an inert N2 atmosphere.
The reaction mixture is stirred at room temperature for
60 hours and then neutralized with a saturated ammonium
chloride solution. The resulting mixture is concentrated
on a rotary e~aporator, diluted with water and extracted
with ethyl acetate. The organic layer is separated,
dried over anhydrous MgSO4 and concentrated to dryness.
Purification of the product by column chromato-
graphy on silica gel with methylene chloride/acetonitrile
(85/15) yields 2-propynyl 6-(4-isopropyl-4-methyl-5-oxo-
2-imidazolin-2-yl)pyridine-5-carboxylate, which after
crystallization from toluene has a mp 188-189.5~C.
1339315
-142-
Utilizing the procedures of Examples 49, 55,
56 and 57 and substituting the appropriate thieno or
furo[3,2-b.]pyridine or thieno or furo[2,3-b]pyridine
compounds, yields the compounds illustrated below.
R~ ~ ~ /N CH3
~ N N - 0
1 B Rg Rlo R mp~C
S H H CH3 215-217
S H H H 220-223.5
(dec)
S H H -CH2C_CH 188-189.5
S H H 11 1 140-142
-CH
CH3
S H H -CH2~=CH2 108-110
S CH3 H H 225~5-227.5
S H Br H 274-276
S H Cl H 266-267
0 H H H 237-244
S H No2 -CH3 201-202.5~C
S H N02 H impure
3o
133931~
- 143-
B Rg R l o R mp~C
S Cl H H 268 (dec)
S H CH3 H 255 - 257
S - (CH2) 4- H 234 - 237
0 H Cl H 239 - 240
O H H CH3 134 - 137
O H Br H 239 - 245
O CH3 H H 174 - 177
~ C2H5 H H 170 - 172
0 C6Hs H H 244 - 245
0 H Cl CH3 137 - 141
r3
0 H H - CH2 0 137 - 141
H H -CH2C_CH 150 - 156
R 9~ ~/N C H 3
N N - =0
B Rlo Rg R mp~C
S H H H 242 - 244
S H Cl H 238 - 239
S H Br H 226 - 227
S H H ~ 156 - 157
- CH2
0 H H H 214 - 223
S Cl H H 266 - 267
1339315
~144-
EXAMPLE 35
Preparation of methyl 5-(4-isopropyl-4-methyl-5-oxo-2-
imidazolidinyl)furo~3,2-b]pyridine-6-carboxylate
~N N~ O
1 0 NaCNBH3
OOCH3
~ ~I H CH(CH3)2
N ~ CH3
N --O
A solution of 22.1 mmol of methyl 5-(4-iso-
propyl-4-methyl-5-oxo-2-imidazolin-2-yl)furo[3,2-b]-
pyridine-6-carboxylate in methanol is cooled to 0~C and
a few drops of methyl orange indicator added. The
solution is stirred and treated with 22.1 mmol of
concentrated hydrochloric acid. The solution is then
treated with 22.1 mmol of sodium cyanoborohydride, and
the pH maintained at ~3 by the additon of 2N methanolic
HCl, stirred overnight, cooled to 0~C and the pH of the
solution adjusted to about O with HCl to decompose
residual NaCNBH3. The pH is thereafter adjusted to 5-6
- with 5N NaOH. The methanol is removed in vacuo and
3~ water added to dissolve inorganic salts. The mixture
is extracted with CH2C12 and the extracts dried and
concentrated to give the title compound.
133331s
-145-
Utilizing the above procedure with the appro-
priately substituted methyl 2-(2-imidazolin-2-yl)-
furo[3,2-b]pyridine-6-carboxylate yields the corres-
ponding 2-(2-imidazolidinyl)furo[3,2-b]pyridine-6-
carboxylate. Similarly, reaction of the appropriately
substituted methyl 2-(2-imidazolin-2-yl)thieno[3,2-b]-
pyridine-6-carboxylate yields the corresponding methyl
2-(2-imidazolinyl)thieno[3,2-b]pyridine-6-carboxylate.
The reaction products are illustrated below:
R 9~ \~O OCH 3
RlO ~ N CH3
N ~ / --CH(CH3 )2
HN ~
Similarly, using the above procedure with the
appropriately substituted methyl 2-(2-imidazolin-2-yl)-
dihydrofuro- or dihydrothieno[3,2-b]pyridine-6-carbo-
xylate yields the corresponding substituted methyl
2-(2-imidazolidinyl)dihydrofuro- or dihydrothieno-
[3,2-b]pyridine-6-carboxylate. The reaction products
are illustrated below:
R ll ~ ~ 0 OCH 3
Rl 2 1~ N/~ N--CH ( CH3 ) 2
3~ HN~
1339315
-146-
B W Rg Rlo mp~C
0 O H H
S S H H
5 S 0 H H
0 S H H
0 O H Cl
S S H Cl
S 0 H Cl
10 0 S H Cl
~ 0 CH3 H
S S CH3 H
S ~ CH3 H
O S CH3 H
0 0 H CH3
S S H CH3
S 0 H CH3
0 S H CH3
~ ~ C2H5 H
20 S S C2H5 H
S ~ C2H5 H
0 S C2H5 H
0 0 H C2H5
S S H C2H5
S 0 H C2H5
0 S H C2H5
~ 0 CH3 CH3
S S CH3 CH3
S ~ CH3 CH3
0 S CH3 CH3
0 0 H Br
S S H Br
S 0 H Br
0 S H Br
1339315
-147-
EXAMPLE 36
Preparation of cis- and trans-methyl 6-(4-isopropyl-4-
methyl-5-oxo-2-imidazolidinyl)thieno[2,3-b]pyridine-5-
carboxylates
COOCH3
~S ~ N ~ / N CH(CH3)2
HN~O
Na CNB H 3
N \ I ~3HOCCHH33
IICH(CH3)2 Hl IICH(CH3)2
Using essentially the szme procedure as
described in Example 58 but substituting methyl 6-(4-
isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl)thieno[2,3-b]-~
pyridine-5-carboxylate for methyl 5-(4-isopropyl-4-
methyl-5-oxo)-2-imidazolin-2-yl)furo[3,2-b]pyridine-6-
carboxylate yields the two products cis-methyl 6-(4-
isopropyl-4-methyl-5-oxo-2-imidazolidinyl)thieno[3,2-b~-
pyridine-5-carboxylate mp 185-186~C and trans-methyl
6-(4-isopropyl-4-methyl-5-oxo-2-imidazolidinyl~thieno-
3~ [2,3-b]pyridine-5-carboxylate mp 145-148~C.
-148_ 133~315
Utilizing the above procedure with the appro-
priately substituted methyl thieno-,dihydrothieno-,
furo- or dihydrofuro[2,3-b]pyridine-5-carboxylate
yields the reaction products illustrated below.
.
Rlo ~ OOCH3
R9 ~ 1 ~1 N CH3 and
B N ~ ~ CH(CH3)2
HN - W
R12 ~ OOCH3
Rll /~ N CH3
B N ~ ~ CH(CH3)2
HN - -W
thus~ OOCH3
0 N ~ / ~ CH(CH3)2 yields
HN~ ==O
25 ~ OOCH3 ~ OOCH3
/ ~ N CH3 and O N ~ N ;
H ¦ IICH(CH3)2 Hll IICH(CH3)2
HN---~-O HN - _ O
mp 170-174~C mp 166-169~C
l3393ls
-149-
EXAMPLE 37
Preparation of trans-6-(4-isopropyl-4-methyl-5-oxo-2-
imidazolidinyl)thieno[2,3-~]pyridine-5-carboxylic acid
OOCH3
H ~H3
HN--O
Na OH
~OOH
N ~CH3
HN--o
Using essentially the same conditions as
those described in Example 32 but substituting trans-
methyl 6-(4-isopropyl-4-methyl-5-oxo-2-imidazolidinyl)-
thieno[2,3-b]pyridine-5-carboxylate for cis-methyl
6-(allyloxy)-2-(4-isopropyl-4-methyl-5-oxo-2-imidazo-
lidinyl)nicotinate gives the product trans-6-(4-isopropyl-
4-methyl-5-oxo-2-imidazolidinyl)thieno[2,3-b]pyridine-
5-carboxylic acid, mp 225-226~C as a sesquihydrate.
3o
133~31~
-150-
Similiary, the furano analogs yield the cor-
responding imidazolidinones below:
OOH
N ~H3
HN - ~
mp 210-218~C
and
OOH
~H CH3
HN _ O
mp 176-178~C
13~9~15
-151-
EXAMPLE 38
Preparation of diethyl 5-acetyl-l,6-dihydro-6-oxo-2,3-
pyridinedicarboxylate
~ ~ ~C2Hs f~~C2H5
CH3 { { H2 { NH2 + O = C ~ { = O
CH--~C2 H5
Sadium acetate
~1 ~
CH3{~/ ~C~2c2H5
o=l~ ~CO 2C2 H5
H
Sodium acetate (30 g, 0.37 mol) is added to
a stirred mixture of diethyl(ethoxymethylene)oxalacetate
(87 g, 0.36 mol) and acetoacetamide (36 g, 0.36 mol) in
absolute ethanol (300 mL). After stirring the reaction
mixture for 30 minutes, the ethanol is distilled off
under reduced pressure, the residue acidified to pH 2
with dilute aqueous hydrochloric acid and the resulting
solid filtered off. Crystallization from an ethanol-
water mixture affords diethyl 5-acetyl-l,6-dihydro-6-
oxo-2,3-pyridinedicarboxylate as crystals mp 200-209~C.
3o
133931~
-152-
EXAMPLE 39
Preparation of diethyl 5-(bromoacetyl)-1,6-dihydro-
6-oxo-2,3-pyridinedicarboxylate
CH3-C ~ 02C2H5 Br2 BrCH2- ~ 02C2H5
0 02C2Hs HBr O 02C2Hs
N N
1O H H
Bromine (8.0 g, 0.050 mol) in 48d HBr is
added dropwise to a stirred solution of diethyl-5-
acetyl-1,6-dihydro-6-oxo-2,3-pyridinedicarboxylate
(14.05 g, 0.05 mol) in 48~ ~Br (200 mL). Upon com-
pletion of this bromine addition the reaction mixture
is poured onto ice (200 g) and the mixture is stirred
until the ice has melted. The crude product is col-
lected by filtration and crystallized twice from an
ethyl acetate-hexane mixture (1/2) affording diethyl
5-(bromoacetyl)-1,6-dihydro-6-oxo-2,3-pyridinedicarboxylate
with mp 141-142~C.
1339315
-153-
EXAMPLE 40
Preparation of diethyl 5-(2-bromo-1-hydroxyethyl)-1,6-
dihydro-6-oxo-2,3-pyridinedicarboxylate and diethyl
2,3-dihydro-3-hydroxy-furo[2,3-b]pyridine-5,6-dicarbo-
5 xylate
B'CH2-C ~ 02C2H5 NaBH4
~ ~ ~ C~2C2H5 i-C3H70H
N
OIH
BrCH2 { H ~ (C2H5)3N
150 ~ ~ C02C2H5
HN
011~C02C2H5
l l~ ~ 02C2H5
0 N
Sodium borohydride (2.54 g, 0.066 mol) is
added in portions over a 30 minute period to a stirred
suspension of diethyl 5-(bromoacetyl)-1,6-dihydro-6-
oxo-2,3-pyridinedicarboxylate (57.2 g, 0.159 mol) at
10-20~C. Upon completion of the sodium borohydride
addition, the reaction mixture is stirred while attaining
room temperature. Ice (100 g) is added and the mixture
stirred until the ice has melted. The mixture is then
concentrated in vacuo and the residue crystalli~ed
twice from an ethyl acetate-hexane mixture to give pure
diethyl 5-(2-bromo-1-hydroxyethyl)-1,6-dihydro-6-oxo-
2,3-pyridinedicarboxylate mp 134-138~C.
1~39315
-154-
Stirring this compound with triethylamine (1.0 mL/g of
solid) in methylene chloride for one hour, followed by
washing the organic solution with dilute hydrochloric
acid, water, brine and drying over anhydrous MgS04
yields the crude furo[2,3-b]pyridine as an oil upon
removin~ the solvent in vacuo. Crystallization from a
cyclohexane-toluene mixture affords pure diethyl 2,3-
dihydro-3-hydroxy-furo[2,3-b]pyridine-5,6-dicarboxylate
mp 73-77~C.
3o
13~9315
-155-
EXAMPLE 41
Preparation of diethyl furo[2,3-b]pyridine-5,6-dicarbo-
xylate
HO~ ,~Cco2c2H5 p-toluene sulfonic acid
C02C2H5 ~ xylene
O N
~(~02C2H5
~ 2C2 Hs
0 N
A xylene solution of the hydroxy-furo compound
obtained in Example 61, t3.7 g) containing para-toluene
sulfonic acid (0.01 g) is heated at reflux for two
hours. The solution is cooled and the xylene solution
decanted off. The residue is extracted with ether and
the extracts combined with the xylene. Distillation of
the solvents gives a yellow solid which is crystallized
from a cyclohexane-toluene mixture to give pure diethyl
furo[2,3-b]pyridine-5,6-dicarboxylate mp 66-77~C.
133931~
-156-
EXAMPLE 42
Preparation of furo[2,3-b]pyridine-5,6-dicarboxylic
acid
02C2Hs KOH ~ ~ 02H
O N 02C2H5 ~ N C02H
1.0
Potassium hydroxide (5.60 g, 85~" 0.087 mol)
in water (5 mL) is added to a stirred suspension of
diethyl furo[2,3-b]pyridine-5,6-dicarboxylate (9.3 g,
0.035 mol) in absolute ethanol (100 mL). The reaction
mixture is heated at 60~C for one hour, then cooled and
anhydrous acetone added. The precipit2te is filtered
off, dried, suspended in dry acetone and treated with
hydrogen chloride to adjust to z pH of 2. Crystal-
lization of the isolated solids from an ethyl acetate-
acetone mixture affords furo[2,3-b]pyridine-5,6-dicarbo-
xylic acid mp 189-192~C.
3o
1339315
-157-
EXAMPLE 43
Preparation of furo[2,3-b]pyridine-5,6-dicarboxylic
. anhydride
02H acetic anhydride
02H
O N
~0
O N
0
Furo~2,3-b]pyridine-5,6-dicarboxylic acid
(6.7 g, 0.032 mol) is heated at 60~C for 30 minutes in
acetic anhydride (l50 mL). The reaction mixture is
cooled to room temperature and concentrated in vacuo .
and the residue triturated with cyclohexane-ether(5:l),
filtered off and dried to give 5.35 g furo[2,3-b]-
pyridine-5,6-dicarboxylic acid anhydride.
3o
-158- 133931~
EXAMPLE 44
Preparation of 6-[(1-carbamoyl-1,2-dimethylpropyl)-
carbamoyl]-furo[2,3-b]pyridine-5-carboxylic acid
~ ~ O +NH2 1 ~ONH2
~ N ~ CH(CH3)2
O2H fH3
ONH ~ --CONH2
O N CH(CH3)2
2-Amino-2,3-dimethylbutyramide (2.1 g,
0.016 mol) is added to a stirred suspension of furo-
[2,3-b]pyridine-5,6-dicarboxylic acid anhydride (3.0 g,
0.016 mol) in tetrahydrofuran (7.5 mL) and the mixture
allowed to stir at room temperature for 16 hours. The
reaction mixture is then stirred at 60~C for one hour,
cooled to room temperature, ether added, and the solid
filtered off and dried to give 5 g of 6-[(1-carbamoyl-
1,2-dimethylpropyl)carbamoyl]furo[2,3-b]pyridine-5-
carboxylic acid mp 192-196~C (dec).
13~9315
-159-
- EXAMPLE 45
Preparation of 6-(4-isopropyl-4-methyl-5-oxo-2-imidazo-
lin-2-yl)furo[2,3-b]pyridine-5-carboxylic acid
c02H fH3 H20
O N ONH--C~ONH2 NaOH
CH(CH3)2
02H CH3
~ CH(CH3)2
O N N--O
A solution containing 6-t(l-carbamoyl-1,2-
dimethylpropyl)carbamoyl]furo[2,3-b]pyridine-5-carbo-
xylic acid (3.8 g, 0.012 mol) in aqueous sodium hydro-
xide 2.4 g, o.o6 mol) in water (40 mL) is stirred at
65~C for three hours. The reaction mixture is then
heated at 75~C for one hour, allowed to cool, poured
into ice, acidified to pH 2-3 and the resulting solid
filtered off and dried. Crystallization from an acetone-
methanol mixture affords pure 6-(4-isopropyl-4-methyl-5-
oxo-2-imidazolin-2-yl)furo[2l3-b~pyridine-5-carboxylic
acid mp 237-244~C.
3o
.
133931~
-160-
EXAMPLE 46
Preparation of 2,3-dihydro-6-(4-isopropyl-4-methyl-5-
oxo-2-imidazolin-2-yl)furo[2,3-_]pyridine-5-carboxylic
acid
02H CH3
CH(CH3)2 5% Pd-C
o N ~ o
~ C02H CH3
~ 1~ ~ ~ CH(CH3)2
o N N --0
A solution of 6-(4-isopropyl-4-methyl-5-oxo-
2-imidazolin-2-yl)furo[2,3-b]pyridine-5-carboxylic acid
(1.7 g 0.056 mol) and 1.0 g (0.0072 mol) potassium
car-oonate in 200 mL 9:1 ethanol:water is added to 100 mg
5~ palladium on carbon catalyst in a 500 mL pressure
bottle. The bottle is fitted to a Parr hydrogenation
apparatus, pressurized to 30 psi, with hydrogen, then
sha~en at room temperature for 10 hours. The catalyst
is removed by filtration through a sintered glass funnel,
and the filtrate concentrated in vacuo to 10 mL. Acidi-
fication of the residue to pH 2 gives a white precipi-
3o tate which is removed by filtration, washed with waterand air dried to give 1.0 g (63%) of 2,3-dihydro-6-(4-
isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl)furo~2,3-b]-
pyridine-5-carboxylic acid as an off-white solid, mp
189-192~C.
133~315
-161-
EXAMPLE 47
Preparation of 4-mercaptoacetyl butyronitrile
CH3CSH + BrCH2CH2CH2CN
CH3--C--S--CH2CH2CH2CN
Thiolacetic acid (49 mL, 0.69 mol) is added
to potassium carbonate (93.4 g, o.68 mol) dissolved in
water (150 mL). Ethanol (260 mL) is added and then
4-bromobutyronitrile is added at 15 to 28~C and the
reaction mixture stirred at room temperature for 16
hours. The resulting inorganic solids are filtered o.r
and the filtrate extracted with toluene. The organic
layer is separated, dried over anhydrous Na2S04 and
concentrated to give the desired 4-mercaptoacetyl
butyronitrile as a yellow oil.
- 133931~
-162-
EXAMPLE 4B
Preparation of dihydrothiophenimine hydrochloride
CH3-C-S-CH2-CH2CH2CN HCl
~ NH HCl
S
Hydrogen chloride is introduced to a cooled
solution of the nitrile in methanol (220 mL) for one
hour and the mixture then stirred at room temperature
for 16 hours. The resulting product is filte~ed off,
washed with ether and dried to give 55.38 g of dihydro-
thiophenimine hydrochloride, mp 189-195~C.
1339315
-163-
EXA~PLE 49
Preparation of dimethyl ~(tetrahydro-2-thienylidene)_
amino]fumarate (and maleate)acid
~ f~2C~3
N~ HCl + C f
C02CH3
~02CH3
N ~C--C02CH3
5 . Dimethylacetylenedicarboxylate (0.45 mL,
0.037 mol) is added to a stirred solution of dihydro-
thiophenimine hydrochloride (0.5 g, 0.0036 mol) in
methanol (60 mL) containing sodium acetate (0.3 g,
0.0036 mol) under an inert N2 atmosphere at -15~C.
After stirring for 16 hours at room temperature, the
solvent is removed on a rotary evaporator and the
resulting mixture separated by column chromatography on
silica gel eluting with a methylene chloride-aceto-
nitrile mixture (19:1) yielding 0.68 g (78% yield) of
2~ the desired mixed isomeric acid esters as a yellow oil.
1339315
-164-
EXAMPLE 50
Preparation of dimethyl 2,3-dihydrothieno[2,3-b]-
pyridine-5,6-dicarboxylate
02CH3 oxalyl chloride
ll DMF
\ ~ ~ /--C02CH3
S N
~{02CH3
,1~ ,~C02CH3
S N
A Vilsmeier reagent is prepared by adding
oxalyl chloride (0.25 mL, 0.0028 mol) to a stirred
solution of DMF (0.22 mL, 0.0028 mol) in 1,2-dichloro-
ethane (50 mL) at room temperature in an inert N2
atmosphere. A 1,2-dichloroethane (50 mL) solution of
dimethyl [(tetrahydro-2-thienylidine)amino~fumarate
(and maleate) (0.0028 mol) is added to the Vilsmeier
reagent and the reaction mixture heated at reflux for
four hours. The reaction mixture is quenched with
water, made basic with sodium bicarbonate and the
organic layer separated and dried over anhydrous Na2S0~.
The solvent is removed in vacuo and the
residue purified by column chromatography on silica
gel, eluting with a methylene chloride-acetonitrile
mixture (19:1). Crystallization from toluene-hexane
affords dimethyl 2,3-dihydrothieno[2,3-b]pyridine-5,6-
dicarboxylate as a white solid with mp 102-103.5~C.
1339315
-165-
EXAMPLE 51
Preparation of 2-isopropyl-2-methyl-5H-imidazo[1',2':1,2]-
pyrrolo[3,4-b]pyridine-3(2~),5-dione
OOH ~ - o
N C~H3 DCC CH3
N ~/ ~ CH(cH3)2 N N CH(cH3)2
HN - - ~
To a solution containing 50.9 g of dicyclo-
hexylcarbodiimide in 600 mL of dry methylene chloride
is added, while stirring, 60 g of the acid at such a
rate that the temperature does not exceed 32~C. After
stirring at room temperature for two and one-half hours,
the mixture is filtered and the filtrate concentrated
to give a white solid. This solid is recrystallized
from methylene chloride to give 57.4 g of the dione, mp
125-128.5~C. The analytically pure dione melts at
132-134~C. The procedure is illustrated in European
Patent Application 0,041,023, published December 16, 1981.
Using essentially the same procedure but
substituting the appropriate acid for 2-(4-isopropyl-
4-methyl-5-oxo-2-imidazolin-2-yl)nicotinic acid, the
following imidazopyrrolopyridine-3,5-diones are prepared.
1339315
- - 1 6 6 -
o o o o ~ o o o
C7 o o ~ ~ U~ ~ o
U~ o o o o o o o
oo o CO ~ oo ,'
a~ o c~ ~ ~ ~ o
~ T
._ Z
i- ~ 2 '-- 2
X--<\ Z ~2~ 2 2 ~ 2 _ -- 2
.~ b'
c~ ~ 2
~ ,2
2 2 ~ r o ~
X ¦ 2~ 2 2 2 2 2 2 2
3 1 0 0 0 0 0 0 V~
l339315
- 1 6 7 -
o o o o
t~ a~ ~ o
C'~
o~ l l l l
~ o o o o
~o ~)
_,
S~ ~ ~
S S S
CS~ C~
2 2
C" 0, 0,
S ~ ~ .
S CS~ C~
~ I O S ~
xl S ~r S S S S
3 1 cr~ U~ o o o o
l3393l5
-168-
EXAMPLE 52
Preparation of 3-isopropyl-3-methyl-5H-imidazo[1',2':1,2]-
pyrrolo[3,4-b]pyridine-2(5H),5-dione
.~\ CH 3
N N CH(cH3)2
NaOAC
Ac20
o
~ CH ( CH 3 ) 2
N N
To a solution containing 0.5 g 2-isopropyl-2-
methyl-5H-imidazo[1',2':1,2]pyrrolo[3,4-b]pyridine-3(2H),
5-dione in 5 mL acetic anhydride is added 50 mg sodium
acetate and the mixture heated at reflux for two hours.
The solvent is removed and the product extracted into
ether to give the rearranged dione, 3-isopropyl-3-methyl-
5H-imidazo[1',2':1,2]pyrrolo[3,4-b]pyridine-2(3H)5-dione
mp 107-115~C.
-169- 133 93 15
EXAMPLE 52-A
5~ 00 HCH
CH(CH3)2
HN----S
10( CF3CO ) 2~
~\ S
15N N
To a solution of the acid in 200 mL toluene
and 100 mL CH2C12 under nitrogen is added with stirring
at -60~C during 0.5 hours, 1.9 g trifluoroacetic anhy-
dride dissolved in 18 mL toluene and 7 mL CH2C12. After
this reaction, 500 mL hexane is added and the mixtu.e
concentrated in vacuo to give a dark green solid. NMR
spectroscopy showed this to consist of 80% of the
desired 2,5-dione and 20% of 3,5-dione.
133931S
-170-
The 2,5-dione was characterized as its water
addition product, mp 162-165~C with structure
~ CH3
~ ~ N CH(CH3)2
~\ ~1~ S
OH
Using essentially this procedure or those
described in Examples 51, 52 and 52-A and starting with
the appropriate acid or 3,5-dione, the following
2,5-diones are obtained.
Y /'~' N R2
Z~\ ~\ ~
N N
W X Y Z Rl R2 mp~C
O H H C3H7 C~3 CH(CH3)2
O H H CH(CH3)2 CH(CH3)2
O H CH(CH3)2 H CH3 CH(CH3)2
O 11 C2H5 H CH3 CH(CH3)2
O H H C2H5 CH3 CH(CH3)2
O H OCH(CH3)2 H CH3 CH(CH3)2
S H H H CH3 CH(CH3)2
S H CH3 H CH3 CH(CH3)2 .
S H C21~5 H CH3 CH(CH3)2
S H -CH=CH-CH=CH- C113 CH(CH3)2 ~-~
c~
W X Y Z Rl R2 mp~C
S H -CH=CH-S- CH3 CH(CH3)2
S H -CH=CH~O- CH3 CH(CH3)2
O H H OCH3 CH3 CH(CH3)2147.0 - 147.5
O H OCH3 H CH3 CH(CH3)2
o H ~3 H ~3 CH(CH3)2
133~31s
-173-
The procedures of Examples 51, 52 and 52-A
may be graphically illustrated as follows:
Y ~ O O H
N ~ / N - R2
HN W
DCC
( C F 3 CO ) ~ O Y~ ~_W AC ~ O/HO A c
when W = S z Rl when W = O
N R2
HOAc/Na OAc
, ~ \
N N
NaBH4
J 3~ Rl
N ~ HN
1339~1~
-174-
EXAMPLE 53
Postemergence herbicidal evaluation of test compounds
The postemergence herbicidal activity of the
compounds of the present invention is demonstrated by
the following tests, wherein a variety of monocotyledo-
nous and dicotyledonous plants are treated with test
compounds dispersed in aqueous acetone mixtures. In
the tests, seedling plants are grown in jiffy flats for
about two weeks. The test compounds are dispersed in
50/50 acetone/water mixtures containing 0.5% TWEEN~ 20,
a polyoxyethylene sorbitan monolaurate surfactant of
Atlas Chemical Industries, in sufficient quantities to
provide the equivalent of about .016 to 10.0 kg per
hectare of active compound when applied to the plants
through a spray nozzle operating at 40 psig for a pre-
determined time. After spraying, the plants are placed
on greenhouse benches and are cared for in the usual
manner, commensurate with conventional greenhouse
practices. From four to five weeks after treatment,
the seedling plants, are examined and rated according
to the rating system provided below. The data obtained
are recorded in Table V below.
% Difference in Growth
Rating System from the Check
25 0 - No Effect ~
1 - Possible effect 1-10
2 - Slight effect 11-25
3 - Moderate effect 26-40
5 - Definite injury 41-60
6 - Herbicidal effect 61-75
7 - Good herbicidal effect 76-90
8 - Approaching complete kill 91-99
9 - Complete kill 100
4 - Abnormal growth, that is, a definite physiological
malformation but with an over-all effect less than
a 5 on the rating scale.
133~
-175-
In most cases the data are for a single test, but in
several instances, they are average values obtained
from more than one test.
Plant Species Used
Barnyardgrass (Echinochloa crusgalli)
Green foxtail (Setaria viridis)
Purple Nutsedge (Cyperus rotundus L.)
Ragweed (Ambrosia artemisiifolia, L.)
10 Quackgrass (Agropyron repens)
Field Bindweed (Convolvulus arvensis L.)
Morningglory (Ipomoea purpurea)
Velvetleaf (Abutilon theophrasti)
Cotton (Gossypium hirsutum, L.)
15 Corn (Zea mays)
Soybean (Glycine max) Bragg
Soybean (Glycine max) Williams
Wheat (Triticum aestivum)
Rice (Oryza sativa) Nato
TABLE V
POST-EMERGEIICE TEST9 ~~ RATES rll KG/~IA
E1AR~IY FOXTA P IIUT ~UACK FLO E ~IRIIGL RAG~E VELVE CORII COTTO RICE SOY~E S~Y~E S WIIE
Compound RATE ARDGR IL SPSEOGE GnA55 IIIO~IDRY SP _____ TLEAf FIELO t~ NATO AN ERAll wr AT ER
3~-Ethyl-1,9b~(and ~)-di- lo.ooo 7.0 0.0 2.0 B.O
hydro-3-methyl-5H-imidazo- 1-000 00 0 0 0 4 0 1 0 7 00 0 0 0 00 0 0 0
[1',2':1,2]pyrrolo[3,4-b]- 250 0.0 0.0 ~.0 0.0 7.0 0.0 0.00.0 0.0
pyridine-2(3H),5-dione .125 0.0 0.0 1.0 0.0 6.0 0.0 0.00.0 0.0
- .0~3 0.0 0.0 4.0 0.0 ~.0 0.0 0.0O.0 0.0
1,9b~L(and ~)-dihydro-3~L- l ooo 9.0 B.O 9.09.09.09.09.0 B O 90 7 5 9090
isopropyl--3--methyl--5H--.soo B.0 7.0 9.09.09.0 B.O 9.0 7.5 9.05.0~ 8.59.0
imidazo[l' 2'-1,2]pyrrolo- .250 2.0 2.0 B.0 9.09.08.09.05.09.0 5.0~ 8.0 B.S
[3,4--b]pyridine--2(311),5-- 125 1 0 0 0 2 0 90 B O lB 09 ~ l S 8 0 4 0'- e o B O ~,
dione .032 0.0 0.0 3.0 4.0 0.0 5.0 0.0 3.0 0.0 1.0 1.0
1.0009.09.0 B.O 9.0 B.0 a.o 9.09.0 2.0 7.0 9.0 4.0 3.0 7.0
1,9b~-Dihydro-3~-isopropyl- .500 9.09.0 B.0 B.0 7.0 8.0 9.0 2.0 7.0 9.0 2.0 2.0 3.0
3,7-dimethyl-5H-imidazo- .250 9.09.08.0 B.O 8.0 4.0 8.0 B.O 1.0 3.0 7.0 2.0 2.0 2.0
[1',2':1,2~pyrrolo[3,4--b]-- B O l O 2 0 3 0 0 0 6806010 7 0 2 0
pyridine-2(3H),5-dione .0320.0 4.0 0.0 0.0 0.0 0.0 4.0 2.0 1.0 2.0 3.0 1.0 0.0 1.0
1 0009.0 9.0 9.0 9.0 9.09.09.09.09.09.0 B.O 9.0 9.0 9.0
(R)-(+)-1,9b ~Dihydro-3 ~ 5009.09.09.09.09.09.09.09.09.09.08.09.09.09.0
isopropyl-3-methyl-5H- .250 9.0 9.0 9.0 9.08.09.08.09.08.09.08.0 ~.09.09.0
. . r I - .125 9.09.09.09.09.09.0 B.09.0 8.0 9.0 7.0 8.09.09.0
lmldazoLl',2 :1,2~pyrrolo-.0~3 B.O 9.08.09.08.09.0 8.0 9.08.0 8.0 ~.0 8.0 8.09.0
[3,4-b]pyridine-2(3H),5- .032 7.0 8.0 7.0 9.0 7.0 9.0 7.0 7.0 2.0 8.0 4.0 8.0 B.O 8.0
dione
~ C.
C~
c~
TA~LE V (Continued)
POS~-E~IERBCIICE rESrS -- RA~ES 11~ KG/IIA
BA11llY FOX-A P llur QU~CK FLD 131!1!11CL RA5'1E \IELVE C~ l CO~O QICE. SO'~LE SO~'OE S UIIE
RATE AnDGR IL SP SEOGE GRASS IIIO~;D R~ Sl' CU- IL~Ar FlELO N IIATO All CR A'l ~I AT EH
Compound ---~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~ ~ ~~~~~ ~~~~~ ~~~~~
(+)--1,9b~--Dihydro--3~--iso-- ~ 9 ~ 9 ~ 9 ~ 9 o 9009090909090
propyl--3--methyl--5H--imidazo-- .2509.09.0 B.09.09.09.0 B.0 B.0 9.09.0 B.09.0 a.o 90
[1',2':1,2]pyrrolo[3,4--b]-- .125 B.0 9.0 ?.09.0 a.o 9.0 ?.0 B.09.08.o 5.o B.0 B.0 8 0
pyridine--2(3~1),5-dione 2 0 ? o 9030~ B 0 30 ? 040
1,9b~--Dihydro--3~--isopropyl-- 0 90 B 070 B 00 ? o B 0 B 090
3,8--dimethyl--51~--lmldaZO-- .2509.09.0 B.04.0 B.0 ?.0 B.0 3.0 5.0 8.0 9.0 3.03.0 3.0
[1',2':1,2]pyrrolo[3,4--b]-- .125 B.09.0 ?.04.0 5.0 2.0 ~.0 1.0 3.0 B.0 9.0 3.0 3.0 4.0 ~--
.0~34.09.04.03.0 2.0 1.0 4.00.0 2.0 6.0 ?.0 2.0 2.0 20 --
pyrlulne-~ cJlone .0320.04.00.00.00.00.0 2.0 0.0 2.02.04.02.02.020
8-(Allyloxy)-1,9b~-dihydro-
3~-isopropyl-3-methyl-5H-
imidazo[l',2':1,2]pyrrolo-
[3,4-b]pyridine-2(3H),5_
dione
10009.0907.09.09.0909.09.0 ?.0 B.06.09.0 B.09.0
,9b~-DihydrO-3o~1SOprOpyl- 50090' 9070909090909.o ?.0 B.0 ~.08.0 B.09.0
3-methyl-5H-imidazo- .250 9.09.04.09.09.09.0 B.0 ~1.07,0 B.0 5.0 8.0 8.0 9.0
[1' 2'-1 2~pyrrolo[3 IJ--b]-- .125 9.09.02.09.0 B.0 9.0 ~.0 B.07.0 ~.0 3.0 B.0 ?.09.0
. ' -- .0639.09.0 2.0 9.0 B.09.0 B.0 O.07.07.03.07.07.0 B.0,
pyrldine-2(3ll) ,5--dlOne .032 l~.0 9.00.0 B.0 B.0 B.0 ~.0 B.03.07.0 2.0 7.06.0 B.0 C~
C~
;:~
TABLE V (Continued)
POST-EMERGEIICE TESTS -- RATE~ Ill KG/HA
~ARIIY FOXTA P NUT ~UACK FLO B ~RIIGL RAGUE VELVE CORII COT~O RICE SO~BE SOr~E S WHE
~lTE ARDGR IL SP SEDGE GRAS~ IIID~:O RY SP ED TLEAF FIEEO N IIATO AN Bn AN ~I AT ER
. Compound ------- ----- ----- ~~~~- ~~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~~~ ~~~~~
7-Ethyl-1,9b~(and ~)-dihydro-I 0OO 9 o 9 o 4 o ~ o 9 o 5 5 B.5 8.0 0.5 1.0 6.0 0.0 0.0 2 0
3~-isopropyl-3-methyl-5H- soo 9.0 9.0 1.5 8.0 9.0 4.0 8.0 3.5 0.5 2.0 6.0 0.0 0.0 1.0
imidazo[l',2':1,2]pyrrolo-.250 B.5 7.0 0.0 2.0 6.0 3.0 5.0 3.0 0.5 1.0 6.0 0.0 0.0 1.0
[3 4-b]pyridine-2(31J) 5- .125 5.5- 4.0 0.0 2.0 2.0 2.0 2.0 1.5 0.5 0.0 4.0 0.0 0.0 0.0
-- -- ' .0~3 1.5 1.0 0.0 2.0 1.0 0.0 0.0 0.0 0.5 0.0 3.0 0.0 0.0 o.o
~ dione .032 1.0 0.0 0.0 2.0 1.0 0.0 0.0 0.0 0.5 0.0 2.0 0.0- 0.0 0.0
1 000 8.0 9 0 8.0 8 0 9.0 8.0 8.0 2.0 2.0 7.0 8.0 4.0 4.0 2.0
1,9b~-Dihydro-3 ~ isopropyl- 500 8.0 7 0 7.0 ~ 0 6.0 7.0 ~.0 0.0 2.0 5.0 7.0 3.0 3.0 2.0
8-methoxy-3-methyl-5H- .250 4.0 5.0 7.0 2.0 5.0 7.0 2.0 0.0 1.0 2.0 6.0 3.0 2.0 0.0 ~--
imidazo[l',2':1,2~pyrrolo- l o l o o o 2 0 3 0~ o o ~ ~ 1 0 2 0 5 o 3
[3,4-b]pyridlne-2(3H),5- 032 1.0 0.0 1.0 0.0 0.0 0.0 0.0 0.0 1.0 1.0 2.0 2.0 1.0 0.0
dione
1.000 ~.0 4.0 2.0 0.0 0.0 2.0 , 0.0 0.0 6.0 4.0 0.0 0.0 0.0
.500 2.0 2.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 ~.0 2.0 0.0 0.0 0.0
1,9b~-Dihydro-3~-isopropyl- .250 1.0 2.0 0.0 0.0 0.0 0.0 0.0 0.0 4.0 0.0 0.0 0.0 0.0
. . 125 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 3.0 0.0 0.0 0.0 0.0
3-methy~ -lmldaZO- 06,3 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3 0 0 0 0 0 0 0 O O
[1',2':1,2~pyrrolo[3,4-b]- .032 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 2.0 0.0 0.0 0.0 0.0
quinoline-2(3H),5-dione
-- 1.000 4.0 9.0 7.0 9.0 4.0 7.0 9.0 4.0 2.0 4.0 5.0 3.0 3.0 5.0
.500 2.0 B.0 3.0 6.0 1.0 5.0 8.0 2.0 2.0 2.0 4.0 2.0 2.0 4.0
8-Chloro-l,9~-dihydro- .250 1.0 ~.0 3.0 6.0 1.0 3.0 8.0 0.0 1.0 1.0 2.0 1.0 2.0 2.0
.125 0.0 6.0 1.0 2.0 0.0 1.0 7.0 0.0 0.0 0.0 1.0 1.0 1.0 1.0
3(~-lSOprOpyl-3-methyl- 0~3 0 0 0 0 0 0 1.0 0.0 0.0 4.0 0.0 0.0 0.0 1.0 0.0 0.0 0.0
5H-imidazo~1',2':1,2]- .032 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 1.0 0.0 0.0 0.0
pyrroloL3,11-b]pyridine- C~
2(3H),5-dione C~
C~
133931~
-179-
EXAMPLE 54
Preemergence herbicidal evaluation of test compounds
The preemergence herbicidal activity of the
compounds of the present invention is exemplified by
the following tests in which the seeds of a variety of
monocotyledonous and dicotyledonous plants are separ2-
tely mixed with potting soil and planted on top of
approximately one inch of soil in separ2te pint cups.
After planting, the cups are sprayed with the selected
aqueous acetone solution containing test compound in
sufficient quantity to provide the equiv21ent of about
0.016 to 10.0 kg per hectare of test compound per cup.
The treated cups are then placed on greenhouse benches,
wat-red and cared for in accordar.ce with conventional
greenhouse procedures. From four to five weeks after
treatment, the tests are terminated and e2ch cup is
ex2mined and rated according to the rating system set
forth 2bove. The herbicidal proficiency of the active
ingredients of the present invention is evident from
the test results which are recorded in Table ~I below.
Where more than one test is involved for a given com-
pound, the data are averaged.
TA~IE VI
PRE-E~lERGE~ICE TESTg ~- RATES I~l KG~HA
8ARrlr FOXTA P NUT nllACI~ FLD B~lRl~GL RAG~IE VELVECOR~I COTTO RICE~ SOY~3E S~YBE S ~HE
RATEARDGR IL SP SEOGE GnASS IIID~O RY SR E~ TLEAFFIELD 9~ ~IATO A\J E~R A~l III AT ER
_______ _____ _____ _____ _____ _____ _____ _____ _____ _____ _____ _____ _____ _____ _____
10.000B.0 8.0 B.0 8.0 8.0
Compound 1.000 3.0 6.0 8.0 7.0 8.0 0.0 3.0 5.0 0.0
3 ~ Ethyl-1,9b~(and l~)-di- ~500 ~-~ ~-~ 8.0 6.0 7.0 0.0 0.0 3.0 0.0
hydro-3-methyl-5H-imidazo- 125 0 0 0 0 0 0 0 0 5 0 0 0 0 0 0 0 0 0
[1',2':1,2]pyrrolo[3,4-b]- 0~3 0 0 0 0 0 0 0 0 0.0 0.0 0.0 0.0 0.0
pyridine-2t3H),5-dione .032 o.a o.o o.o o.o o.o o.o o.o o.o o.o
8.000 9.0 9.0 9.0 B.O 8.0
2 000 9 0 9 0 9 0 9.0 9.0 8.0 9.0 9.0 8.0 9.0 8.0 8.0 9.0
1,9b~(and ~)-dihydro-3~- l ooo 9 0 9 0 9 0 9.0 8.0 8.0 9.0 9.0 8.0 9.0 B.07.09.0
isopropyl-3-methyl-5H- .500 8.0 9.0 9.0 9.0 8.5 8.5 B.5 7.5 8.5 9.0 8.0 a.o 9.0
imidazo[l',2':1,2]pyrrolo-.250 8.0 8.0 8.0 9.0 8.5 8.0 8.5 6.5 7.5 8.0 7.0 6.5 8.0
r I -~ . . .125 7.0 5.5 7.0 9.0 8.5 6.5 8.5 3.5 6.0 7.5 5.0 5.5 7.0
L3,~1-b~pyrldlne-2(3~1),5- .06~ 3.0 2.5 4.0 8.5 5.5 3.0 8.0 2.0 2.5 4.5 2.0 2.5 5.0
dione .0~2 1.0 1.0 2.0 6.0 0.5 1.0 5.5 0.0 3.5 2.0 0.0 0.0 3.0
.016 0.0 0.~0 9.0 0.0 0.0 4.0 0.0 3 0 3.0 0.0 0.0
1,9b~-Dihydro-3~-isopropyl-1.000 9.0 9.0 9.0 9.0 8.0 9.99.0
3,7-dimethyl-511-imidazo- ~750 9.0 9.0 8.0 9.09.09.0 9.0
[1',2':1,2]pyrrolo[3,4-b]- 9 0 8 0 8 5 8 5 8 0 8 5 90 4 0 8 0 9 0 9
pyridine-2(311),5-dione .125 8.5 9.0 7.5 4.0 9.0 4.0 8.0 e.s 2.0 4.0 8.0 2.0 2.0 5.0
.0~3 8.0 6.0 3.0 4.0 7.0 1.0 8.0 3.0 2.0 3.0 6.0 2.0 7.0 2.0
.032 3.0 4.0 2.0 2.0 ~.0 0.0 2.0 1.0 2.0 2.0 2.0 2.0 ".O 1.0
(R)-(+)-1,9b~-Dihydro-3f3- .01~ o.o o.o 1.0 2.0 0.0 0.0 0.0 0.0 2.0 2.0 2.0 2.0 2.0 1.0
isopropyl-3-methyl-5~1-
. . r ~ -- .500 9.0 9.0 9.09.09.0 9.0 9.0 9.0 9.0 9.0 9.0 9.0 9.n 9.0
lmldaZOLl~,2~ :1,2Jpyrrolo- .250 9.0 9.09.09.0 9.0 9.0 9.0 9.0 9.0 9.0 17.0 ~.0 B.O 9.0
[3,4-b]pyridine-2(311),5- .125 9.0 9.09.09.09.09.09.09.09.09.09.0 8.0 7.0 9.0
di -- .063 8.0 9.0 8.0 9.0 7.0 9.0 8.0 8.0 9.0 9.0 ~.0 7.0 7.0 9.0
one .032 3.0 9.0 7.0 9.0 7.0 8.0 3.0 7.0 7.0 7.0 5.0 6.0 4.0 9.0
.016 0.0 4.0 3.0 8.0 ~.0 7.0 0.0 7.0 2.0 2.0 4.0 3.0 3.0 7.0
e~ ~
TABLE VI (Continued)
PIIE-EME175EIICE TESTS -- RATES IN KGOIA
8Aal~Y F~XTA P IIUr ~UACK FLO ~ ?11GL ~AGIIE VLLVE COnll .COTTO ~rCE. S~IBE CO~E S ~IIIE
~ATE ~.RDGR lL SP SEOGE GnA55 IIIL~WO llr s~- ED TLEAF FIElO ~ A~O All ~R All III A~ ER
_______ _____ _____ _____ _____ _____ _____ _ ___ _____ _____ _____ _____ _____ _____ _____
Compound
(+)-1 9b~-Dihydro-3~-iso- .5009.09.09.09.09.09.09.09.09.09.09.09.0 B.09.0
- ' . . .2509.09.07.09.09.09.09.09.09.09.09.0 B.O B.O 9.0
prOpyl-3-methyl-5H-lmldaZO- .125 B.O 9.09.09.09.09.0 B.O 9.09.09.0 ~.00.07.09.0
[1',2':1,2]pyrrolo[3,4--b]-- .063 B.O 9.07.0 B.09.08.07.08.07.00.0 4.0 3.0 6.0 8.0
pyridine-2(31l),5-dione .032 3.0 B.0 4.0 8.09.00.0 3.0 9.05.0 4.0 3.0 7.0 2.0 7.0
.016 0.0 q.O 2.07.08.02.02.0 6.0 3.0 3.0 2.07.02.07.o
1,9bl3-Dihydro-3~-isopropyl- 90 90 90909 o 7 0 9080 3 0 909020
3,8-dimethyl-5H-imidazo- .1250.09.0 B.O 9.0 û.0 7.0 ~.0 7.04.0 5.0 6.0 2.02.00.0
[1' ,2~ :1,2]pyrrolo[3,11-b]- .063 0.0 7.0 6.0 7.0 5.0 4.0 0.0 5.0 3.0 4.0 4.01.02.0 3.0 ~--
pyridine-2(3H),5-dione .032 0.0 4.04.03.0 5.0 0.0 3.03.02.0 2.0 3.0 1.0 2.0 2.0 ~~
8-(Allyloxy)-1,9b~-dihydro-
3~-isopropyl-3-methyl-5H-
imidazo[l',2':1,2]~?yrrolo- ~52~50 1 0 o o 3-0 2-0 1;0 2 0 2 0 0-0 0 ~ ~ ~ ~ ~ 1 0 o.o o o
[3,4-b]pyridine-2(3H),5-
dione
5009.09.09.09.09.09-o 9-o 9-09-09-08-09.0 B.09.0
1,9b~-Dihydro-3(x-isopropyl- 250 9.09.07.09.09.09.09.09.09.09.07.09.08.09.0
3-methyl-5H-imidazo- .o635 9-09-0 6-0 0-0 90 ~ 0 ~ o 90900 ~ ~3 0 4 0 4 0 0 0 ,~
[1~,2~:1,2~pyrrolo[3,11-b]- 032 708020707.00.00.09.0 3.0 3.0 3.0 5.0 2.07.0
pyridine-2(3H),5-dione016 207000 7 0 7.0 7.0 7.0 7.0 2.0 2.0 2.0 2.07.0
C~
c~n
TABL~ VI (Continued)
PI~E-EMERGEIICE TESrS -- RATES IN t~G~HA
t3ARtlY FOX~ P ~IUT QUACK FLD ~3 ~1RtlGL RAGIIE ~/ELVE COR~I COTTO RICE SOr~E ~)Y~E S ~IHE
R~TE ARnGRIL SP SEOGE GRASS IIIDIID RY SP EO TLEAF FIElO N NATO ~ T E~
7-Ethyl-1,9b~(and ~)-dihydro- l.ooo 9.0 B.O 9.0 9.0 9.0 9.0 9.0
3 ~ isopropyl--3-methyl--5~i--~500 B.5 9~0 B.O 9.0 9.0 7.5 9.0 7.5 3.0 4.0 9.0 0.0 1.07.0
imidazo[l' 2'-1 2]pyrrolo- .250 7.0 9.0 5.5 B.O B.5 2.5 7.0 7.0 2.0 2.0 9.0 0.~ ).02.0
.125 ~.0 9.0 3.0 5.0 B.O 0.5 4.5 4.0 2.0 0.0 6.0 0.0 0.00.0
[3~4~bJpyridine-2(3H)~5- .Ot~3 2.0 1!~.0 0.5 2.0 5.0 0.0 0.0 0.0 1.0 0.0 3.0 0.0 0.00.0
dione .032 0.0 2.0 0.0 0.0 0.0 0.0 0.0 1.0 0.0 2.0 0.0 0.00.0
.ol~ o.o a.o o.o o.o o.o o.o o.o o.o l.o o.o 2.0 0.0 0.00.0
1,9b ~Dihydro-30~isopropyl- .500 9.0 9.0 9.0 9.0 9.0 9.0 9.0 7.0 7.0 B.O t.O 5.0 4.07.0
8--methoxy--3--methyl--51i--.250 B.O 9.0 ~.0 7.0 7.0 B.O 8.0 4.0 5.0 B.O 4.0 3.07.0
-- .125 8.0 6.0 7.0 7.0 B.O 7.0 5.0 2.0 3.0 5.0 3.0 1.0 1.05.0 ~
imidazo[l',2':1,2]pyrrolo- .0~3 2.0 1!~.0 4.0 2.0 6.0 ~.0 2.0 1.0 3.0 2.0 2.0 0.0 1.02.0 w
[3,4-b]pyridine-2(3H),5- .032 0.0 0.0 1.0 0.0 3.0 3.0 0.0 0.0 2.0 2.0 2.0 0.0 0.01.0
dione .ol~ o.o o.o o.o o.o o.o o.o o.o o.o 2.0 2.0 1.0 0.0 0.01.0
.500 B.O 5.0 6.0 0.0 4.0 0.0 r.o 4.0 0.0 0.0 0.0 0.0 0.00.0
1,9b ~Dihydro-3 ~ isopropyl- 250 7 0 5 0 2 0 0 0 3 0 0 0 7 0 3 0 o 0 0 0 0 0 0 0 0 00 o
3-methyl-5H-imidazo- .0~3 0.0 0.0 0.0 0.0 0.0 0.0 0.0 ~.~ ~-~ ~-~ ~-~ ~-~ ~-~ ~-~
[1',2':1,2~pyrrolo[3,4-b]- .032 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.00.0
quinoline-2(3i-l),5-dione .ol~ o.o o.o o.o o.o o.o o.o o.o o.o o.o o.o o.o o.o o.o o.o
.500 7.0 9~0 9.0 9.0 9.0 7.0 9.0 5.0 ~.0 2.0 4.0 0.0 1.0 9.0
8-Chloro-l,9 ~dihydro- 4 0 ~ 0 7 0 4 0 5 0 B O 2 ~ 3 0 1 0 2 0 0 0
3 ~ isopropyl-3-methyl-.0~3 0.0 1.0 2.0 7.0 0.0 2.0 3.0 0.0 2.0 0.0 1.0 0.0 0.0 4.0
5H-imidazo[l',2'-1,2]- .032 0.0 0.0 1.0 ~.0 0.0 1.0 0.0 0.0 1.0 0.0 1.0 0.0 0.0 2.0
- -- r I ~ . . .Olt~ 0 0 0 0 0.0 3.0 0.0 0.0 0.0 0.0 0.0 0.0 1.0 0.0 0.0 2.0 C.l:)
pyrroloL3,~-bJpyrldlne- ~ ~ C~
2(3H),5-dione cS~
c~
TA~LE VI ~Continued)
PRE-E~IERGEIICE TESTS -- RATES I~l KG~IA
~ BARllr FOXTA P NUt qlJACK FLD El ~IQIIGL RAGIIE VELVE CORN COTTO RICE SOY13E SOYaE S I~IIE
RATE AROGR IL SP SEDGE GRASS IIIO~ID RY SP EO TLEAF FIELO ~l ~IATO ArJ I~R Al~ ~II AT ER
7-Ethyl-1,9b~-dihydro-3~- .500 9.0 9.0 8.0 2.0 9.0 7.0 9.0 t~.0 3.0 2.0 9.0 0.0 0.0 ~.0
isopropyl-3-methyl-5H-.250 9.0 9.0 7.0 1.0 9.0 7.0 7.0 ~1.0 2.0 1.0 9.0 0.0 0.0 8.0
imidazo~l',2':1,2]pyrrolo- ~ 1 O ~ O 4 0 0 0 42 o 63 o 1 0 0 0 7 0
-[3,4-b]pyridine-2(3H),5- .032 2.0 3.0 0.0 0.0 1.0 0.0 0.0 1.0 0.0 0.0 2.0 0.0 0.0 0.0
dione ~01~ ~-~ ~-~ ~-~ ~.o o.o o.o o.o o.o o.o o.o 2.q 0.0 0.0 0.0
.500 9.0 9.0 7.0 0.0 7.0 8.0 9.0 ~.0 2.0 9.0 0.0 2.0 B.0
7-Ethyl-1,9b~-dihydro-.250 9.0 9.0 ~.0 0.0 ~.0 7.0 9.0 7.0 2.0 2.0 7.0 0.0 1.0 3.0
, _ , 1.125 9.0 9.0 3.0 0.0 4.0 2.0 4.0 3.0 2.0 1.0 7.0 0.0 0.0 1.0 o~
sopropyl-~-rnetrlyl- .063 4.0 6.0 1.0 0.0 1.0 0.0 2.0 0.0 1.0 0.0 ~.o 0.0 0.0 0.0
5H-imidazo[1',2':1,2]- .032 1.0 2.0 1.0 0.0 0.0 0.0 0.0 0.0 1.0 0.0 3.0 0.0 0.0 0.0
pyrrolo[3,4-b]pyridine- .016 0.0 0.0 1.0 0.0 0.0 0.0 0.0 0.0 1.0 0.0 2.0 0.0 0.0 0.0
2(3H),5-dione 500 9 o 9 o 9 o 9 o 9 o ~ o 7 o
,250 7.0 0.0 9.0 9.a 9.0 ~.0 ~.0 9.0 7.0 8.0 7.0 7.0 9.0
.125 ~.0 ~.0 ~.0 7.0 7.0 6.0 2.0 7.0 9.0 3.0 ~.0 5.0 4.0 9.0
,9b,~-DlhydrO-3~1SO-Ot~3 1.0 2.0 2.0 7.0 6.0 2.0 1.0 ~.0 ~.0 1.0 2.0 3.0 2.0 4.0
propyl-3-methyl-2-thio-.032 0.0 1.0 0.0 2.0 0.0 0.0 0.0 3.0 3.0 0.0 1.0 1.0 1.0 2.0
5H-imidazo[1',2':1,2]- .ol~ o.o o.o . o.o o.o o.o o.o o.o 1.0 l.o o.o o.o o.o o.o o.o
pyrrolo[3,4-b]pyridine-
2(31~),5-dione
:;~
c~