Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2~247~
ME~HOD FOR MAKING ARSENIC ACID
This invention relates to a method for making arsenic
acid, and, more particularly, to a method for making
arsenic acid from soluble arsenate salts using
electrolysis with membranes.
BACKGROUND OF T~E INVENTION
Arsenic acid can be readily prepared by dissolving
arsenic pentoxide in water or by dissolving arsenic
trioxide in the presence of an oxidizing agent. The
oxides of arsenic are often prepared from solutions
obtained in the metallurgical treatment processes of
arsenical materials such as ores, concentrates, fumes,
speisses, slags, residues, flue dusts and the like.
Depending on the arsenical material being treated, these
processes often include a leach with a lixiviant
yielding a solution that contains arsenic in the form of
an arsenite or arsenate. The leach may be carried out at
atmospheric pressure, elevated pressures, at elevated
temperatures and, in many cases, ln the presence of
an oxygen-containing gas. Concurrent wi~h the leaching
of arsenic, other metals are dissolved that contaminate
the solution and, ultimately, also contaminate the
arsenic product prepared from such a solution. In many
cases, the arsenic-containing solution is treated for the
formation of arsenic compounds that may be disposed in
~ :
479
an acceptable form.
The leaching of arsenical materials, the oxidation
of trivalent to pentavalent arsenic and the preparation
of arsenic compounds are well documented. The prior
art, however, is silent on the process according to the
present invention according to which arsenic acid is
prepared from a soluble arsenate salt solution by
electrolysis with membranes.
5UMMARY OF THE INVENTION
I have now discovered that an impure water-soluble
arsenate salt can be treated for at least partial
conversion to arsenic acid. More specifically, I have
discovered that by subjecting a soluble arsenate salt
solution to electrolysis in a cell containing an
arrangement of electrodes and cationic membranes or
electrodes and cationic and anionic membranes, arsenic acid
and a hydroxide may be formed and recovered. The
soluble arsenate salt solution may contain arsenic as
sodium, potassium, lithium or ammonium arsenate.
Depending on the configuration of the cell, arsenic acid
containing substantially none to a reduced amount of
sodium, potassium, lithium or ammonium and impurities
may be recovered. The arsenate salt solution may be a
leach solution containing arsenate or arsenite, the
latter being easily oxidized to arsenate prior
29L7~
to electrolysis. Most commonly, the arsenate solution
contains sodium arsenate. The arsenic acid-containing
solution that is recovered can be used in the
manufacture of arsenic compounds having a reduced
S impurity content. The hydroxide-containing solution,
i.e., concurrently generated sodium, potassium, lithium
or ammonium hydroxide, i5 also recovered, and can be
recycled to replace at least a portion of any hydroxide
input required for generating the soluble arsenate.
Generally, electrolysis is carried out in an
electrolysis unit that comprises a stack of two- or
three-compartment cells. The unit may comprise at
least one electrode/membrane group consisting of either
a bipolar ~r a monopolar electrode in combination with
either cationic or cationic and anionic membranes. The
at least one electrode/membrane group is arranged between
two terminal electrodes with or without adjacent
terminal membranes. Hydroxide and acid compartments are
defined between electrodes and membranes, and diluate
compartments may be defined between membranes. Thus, there
are four configurations of the electrolysis cell that can
be used in the present invention: a three-compartment,
bipolar electrode cell, a three-compartment, monopolar
~ electrode cell; a two-compartment, bipolar electrode cell,
and a two-compartment, monopolar electrode cell.
,
When using a particular configuration for a cell,
:: ~
.
aqueous arsenate salt solution is fed into diluate or acid
compartments of an electrolysis unit while a direct
electric current is applied between either the terminal
or all electrodes. The application of current causes at
least a portion of the arsenate anions to pass through
any anionic membranes to concentrate in acid compartments
where oxygen is evolved at the anodic electrode surfaces
resulting in the formation of arsenic acid.
Simultaneously, at least a portion of the alkali metal and
ammonium cations pass through any cationic membranes to
concentrate in the hydroxide compartments where hydrogen
is evolved at the cathodic electrode surfaces resulting in
the formation of alkali metal hydroxide or ammonium
hydroxide. By selecting monovalent permselective cationic
membranes, the passage of any present di- and multi-valent
cations to the hydroxide compartments is minimized.
Solutions are circulated through the compartments in an
electrolytic cell, and arsenic acid with a reduced
concentration of monovalent alkali metal and ammonium
cations, and substantially pure hydroxide are withdrawn as
products.
It is an cbject of the present invention to provide a
method ~or making arsenic acid. It is another object to
provide a method for converting at least a portion of
a soluble arsenate to arsenic acid and a hydroxide by
electrolysis with membranes. It is a further object to
provide a method for treating metallurgical leach
solutions containing arsenic by electrolysis with
membranes for the recovery of arsenic acid and a
hydroxide. These and other objects of the present
invention will become clear from the following detailed
description.
Accordi,ngly, there is provided a method for the
production of arsenic acid which comprises the steps of
passing an aqueous solution of an arsenate salt
through cells of an electrolysis unit containing
electrodes and membranes, said unit comprising terminal
electrodes and at least one electrode/membrane group
arranged between said terminal electrodes, each said
group consisting of at leact one membrane adjacent an
intermediate electrode; at least defining a hydroxide
compartment and an acid compartment hetween membranes and
said electrodes; applying an electrical current between
said electrodes at a value such that the value of
the corresponding current density is in the range of about
10 to 4,500 A/m2; passing flows of solutions through said
cells; forming hydroxide-containing solution in said
hydroxide compartment; forming arsenic acid-containing
solution in said acid compartment; withdrawing
hydroxide-containing solution from said hydroxide
compartment; withdrawirlg arsenic acid containing solution
from said acid compartment; and recovering hydroxide
solution and arsenic acid-containing solution.
2~q~479
~RIEF DESCRIPTION OF DRAWINGS
The invention will now be described with reference to
the accompanying drawings wherein:
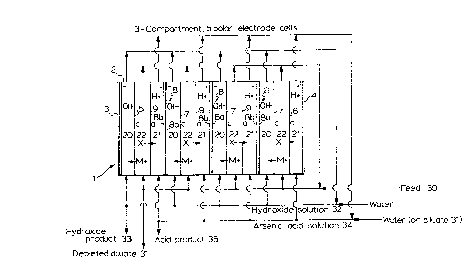
Figure 1 is a schematic of a first configuration of
an electrolysis with membranes unit used in the method of
the invention;
Figure 2 is an alternative of the schematic of Figure 1;
Figure 3 is a second alternative of the schematic of
Figure 1;
Figure 4 is a third alternative of the schematic of
Figure l; and
wherein M~ is a monovalent cation (K+, Na+, Li+ or NH4+),
X~ is an anion (Aso43-, HAsO42- or H2AsO4-),
H+ is a hydrogen ion,
OH~ is a hydroxyl anion,
c is a cationic membrane,
a is an anionic membrane,
- is a cathode or a cathodic surface, and
: + is an anode or an anodic surface.
In the Figures and the following detailed description,
: like numbers refer to like parts.
DETAILED DESCRIPTION
. The method of the present invention is applicable to
aqueous ~olutions containing water-soluble arsenate salts,
~2~
as well as water-soluble arsenite salts that have been
oxidized to the arsenate form. The water-soluble
arsenites and arsenates are those containing monovalent
cations such as sodium, potassium, lithium and
ammonium. The method is particularly applicable to
aqueous arsenate salt-containing solutions derived from
the metallurgical processing of arsenic-containing
materials, arsenical products and intermediates capable
of forming soluble arsenates and arsenites. The
treatment of specific interest is that in which a leach
of arsenical material is carried out under alkaline
conditions to yield a solution containing arsenites
and/or arsenates as well as dissolved impurity cations,
such as for example Zn, Pb, Sb, Ni, Co, and Fe. ~ny
arsenite in solution is oxidized to arsenate prior to
subsequent treatment of the solution by any one of a
number of well-known methods. The arsenate solution
may contain a mixture of mono-, di-, and tri-alkali
metal or ammonium arsenates. Usually, the arsenate
solution treated according to the method of the present
invention is a sodium arsenate solution, also
containing impurity cations.
The arsenate solution is fed to an electrolysis cell
with membranes wherein the arsenate is partly or
completely converted into arsenic acid and a hydroxide
according to the following simplified equations for a
.
:
2~ 7~
tri-metal arsenate:
M3As04 ~ H20 ---> ~M2ASO + MOH
M3As04 ~ 2H2~ -~~' H2MAsO4 + 2MOH
~3AsO4 + 3H20 --> H3As04 + 3~0H
wherein M represents the monovalent cation of
sodium, potassium, lithium or ammonium. Any mono- or
di-hydrogen arsenate reacts similarlyO
Electrolysls with membranes may yield an arsenic
acid solution that contains substantially none of the
monovalent cations and is also substantially free of
impurity cations. Electrolysis with membranes may
also yield a solution containing arsenic acid and a
reduced content of the monovalent cation and
impurities. The proportion of arsenic acid in the
arsenic acid product solutisn depends on the
electrode/membrane configuration of the electrolysis cellJ
to be explained.
The electrolysis of aqueous arsenate salt solution
is carried out by passing arsenate solution to cells of
an electrolysis unit containin~ electrodes and membranes.
The electrolysis unit comprises terminal electrodes and at
least one electrode/membrane group arranged between the
terminal electrodes. Each electrode/membrane group
consists of one membrane or two membranes adjacent an
intermediate electrode. At least a hydroxide compartment
and an acid compartment are defined between membranes
~jO02479
and the electrodes. A diluate compartment may be defined
between membranes. The intermediate electrode of an
electrode/membrane group may be either a bipolar or a
monopolar electrode. The membranes may be suitable
cationic membranes or suitable cationic and anionic
membranes. A direct electrical current is applied between
either the terminal electrodes or between all electrodes
in an electrolysis cell. Hydroxide-containing solution
is formed in a hydroxide compartment and arsenic acid-
containiny solution is formed in an acid compartment.
Hydroxide-containing solution and arsenic acid-containing
solution are circulated through their respective
compartments and portions are withdrawn from the
circulating solutions as products, The specific
electrode and membrane configuration and type used in an
electrolytic cell depend on the desired degree of purity of
the arsenic acid product. Four possi~le configurations of
an electrolysis unit employing different electrode and
membrane configurations and types are described in detail
with reference to Figures 1, 2, 3 and 4. The figures show
electrolysis units each having four cells.
With reference to Figure 1, the electrolysis unit,
generally indicated with 1, comprises 3-compartment bipolar
electrode cells. The unit comprises a housing 2
containing a terminal cathode 3 and a terminal anode 4
positioned at opposite ends of housing 2. The terminal
electrodes 3 and 4 are connected to a source ~not shown)
2479
of direct electri~al current. The terminal anode 4 is made
of an acid-resistant material such as, for example, lead,
lead alloys of silver, antimony or calcium, platinum or
platinum-coated, iridium or iridium oxide-coated valve
metals. The terminal cathode 3 is made of an
alkali-resistant material such as, for example, copper,
lead, nickel, iron, steel, tin~ silver, ~raphite, gold,
platinum, palladium or platinum-plated titanium, iridium or
iridium oxide, zirconium or niobium, or alloys of lead or
nickel. Adjacent and closely spaced from cathode 3 and
anode 4 are a terminal cationic membrane 5 and a terminal
anionic membrane 6, respectively. Between terminal
membranes S and 6 is positioned, in closely spaced
relation, at least one electrode/membrane group.
Preferably a plurality of electrode/membrane groups is
used (three are shown). Each electrode/membrane group
consists of a cationic membrane 7, an intermediate
electrode, i.e., a bipolar electrode 8 having a cathodic
side 8a and an anodic side 8b, and an anionic membrane 9,
in that order. The number of electrode/membrane groups
depends on the desired capacity of the cell and may be
limited by desired and practical cell voltages, and by
optimum cell design for performance efficiency. The
electrode/membrane groups and the terminal membranes and
25 ~ terminal electrodes may form a stack in housing 2. A
hydroxide compartment 20 is defined between each of the
cationic membrane~ 7 and the cathodic sides 8a of bipolar
electrodes 8, as well as between terminal cathode 3 and
:: :
7~
terminal cationic membrane 5. An acid compartment 21 is
defined between each of the anionic membranes 9 and the
anodic sides 8b of bipolar electrodes ~, as well as between
terminal anode 4 and terminal anionic membrane 6. A
diluate compartment 22 is defined between each of the
cationic membranes 7 and the anionic membranes 9, as well
as between the termlnal membranes 5 and 6 and the adjacent
anionic membrane 9 and the adjacent cationic membrane 7,
respectively.
The cationic membranes 5 and 7 are suitable monovalent
cation permselective membranes such as those that have,
for example, strongly acidic active groups and a membrane
matrix of a styrene di-vinyl benzene co-polymer on a
polyvinyl chloride base, the active groups being sulfonic
acid radicals lR-SO3H). Suitable cationic membranes include
sulfonated or carboxylated per fluorocarbon membranes.
Suitable membranes 5 and 7 are treated SelemionTM CMR,
SelemionTM CSV, SelemionTM CSR, SelemionTM CMT and
especially, treated SelemionTM CMF membranes, manufactured
by the Asahi Glass Company of Japan, and equivalent
membranes manufactured by other companies.
Suitable anionic membranes 6 and 9 are those that are
permselective for anions, and may include SelemionTM AMV,
SelemionTM ASR, SelemionTM AAV and Selemion AMT membranes.
Other, simllarly suitable anionic membranes may be used to
yield the desired result.
2Ç~247~
Each bipolar electrode 8 has a cathodic side 8a and an
anodic side 8b, and is made from a suitable, electrically
conductive material or composite that, when the direct
current is applied between the terminal electrodes 3 and
4, causes evolution of oxygen at the anodic side 8b and
hydrogen at the cathodic side 8a. Suitable materials for
the bipolar electrodes comprise, Eor example, metals such
as lead, alloys such as antimony-lead, silver-lead or
calcium-lead; and composites such as titanium coated with
a noble metal~ or a metal with a cathodic side of,
for example, nickel and an anodic side of platinum, or
platinum-plated niobium, tantalum, titanium or
zirconium, iridium or iridium oxide-coated titanium or a
bimetallic electrode with a cathodic side of steel and
an anodic side of any of the suitable anodes listed above.
With reference to Figure 2, the electrolysis unit
comprises 3-compartment, monopolar electrode cells similar
to the cell descxibed with reference to Figure 1, but
wherein the bipolar electrodes have been replaced with
monopolar electrodes, and the sequence of membranes has
been changed. The cell, generally indicated with 1,
comprises a housing 2, two terminal anodes 4 placed at
: opposite ends of housing 2. Ad~acent each of the terminal
anodes 4 is positioned a terminal anionic membrane 6.
Between the membranes 6 are positioned, in closely spaced
relation, at least three electrode/membrane groups ~as
~~~o~~
shown) consisting of intermediate electrodes, i.eO anodes
10 alternating with cathodes 11; the anodes and cathodes
have two anionic membranes 9 or two cationic membranes 7,
one on either side, respectivelyO The number of electrode
membrane groups depends on the desired capacity of the
unit. The anodes 10 alternatin~ with cathodes 11 are
arranged between the terminal anodes 4 such that a
cationic membrane 7 is adjacent a terminal anionic membrane
6. Terminal anodes 4, anodes 10 and cathodes 11 are all
connec~ed to a source of direct electrical current (not
shown). The anodes and cathodes may be made of the same
materials as recited for the terminal electrodes in the
description of the unit of Figure 1. The cationic
membranes are suitable monovalent cation permselective
membranes. The suitable cationic and anionic membranes
may be the same as those recited in the description of the
unit of Figure 1.
A hydroxide compartment 20 is defined between each
cationic membrane 7 and a cathode 11. An acid compartment
21 is defined between each anionic membrane 9 and anode 10
and between each ~erminal anionic membrane 6 and a
terminal anode 4. A diluate compartment 22 is defined
between a cationic membrane 7 and an anionic membrane 9 and
: between a cationic membrane 7 and a terminal anionic membrane 6~
In various alternative embodiments (not shown) of the unit
~!~1[32479
according to Figure 2, the unit may, for example, have
terminal cathodes 3 with adjacent terminal cationic
membranes 5. The smallest 3-compartment cell unit
possible may consist of a cathode and an anode with a
cationic and an anionic membrane between the electrodes.
The operations of a unit according to Figure l or Figure
2 are similar. Soluble arsenate solution 30, which must
be essentially free of solids, i5 fed to a flow of diluate
recirculating through diluate compartments 22 of unit l.
A portion of the circulating diluate may be removed from
the process as depleted diluate 31. ~ direc~ electrical
current is applied between either the terminal electrodes
or all anodes and cathodes in the unit, as applicable.
The monovalent cations from the arsenate solution pass
through the cationic membranes, and concentrate as
hydroxide in hydroxide compartments 20. The hydroxide
solution 32 is passed and recirculated through the
hydroxide compartments 20, and a portion is removed and
recovered as hydroxide product 33. The arsenate ions pass
through the anionic membranes and concentrate as arsenic
acid in acid compartments 21. The arsenic acid solution 34
is passed and recirculated through compartments 21~ and a
portion is recovered as arsenic acid product 35. Water may
be added to the acid compartments 21 and/or hydroxide
compartments 20, a5 necessary, and is conveniently added to
circulating solution 32 and/or 34, to give the desired acid
or hydroxide concentrations in the products 35 and 33,
~,~02~L7~
respectively. Instead of water, depleted diluate 31 may be
added to acid compartments 21.
With reference to Figure 3, the electrolysis unit comprises
2~compartment, bipolar electrode cells similar to the cells
described with reference to Figure 1 but wherein the
anionic membranes have been deleted. The unit, generally
indicated with 1, comprises a housing 2~ a terminal cathode
3 and a terminal anode 4 placed at opposite ends of housing
2, and at least one electrode/membrane group. Each
electrode/membrane group consists of a cationic membrane 7
and an intermediate electrode, i.e., a bipolar electrode
8 having a cathodic side 8a and an anodic side 8b. A
terminal (additional) cationic membrane 5 is positioned
between terminal anode 4 and the cathodic side 8a of the
adjacent bipolar electrode 8. The number of electrode
membrane groups depends on the desired capacity of the
cell. A hydroxide compartment 20 is defined between a
cationic membrane 7 and cathodic side 8a of a bipolar
electrode 8, between a cationic membrane 7 and terminal
cathode 3, and between terminal cationic membrane 5 and
the cathodic side 8a of adjacent bipolar electrode 8. ~n
acid compartment 21 is formed between each cationic
membrane 7 and the anodic side 8b of a bipolar electrode
8 and between terminal cationic membrane 5 and terminal
anode 4.
The terminal electrodes 3 and 4 and the bipolar electrodes
29L~
16
8 may be made of the same materials as those of the unit
described with reference to Figure 1. The cationic
membranes 7 and 5 are monovalent cation permselective
membranes, which may be chosen from those described for
use in the unit of Figure 1.
With reference to Figure 4, the electrolysis unit
comprises 2-compartment, monopolar electrode cells similar
to the cells described with reference to Figure 3 but
wherein the bipolar electrodes have been replaced with
monopolar electrodes. The unit, generally indicated with
1~ comprises a housing 2, two terminal anodes 4 positioned
at opposite ends of housing 2 and a number of
electrode/membrane groups. Each group consists of a
cationic membrane 7 and an intermediate electrode.
Intermediate electrodes are anodes 10 alternating with
cathodes 11, cathodes being adjacent the terminal anodes
4. A terminal cationic membrane 5 is positioned between
one of the terminal anodes 4 and its adjacent cathode 11.
Thus, a unit according to Figure 4 contains alternating
anodes and cathodes with cationic membranes positioned
between the electrodes. A hydroxide compartment 20 is
formed between cationic membrane 5 and 7 and a cathode 11.
An acid compartment 21 is formed between a cationic
membrane 7 and an anode 10, and between membranes 5 and 7
and a terminal anode 4~ The anodes and cathodes may be
made of the same material as recited in the description of
the cell of Figure 1. The cationic membranes are suitable
247~31
monovalent cation permselective membranes such as those
described in the description of the cell of Figure 1.
In various alternative embodiments (not shown~ of the unit
according to Figure 4, the unit may, for example, have
terminal cathodes with alternating anode and cathode
intermediate electrodes, anodes being adjacent the
terminal cathodes, and cationic membranes between all
electrodes. The 2-compartment cell unit may also consist
of one cathode and one anode with one cationic membrane,
or of two terminal electrodes (cathodes or anodes~, an
anode or a cathode as intermediate electrode as applicable,
with cationic membranes between all electrodes.
The operations of a unit according to Figures 3 or 4 are
; similar. Soluble arsenate solution 30 is fed into an
arsenic acid-containing solution 34 that is formed in and
is passed and recirculated throu~h acid compartments 21.
A direct electrical current is applied to either the
terminal electrodes or to all anodes and cathodes, as
applicable, and electrolysis takes place. Monovalent
~ cations~from the arsenate solution pass through the
cationic membranes, and form a hydroxide solution 32 in
hydroxide compartments 20. Hydroxide solution 32 is passed
and recirculated through hydroxide compartments 2Q, and a
portion is removed and recovered as hydroxide product 33.
Water may be added to the circulating hydroxide solution
32 as required to give the desired hydroxide
7~
18
concentration in product 33. Arsenate ions substantially
remain in acid compartments 21 and form arsenic acid, and
the arsenic acid-containing solution 34 is recirculated,
a portion of the circulating solution being removed as
acid product 35.
The electrolysis using any one of ~he above-described cell
configurations is carried out at temperatures in the range
of from just above the freezing temperature of solution to
as high as 60~C, and preferably, at temperatures in the
range of from ambient to about 50~C. The electrical current
applied between the terminal electrodes or all electrodes
may be equivalent to a current density in the range of
about lO to 4,500 A/m2. 4,500 A/m2 is a practical upper
limit, as higher values result in excessive heat
generation. Below 10 A/m2 the rate of electrolysis is too
low. The current density values may be constant or varied,
depending on whether the arsenate solution is treated
batchwise or in a continuous operation. The current
~ density is preferably chosen in the range of about 200 to
4,000 A/m2. Above about 500 ~/mZ, cooling of one or more of
the circulating solutions may be necessary. The lower
values of the current density may be selected to achieve
lower cation concentration in the arsenic acid-containing
solution that can be recovered from a 2-compartment
configuration. The flows through the diluate, acid and
hydroxide compartments should be substantially balanced to
a~oid damage to the membranes due to excessive differential
9;7~
19
pressure. Damage is avoided when the flow rates are
adjusted such that the differential pressure across the
membranes does not exceed about 150 kPa. The acid and
hydroxide streams may be self circulating aided by the gas
evolution. The feed rate of the feed solution may be
selected such that the equivalent amount of arsenic in the
feed is in the range of about 2 to 60 g/min.m2 of membrane
area. The value selected is dependent on the value of
the current density and the desired degree of conversion of
the arsenate to arsenic acid and hydroxide.
Depending on the configuration of the electrolysis unit,
the arsenic acid product is substantially free of metal
cations or ammonium ions. The units of the configurations
described with reference to Figures l and 2 (3-compartment
cells) may give substantially pure products, the arsenic
acid product 35 containing substantially no monovalent
cations, typically less than l g/L, from the arsenate in
the feed solution 30. Less than complete conversion may
still yield a pure arsenic acid-containing solution. The
impurity content is also substantially reduced. The units
of the configurations of Figures 3 and 4 (2-compartment
cells) give usually only partly pure products, and the
arsenic acid~containing product 35 contains monovalent
cations in a concentration reduced from that in the feed
30. Thus, for example, the acid product 35 may contain
a concent~ation of monovalent cations in the range of
about 0.1 to 50 g/L, when treating feed solutions
2~L~9
containing about 30 to 100 g/L of monovalent cationsO The
impurity content is also reduced. The configurations of
the units using bipolar electrodes operate at a lower
current and at a higher potential at the current rectifier,
S and with a potentially higher efficiency than the
configurations with monopolar electrodes.
The performance of a three-compartment electrolysis cell
unit may be improved by maintaining the concentration of
the arsenate in the diluate compart~ent(s) 22 of a cell at
a relatively high value. When the concentrations are
maintained at a relatively high value such as for example,
30 g/L or higher, i,e., are not allowed to decrease
substantially below that value, the unit may be operated
at a substantially constant high current density of above
about 2000 A/m2, the current density need not decrease
during operation and provides high current efficiencies.
High concentrations may be maintained by a number of
methods that include electrodialysis, crystallization,
evaporations or other suitable methods. When using
electrodialysis, a flow from the solution circulating
through diluate compartment~s) 22 of an electrolysis unit
is passed through an electrodialysis unit for
concentration, and the concentrated solution is returned to
~ the circulat.ing solution. When using crystallization, the
25 ~ feed solution is~subjected to crystallization by a known
method. The mother liquor from the crystallization is fed
to diluate compartment(s) 22 of the electrolysis unit 1
:: :
: ~:
The crystals are retained and are dissolved at a controlled
rate in circulating diluate of the electrolysis unit,
thereby replenishing the arsenate tenor and maintaining it
at an adequately high value.
It is understood that concentration not only increases
performance but also allows treatment of dilute arsenate
solutions.
The invention will now be illustrated by means of the
following non-limitative examples.
Example l
An electrolysis unit comprlsing three, three-compartment
cells with bipolar electrodes was used for electrolysis of
an impure sodium arsenate solution. The electrodes
including the terminal electrodes were made of silver~lead
alloys. The electrode-membrane arrangement was as shown in
Figure l.
SelemionTM CMR cationic membranes and SelemionTM AMV anionic
membranes were used. In a first test, 8L of the sodium
arsenate solution was circulated through the diluate
compartments, and water was initially circulated through
the acid and the hydroxide compartments at a velocity of
0.5 cm/s. The current was gradually increased to obtain
225 A/m2 through the cells.
22
Oxygen and hydrogen were evolved at the anode and cathode
respectively. Samples were taken from the three
circulating streams and analysed. The results are ~hown in
Table I.
Table I
Time As Na NaOH H3As04 Pb
Stream h g/L g/Lg/L g/L 9,~
Feed/Diluate 0 53 61 21 0 0.41
Acid/Anolyte 0 0 0 0 3 0
Hydroxide/ 0 0 0 0 0
Catholyte
Diluate 7 49 50 - ~ 0.22
Acid/Anolyte 7 160.02 - 30.5 0.04
Hydroxide/ 7 0.005 16.831 - 0.001
Ca~holyte
Diluate 55 52 0.8 - 98 0.05
Acid 55 51 0.1 - 97.50.14
Hydroxide 55 0.1 60 108 - 0.003
The feed/diluate circulation decreased in volume by 2.8L
over the 55 hours, with 2.6L being gained in the catholyte
and 0.2L in the anolyte. Back-diffusion of arsenic acid to
the diluate stream from the acid stream was appar~nt as the
acid concentration increased.
Cathodic and anodic current effciencies were 92~ and 100%
respectively, after 7 hours and 50~ and 33%*, respectively
after 55 hours of operation.
~2~
23
*A value of 65% is obtained if acid in diluate was
considered.
Some white Pb-containing solids formed in the diluate
recirculation, due to precipitation of the original Pb
content in the feed with the decrease in p~. Some
corrosion of the lead electrodes was observed; and was
particularly severe on the cathodic surfaces.
In a second test, the acid and hydroxide circulation
streams were initially made-up using diluted products from
the first test. Starting circulating solutions were 8L for
the diluate compartments, and 5~5L each for the acid and
hydroxide compartments. The results are shown in Table II.
Table II
Time As Na NaOH H3AsO4Pb
Stream h g/L g/L g/L g/L ~/L
Feed 0 47 53 17 - 0.49
Acid/Anolyte 0 9 0 0 17 0
Hydroxide/ 0 0 6 10 0 0
Catholyte
Diluate 68 40 5 0 64 0.06
Acid product 68 50 0.05 0 99 0~005
~ydroxide 68 0.02 62 110 0 0.003
product
Current efficiency: Cathodic: 60.5%
25Anodic: 38% or
62~ considering acid in the
diluate~
2~7~
24
Example 2
Four, three-compartment, monopolar electrode cells were
used as shown in Figure 2 with SelemionTM CMF cationic and
SelemionTM AAV anionic membranes. The monopolar electrodes
consisted of silver-lead alloy anodes and 316 stainless
steel cathodes. In a first test, a 20L portion of feed
solution was treated over 120 hours at a current density of
initially 500A/m2 but decreased to 250A/m2 as the sodium
concentration in the feed decreased from 45 g/L to 5.6 g/L.
The acid and alkali products yenerated were analyzed and
the results are shown:
Table III
As Na NaOH H3As04Pb
5tream g/L ~/L g/L g/L g/L
Acid Pxoduct 77 0.2 - 146 0.02
Alkali Product 0.1 114 200 - <0.001
Dilute 31 5.6 ~ 47 0.005
Current efficiency: Cathodic - 45%
Anodic - 48%
White Pb-containing solids formed in the diluate
circulation stream due to precipitation of the original
lea~ content in the feed.
In a second test, starting with 19.5L of feed solution
containi.ng 54 g/L As, 57 g/L Na and 170 mg/L Pb, the hot
feed solution was cooled from 80~C to 30~C, whereupon some
7g
sodium arsenate crystals formed in the feed tank. The feed
solution was circulated through the middle compartment of
the unit which was operated at 500A/m2 for 65 hours. ~he
temperature was maintained between 35 and 40~C by cooling.
The current efficiency was found to decrease with time as
the sodium and arsenic concentrations in the diluate
compartments decreased. The sodium concentrations and
cathodic current efficiencies after 48 hours were 29 g~L
and 80%, and after 65 hours 21 g/L and 57.5%.
At the 65th hour the circulating strams assayed as shown in
Table IV.
Table IV
As Na NaOH H3As04Pb
g/Lg/L g/L g/L g/L
Diluate 38 21 - -0.02
Caustic product 0.1 124 215 - <0.001
Acid product 62 - - 1150.005
Example 3
A five-cell, two-compartment bipolar cell unit in stack
confi~uration was assembled with SelemionTM CMF cationic
membranes with an effective membrane area of 860 cm2.
Bipolar electrodes each with a platinum-coated titanium
anodic surface and a stainless steel cathodic surface were
employed. The terminal anode was made of platinum-coated
titanium and the terminal cathode was made of stainle~s
2479
26
steel. A number of tests were conducted with a sodium
arsenate feed solution that was circulated through the
anode compartments. Cartridge filters were provided in the
recirculating anolyte stream. Both anolyte and catholyte
were circulated at a linear velocity of 0.9 cm/s.
The feed solution contained 81 g/L As, 85 g/L Na and 0.78
~/L Pb. Some test parameters and results are tabulated in
Table V.
Table V
Current
Density Unit Cell As Na H3As04 NaOH Pb
Test A/m2 Voltage V Products ~ g/L ~/L g/L ~
1 205 5 Acid:110 4.1200 ~ 0.03
Alkali:5 74 - 126 0
2 1000 5 Acid:119 8.8207 - -
Alkali:6 84 - 126
3 1000 4.8 Acid:115 14 190 - 0.03
Alkali:2 72 - 125 0.003
The results show that, at a constant cell voltage of about
5V, the possible operating current depended on the sodium
concentration in the anolyte (acid product). At 4.1 g/L
Na, the unit could be operated at 205A/m2 and at 8.8 g/L Na
at l,000A/m2.
The sodium concentration in the anolyte is partly increased
by some water loss due to electro-osmosis, but may also be
increased by other means (see also the following examples).
~29~9
Example 4
A single three-compartment cell was assembled using a
SelemionTM CMF cationic and a SelemionTM AMT anionic
mernbrane, a platinum-coated titanium anode and a stainless
steel cathode. A feed solution initially containing 85 g/L
Na, 90 g/L As and 0.7 g/L Pb was circulated at a velocity
of 0.5 cm/s through the diluate compartment. The current
density was 3,000 A/m2. The temperature was maintained at
60~C by cooling the circulating acid and diluate compartment
streams. The acid product was generated at 12O8L/h.m~ and
the alkali product at 13.2L/h.m2. The compositions of the
acid and alkali products are shown in Table VI.
Table VI
As Na NaOH ~3As04 Pb
~/L ~/L g/L g/L ~/L_
Acid product 56.3 0.15 107 0.04
Alkali product0.06 92.5 160 ~ 0
With the sodium in the diluate compartment maintained at 50
g/L and As at 60 g/L, due to water loss by electro-osmosis
to the acid and hydroxide compartments, anodic and cathodic
current efficiencies of 84% and 80.4%, respectively, were
achieved. The current efficiencies did not decrease over
the 72 hour duration of the test. The cell voltage was
measuxed at 7.7 Volts.
a~ 2gL79
28
Example 5
This example illustrates that maintaining a high
concentration of arsenate in the diluate compartments of a
three-compartment cell electrolysis unit results in
improved performance.
To maintain the high concentration, an electrodialysis unit
is positioned in the s~ream circulating around and through
the diluate compartments of the electrolysis unit. The
feed solution to the electrolysis unit is first fed to the
electroldialysis unit together with the diluate from the
electrolysis unit. The feed solution contains 54 g/L
arsenic and 62 g/L sodium and is fed at 1350 mL/h to the
diluate compartment of the electrodialysis unit, which also
receives the diluate stream from the electrolysis unit.
The diluate flow from the electrolysis unit containing 38
g/L As and 36 g~L ~a is fed to the electrodialysis at 700
mL/h. The electrodialysis unit contains alternating
SelemionTM CMV cationic and SelemionTM AMV anionic membranes
with an effective membrane pair surface area of 5350 cm2.
The electrodialysis unit is operated at 340A/m2 and at 50~C.
The diluate from the electrodialysis unit contains 20 g/L
arsenic and 10 ~/L sodium and is removed at 1050 mL/h as
ef~luent.
A concentrate ~rom the electrodialysis unit contains 81 g/L
arsenic and 77 g/L sodium and is fed at 1000 mL/h to the
electrolysis unit. The electrolysis unit is a three-
29
compartment bipolar unit employing 620 cm2 of effective area
of SelemionTM C~F cationic and SelemionTM AMT anionic
membranes, and is operated at a cell voltage of 6Y, a
constant current density of 2250 A/m2 and at a temperature
of 50~C. The alkali product is generated at 154 g/h NaOH
and the acid product at 143 g/h H3AsO4.
From the results of the above examples it can be seen that
the electrolysis of an arsenate solution by
electrolysis with membranes in a two- or three-
compartment bipolar or monopolar electrode unit is
effective in producing an arsenic acid solution containing
low concentrations of sodium and impurities such as lead
and producing a hydroxide solution similarly
containing, low concentrations of arsenic and impurities
such as lead. Best results are obtained wi~h a three-
compartment cell unit which yields substantially pure
arsenic acid and hydroxide solutions at high current
efficiencies.
It i~ understood that variations and modifications may be
made in the embodiments of the invention without departing
from the scope and purview of the appended claims.