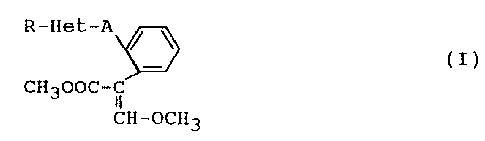Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2000362
1
METHYL a-ARYL_ACRYLATES SUBSTITUTED BY A
HETEROCYCLIC RADICAL ANI? TH,~;IR USE
The present invention relates to useful novel methyl
a-arylacrylates substituted by a heterocyclic radical, which
have a fungicidal and insecticidal action, and fungicides and
insecticides which contain these compounds.
It is known that methyl acrylates, for example
l0 methyla-[2-(benzoxazol-2'-yloxy)-phenyl]-(3-methoxyacrylate
(EP-256,667) can be used as insecticides. However, its
insecticidal action is unsatisfactory.
It has been found that methyl a-arylacrylates
substituted by a heterocyclic radical of the general formula:
R-Het-A
/ \
(I)
CH300C- i
CH-OCH3
where:
R is C1-C8-alkyl, C2-C8-alkenyl, C1-C4-haloalkyl, C3-C6-
cycloalkyl, C1-Cq-alkoxy, C1-C4-alkylcarbonyl, C1-CQ-
alkoxycarbonyl, halogen or aryl, the aromatic ring of the aryl
being unsubstituted or substituted by C1-Cg-alkyl, C3-C6-
cycloalkyl, C1- or C2-haloalkyl, C1-C4-alkoxy, halogen, cyano
or nitro,
Het is a five-membered heteroaromatic ring which has from 1
to 3 heteroatoms selected from the group consisting of oxygen,
sulfo and nitrogen, and which, when the heteroatom is nitrogen
atom, is unsubstituted or substituted by methyl at said
nitrogen Het being bonded to A via a carbon atom and
A is ethenylene, ethylene, methyleneoxy or methylenethio,
have an excellent fungicidal and insecticidal action, which
is better than that of the known methyl acrylates.
2ooo3s2
la
The radicals mentioned in the general formula may
have, for example, the following meanings:
R may be, for example C1-C8-alkyl, in particular
Cl-C4-alkyl (eg. methyl, ethyl, n-propyl, isoproyl, n-butyl,
isobutyl, sec-butyl, tert-butyl, n-pentyl, iso-pentyl, sec-
pentyl, tert-pentyl, neopentyl, hexyl, hetatvl
'~..:~ ..
.w
~0(~~36~'
- 2 - O.Z. 0050/40288
or octyl ) , CZ-Ce-alkenyl, in particular C2- or C3-alkenyl
(eg. vinyl, allyl, propenyl or isopropenyl), C1-C4-halo-
alkyl (eg. trifluoromethyl, 2,2,2-trifluoroethyl, penta-
fluoroethyl, chloromethyl, dichloromethyl or trichloro-
methyl), C3-C6-cycloalkyl (eg. cyclopropyl, cyclobutyl,
cyclopentyl or cyclohexyl), C1-C4-alkoxy (eg. methoxy,
ethoxy, n-propoxy, isopropoxy, n-butoxy, isobutoxy, sec-
butoxy or tert-butoxy), C1-C4-alkylcarbonyl (eg. acetyl,
propanoyl, butanoyl, pentanoyl), C1-C4-alkoxycarbonyl (eg.
methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl or but-
oxycarbonyl), halogen (eg. fluorine, chlorine or bromine),
or aryl (eg. phenyl), and the aromatic ring may be
unsubstituted or substituted by one to three of the
following radicals:
C1-Ce-alkyl, in particular C1-C4-alkyl (eg. methyl, ethyl,
n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-
butyl, n-pentyl, isopentyl, sec-pentyl, tert-pentyl, neo-
pentyl, hexyl, heptyl or octyl), C3-C6-cycloalkyl (eg.
cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl), C1-
or CZ-haloalkyl (eg. trifluoromethyl, 2,2,2-trifluoro-
ethyl, pentafluoroethyl, chloromethyl, dichloromethyl or
trichloromethyl), C1-C4-alkoxy (eg. methoxy, ethoxy,
propoxy or butoxy), halogen (eg. fluorine, chlorine or
bromine), cyano or vitro.
Het may be, for example, a five-membered hetero-
aromatic ring which is unsubstituted or substituted by
methyl at a nitrogen atom and has 1 to 3 heteroatoms,
such as oxygen, sulfur or nitrogen (eg. pyrrolyl, furyl,
thienyl, pyrazolyl, imidazolyl, 1,2,4-triazolyl, oxazol-
yl, N-methylpyrazolyl, thiazolyl, isoxazolyl, isothiazol-
yl, 1,3,4-oxadiazolyl, 1-N-methyl-1,2,4-triazolyl, 1,3,4-
thiadiazolyl, 1,2,4-oxadiazolyl or 1,2,4-thiadiazolyl),
and is bonded to A via a carbon atom.
A may be, for example, ethenylene (-CH=CH-),
ethylene (-CHZ-CH2-), methyleneoxy (-CHZ-0-) or methylene
thio ( -CHZ-S- ) .
The novel compounds can be prepared, for example,
- 3 - O.Z. 0050/40288
by the following processes:
The compounds of the general formula I a (where
R and Het have the abovementioned meanings and A is
ethenylene) and of the general formula I b (R and Het
have the abovementioned meanings and A is methyleneoxy)
are obtained from the methyl a-aryl-!i-hydroxyacrylate
derivatives of the general formula V which are sub-
stituted by a heterocyclic radical and which may occur in
equilibrium with the formyl derivatives VI, by reaction
with an alkylating agent (eg. dimethyl sulfate or methyl
iodide) in the presence of a base (eg. potassium car-
bonate or sodium carbonate) in a diluent (eg. acetone).
In the formulae below, L is a leaving group (eg. methyl-
sulfate or iodide).
R-Het-A ~ , ~ R-Het-A
CHz00C-C V CH;OOC-CH V[
II
CH-OH
H
H,C-L
---~ R-Het-A ~ , Ia: A = -CH=CH_
CH 300C- i I b : A = -CH 2-~C-
CH--0CH 3
The heterocyclic methyl a-aryl-!3-hydroxyacry-
lates of the general formula V where A is ethenylene or
methyleneoxy are obtained from methyl phenylacetates of
the general formula II which are substituted by a hetero-
cylic radical by reaction with methyl formate using a
base (eg. sodium hydride, lithium diisopropylamide or
sodium methylate) in an inert solvent, eg. diethyl ether
or tetrahydrofuran (cf. Ann. Chem. 424 (1921), 214).
R-Het-A ~ , --. R-Het-A ~ ,
CHj00C-CHZ II CH300C-C
II V
CH-OH
A = -cH=cH-, -cH2-o-
20Q~362
- 4 - O.Z. 0050/40288
The methyl phenylacetates IIa (A - ethenylene)
which are substituted by a heterocyclic radical and are
required as starting compounds are prepared by reacting
a methyl 2-formylphenylacetate III with a methanephos-
phonic ester of the general formula IV (where R and Het
have the abovementioned meanings and R1 is methyl or
ethyl). The reaction is carried out in a conventional
manner (cf. for example J. Am. Chem. Soc. 83 (1961),
1733). The starting materials are usually used in a
stoichiometric ratio. An excess of up to 10~ by weight
of one of the two reactants over and above the stoichio-
metric amounts is possible. The reaction is advan-
tageously carried out in an inert solvent or diluent (eg.
diethyl ether, tetrahydrofuran, methyl tert-butyl ether,
ethylene glycol dimethyl ether, toluene or dimethyl sul-
foxide) in the presence of an equivalent amount of a base
(eg. sodium hydride, sodium amide, potassium tert-
butylate, sodium methylate, butyllithium, phenyllithium,
sodium bis-trimethylsilylamide or methylsulfinylmethyl-
sodium). The reactions usually take place at from -70 to
+30°C. Since they take place with evolution of heat in
some cases, it may be advantageous to provide a means of
cooling.
0
R-Het-CH2-P)-(OR1)Z + O~C r v
H
I~ CH300C-CHZ III
R-Het-CH=CH
CH300C-CHZ IIa
Methyl 2-formylphenylacetate III is obtained by
esterifying 2-formylphenylacetic acid VII with methanol
under standard conditions. 2-Formylphenylacetic acid VII
is prepared in a simple manner by ozonolysis of the tri-
methylsilyl enol ether VIII of 2-indanone IX (Tetrahedron
Lett. ~ (1984), 3659; Tetrahedron 43 (1987), 2075).
2~U036~
- 5 - O.Z. 0050/40288
C ~ -------~ \ j ~ C-0 S i Me 3 -----
IX VIII
0
0
C~H I I
I ~ C~H
~COZH ~ I CO CH
2 3
VII III
The methanephosphonic esters substituted by a
heterocyclic radical, of the general formula IV (where R
and Het have the abovementioned meanings and R1 is methyl
or ethyl) are obtained by reacting a methylhalogen com-
pound X which contains a five-membered heteroaromatic
ring and is of the general formula R-Het-CHZ-Z (where Z
is chlorine or bromine) with trimethyl phosphite or tri-
ethyl phosphite P(OR1)3 (cf. Methoden der organischen
Chemie, Volume 12/1, page 443, Thieme, Stuttgart 1963).
The methyl phenylacetates II b substituted by a
heterocyclic radical (A - methyleneoxy), which are
required as starting compounds, are prepared by reacting
a methylhalogen compound X containing a five-membered
heteroaromatic ring (X = chlorine or bromine) with methyl
ortho-hydroxyphenylacetate XI.
I XI
H
CH 2
I
COOCH;
The reaction can be carried out by a procedure in
which stoichiometric amounts of the starting compounds X
and XI in an inert solvent or diluent (eg. acetone,
acetonitrile, dimethyl sulfoxide, dioxane, dimethylform-
amide, N-methylpyrrolidone, N,N~-dimethylpropyleneurea or
pyridine) are reacted with the addition of an equivalent
amount of a base (eg. sodium carbonate or potassium
carbonate).
2000362
- 6-- O.Z. 0050/40288
In an alternative procedure, the methyl ortho
hydroxyphenylacetate XI can first be converted with a
base (eg. sodium hydroxide or potassium hydroxide) into
the corresponding sodium or potassium phenolate and the
latter then reacted, in an inert solvent or diluent (eg.
dimethylformamide), with the methylhalogen compound X
containing a five-membered heteroaromatic ring to give
the methyl phenylacetates II b substituted by hetero-
cyclic radicals.
A second process is available for the preparation
of the novel compounds of the general formula I a (where
R and Het have the abovementioned meanings and A is
ethenylene). In this process, an aldehyde which contains
a five-membered heteroaromatic ring and is of the general
formula XII (where R and Het have the abovementioned
meanings) is reacted with dimethyl 2-(!3-methoxy-a-
methoxycarbonylvinyl)-benzylphosphonate XIII (cf. J. Am.
Chem. Soc. 83 (1961), 1733).
0 0
R-Het-C\ + (H3C0) 1-PI-CHz
H CH j00C- i
XII CH-OCH3
R-Het-CH=CH
CH 300C-il I a
CH-OCH3
Dimethyl 2-(8-methoxy-a-methoxycarbonylvinyl)-
benzylphosphonate XIII is disclosed in DE-3 519 280 and
DE-3 545 318.
The novel compounds of the general formula I c
(where R and Het have the abovementioned meanings and A
is ethylene) are obtained by selective reduction of the
novel compounds of the general formula I a (where R and
Het have the abovementioned meanings and A is ethenyl-
ene). The reduction is usually carried out catalytically
with hydrogen (cf. Methoden der organischen Chemie,
2000362
- 7 - O.Z. 0050/40288
Volume 5/2 b, page 264, Thieme, Stuttgart 1981).
R-Het-CH=CH ~ , --. R-;let-CHZ-CHZ
CH 3000-II CH 300C-C
CH-OCH 3 CH-~'JCH 3
Ia Ib
For the preparation of the novel compounds of the
general formula I as claimed in claim 1 by the process
described above, methylhalogen compounds X containing a
five-membered heteroaromatic ring and aldehydes XII con-
taining a five-membered heteroaromatic ring are required
as educts. These compounds are either known or can be
prepared by known processes. Appropriate preparation
processes are described in, for example, J. Chem. Soc.
(C), 1970, 2563; Synth. Common. 13 (1983), 741; J. Org.
Chem. 50 (1985), 5272; Acta Chem. Scand. 24 (1970), 99;
Acta Chem. Scand. 2~C ( 1972 ) , 1851; J. Chem. Soc . 1961,
2733; Liebigs Ann. Chem. 1985, 1377; J. Heterocyclic
Chem. 23 (1986), 1535; Synthesis 1982, 318; Eur. J. Med.
Chem. 19 (1984), 285; Chem. Pharm. Bull. 34 (1986), 2840;
Liebigs Ann. Chem. 717 (1968), 148; Heterocycles 26
(1987), 947; Tetrahedron 43 (1987), 235; J. Chem. Soc.,
Perkin Trans. I, 1976, 570; Chem. Ber. 106 (1973), 3345;
J. Org. Chem. 43 (1978), 3736; J. Org. Chem. 43 (1978),
3742; J. Indian Chem. Soc. 64 (1987), 314; Chem. Ber. 121
(1988), 723; DE-3118258; Chem. Ber. 101 (1968), 3872.
The novel compounds of the general formula I as
claimed in claim 1 may occur as E or Z isomers at the
double bonds (methyl 8-methoxyacrylate group and side
chain for A - ethenylene). The stereoisomers can be
separated, for example, by column chromatography or iso-
lated in pure form on the basis of their solubility
differences. The pure isomers can be converted into one
another by known methods. The pure isomeric compounds
and their mixtures are embraced by the present invention.
2000362
- 8 - O.Z. 0050/40288
Regarding the use of the novel compounds as fungicides
and insecticides, both the diastereomer mixtures and the
pure isomeric compounds as well as their mixtures ob-
tained in the synthesis are suitable.
The Examples which follow illustrate the syn-
thesis of the novel compounds.
EXAMPLE 1
Methyl alpha-2-[2'-(3 " -cyclopropylisoxazol-5 " -yl)-ethen-
1'-yl]-phenyl-!3-methoxyacrylate
rH,
H ZC-CH
I
N~0
CH 300C- i
CH-OCH 3
a) 30.3 g (0.30 mole) of triethylamine are added to
a solution of 36.5 g (0.28 mole) of 2-indanone and 32.4 g
(0.30 mole) of trimethylchlorosilane in 300 ml of tetra-
hydrofuran at room temperature while stirring. Stirring
is continued for a further 3 hours at room temperature
(20°C), the precipitate which has separated out is
filtered off under suction and the filtrate is evaporated
down. The residue is purified by distillation (53°C, 0.3
mbar). 40.6 g (72%) of 2-trimethylsilyloxy-1H-indene are
obtained as a colorless liquid in this manner.
b) 40.0 g (0.20 mole) of 2-trimethylsilyloxy-1H-
indene are dissolved in a mixture of 500 ml of methanol
and 150 ml of methylene chloride, and 14.0 g (0.30 mole)
of ozone are added in the course of 5 hours at -70°C.
After removal of excess ozone with nitrogen, 150 ml (2.0
moles) of dimethyl sulfide are added and the mixture is
stirred overnight at room temperature. Thereafter, the
solution is evaporated down and the residue is taken up
in NaHC03 solution. The aqueous phase is washed with di-
ethyl ether and brought to pH 2 with dilute HCl. The
mixture is then extracted with diethyl ether and the com-
2000362
- 9 - O.Z. 0050/40288
biped organic phases are dried over MgSO, and evaporated
down. 24.1 g (75%) of 2-formylphenylacetate are obtained
in the form of colorless crystals (mp.: 103-105°C).
c) 24.0 g (0.15 mole) of 2-formylphenylacetic acid
and 0.1 g of p-toluenesulfonic acid in 250 ml of methanol
are refluxed for 2 hours. Thereafter, the solution is
evaporated down, the residue is taken up in diethyl ether
and the solution is washed with dilute HC1. The organic
phase is separated off, dried over MgSO, and evaporated
down. The residue is purified by distillation (90°C, 0.4
mbar). 19.3 g (74%) of methyl 2-formylphenylacetate are
obtained as a colorless liquid in this manner.
d) 43.3 ml of a 1.5 molar solution of n-butyllithium
in hexane (0.065 millimole) are added dropwise to a solu
tion of 15.6 g (0.06 mole) of diethyl 3-cyclopropyl
isoxazol-5-ylmethanephosphonate in 50 ml of tetrahydro-
furan at 20°C. The mixture is stirred for 20 minutes at
20°C, after which a solution of 10.7 g (0.06 mole) of
methyl 2-formylphenylacetate in tetrahydrofuran is added
dropwise at this temperature. The reaction mixture is
stirred overnight, poured onto ice water and extracted
with methyl tert-butyl ether. The organic phases are
washed with water, dried over MgSO, and evaporated down.
The residue is chromatographed over silica gel (8 : 2
cyclohexane/ethyl acetate). 8.0 g (47%) of methyl 2-[2'-
(3 " -cyclopropylisoxazol-5 " -yl)-ethen-1'-yl]-phenyl-
acetate are obtained as a colorless oil.
1H-Nl~t (CDC13) : 0.87 (m, 2H) ; 1.03 (m, 2H) ; 3. 68 (s, 3H) ;
3.77 (s, 2H); 5.93 (s, 1H); 6.80 (d, 1H); 7.25-7.62 (m,
4H); 7.52 (d, 1H).
e) A mixture of 6.0 g (0.02 mole) of methyl 2-[2'-
(3 " -cyclopropylisoxazol-5 " -yl)-ethen-1'-yl]-phenyl-
acetate, 2.8 g (0.05 mole) of methyl formate and 50 ml of
diethyl ether is added dropwise to a suspension of 0.76 g
(0.03 mole) of sodium hydride in 30 ml of diethyl ether
at room temperature. The mixture is stirred for 12 hours
at room temperature, after which hydrolysis is carried
2000362
- 10 - O.Z. 0050/40288
out with ice water. The aqueous phase is brought to pH
4 with dilute HC1 and extracted with diethyl ether. The
combined ether phases are dried over MgS04 and evaporated
down. 5.4 g (82%) of methyl alpha-2-[2'-(3 " -cyclo-
propylisoxazol-5 " -yl)-ethen-1'-yl]-phenyl-!i-hydroxy-
acrylate are obtained as a colorless oil.
f ) 5 . 4 g ( 0 . 02 mole ) of the methyl acrylate obtained
under e) , 2 . 4 g ( 0 . 02 mole) of potassium carbonate and
2.2 g (0.02 mole) of dimethyl sulfate in 60 ml of acetone
are stirred for 12 hours at room temperature. There-
after, the solution is filtered off from the precipitate,
the filtrate is evaporated down and the residue is taken
up in diethyl ether . The organic phase is washed with
dilute NH40H solution, dried over MgS04 and evaporated
down. The residue is purified by chromatography over
silica gel (8 : 2 cyclohexane/ethyl acetate). The oil
obtained is covered with a layer of diisopropyl ether and
is crystallized by trituration. 4.5 g (80%) of methyl x-
2-[2'-(3 " -cyclopropylisoxazol-5 " -yl)-ethen-1'-yl]-
phenyl-!3-methoxyacrylate are obtained in the form of
colorless crystals (mp.: 109-111°C, compound No. 122).
EXAMPLE 2
Methyl alpha-2-[2'-(N-para-chlorophenylpyrrol-3 " -yl)-
ethen-1'-yl]-phenyl-!i-methoxyacrylate
I
~CH=CH
l N I CH 300C-C
I(
p-C1-C6H4 Ct+--OCH3
A solution of 13.7 g (0.04 mole) of dimethyl 2-
(!i-methoxy-a-methoxycarbonylvinyl)-benzylphosphonate and
9.0 g (0.04 mole) of N-para-chlorophenyl-pyrrol-3-yl-
carboxaldehyde in 100 ml of dimethylformamide is added
dropwise to a suspension of 1.1 g (0.04 mole) of sodium
hydride in 50 ml of dimethylformamide at 0°C, while
stirring. Stirring is continued for a further hour at
0°C and for 12 hours at room temperature. Thereafter,
2000362
- 11 - O.Z. 0050/40288
the mixture is hydrolyzed with ice water and extracted
with diethyl ether. The organic phase is dried over
MgS04. On evaporation of the ether phase, crystallization
begins. 5.4 g (31%) of the title compound were obtained
in the form of colorless crystals in this manner (mp..
146-147°C, compound No. 8).
EXAMPLE 3
Methyl alpha-2-[2'-(N-para-chlorophenylpyrrol-3 " -yl)-
eth-1'-yl]-phenyl-B-methoxyacrylate
4.2 g (0.01 mole) of methyl alpha-2-[2'-(N-para-
chlorophenylpyrrol-3 " -yl)-ethen-1'-yl]-phenyl-1i-methoxy-
acrylate (cf. Example 2) are dissolved in 100 ml of
tetrahydrofuran and hydrogenated in the presence of 1.0 g
of Pd/C (10% strength) under 0.05 bar hydrogen gage
pressure and at 0°C. After the absorption of 220 ml of
hydrogen, the mixture is filtered and the organic phase
is evaporated down. The residue is chromatographed over
silica gel (toluene). The oil obtained is covered with
a layer of diisopropyl ether and crystallized by tritura-
tion. 1.9 g (45%) of the title compound are obtained in
the form of colorless crystals (mp.: 115-116°C, compound
No. 14).
The following compounds can be prepared in a
similar manner:
2000362
880667
12 O.Z. 0050/40288
Table 1
R-Het-A ~-~ ( I )
CH 300C-i lI
CH-OCHg
Compounds of the general formula I.
The first configuration statement refers to the methyl ~i-methoxyacrylate
group, and the second to the ethenylene group in the compounds of the
general formula I a (A = ethenylene).
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
1 C6H5 1 I
I CH=CH-
N
2 4-C1-C6H4 1 I
I CH=CH-
N
3 C6H5 1 I
I CH -CH -
N
2 2
4 4-C1-C6H4 1 I
I CH -CH -
N
2 2
5 C6H5 1 I
I CH -0-
N
2
6 4-C1-C6H4 1 I
I C
-0-
N
H
2
CH=CH- 133-135 (E, E)
1 I
I
7 c6H5 N
CH=CH-
1 I 146-147 (E, E)
I
8 4-C1-C6H4 N
CH=CH-
9 H4 1 I
4-Br-C I
6 N
CH=CH-
4-OCH3-C6H41 I N I
2 0 0 0 3 6 2 880667
13 O.Z. 0050/40288
Table 1 (contd.)
No. R Position of Het-A- mp.: (C)
R on Het (Isomer)
CH=CH-
11 4-N02-C6H4 1 I N I 142-143 ( E, E )
~CH=CH-
12 2, 6-(CH3) 2-C6H31 I N I
CH2-CH2-
13 C6H5 1 I
I
N
CH2-CH2- 115-116 (E)
14 4-C1-C6H4 1 I N I
CH2-CH2-
15 H4 1 I
4-er-C I
6 N
CH2-CH2-
16 4-OCH3-C6H4 1 I
I
N
CH2-CH2-
17 -C6H4 1 I
4-N0 (
2 N
CH2-CH2-
18 6-(CH3) 2-C6H3 1 I
2 I
, N
CH2-0-
19 C6H5 1 I
I
N
CH2-0-
20 4-C1-C6H4 1 I
I
N
CH2-0-
21 H 1 I
4-Br-C I
6 N
4
CH2-0-
22 H4 1 I
-C I
4-OCH
6 N
3
CH2-0-
H 1 I
I
23 4 N
4-N02-C6
880667
14 O.Z. 0050/40288
Table 1 (contd.)
No. R Position of Het-A- mp (C)
R on Het (Isomer)
~CH2-0-
24 6-(CH3) 2-C6H3 1 I
2 I
, N
25 (CH3)2CH 5 I p I CH=CH-
26 cyclo-C3H5 5 I p I CH=CH-
27 4-Cl-C6H4 5 I p I CH=CH-
28 4-OCH3-C6H4 5 I I
CH=CH-
29 (CH3) 2CH 5 I p I CH -CH
2 2
30 cyclo-C3H5 5 I I CH2-CH2
31 4-Cl-C6H4 5 I I CH2_CH2
32 4-OCH3-C6H4 5 I p I CH -CH
2 2
33 (CH3)2CH 5 I p I CH2-0-
34 cyclo-C3H5 5 I p I CH -0-
2
35 4-Cl-C6H4 5 I p I CH -0-
2
36 4-OCH3-C6H4 5 I I CH2-0-
880667
15 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp (C)
of
R on Het (Isomer)
37 (CH3)2CH 4 I 0 I CH=CH-
38 cyclo-C3H5 4 I 0 I CH=CH-
39 4-Cl-C6H4 4 I 0 I CH=CH-
40 4-OCH3-C6H44 I 0 I CH=CH-
41 (CH3)2CH 4 I 0 I CH -CH
2 2
42 cyclo-C3H5 4 I 0 I CH -CH
2 2
43 4-Cl-C6H4 4 I 0 I CH -CH
2 2
44 4-OCH3-C6H44 I 0 I CH -CH
2 2
45 (CH3)2CH 4 I 0 I CH -0-
2
46 cyclo-C3H5 4 I I
CH2-0-
47 4-Cl-C6H4 4 I I
CH2-0-
48 4-OCH3-C6H44 I I
CH2-0-
49 (CH3)2CH
5 I S I CH=cH-
2000362 T '~ ~ 880667
O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp~ (C)
of
R on Het (Isomer)
50 cyclo-C3H5 5 ~ g I CH=CH-
51 4-CI-C6H4 5 ~ g ~ CH=CH-
52 4-OCH3-C6H4 5 ~ g I CH=CH-
53 (CH3)2CH 5 I
I CH -CH -
S
2 2
54 cyclo-C3H5 5 ( g I CH -CH -
2 2
55 4-Cl-C6H4 5 ~ g I CH -CH -
2 2
56 4-OCH3-C6H4 5 ~ g I CH -CH -
2 2
57 (CH3)2CH 5 ~ g I CH -0-
2
58 cyclo-C3H5 5 ~ g ~ CH -o-
2
59 4-C1-C6H4 5 ~ g I CH -0-
2
60 4-OCH3-C6H4 5 ~ g ~ CH -0-
2
61 (CH3)2CH 4 ~ g I CH=CH-
62 cyclo-C3H5 4 ~ 5 ~ CH=CH-
2000362 880667
17 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
63 4-Cl-C6H4 4 I I
S CH=CH-
64 4-OCH3-C6H4 4 I I
S CH=CH-
65 (CH3)2CH 4 I S I CH -CH -
2 2
66 cyclo-C3H5 4 I S I CH -CH -
2 2
67 4-Cl-C6H4 4 I S I CH -CH -
2 2
68 4-OCH3-C6H4 4 I S I CH -CH -
2 2
69 (CH3)2CH 4 ~I I
'S~CH2-0-
70 cyclo-C3H5 4 I S I CH -0-
2
71 4-Cl-C6H4 4 I S I CH -0-
2
72 4-OCH3-C6H4 4 I S I CH -0-
2
CH=CH-
73 (CH3)2CH 5 I S I
CH=CH-
74 cyclo-C3H5 5 I S
CH=CH-
75 4-Cl-C6H4 5 I S I
2000362 ~'~! 880667
O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
CH=CH-
76 4-OCH3-C6H4 5 i
I
S
CH2-CH2-
77 (CH3)2CH 5 I
I
S
CH2-CH2-
78 cyclo-C3H5 5 I
(
S
CH2-CH2-
79 4-C1-C6H4 5 I
I
S
CH2-CH2-
80 4-OCH3-C6H4 5 I
I
S
CH2-0-
81 ( CH3 ) 2CH 5 I S I
CH2-0-
82 cyclo-C3H5 5 I
I
S
CH2-0-
83 4-Cl-C6H4 5 I
(
S
CH2-0-
84 4-OCH3-C6H4 5 I
I
S
CH=CH-
85 C6H5 1 I
I
N
N
CH=CH- 120-122 (E, E)
86 4-CH3-C6H4 1 N~ N I
CH=CH- 141-143 (E, E)
87 4-C1-C6H4 1 NI N I
CH=CH-
H 1 I
C
88 4 N~ N
6
4-OCH3-
2000362 880667
1g O.Z. 0050/40288
Table 1 (contd.)
No. R Position of Het-A- mp (C)
R on Het (Isomer)
CH2-CH2-
89 1 I
H I
c
5 N
6 N
CH2-CH2-
90 4-CH ~
-C(H4 1 I
3 N
N
CH2-CH2-
91 H4 1 I
4-Cl-C I
6 N
N
CH2-CH2-
92 -C6H4 1 ~
4-OCH I
3 N
N
CH2-0-
93 1 I
H )
C
5 N
6 N
CH2-0-
94 H4 1 I
-C I
4-CH
6 N
3 N
CH2-0-
95 H4 1 I
4-Cl-C I
6 N
N
CH2-0-
96 -C6H4 1 ~
4-OCH I
3 N
N
CH=CH-
97 C6H5 5 I N IN
I
CH3
CH=CH-
98 cyclo-C3H5 5 I N IN
I
CHg
CH=CH-
99 4-C1-C6H4 5 I N IN
I
CH3
2000362 880667
20 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
CH=CH-
100 4-OCH3-C6H45 (
I
N
N
I
CHg
CH2-CH2-
101 (CH3)2CH 5 I
I
N
N
I
CH3
CH2-CH2-
102 cyclo-C3H5 5 I N IN
I
CH3
CH2-CH2-
103 4-C1-C6H4 5 I
I
N
N
I
CH3
CH2-CH2-
104 4-OCH3-C6H45 I N IN
I
CHg
CH2-0-
105 (CH3) 2CH 5 I
~
N
N
I
CH3
CH2-0-
106 cyclo-C3H5 5 I N IN
I
CH3
CH2-0-
107 4-C1-C6H4 5 I N IN
I
CH3
CH2-0-
108 4-OCH3-C6H45 I N IN
I
CH3
2000362
880667
21 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
109 C6H5 1 N~~CH=CH-
~ J
CH=CH-
110 4-CH3-C6H4 1 N
N CH=CH-
111 4-Cl-C6H4 1
N CH=CH-
112 4-OCH3-C6H41
N CH2-CH2-
113 H 1
C
5
6
114 -C6H4 1 N~~CH2-CH2-
4-CH
3
N CH2-CH2-
115 4-C1-C6H4 1
116 4-OCH3-C6H41 N~~CH2-CH2-
N CH2-0-
117 H 1
C
5
6
N CH2-0-
118 -C6H4 1
4-CH
3
N CH2-0-
119 H4 1
4-Cl-C
6
N CH2-0-
120 H4 1
-C
4-OCH
6
3
121 (CH3)2CH 3 I I 90- 91 (E, E)
N~0 CH=CH-
200Q~62
880667
22 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
122 cyclo-C3H5 3 I I 109-111 (E,
E)
N~0 CH=CH-
123 4-Cl-C6H4 3 I I 161-163 (E,
E)
N~0 CH=CH-
124 4-OCH3-C6H43 I I 130-132 (E,
E)
N~0 CH=CH-
125 4-CH3-C6H4 3 I I 138-140 (E,
E)
N~0 CH=CH-
126 4-CN-C6H4 3 I I 163-165 (E,
0 CH=CH- E)
~
N
-
127 2,6-F2-C6H33 I I 126-128 (E,
E)
N~0 CH=CH-
128 C02C2H5 3 I I
N~0 CH=CH-
129 ( CH3 ) 3 I
2CH I CH
-CH
N
0
Z
p
130 cyclo-C3H5 3 I I
-
-CH
CH
2
2
N~0
131 4-C1-C6H4 3 I I CH -CH -
N
2 2
132 4-OCH3-C6H43 N
I CH -CH -
~0
2 2
133 4-CH3-C6H4 3 I
I CH -CH -
N
0
2 2
134 4-CN-C6H4 3 N~ 0 I CH -CH -
2 2
200036
880667
23 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
135 2,6-F2-C6-H4 3 I I
N~0 CH2-CH2-
136 C02C2H5 3 N..p I CH -CH -
2 2
137 (CH3)2CH 3 N
I CH -0-
~0
2
138 cyclo-C3H5 3 N~ 0 I CH -0-
2
139 4-Cl-C6H4 3 NI I CH -0-
2
140 4-OCH3-C6H4 3 N~ 0 I CH2-0-
141 4-CH3-C6H4 3 NI I CH -0-
2
142 4-CN-C6H4 3 N~0 I CH -0-
2
143 2, 6-F2-C6H4 3 N~ 0 I CH -0-
2
144 C02C2H5 3 N~ 0 I CH2-0-
CH=CH-
145 (CH3)2CH 5 I O IN
CH=CH-
146 cycio-C3H5 5 I 0 ~N
CH=CH-
147 4-Cl-C6H4 5 I IN
~ooo~s~~
880667
24 O.z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp (C)
of
R on Het (Isomer)
CH=CH-
14$ 4-OCH3-C6H45 I 0 IN
CH2-CH2
149 (CH3)2CH 5 I
~
0
N
CH2-CH2
150 cyclo-C3H5 5 I 0 ~N
CH2-CH2
151 H4 5 I I
4-C1-C
6 N
CH2-CH2
152 4-OCH3-C6H45 I 0 IN
CH2-0-
153 (CH3)2CH 5 I
~
N
0
CH2-0-
154 cyclo-C3H5 5 IOIN
CH2-0-
155 4-C1-C6H4 5 I 0 IN
CH2-0-
156 4-OCH3-C6H45 I 0 ~N
157 (CH3)2CH 3 nI I
~CH=CH-
N~
158 cyclo-C3H5 3 I I
NHS CH=CH-
159 4-C1-C6H4 3 I
I
N
S
CH=CH-
3 I I
160 4-OCH3-C6H4 NHS CH=CH-
2,,DQ~36~ 880667
25 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
161 ( CH3 ) 3 ~
2CH I CH -CH
N
g
2 2
162 cyclo-C3H5 3 N~ g ~ CH2-CH2
163 4-Cl-C6H4 3 N~ g ~ CH2-CH2
164 4-OCH3-C6H43 N~ g I CH2-CH2
165 ( CH3 ) 3 NI g I CH -0-
2CH 2
166 cyclo-C3H5 3 NI S I CH2-0-
167 4-C1-C6H4 3 N~ g I CH2-0-
168 4-OCH3-C6H43 NI g I CH2-0-
169 CH 2 N~~CH=CH-
) ~
(CH
3 ~
2
CH=CH-
170 H 2 N
clo-C
c
5
3
y
N CH=CH-
171 H 2
4-C1-C
4
6
N CH=CH-
172 H4 2
-C
4-OCH
6
3
2 ~CH2-CH2
N
173 (CH3)2CH ~
zoooas~ 880667
26 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
N CH2-CH2
174 cyclo-C3H5 2 I I
175 4-Cl-C6H4 2 N~~CH2-CH2
N CH2-CH2
176 -C6H4 2
4-OCH
3
N CH2-0-
177 CH 2
(CH
)
3
2
N CH2-0-
178 H 2
cyclo-C
5
3
N CH2-0-
179 H4 2
4-Cl-C
6
N CH2-0-
180 -C6H4 2
4-OCH
3
N CH=CH-
181 CH 2
(CH
)
3
2
N CH=CH-
182 H 2
clo-C
c
3
5
y
N CH=CH-
183 4-C1-C 2
H
6
4
N CH=CH-
184 -C 2
H4
4-OCH
6
3
185 CH 2 N~~CH2-CH2
(CH
)
2
3
N CH2-CH2
186 cyclo-C3H5 2
200Q362
880667
27 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mP~ (C)
of
R on Het (Isomer)
N CH2-CH2
187 H 2
4-Cl-C
4
6
N CH2-CH2
188 H4 2
-C
4-OCH
6
3
N CH2-0-
189 CH 2
)
(CH
3
2
N CH2-0-
190 H 2
clo-C
c
5
3
y
N CH2-0-
191 H 2
4-C1-C
4
6
N CH2-0-
192 H4 2
-C
4-OCH
6
3
N
193 (CH3)2CH 3 N
~CH=CH-
N
I
CHg
N
194 cyclo-C3H5 3 N
N
CH=CH-
I
CHg
N
195 4-Cl-C6H4 3 N
N
~CH=CH-
I
CH3
N
196 4-OCH3-C6H43 N
N
~CH=CH-
I
CH3
N
197 (CH3)2CH 3 NCH -CH
N
I 2 2
CH3
~QQ~03f~~
880667
28 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
N
198 cyclo-C3H5 3 N
CH -CH
N
I 2 2
CH3
N
199 4-Cl-C6H4 3 N~
CH2-CH2
N
I
CH3
N
200 4-OCH3-C6H43 N~
CH2-CH2
N
(
CH3
N
201 (CH3)2CH 3 NCH -0-
N
I 2
CHg
N
202 cyclo-C3H5 3 N~
CH2-0-
N
I
CH3
N
203 4-Cl-C6H4 3 N~
CH2-0-
N
I
CH3
N
204 4-OCH3-C6H43 N~
CH2-0-
N
I
CH3
205 (CH3 ) 2CH 5 91 92 ( E, E )
N~
I~
0
CH=CH-
N-N
206 cyclo-C3H5 5 ~O~CH=CH-
N-N
207 4-Cl-C6Ht, 5
CH=CH-
2ooo~s~ 880667
29 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
N-N
208 4-OCH3-C6H45 ~O~CH=CH-
N-.N
209 (CH3)2CH 5 ~
-CH
~
CH
O
2 2
N--N
210 cyclo-C3H5 5 ~p~CH -CH
2 2
N-N
211 4-C1-C6H4 5 ~O~CH -CH
2 2
N-N
212 4-OCH3-C6H45
CH2-CH2
N-N
213 (CH3)2CH 5 '0~CH -0-
2
N--IN
214 cyclo-C3H5 5 ~O~CH -0-
2
N-.N
215 4-C1-C6H4 5 ~p~CH -0-
2
N-.N
216 4-OCH3-C6H45
CH2-0-
N-N
217 (CH3)2CH 5 'S~CH=CH-
N--N
218 cyclo-C3H5 5 ~S~CH=CH-
N.-N
219 C2H50 5
I S I CH=CH-
H 5 152-153 (E, E)
4-C1-C N~
~
220 4 CH=CH-
6 S
204~t~~fi~
880667
30 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
N-N
221 4-OCH3-C6H45 ~
~
CH-
S
CH=
N-N
222 (CH3)2CH 5 ~
~CH -CH
S
2 2
N-N
223 cyclo-C3H55 ~
~CH -CH
S
2 2
N-N
224 C2H50 5 '
~CH -CH
S
2 2
N-N
225 4-C1-C6H4 5 ~
~CH -CH
S
2 2
N-N
226 4-OCH3-C6H45 ~
~CH -CH
S
2 2
N-N
227 (CH3)2CH 5 ~
~CH -0-
g
2
N-.N
228 cyclo-C3H55 ~
~CH -0-
S
2
N-N
229 C2H50- 5 -0-
~
~
CH
S
2
N~~-IN
230 4-C1-C6H4 5 ~
~CH -0-
S
2
N--~N
231 4-OCH3-C6H45 ~S~CH -0-
2
232 CH 5 ~~CH=CH-
)
(CH
3 ~
2
CH=CH-
N
233 cyclo-C3H55
880667
31 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.c (C)
of
R on Het (Isomer)
CH=CH-
N
--
234 4-Cl-C6H4 5 ~
~
N CH=CH-
235 4-OCH3-C6H45
N CH2-CH2
236 (CH3)2CH 5
N CH2-CH2
237 cyclo-C3H55
N CH2-CH2
238 4-Cl-C6H4 5
N CH2-CH2
239 -C6H4 5
4-OCH
3
N CH2-0-
240 CH 5
)
(CH
3
2
N CH2-0-
241 cyclo-C3H55
N CH2-0-
242 4-Cl-C6H4 5
N CH2-0-
243 4-OCH3-C6H45
N
244 (CH3)2CH 3 O~
CH=CH-
N
N
245 cyclo-C3H53 O~
CH=CH-
N
N
3 ~I 111-113 (E, E)
246 4-Cl-C6H4 N~0 CH=CH-
2oo~~s~ 880667
32 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
N
247 4-OCH3-C6H4 3
N~0 CH=CH-
248 (CH3)2CH 3
-CH
CH
2
2
N~0
N
249 cyclo-C3H5 3 N.
~
~~
CH -CH
.
p
.
2 2
N
250 4-Cl-C6H4 3 O~
CH -CH
N
2 2
251 4-OCH3-C6H4 3 ~~
~
~
0
N
.,,
CH -CH
2 2
252 (CH3)ZCH 3 ~~CH -0-
N
2
N
253 cyclo-C3H5 3 O~
CH -0-
N
2
N
254 4-C1-C6H4 3 O~
CH -0-
N
2
N
255 4-OCH3-C6H4 3 O~
CH -0-
N
2
N
256 (CH3)2CH 3 ~~
CH=CH-
N
N
257 cyclo-C3H5 3 r S~
CH=CH-
N
N
258 4-Cl-C6H4 3
N'~S CH=CH-
N
259 4-OCH3-C6H4 3
NHS CH=CH-
20UtD362
88066
33 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
N
260 (CH3)2CH 3 ~~C
-CH
N
2
H2
N
261 cyclo-C3H53 r S~
CH2-CH2
N
N
262 4-Cl-C6H4 3 ~~
N
CH -CH
2 2
N
263 4-OCH3-C6H43 ~j~
N
CH -CH
2 2
N
264 (CH3)2CH 3 ~~CH -0-
N
2
N
265 cyclo-C3H53 ~~
N
CH -0-
2
N
266 4-Cl-C6H4 3 S~
CH -0-
N
2
N
267 4-OCH3-C6H43 ~~
CH -0-
2
CH=CH- gl- 92 E)
I (E
268 2-F-C6H4 1 NI ,
CH=CH- 138-139 (E,
269 3-F-C6H4 1 I I E)
N
CH=CH- 13$ (E,
270 H 1 I I E)
4-F-C
4 N
6
CH=CH- 141-142 (E,
271 3-Cl-C6H4 1 N I E)
CH=CH-
1 I 124-125 (E,
E)
272 4-CH3-CgH4 N
2000362 88066
34 O.Z. 0050/40288
Table 1 (contd.)
No. R Position Het-A- mp.: (C)
of
R on Het (Isomer)
CH=CH-
I 105-106 (E
E)
273 4-t-C4Hg-C6H41 NI ,
CH=CH- 116-117 (E,
1 I I E)
274 2, 6-Fy-C6H3 N
CH=CH-
I I 147-149 E)
(E
275 3, 4-C1 Z-C6H31 N ,
CH=CH- 115-117 (E,
276 4-F-C6H4 1 I E)
I
N
N
CH=CH- 138-140 E)
I (E
I
277 4-Br-C6H4 1 N ,
N
278 CH 3 3 I 155-157 (
I E,
E
)
CH=CH-
N
0
3 I I 117-119 (E,
E)
279 C6H5 N~0 CH=CH-
280 2-C1-6-F-CgH33 I I oil (E,
E)
N~0 CH=CH-
oil (E)
3 I
281 CH C H z-0-
NI 0
N-N
282 H4 II I 147 (E,
-C ~ E)
4-CH ~
g CH=CH-
3 O
N-.N
283 H ~~ I 144 (E,
C ~ E)
~
S CH=CH-
g O
2ood3s~
35 O.z. 0050/40288
Table 2
NMR data of selected compounds from Table 1. The chemical shift (8) is
given in ppm relative to tetramethylsilane. CDC13 was used as solvent.
Compound no. 8
3.70 (s, 3H); 3. 80 (s, 3H); 6.53 (d, 1H); 6.80 (d, 1H); 6.95 (d, 1H);
7.00 (d, 1H); 7.10 (s, 1H); 7.15 - 7.70 (m, 8H); 7.63 (s, 1H).
Compound no. 14
2.78 (m, 4H); 3.73 (s, 3H); 3.86 (s, 3H); 6.21 (d, 1H); 6.82 (s, 1H); 6.98
(d, 1H); 7.11 - 7.42 (m, 8H); 7.63 (s, 1H).
Compound no. 122
0.82 (m, 2H) ; 1 .00 (m, 2H) ; 2.00 (m, 1H) ; 3.68 (s, 3H) ; 3.80 (s, 3H) ;
5.88
(s, 1H) ; 6.84 (d, 1H) ; 7.17 - 7.68 (m, 4H) ; 7.28 (d, 1H) ; 7.65 (s, 1H) .
Compound no. 123
3.70 (s, 3H); 3.82 (s, 3H); 6.50 (s, 1H); 6.92 (d, 1H); 7.19-7.79 (m, 9H);
7.68 (s, 1H).
Generally speaking, the novel compounds are extremely effective on a broad
spectrum of phytopathogenic fungi, in particular those from the Asco-
mycetes and Basidiomycetes classes. Some of them have a systemic action
and can be used as foliar and soil fungicides.
The fungicidal compounds are of particular interest for controlling a
large number of fungi in various crops or their seeds, especially wheat,
rye, barley, oats, rice, Indian corn, lawns, cotton, soybeans, coffee,
sugar cane, fruit and ornamentals in horticulture and viticulture, and in
vegetables such as cucumbers, beans and cucurbits.
The novel compounds are particularly useful for controlling the following
plant diseases:
Erysiphe graminis in cereals,
Erysiphe cichoracearum and Sphaerotheca fuliginea in cucurbits,
Podosphaera leucotricha in apples,
Uncinula necator in vines,
Puccinia species in cereals,
Rhizoctonia species in cotton and lawns,
Ustilago species in cereals and sugar cane,
Venturia inaequalis (scab) in apples,
~ooo3s~
36 O.Z. 0050/40288
Helminthosporium species in cereals,
Septoria nodorum in wheat,
Botrytis cinerea (gray mold) in strawberries and grapes,
Cercospora arachidicola in groundnuts,
Pseudocercosporella herpotrichoides in wheat and barley,
Pyricularia oryzae in rice,
Phytophthora infestans in potatoes and tomatoes,
Fusarium and Verticillium species in various plants,
Plasmopara viticola in grapes,
Alternaria species in fruit and vegetables.
The compounds are applied by spraying or dusting the plants with the
active ingredients, or treating the seeds of the plants with the active
ingredients. They may be applied before or after infection of the plants
or seeds by the fungi.
The novel substances can be converted into conventional formulations such
as solutions, emulsions, suspensions, dusts, powders, pastes and granules.
The application forms depend entirely on the purposes for which they are
intended; they should at all events ensure a fine and uniform distribution
of the active ingredient. The formulations are produced in known manner,
for example by extending the active ingredient with solvents and/or
carriers, with or without the use of emulsifiers and dispersants; if water
is used as solvent, it is also possible to employ other organic solvents
as auxiliary solvents. Suitable auxiliaries for this purpose are solvents
such as aromatics (e. g., xylene), chlorinated aromatics (e. g., chloro-
benzenes), paraffins (e. g., crude oil fractions), alcohols (e. g., meth-
anol, butanol), ketones (e. g., cyclohexanone), amines (e. g., ethanolamine,
dimethylformamide), and water; carriers such as ground natural minerals
(e. g., kaolins, aluminas, talc and chalk) and ground synthetic minerals
(e. g., highly disperse silica and silicates); emulsifiers such as nonionic
and anionic emulsifiers (e. g., polyoxyethylene fatty alcohol ethers, alkyl
sulfonates and aryl sulfonates); and dispersants such as lignin, sulfite
waste liquors and methylcellulose.
The fungicidal agents generally contain from 0.1 to 95, and preferably
from 0.5 to 90, wt% of active ingredient. The application rates are from
0.02 to 3 kg or more of active ingredient per hectare, depending on the
type of effect desired. The novel compounds may also be used for protect-
ing materials, for example against Paecilomyces variotii.
The agents and the ready-to-use formulations prepared from them, such as
solutions, emulsions, suspensions, powders, dusts, pastes and granules,
are applied in conventional manner, for example by spraying, atomizing,
dusting, scattering, dressing or watering.
zooo~s~
37 O.Z. 0050/40288
Examples of formulations are given below.
I. 90 parts by weight of compound no. 8 is mixed with 10 parts by weight
of N-methyl-a-pyrrolidone. A mixture is obtained which is suitable for
application in the form of very fine drops.
II. 20 parts by weight of compound no. 14 is dissolved in a mixture
consisting of 80 parts by weight of xylene, 10 parts by weight of the
adduct of 8 to 10 moles of ethylene oxide and 1 mole of oleic acid-N-
monoethanolamide, 5 parts by weight of the calcium salt of dodecylbenzene-
sulfonic acid, and 5 parts by weight of the adduct of 40 moles of ethylene
oxide and 1 mole of castor oil. By pouring the solution into water and
uniformly distributing it therein, an aqueous dispersion is obtained.
III. 20 parts by weight of compound no. 122 is dissolved in a mixture
consisting of 40 parts by weight of cyclohexanone, 30 parts by weight of
isobutanol, 20 parts by weight of the adduct of 40 moles of ethylene oxide
and 1 mole of castor oil. By pouring the solution into water and finely
distributing it therein, an aqueous dispersion is obtained.
IV. 20 parts by weight of compound no. 8 is dissolved in a mixture con-
sisting of 25 parts by weight of cyclohexanol, 65 parts by weight of a
mineral oil fraction having a boiling point between 210 and 280°C, and
10 parts by weight of the adduct of 40 moles of ethylene oxide and 1 mole
of castor oil. By pouring the solution into water and uniformly distribut-
ing it therein, an aqueous dispersion is obtained.
V. 80 parts by weight of compound no. 14 is well mixed with 3 parts by
weight of the sodium salt of diisobutylnaphthalene-a-sulfonic acid,
10 parts by weight of the sodium salt of a lignin-sulfonic acid obtained
from a sulfite waste liquor, and 7 parts by weight of powdered silica gel,
and triturated in a hammer mill. By uniformly distributing the mixture in
water, a spray liquor is obtained.
VI. 3 parts by weight of compound no. 122 is intimately mixed with
97 parts by weight of particulate kaolin. A dust is obtained containing 3~
by weight of the active ingredient.
VII. 30 parts by weight of compound no. 8 is intimately mixed with a
mixture consisting of 92 parts by weight of powdered silica gel and
8 parts by weight of paraffin oil which has been sprayed onto the surface
of this silica gel. A formulation of the active ingredient is obtained
having good adherence.
zooo3sz
38 O.Z. 0050/40288
VIII. 40 parts by weight of compound no. 14 is intimately mixed with
parts by weight of the sodium salt of a phenolsulfonic acid-urea-
formaldehyde condensate, 2 parts of silica gel and 48 parts of water to
give a stable aqueous dispersion. Dilution in water gives an aqueous
5 dispersion.
Ix. 20 parts by weight of compound no. 122 is intimately mixed with
2 parts by weight of the calcium salt of dodecylbenzenesulfonic acid,
8 parts by weight of a fatty alcohol polyglycol ether, 2 parts by weight
10 of the sodium salt of a phenolsulfonic acid-urea-formaldehyde condensate
and 68 parts by weight of a paraffinic mineral oil. A stable oily
dispersion is obtained.
In these application forms, the agents according to the invention may also
be present together with other active ingredients, for example herbicides,
insecticides, growth regulators, and fungicides, and may furthermore be
mixed and applied together with fertilizers. Admixture with other fun-
gicides frequently results in an increase in the fungicidal Spectrum.
The following list of fungicides with which the novel compounds may be
combined is intended to illustrate possible combinations but not to impose
any restrictions.
Examples of fungicides which may be combined with the novel compounds are:
sulfur,
dithiocarbamates and their derivatives, such as
ferric dimethyldithiocarbamate,
zinc dimethyldithiocarbamate,
zinc ethylenebisdithiocarbamate,
manganese ethylenebisdithiocarbamate,
manganese zinc ethylenediaminebisdithiocarbamate,
tetramethylthiuram disulfides,
ammonia complex of zinc N,N'-ethylenebisdithiocarbamate,
ammonia complex of zinc N,N'-propylenebisdithiocarbamate,
zinc N,N'-propylenebisdithiocarbamate and
N,N~-polypropylenebis(thiocarbamyl) disulfide;
vitro derivatives, such as
dinitro(1-methylheptyl)-phenyl crotonate,
2-sec-butyl-4,6-dinitrophenyl 3,3-dimethylacrylate,
2-sec-butyl-4,6-dinitrophenyl isopropylcarbonate and
diisopropyl 5-nitroisophthalate;
20Q4~~~
39 O.Z. 0050/40288
heterocyclic substances, such as
2-heptadecylimidazol-2-yl acetate,
2,4-dichloro-6-(o-chloroanilino)-s-triazine,
0,0-diethyl phthalimidophosphonothioate,
5-amino-1-[-bis-(dimethylamino)-phosphinyl]-3-phenyl-1,2,4-triazole,
2,3-dicyano-1,4-dithioanthraquinone,
2-thio-1,3-dithio[4,5-b]quinoxaline,
methyl 1-(butylcarbamyl)-2-benzimidazolecarbamate,
2-methoxycarbonylaminobenzimidazole,
2-(fur-2-yl)-benzimidazole,
2-(thiazol-4-yl)benzimidazole,
N-(1,1,2,2-tetrachloroethylthio)-tetrahydrophthalimide,
N-trichloromethylthiotetrahydrophthalimide,
N-trichloromethylthiophthalimide,
N-dichlorofluoromethylthio-N', N'-dimethyl-N-phenylsulfuric acid diamide,
5-ethoxy-3-trichloromethyl-1,2,3-thiadiazole,
2-thiocyanatomethylthiobenzothiazole,
1,4-dichloro-2,5-dimethoxybenzene,
4-(2-chlorophenylhydrazono)-3-methyl-5-isoxazolone,
2-thiopyridine 1-oxide,
8-hydroxyquinoline and its copper salt,
2,3-dihydro-5-carboxanilido-6-methyl-1,4-oxathiyne,
2,3-dihydro-5-carboxanilido-6-methyl-1,4-oxathiyne 4,4-dioxide,
2-methylfuran-3-carboxanilide,
2,5-dimethylfuran-3-carboxanilide,
2,4,5-trimethylfuran-3-carboxanilide,
2,5-dimethyl-N-cyclohexylfuran-3-carboxamide,
N-cyclohexyl-N-methoxy-2,5-diethylfuran-3-carboxamide,
2-methylbenzanilide,
2-iodobenzanilide,
N-formyl-N-morpholine-2,2,2-trichloroethylacetal,
piperazine-1,4-diylbis-(1-(2,2,2-trichloroethyl)-formamide),
1-(3,4-dichloroanilino)-1-formylamino-2,2,2-trichloroethane,
2,6-dimethyl-N-tridecylmorpholine and its salts,
2,6-dimethyl-N-cyclododecylmorpholine and its salts,
N-[3-(p-tert.-butytphenyl)-2-methylpropyl]-cis-2,6-dimethylmorpholine,
N-[3-(p-tert.-butylphenyl)-2-methylpropyl]-piperidine,
1-[2-(2,4-dichlorophenyl)-4-ethyl-1,3-dioxolan-2-ylethyl]-1H-1,2,4-
-triazote,
1-[2-(2,4-dichlorophenyl)-4-n-propyl-1,3-dioxolan-2-ylethyl]-1H-1,2,4-
-triazole,
N-(n-propyl)-N-(2,4,6-trichlorophenoxyethyl)-N'-imidazolyl-urea,
1-(4-chlorophenoxy)-3,3-dimethyl-1-(1H-1,2,4-triazol-1-yl)-butan-2-one,
200036
40 0.1. 0050/40288
1-(4-chlorophenoxy)-3,3-dimethyl-1-(1H-1,2,4-triazot-1-yl)-butan-2-ol,
1-(4-phenylphenoxy)-3,3-dimethyl-1-(1H-1,2,4-triazol-1-yl)-2-butanol,
a-(2-chlorophenyl)-a-(4-chlorophenyl)-5-pyrimidinemethanol,
5-butyl-(2-dimethylamino-4-hydroxy-6-methylpyrimidine,
bis-(p-chlorophenyl)-3-pyridinemethanol,
1,2-bis-(3-ethoxycarbonyl-2-thioureido)-benzene,
1,2-bis-(3-methoxycarbonyl-2-thioureido)-benzene,
and various fungicides, such as
dodecylguanidine acetate,
3-[3-(3,5-dimethyl-2-oxycyclohexyl)-2-hydroxyethyl]-glutaramide,
hexachlorobenzene,
OL-methyl-N-(2,6-dimethylphenyl)-N-fur-2-yl alanate,
methyl DL-N-(2,6-dimethylphenyl)-N-(2~-methoxyacetyl)-alanate,
N-(2,6-dimethylphenyl)-N-chloroacetyl-DL-2-aminobutyrolactone,
methyl DL-N-(2,6-dimethylphenyl)-N-(phenylacetyl)-alanate,
5-methyl-5-vinyl-3-(3,5-dichlorophenyl)-2,4-dioxo-1,3-oxazotidine,
3-[3,5-dichlorophenyl]-5-methyl-5-methoxymethyl-1,3-oxazolidine-2,4-dione,
3-(3,5-dichlorophenyl)-1-isopropylcarbamylhydantoin,
N-(3,5-dichlorophenyl)-1,2-dimethylcyctopropane-1,2-dicarboximide,
2-cyano-[N-(ethylaminocarbonyl)-2-methoximino]-acetamide,
1-[2-(2,4-dichlorophenyl)-pentyl]-1H-1,2,4-triazole,
2,4-difluoro-a-(1H-1,2,4-triazol-1-ylmethyl)-benzhydryl alcohol,
N-(3-chloro-2,6-dinitro-4-trifluoromethylphenyl)-5-trifluoromethyl-3-
chloro-2-aminopyridine, and
1-((bis-(4-fluorophenyl)-methylsilyl)-methyl)-1H-1,2,4-triazole.
Use examples
For comparison purposes, the compound methyl a-2-(6-chloropyrazin-2-oxy)-
phenyl-a-methoxyacrylate (C) disclosed in EP-260,794 was used.
Use Example 1
Action on Plasmopara viticola
Leaves of potted vines of the Miiller-Thurgau variety were sprayed with
aqueous suspensions containing (dry basis) 80% of active ingredient and
20~ of emulsifier. To assess the duration of action, the plants were set
up, after the sprayed-on layer had dried, for 8 days in the greenhouse.
Then the leaves were infected with a zoospore suspension of Plasmopara
viticola. The plants were first placed for 48 hours in a water vapor-
saturated chamber at 24°C and then in a greenhouse for 5 days at from
20
to 30°C. To accelerate and intensify the sporangiophore discharge, the
plants were then again placed in the moist chamber for 16 hours. The
extent of fungus attack was then assessed on the undersides of the leaves.
2000362
41 O.Z. 0050/40288
The results of this experiment show that active ingredients 14, 86, 87,
122, 123, 124, 125, 126, 220, 270, 276, 277, 278, 279 and 280, applied as
0.0125wt% spray liquors, have a better fungicidal action (95%) than prior
art comparative agent C (60%).
Use Example 2
Action on Septoria nodorum
Wheat plants of the "Friihgold" variety were sprayed to runoff at the
one-leaf stage with aqueous formulations consisting (dry basis) of 80% of
active ingredient and 20% of emulsifier. 20 hours after the sprayed-on
layer had dried, the plants were inoculated with an aqueous spore suspen-
sion of Septoria nodorum until droplets formed, and placed for a week in a
climatic cabinet at from 17 to 19°C and a relative humidity of approx.
90
to 95%. The spread of the symptoms was then assessed.
The results of this experiment show that active ingredients 8, 14, 86, 87,
121, 205, 246, 268, 269, 272, 277, 280, 281 and 282, app i i ed as 0 . 05wt%
spray liquors, have a very good fungicidal action (95%).
Use Example 3
Action on Pyrenophora teres
Barley seedlings of the "Igri" variety were sprayed to runoff at the
two-leaf stage with aqueous suspensions consisting (dry basis) of 80% of
active ingredient and 20% of emulsifier. After 24 hours the plants were
inoculated with a spore suspension of the fungus Pyrenophora teres, and
set up for 48 hours in a high-humidity climatic cabinet at 18°C. The
plants were then cultivated for a further 5 days in the greenhouse at 20
to 22°C and a relative humidity of 70°C. The extent of fungus
spread was
then assessed.
The results of this experiment show that active ingredients 86, 87, 125,
126, 205, 246, 276, 277, 278 and 280, applied as 0.0125wt% spray liquors,
have a better fungicidal action (95%) than prior art active ingredient C
( 55fo) .
The novel compounds are also suitable for effectively combating pests from
the class of insects, mites and nematodes. They may be used as pesticides
in crop protection, and in the hygiene, stores protection and veterinary
sectors.
2~oo3s~
42 O.Z. 0050/40288
Examples of injurious insects from the Lepidoptera order are Agrotis
ypsilon, Agrotis segetum, Alabama argillacea, Anticarsia gemmatalis,
Argyresthia conjugella, Autographa gamma, Bupalus piniarius, Cacoecia
murinana, Capua reticulana, Cheimatobia brumata, Choristoneura fumiferana,
Choristoneura occidentalis, Cirphis unipuncta, Cydia pomonella,
Dendrolimus pini, Diaphania nitidalis, Diatraea grndiosella, Earias
insulana, Elasmopalpus lignosellus, Eupoecilia ambiguella, Evetria
bouliana, Feltia subterranea, Galleria mellonella, Grapholita funebrana,
Grapholita molesta, Heliothis armigera, Heliothis virescens, Heliothis
zea, Hellula undalis, Hibernia defoliaria, Hyphantria cunea, Hyphantria
cunea, Hyponomeuta malinellus, Keifferia lycopersicella, Lambdina
fiscellaria, Laphygma exigua, Leucoptera coffeella, Leucoptera scitella,
Lithocolletis blancardella, Lobesia botrana, Loxostege sticticalis,
Lymantria dispar, Lymantria monacha, Lyonetia clerkella, Malacosoma
neustria, Mamestra brassicae, Orgyia pseudotsugata, Ostrinia nubilalis,
Panolis flames, Pectinophora gossypiella, Peridroma saucia, Phalera
bucephala, Phthorimaea operculella, Phyllocnistis citrella, Pieris
brassicae, Plathypena scarbra, Plutella xylostelia, Pseudoplusia
includens, Phyacionia frustrana, Scrobipalpula absoluta, Sitotroga
cerelella, Sparganothis pilleriana, Spodoptera frugiperda, Spodoptera
tittoralis, Spodoptera litura, Thaumatopoea pityocampa, Tortrix viridana,
Trichoplusia ni and Zeiraphera canadensis.
Examples from the Coleoptera order are Agrilus sinuatus, Agriotes
lineatus, Agriotes obscurus, Amphimallus solstitialis, Anisandrus dispar,
Anthonomus grandis, Anthonomus pomorum, Atomaria linearis, Blastophagus
piniperda, Blitophaga undata, Bruchus rufimanus, Bruchus pisorum, Bruchus
lentis, Byctiscus betulae, Cassida nebulosa, Cerotoma trifurcata,
Ceuthorrhynchus assimilis, Ceuthorrynchus napi, Chaetocnema tibialis,
Conoderus vespertinus, Crioceris asparagi, Diabrotica longicornis,
Diabrotica 12-punctata, Diabrotica virgifera, Epilachna varivestis,
Epitrix hirtipennis, Eutinobothrus brasiliensis, Hylobius abietis, Hypera
brunneipennis, Hypera postica, Ips typographus, Lema bilineata, Lema
melanopus, Leptinotarsa decemlineata, Limonius californicus, Lissorhoptrus
oryzophilus, Melanotus communis, Meligethes aeneus, Melolontha
hippocastani, Melolontha melolontha, Onlema oryzae, Ortiorrhynchus
sulcatus, Otiorrhynchus ovatus, Phaedon cochleariae, Phyllotreta
chrysocephala, Phyllophaga sp., Phyllopertha horticola, Phyllotreta
nemorum, Phytlotreta striolata, Popillia japonica, Sitona lineatus and
Sitophilus granaria.
Examples from the Diptera order are Aedes aegypti, Aedes vexans,
Anastrepha ludens, Anopheles maculipennis, Ceratitis capitata, Chrysomya
bezziana, Chrysomya hominivorax, Chrysomya macellaria, Contarinia
sorghicola, Cordylobia anthropophaga, Culex pipiens, Dacus cucurbitae,
z~oaa~s~
43 O.Z. 0050/40288
Daces oleae, Dasineura brassicae, Fannia canicularis, Gasterophilus
intestinalis, Glossia morsitans, Haematobia irritans, Haplodiplosis
equestris, Hylemyia platura, Hypoderma lineata, Liriomyza sativae,
Liriomyza trifolii, Lucilia caprina, Lucilia cuprina, Lucilia sericata,
Lycoria pectoralis, Mayetiola destructor, Musca domestica, Muscina
stabulans, Oestrus ovis, Oscinella frit, Pegomya hysocyami, Phorbia
antiqua, Phorbia brassicae, Phorbia coarctata, Rhagoletis cerasi, Rhago-
letis pomonella, Tabanus bovines, Tipula oleracea and Tipula paludosa.
Examples from the Thysanoptera order are Frankliniella fusca,
Frankliniella occidentalis, Frankliniella tritici, Scirtothrips citri,
Thrips oryzae, Thrips palmi and Thrips tabaci.
Examples from the Hymenoptera order are Athalia rosae, Atta cephalotes,
Atta sexdens, Atta texana, Hoplocampa minuta, Hoplocampa testudinea,
Monomorium pharaonis, Solenopsis geminata and Solenopsis invicta.
Examples from the Heteroptera order are Acrosternum hilare, Blissus
leucopterus, Cyrtopeltis notatus, Dysdercus cingulatus, Dysdercus
intermedius, Eurygaster integriceps, Euchistus impictiventris,
Leptoglossus phyllopus, Lygus lineolaris, Lygus pratensis, Nezara
viridula, Piesma quadrata, Solubea insularis and Thyanta perditor.
Examples from the nematode class are root-knot nematodes, e.g.,
Meloidogyne hapla, Meloidogyne incognita and Meloidogyne javanica,
cyst-forming nematodes, e.g., Globodera rostochiensis, Heterodera avenae,
Hetrodera glycinae, Heterodera schatii, Hetrodera triflolii, stem and leaf
eelworms, e.g., Belonolaimus longicaudatus, Ditylenchus destructor,
Ditylenchus dipsaci, Heliocotylenchus multicinctus, Longidorus elongates,
Radopholus similis, Rotylenchus robustus, Trichodorus primitives, Tylen-
chorhynchus claytoni, Tylenchorhynchus dubius, Pratylenchus neglectus,
Pratylenchus penetrans, Paratylenchus curvitatus, Partylenchus goodeyi.
For combating pests, the active ingredient concentrations in the finished
formulations may vary over a wide range. Generally, they are from 0.0001
to 10, and preferably from 0.001 to 0.1, 96.
The active ingredients may also successfully be used in the
ultra-low-volume (ULV) method, where it is possible to apply formulations
containing more than 95wt% of active ingredient, or even the active
ingredient without additives.
In the open, the amount of active ingredient applied is for example from
0.01 to 10, particularly from 0.1 to 1.0, kg/ha.
2000362
44 O.Z. 0050/40288
For comparison purposes, the compounds 2-(p-methoxy-a-methoxycarbonyl-
vinyl)-4'-chlorostilbene (A) and methyl a-[2-(benzoxazol-2'-yloxy)-
phenyl)-(i-methoxyacrylate (B) disclosed in EP 178,826 and EP 256,667 were
used.
Use Example 4
Prodenia litura
Type of experiment: Effect of ingested food
The experiment was carried out in 250 ml plastic beakers. Two caterpillars
were placed in each vessel, and pieces of Indian corn plants which had
been previously dipped for 5 seconds into aqueous formulations of the
active ingredients were proffered as food. The amount of active ingredient
is given in ppm. The kill rate was assessed in % after 24 hours.
Active ingredient no. Prodenia
ppm Ki l l rate (9'0)
8 40 100
123 200 80
A 1000 0
g 1000 80
use Example 5
Musca domestics
Type of experiment: Continuous contact action
Both tops and bottoms of a glass dish 10 cm in diameter were wetted with a
total of 1 ml of acetonic solutions of the active ingredients. The amount
of active ingredient is given in ppm. After the solvent had evaporated, 10
flies were introduced into each dish, each dish was closed and the animals
in supine position were counted after 4 hours and the kill rate was
determined in 96.
Active ingredient no. Musca
ppm K i 1 l rate (9'0)
8 2 100
14 2 100
A 2 0
g 4 80
2~oQ3s2
45 O.Z. 0050/40288
Use Example 6
Plutella maculipennis
Type of experiment: Contact action
Young cabbage leaves were dipped for 3 seconds into aqueous formulations
of the candidate compounds (amount of active ingredient in ppm) and placed
in a glass dish (10 cm in diameter) on a circular filter paper (9 cm in
diameter) moistened with 0.5 ml of water. 10 caterpillars of the fourth
larval stage were then placed on each leaf and the dishes were closed. The
kill rate was assessed in 96 after 4$ hours.
Active ingredient no. Plutella
ppm Ki l l rate (9~0)
g 100 100
14 1000 100
123 200 100
p 1000 0
B 200 0
1000 80
Use Example 7
Ornithodorus moubata
Type of experiment: Contact action
Young ticks (1.5 to 2 mm in diameter) which had sucked blood once were
individually picked up by means of a suction tube. A strong light source
drove the active animals from the discarded exoskeleton remains.
5 ticks were placed in paper bags, and the bags were dipped for 5 seconds
in aqueous active ingredient formulations (amounts of active ingredient
given in ppm). The bags were then suspended and the action was assessed
after 48 hours by holding the bags up to a strong light source (60 watt
bulb); the animals still living attempted to escape and were easy to
recognize from their movements. The temperature was kept at about 25°C.
The kill rate was determined in 96.
20aQ362
46 O.Z. 0050/40288
Active ingredient no, Ticks
ppm K i 11 rate (9'a)
g 400 80
14 1000 0
5 121 1000 80
125 1000 60
279 1000 60
A 1000 0
g 1000 0
Use Example 8
Tetranychus telarius; contact action; spray experiment
Potted bush beans exhibiting the first pair of leaves were sprayed to
runoff with aqueous formulations of the active ingredients. The plants
were sprayed from all sides with a total of 50 ml of spray liquor. The
plants were under heavy mite attack and numerous eggs had been laid on
them.
The action was assessed after 5 days by means of a binocular magnifying
glass, care being taken to ascertain whether animals of all development
stages were killed. For the 5 days of the experiment, the plants were
subjected to normal greenhouse conditions.
Active ingredient no. ppm Kill rate (9~0)
121 100 100
125 40 80
126 400 100
279 100 100
g 1000 0
40