Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
20(142~6 ~ ~
. ~`~ ..
422-330A - ~
MM:570
PRODUCTION OF AMMONIA FROM .::-`
HYDROCARBONACEOUS FEEDSTOCK
Field of the Invention :-~
The present invention rela~es to the production of :~
ammonia from hydrocarbonaceous feedstocks by a process which
includes the partial oxidation of a feedstock to produce a .
hydrogen-rich synthesis gas, which is further processed and
fed into an ammonia-synthesis loop.
Description of the Prior Art
Ammonia has been produced by reacting hydrogen and ` ~ .
nitrogen under ammonia-producing conditions in a so-called
ammonia-synthesis loop according to the equation~
N2 + 3H2 <---> 2NH3 (1) ~. :.
Hydrocarbonaceous feedstocks, such as natural gases
recovered from sites near petroleum deposits, are
convenient sources of hydrogen for use in ammonia synthesis. :
Typically, natural gases contain, as their principle
constituent, methane, wLth minor amounts of ethane, propane .`~
and butane. Also included in the conversion in some
instances, may be low-boiling liquid hydrocarbons.
In order to convert a hydrocarbonaceous feedstock
into a hydrogen-containing stream suitable for introduction
to an ammonia synthesis loop, the feedstock is first ~ ... :
converted into a synthesis gas containing a ma~or amount of
hydrogen, together with minor amounts of carbon monoxide,
carbon dioxide and methane. Steam reforming process
exemplified for methane by the endothermic equation: :`` :`
CH4 + H20 ---> CO + 3H~ (2)
and partial oxidation process exemplified for methane by the
exothermic equation
CH4 + ~o2 ---> CO + 2H2 (3)
or a combination of steam reforming and oxidation have been .
employed to produce synthesis gas. The steam reforming
,' ~:.',';;;",:,,
200'~Z~G
process equation shows production of an additional mole of -~
hydrogen compared to the partial oxidation process equation.
The synthesis gas produced by steam reforming and/or partial
oxidation is treated, such as by a water shift reaction -
C0 + H20 ---> CO2 + H2 (4)
and a carbon oxide removal process, to produce a hydrogen
feed to the ammonia synthesis loop.
The most commonly employed method for converting
hydrocarbonaceous feedstocks to synthesis gas includes
catalytic steam reforming, for example the above equation
(2). In this process, the hydrocarbonaceous feedstock is `;~
reacted with steam in the presence of a catalyst, usually a
nic~el-containing catalyst, at a temperature between about
1200P (650C) and 1900F (1040C). This reaction ha~ been
performed in catalyst filled tubes within a furnace. The
hydrocarbons react with steam under these conditions to
produce carbon monoxide and hydrogen. Catalytic steam
reforming is an expensive process to carry out. Not only is
the nickel-containing catalyst very expensive, but also, the ;~
reactions are highly endothermic. Consequently, a great ;
deal of energy must be provided to drive the reaction.
Frequently, air is provided to the reforming
reaction in order to provide energy through partial
oxidation of hydrocarbons and thu~ reduce the external heat
requirements. Air xeforming can also be performed as a
secondary reforming step to reduce unreacted methane
(methane slippage) to less than one percent on a volumetric
basis. Upon exiting the primary steam reformer, the `~
unreacted methane is converted in the secondary steam ~ ~
reformer by the in~ection of air, whereby the heat of .~ 1.
reaction is supplied by the combustion of methane, hydrogen `
and carbon monoxide. The quantity of internal combustion ;~
air in~ection is such that it will supply the nitrogen ~ `~
requirements of the ammonia synthesis loop. Carbon oxides, ~ :
~' :. ..,'
, :
., - - - . . .
-- 20042~
:
- 3 -
which are poisonous to the ammonia synthesis catalyst must
be removed from the exit gas of the secondary reformer prior ~ -to entry into the ammonia synthesis loop.
Synthesis gas production for ammonia synthesis may
also be carried out autothermally in an autothermal reactor
by adding an oxidant such as air to the steam and
hydrocarbon mixture. The endothermic heat of reaction is
supplied by the exothermic combustion reactions:
CH~ + 2O2 ---> CO~ + 2HzO (5)
H2 + ~ O2 ---> H20 (6)
CO + ~ O2 ---> COz (7) -~`~
The autothermal reactor typically consi6ts of two
catalyst beds, the first bed providing a high outlet
temperature sufficient for steam reforming in the second
bed. Alternatively, the reactants can be partially reformed ~- -
in a steam reforming furnace and enter the autothermal
reactor at a temperature sufficiently high to ignite
spontaneously with the er.tering oxygen, thus producing a -~higher temperature sufficient for reforming in the
downstream catalyst bed. Following autothermal reforming,
carbon oxides are removed so that the synthesis gas can be
provided to the ammonia synthesis loop. Autothermal ~;~
reforming generally takes place at relatively low
throughputs. The process is carried out at space velocities
of the order of 8,000 hr-1 to 12,000 hr-~. "Space velocity" ``
can be defined as the volumetric hourly rate of throughput .
per volume of catalyst. All figures quoted herein refer to
the volumetric hourly rate at standard conditions of `;
temperature and pressure.
The foregoing procedures for producing synthesis
gas suitable for use in ammonia production have the -~
drawbacks of requiring expensive catalysts; large volumes of
catalyst; relatively low rates of throughput; equipment that ; -~
is expensive and, in some cases, takes up excessive amounts ~.
, .: :: .'
' `', ~ ~` -'',
'",
ZO(~12~6
of space; andl in some cases, requires unacceptably large
amounts of enerqy to drive the process. -~
Partial oxidation o~ hydrocarbonaceous feedstocks
represents one alternative to steam reforming in the
production of synthesis gas. ~ost of the partial oxidation ~ ~-
processes that have been employed commercially are
non-catalytic processes. Non-catalytic partial oxidation
reactions, however, are relat vely inefficent. They operate
at high temperatures, i.e.~ ir. the range of 2,200F (1200C
to 2, 800F ( 1500C) and requi-e large amounts of oxygen.
Typically, the oxygen-to-carbcn ratio required in
non-catalytic partial oxidaticn is greater than 0.8:1 and ~ `
often greater than 1:1. Furt~.ermore partial oxidation
produces free carbon. - i
U.S. Patent 4,390,3~7 issued to Dille at al.
describes a process for the p-oduction of synthesis gas by
the non-catalytic partial oxication of a liquid
hydrocarbonaceous fuel. The ..ydrocarbonaceous feedstock is
reacted with a free oxygen-co-.taining gas in the presence of
steam at an autogenously main-ained temperature within the
range of 1700F (930C) to 30C0F (1650C) at a pressure in
the range of about 1 to 23 at-ospheres absolute (1 to 23
bar). The oxyqen-to-carbon molar ratio is said to be from
0.7:1 to 1.5sl. Steam is mix~d with the hydrocarbon stream
to moderate the temperature. Generated carbon soot prevents
damage to the refractory lini~.g of the generator. A water
quench and scrub removes the ree carbon.
U.S. Patent 3,890,1 3 issued to Child et al, ~ ~
describes the production of a methane-rich steam in which ~ -
non-catalytic partial oxidation of a hydrocarbonaceous
feedstock i6 carried out in the presence of steam and ~ -
oxygen. The ratio of free oxygen in the oxidant to carbon
in the feedstock i8 in the range of 0.8:1 to 1.5:1.
Particulate carbon is removed from the effluent gas stream
', ,~ -,......
....
- 20~Z~6 -
- 5 ~
in a ga~ cleaning zone. The product synthesis gas is - ;
sub~ected to a water gas shift reaction to increase the
amount of hydrogen in the gas. ~ ~ ~
U.S. Patent 3,927,998 issued to Child et al., ~ ~ -
relates to the production of a methane rich stream by the
partial oxidation of a hydrocarbonaceous fuel employing a
steam to fuel weight ratio of 2.2:1 to 2.9:1 and an
oxygen-to-carbon molar ratio of 0.8:1 to 0.84:1. The
partial oxidation is carried out in the absence of
catalysts. The synthesis gas is cooled and water, carbon -~ .
dioxide, particulate carbon and other impurities are
removed. The hydrogen and carbon monoxide in the gas are
reacted in a catalytic methanation zone to produce a
methane-rich stream.
Conversion efficency of oxidation processes can
generally be improved by the use of catalysts; but where the
oxidation process in only partial, i.e. with insufficient ~ ~`
oxygen to completely oxidize the hydrocarbon, then the . -
catalyst is sub~ect to carbon deposit and blockage. ~ i
However, U.S. Patent 4,087,259, issued to Fujitani `.,.`.'.~'',;,"~!''et al., describes employment of a rhodium catalyst in a
process wherein liquid hydrocarbonaceous feedstock is
vaporized and then partially oxidized in contact with the ~ ~
rhodium catalyst at a temperature in the range of 690 to ~u
900C with optional steam added as a coolant at rate not
more than 0.5 by volume relative to the volume of the liquid
hydrocarbon in terms of the equivalent amount of water. The
rhodium catalyst enables partial oxidation without causing
deposition of carbon, but at temperatures greater than -~;
900C, thermal decomposition occurs producing ethylene or -:
acetylene impurities. When steam is added, the quantity of
hydrogen produced is increased while the yield of carbon
monoxide remains constant due to catalytic decomposition of ~," "~.~",
the steam to hydrogen gas and oxygen. A "LHSV" (Liquid
`'. ' , ~, :.' '.". '
.,. ' ' ' "," . "4 ,' .';~ .
: '' ', ~,: '
Z00~2~,
- 6 -
Hourly Space Velocity) from 0.5 to 25 l/hour is disclosed;
particularly, a high yield from partial oxidation of
gasoline vapor, without steam, is produced at a temperature
of 725C and at a L~SV of 2~, and with steam, is produced at
temperatures of 700C and 800C and at a LHSV of 2. -~ -
In order to obtain acceptable levels of conversion
using catalytic reforming processes of the prior art it has
generally been necessary to use space velocities below about ~ ~n~`12,000 hr- . For example, U.S. Patent No. 4,522,894,
issued to Hwang et al., describes the production of a
hydrogen-rich ~as to be used as fuel for a fuel cell. The
process reacts hydrocarbon feed with steam and an oxidant in
an autothermal reformer using two catalyst zones. The total
hourly space velocity is between 1,960 hr-l and 18,000 hr- .
Because the prior art processes must be carried out at low : ~ `
space velocity, catalytic reactors of the prior art have had ~ ~
to have large catalyst beds in order to achieve the ;
throughputs desired in commercial operation. This increases
the size and cost of the reactor.
It is an ob~ect of the present invention to -~
provide a process for the production of ammonia from~ ;
hydrocarbonaceous feedstock which i8 energy efficient, is
capable of using low cost catalysts and employs relatively ;
small equipment volume to achieve commercially acceptable
throughput.
It is a further ob~ect of the invention to provide ~ ~ -
a process for the production of ammonia from hydrocarbona~
ceous feedstock with a relatively low oxygen demand, thereby ;~
increasing throughput of hydrocarbonaceous feed.
These and other ob~ects of the invention are
achieved by a process which is described below.
.:' .. .. .
. . ' ' ' ' ' ', ' , .
- ZO~Zl~
Summary of the Invention
This invention provides a process for the ~ :
production of ammonia in which a hydrogen-rich synthesis gas
is generated by the catalytic partial oxidation of a
hydrocarbonaceous feedstock, such as natural gas, with an
oxidant stream under temperature and steam conditions
producing essential no free carbon at a space velocity in
the range from 20,000 hour-l to 500,000 hour-l; treatment of
the resultant synthesis gas to remove components other than :~
hydrogen and nitrogen; ad~ustment of the nitrogen content of
the hydrogen-containing stream; and reaction of the hydrogen ~::: h
and nitrogen to produce ammonia.
In one embodiment, the invention provides a
process for producinq ammonia from hydrocarbonaceous
feedstock which comprises~
(a) introducing to a catalytic partial oxidation zone :
an essentially completely mixed gaseous mixture of ::
a hydrocarbonaceous feedstock, oxygen or an
oxygen-containing gas and, optionally, steam in :::::~
which the steam-to-carbon molar ratio is from 0:1
to 3.0:1 and the oxygen-to-carbon molar ratio is
from 0.3:1 to 0.8:1, said mixture being introduced
to the catalytic partial oxidation zone at a : : :
temperature not lower than 200F (93C) below its
catalytic autoignition temperature;
(b) partially oxidizing the hydrocarbonaceous :;-
feedstock in the catalytic partial oxidation zone
at a temperature equal to or greater than a .
minimum non-carbonizing temperature selected in
the range from 1600F (870C) to 1900F (1030C) :.
as a linear function of the steam-to-carbon molar
ratio being equal to or greater than a ratio in a
corresponding range from 0.4:1 to 0:1 to produce a:.
synthesis gas contain hydrogen, carbon monoxide
,.. ,, ,. ,, .., .
:- zoo'~
- 8 -
and carbon dioxide by passing the mixture through
a catalyst capable of catalyzing the partial ;
oxidation of the hydrocarbons at a space velocity
in a range from 20,000 hour-l to 500,000 hour- ,
said catalyst having a ratio of geometric surface
area to volume of at least 5 cm2/cm3;
(c) contacting the synthesis gas with a shift catalyst
under water gas shift reaction conditions which ~ n
cause carbon monoxide to be converted to carbon
dioxide and hydrogen;
(d) removing carbon dioxide from the gas stream; `;~
.
(e) ad~usting the nitrogen content of the carbon
dioxide free gas stream to obtain a
hydrogen-to-nitrogen molar ratio between about 2:1
and 4:1; and
(f) reacting the hydrogen and nitrogen in the ad~usted ~`
gas stream under ammonia-producing conditions to
produce ammonia.
Brief Descri~tion of the Drawin~s
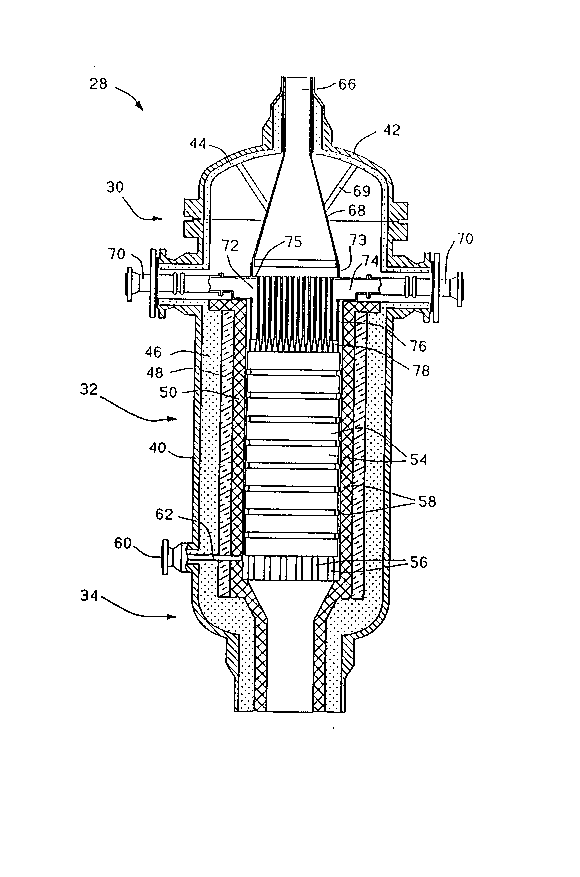
Fig. 1 is an elevated cross-section view of a
partial oxidation reactor having at its input a mixer and
distributor suitable for introducing the reactants to the ;~
catalyst bed for use in the process of the invention.
Fig. 2 is an enlarged elevational cross-section
view of a broken-away portion of the mixer and distributor ; j ~
of Fig. 1. , ;~,
Fig. 3 i8 a top view of a broken-away quarter
section of the mixer and distributor of Fig. 1.
Fig. 4 is a bottom view of a broken-away quarter : ;~
section of the mixer and distributor of Fig. 1. `
Fig. 5 is a diagrammatic elevational
cross-sectional illustration of a broken-away portion of the `~
'' ;',:: .'' ~'.''-.';,
.,',~,.,',~'- ........
200'~2~-
mixer and feeder of Figs. 1 and 2 showing critical
dimensions.
Fig. 6 is a block flow diagram of one embodiment
in accordance with the process of the invention for ammonia
production using air as an oxidant in catalytic partial
oxidation, and using cryogenic removal of nitrogen prior to ~ - `
the ammonia synthesis loop.
Fig. 7 is a block flow diagram of a modified
embodiment similar to Fig. 6, but employing pressure swing
adsorption to ad~ust nitrogen content prior to the ammonia
synthesis loop.
Fig. 8 is block flow diagram of another modified
embodiment similar to Fig. 6, but using oxygen-enriched air
in the catalytic partial oxidation step without the
ad~ustment of nitrogen content prior to the ammonia
synthesis loop.
Fig. 9 i8 a block flow diagram of a still further ;
modified embodiment of the process of the invention for
producing ammonia which employs oxygen or oxygen-rich gas as
the oxidant in the catalytic partial oxidation steps and is ~ -~
designed for low-capital cost.
Fig. 10 is a block flow diagram of yet another
modified embodiment similar to Fig. 9 but is designed for
low energy consumption.
Fig. 11 is a graph plotting oxygen-to-carbon molar
ratio vs. steam-to-carbon molar ratio for three different ` ~
operating temperatures at an operating pressure of 400 psig. ~ -~-;',`i
~2760 KPa). ';
Fig. 12 i8 a graph plotting the hydrogen-to-carbon ~ ~ ~
monoxide molar ratio in the catalytic partial oxidation ~ s
reaction product V8. the steam-to-carbon molar ratio for
three different operating temperatures at an operating `
pressure of 400 psig. (2760 KPa).
.,....................................................................... ... ,,, ",~,"~,,j,,
20~
- 10 --
Fig. 13 is a graph plotting the volume % methane
in the catalytic partial oxidation product vs. the
steam-to-carbon molar ratio for thrée different operating
temperatures at an operating pressure of 400 psig. (2760
XPa).
Fig. 14 is a graph plotting the volume % carbon
dioxide in the catalytic partial oxidation product vs.
steam-to-carbon molar ratio for three different operating
temperatures at an operating pressure of 400 psig. (2760
KPa).
Fig. 15 is a graph plotting the molar ratio of
total hydrogen and carbon monoxide in the product to total
hydrogen and carbon in the feedstock vs. steam-to-carbon
molar ratio for three different operating temperatures at an
operating pressure of 400 psig. (2760 KPa). ~ .
Fig. 16 is a detailed process flow diagram of a ~ ;
first portion of a process in accordance with the invention.
Fig. 17 is a detailed process flow diagram of a
second portion of the process of Fig. 16.
... : .: :.: :.
Detailed Descri~tion of the Preferred Embodiments
The process of the present invention can be used ~- ;
to produce ammonia from any gaseous or low-boiling
hydrocarbonaceous feedstock. Typically, a gaseous
hydrocarbonaceous feedstock used to produce synthesis gas is
a gas containing principally methane such as natural gas ;j
having the following approximate composition: methane, 93%;
eth;ne, 5%; propane 1.5%; butane and higher hydrocarbons,
In general, the process of the invention involves
the steps of catalytic partial oxidation of hydrocar~
bonaceous feedstock under temperature and water content
conditions to produce synthesis gas without free carbon; ; ~ ;
treatment of the resultant synthesis gas to remove
~ '' . ,.', ."',-..
`- 200~Z~-
components other than hydrogen and nitrogen (e.g., carbon
oxides) and to recover a carbon dioxide stream; adjustment
of the nitrogen content of the hydrogen-containing stream;
and reaction of the hydrogen and nitrogen to produce
ammonia. ;~
CatalYtic Partial Oxidation Of
Hydrocarbonaceous Feedstock -
One particular aspect of the invention is the ~ `
substantial capital cost savings and/or advantageous
operating economy resulting from the employment of catalytic ~ ;
partial oxidation to produce the raw synthesis gas employed -~ m-
in the ammonia producing process. This is made possible by
the discovery that catalytic partial oxidation performed at
a temperature equal to or greater than a minimum non-carbon-
forming temperature selected in the range from 1600F
(870C) to 1900F (1040C) as a linear function of the
steam-to-carbon molar ratio being equal to or greater than a
ratio in a corresponding range from 0.4:1 to 0:1 and at a ~b~
space velocity in the range from 20,000 hour- to 500,000
hour-l produces essentially no free carbon deposits on the
catalyst. Further, it is found that products of the partial
catalytic oxidation in the process of the invention consist
essentially of hydrogen, carbon monoxide and carbon dioxide ;:
at oxidation temperatures equal to or qreater than the
minimum temperature, rhodium catalysts are not required to '~','.. '"`!~,""",'-"''-'"`~'
prevent carbon formation. For example in Fig. 11, dotted
line 25 represents a generally linear function which, at a
steam/carbon ratio of 0, corresponds to a minimum partial ,~
oxidation temperature of about 1900F (1040C), and at a
30 steam/carbon ratio of 0.4 corresponds to a minimum partial
oxidation temperature of about 1600F (870C); favorable
catalytic partial oxidation without producing free carbon
occurs at temperatures and steam/carbon ratios equal to or -
greater than points on the line. Further, lower minimum
' '' '~' "'.'. '''' ~' "'', '.
' - ' ' '~ ' ' . ' ' ' ' '
"'" ',,' " ~
Z00~2~
temperatures at corresponding steamtcarbon ratios greater
than 0.4 can be extrapolated from the linear function
represented by line 25.
In the catalytic partial oxidation step of the
process of the invention, reactant gases are introduced to
the catalytic partial oxidation reaction zone, i.e. the
catalyst bed, at an inlet temperature not lower than 200F
(93C) below the catalytic autoignition temperature of the
feed mixture. Preferably the reactant gases are introduced
at a temperature at or above the catalytic autoignition
temperature of the mixture. The reactants should be
completely mixed prior to the reaction. Introducing the
thoroughly mixed reactant gases at the proper temperature
ensures that the partial oxidation reactions will be mass
transfer controlled. Consequently, the reaction rate is
relatively independent of catalyst activity, but dependent ; ;
on the surface-area-to-volume ratio of the catalyst. It is -~
possible to use any of a wide variety of materials as a
catalyst, provided that the catalyst has the desired -
surface-area-to-volume ratio. It is not necessary that the `i
catalyst have specific catalytic activity for steam ~ -
reforming. Even materials normally considered to be
non-catalytic can promote the production of synthesis gas
herein when used as a catalyst in the proper configuration. ~ -
The term "catalyst", as used herein, is intended to
encompass such materials. -
The catalytic partial oxidation step can be ~ -
understood with reference to the figures. The catalytic ;~ r
partial oxidation zone is typically the catalyst bed of a
reactor such as that indicated generally in Fig. 1 at 28.
The reactor 28 includes an input mixing and distributor
section indicated generally at 30 which mixes the feedstock
with an oxidant and distributes the mixture to the entrance
of a catalytic reactor section indicated generally at 32. In
.',: ,. ., .' '', .,
: :: ~"'.',
' '' '"',
Z0(:~2i.6 - ~
- 13 - ~
: ~- - ~ :,
the catalytic reactor section 32, the feedstock is partially -
oxidized to produce a product which is then passed through
the exit section indicated generally at 34. ;
The reactor includes an outer shell 40 of
structural metal such as carbon steel with a top 42 secured
thereon by bolts ~not shown) or the like. A layer 44 of
insulation, such as 2300F (1260C) BPCF ceramic fiber
insulation, is secured to the inside of the upper portion of
the shell 40 including the top 42. In the lower portion of
the mixing section 30, in the reactor section 32 and in the `~
outlet section 34, there are secured insulating layers 46,
48 and 50 on the inside of the shell. The layer 46 is a
castable or equivalent insulation such as 2000F (1090C)
ceramic insulation. The layer 48 is also a castable or ~ -
equivalent layer of insulation but containing 60~ alumina ;
for withstanding 3000F (1650 C). The internal layer 50 is
a refractory or equivalent layer such as g7~ alumina with ~ -
ceramic anchors or 97~ alumina brick for withstanding the ~-
interior envlronment of the reactor section. -~
The catalytic reactor section 32 contains one or
more catalyst discs 54. As shown, the reactor contains a
sequence of discs 54 separated by high alumina rings 58 ~- };~ ;;
between each ad~acent pair of discs. The stack is supported :;"
by a grill with high alumina bars 56. A sample port 60 is ; ~;
formed in the lower end of the reaction section and has a :
tube, such as type 309 stainless steel tube 62, extending -i
below the bottom refractory disc 54 for withdrawing samples
of the product. ~-A~
The outlet section 34 is suitably formed for being
connected to a downstream heat recovery boiler (not shown) - ~
and/or other processing equipment. ` ~ -
The catalyst comprises a high surface area
material capable of catalyzing the partial oxidation of the
hydrocarbonaceous feedstock. The catalyst is in a
. " ~ ,
, . . . . . :
20(~23L~
configuration that provides a surface area to volume ratio
of at least 5 cm2/cm3. Preferably, the catalyst has a
geometric surface area to volume ratio of at least 20 ~`
cm2/cm3. While there is no strict upper limit of surface
area to volume ratio, it normally does not exceed about 40
cm2/cm3. A wide variety of materials can be used in the `
construction of the catalyst including materials not
normally considered to have catalytic activity, provided
that the catalyst configuration has the desired surface area
to volume ratio.
The catalyst disc 54 can be, for example, a ; ~ -
monolithic structure having a honeycomb type cross-sectional :
configuration. Suitable monolithic structures of this type -~ :
are produced commercially, in sizes smaller than those used -
in the process of the invention, as structural substrates
for use in the catalytic conversion of automobile exhausts
and as catalytic combustion chambers of gas turbines or for
catalytic oxidation of waste streams. Typically, the -~
monolithic structure is an extruded material containing a
plurality of closely packed channels running through the ~ `-
length of the structure to form a honeycomb structure. The ,
channels are typically square and may be packed in a density
as high as 1,200 per square inch of cross section. The
monolithic structure can be constructed of any of a variety
of materials, including cordierite (MgO/Al203/SiO2), Mn/MgO
cordierite (Mn-MgO/Al203SiO2), mullite (Al203/SiO2), mullite ,,, ,' .. ,i, i
aluminum titanate (Al203/SiO2-(Al,Fe)203/TiO2), zirconia -~
spinel (ZrO2/MgO/Al203), spinel (MgO/Al203), alumina (Al203)
and high nickel alloys. The monolithic catalyst may consist ~ -
solely of any of these structural materials, even though
these materials are not normally considered to have ;~- `
catalytic activity by themselves. Using honeycombed
substrate~, surface area to volume ratios up to 40 cm2/cm3
or higher can be obtained. Alternatively, the monolithic `
; ' ''''~'` ~."
20~'~2~
-
substrate can be coated with any of the metals or metal
oxides known to have activity as oxidation catalysts. These
include, for example, palladium, platinum, rhodium,
irridium, osmium, ruthenium, nickel, chromium, cobalt,
cerium, lanthanum and mixtures thereof. Other metals which ``~`'
can be used to coat the catalyst disc 54 include noble ;~
metals and metals of groups IA, IIA, III, IV, VB, VIB, or
VIIB of the periodic table of elements.
The catalyst discs S4 may also consist of `
structural packing materials, such as that used in packing `~
absorption columns. These packing materials generally =
comprise thin sheets of corrugated metal tightly packed `~
together to form elongate channels running therethrough.
The structural packing materials may consist of corrugated
sheets of metals such as high temperature alloys, stainless
steels, chromium, manganese, molybdenum and refractory
materials. These materials can, if desired, be coated with -
metals or metal oxides known to have catalytic activity for ~ ` `-
the oxidation reaction, such as palladium, platinum,~ `
rhod~um, irridium, osmium, ruthenium, nickel, chromium,
cobalt, cerium, lanthanum and mixtures thereof. `
The catalyst discs 54 can also consist of dense `
wire mesh, such as high temperature alloys or platinum mesh. `:~
If desired, the wire mesh can also be coated with a metal or
metal oxide having catalytic activity for the oxidation
reaction, including palladium, platinum, rhodium, irridium,
osmium, ruthenium, nickel, chromium cobalt, cesium,
lanthanum and mixtures thereof. ~ :
The surface area to volume ratio of any of the
aforementioned catalyst configurations can be increased by
coating the surfaces thereof with an aqueous slurry ;~
containing about 1% or less by weight of particulate metal
or metal oxide such as alumina, or metals of groups IA, IIA,
III, IV, V8, VIB and VIIB and firing the coated surface at ~ ;
. ':
: . . ',
. ;.. . . ., .. , i ., ~ , .. ..... .
20(~'~Z3L~
: `. `
- 16 -
. . ~..'...''-.,.' .
high temperature to adhere the particulate metal to the -~ ~ -
surface, but not so high as to cause sintering of the
surface. The particles employed should have a BET
(Brunnauer-Emmett-Teller) surface area greater than about 10
m2/gram, preferably greater than about 200 m2/gram. -~
In the practice of the invention, a gaseous ~ ~
mixture of hydrocarbonaceous feedstock, oxygen or an ` `
oxygen-containing gas, which can be air, oxygen-enriched ``
air, or other oxygen-rich gas, and, optionally, steam is ~' ''""'"'.""`''~.'!~`''`
introduced into the catalytic partial oxidation zone at a ~ ,
temperature not lower than 200F (93C) below its catalytic
autoignition temperature. Preferably, the gaseous mixture
enters the catalytic partial oxidation zone at a temperature
equal to or greater than its catalytic autoignition
I5 temperature. It is possible to operate the reactor in a ; ~ `
mass transfer controlled mode with the reactants entering
the reaction zone at a temperature somewhat below the
autoignition temperature since the heat of reaction will ',,.',~.. '.'i,i~,
provide the necessary energy to raise the reactant ...
temperature within the reaction zone. In such a case,
however, it will generally be necessary to provide heat
input at the entrance to the reaction zone, for example by a
sparking device, or by preheating the contents of the
reactor, including the catalyst, to a temperature in excess ;
of the autoignition temperature prior to the introduction of ~ `
the reactants to initiate the reaction. If the reactant
temperature at the input to the reaction zone is lower than
the autoiginition temperature by more than about 200 F
(93C), the reaction becomes unstable.
When the reactant mixture enters the catalytic
partial oxidation zone at a temperature exceeding its
autoignition temperature, it is necessary to introduce the ~ `
mixture to the catalyst bed immediately after mixing; that
is, the mixture of hydrocarbonaceous feedstock and oxidant
~00425
- 1 7
should preferably be introduced to the catalyst bed before
the autoignition delay time elapses. It is also essential
that the gaseous reactants be thoroughly mixed. Failure to
mix the reactants thoroughly reduces the quality of the
product and can lead to overheating. A suitable apparatus ~ `
for mixing and distributing the hydrocarbonaceous feedstock `~`and oxygen or oxygen-containing gas so as to provide `~thorough mixing and to introduce the heated reactants into
the reaction zone in a sufficiently short time is
illustrated in Figs. 1-5 and described in more detail in
copending commonly assigned patent Application Serial No.
085,159, filed August 14, 1987 in the names of J.D.
Korchnak, M. Dunster and J.H. Marten. `~
Referring to Fig. 1, one of the feed gases, i.e.
hydrocarbonaceous gas or oxygen-containing gas, is
introduced into the input section 30 through a first inlet ~ ` `;
port 66 through the top 42 which communicates to an upper
feed cone 68 which forms a first chamber. The cone 68 is ~ ~ -fastened by supports 69 in the top 42. The other feed gas
i8 introduced into the input section 30 through second
inlets 70 extending through side ports of the shell 40 and
communicating to a second chamber 72 which is interposed
between the upper chamber 68 and the inlet of the catalyst
reaction section 32. A ring 73 mounted on the central
portion of an upper wall 75 of the chamber 72 sealingly ~`
engages the lower edge of the cone 68 so that the wall 75 ~ ~ `forms a common wall between the upper chamber 68 and lower
chamber 72. The chamber 72 has an upper outer annular ;
portion 74, see also Figs. 2 and 3, which is supported on
the top surface of the refractory layer 50. A lower portion
of the chamber 72 has a tubular wall 76 which extends
downward in the refractory sleeve 50. The bottom of the
chamber 76 is formed by a cast member 78.
~"''.'' -
` :' '' "
200'~ 6
. . . ~ ,
- 18 -
Optionally, steam can be introduced into either or
both of the hydrocarbonaceous feedstock and oxygen or - .
oxygen-containing gas. The gases are fed to the reactor in
relative proportions such that the steam-to-carbon molar ~ -
ratio is from 0:1 to 3.0:1, preferably from 0.3:1 to 2.0:1. ~--
The oxygen-to-carbon ratio is from 0.4:1 to 0.8~
preferably from 0.45:1 to 0.65:1. - `
The reactant mixture preferably enters the ` `
catalytic reactor section 32 at a temperature at or above
its autoignition temperature. Depending on the particular
proportions of reactant gases, the reactor operating
pressure and the catalyst used, this will generally be ~ ^"
between about 550P (290C) and 1,100F (590C) Preferably,
hydrocarbonaceous feedstock and steam are admixed and heated
to a temperature from 650F (340C) to 1,200F (650C) prior
to passage through inlet port(s) 70 or 66. Oxygen or , `,~',"~!, "~'' m
oxygen-containing gas, such as air, is heated to a ~``
temperature from 150F (65C) to 1200F (650C) and passes
through the other inlet port(s) 66 or 70.
Referring to Figs. 2, 3 and 4, the mixing and
distributing means comprises a plurality of elongated tubes
80 having upper ends mounted in the upper wall 75 of the
chamber 72. The lumens of the tubes at the upper end
communicate with the upper chamber 68. The bottom ends of -
the tubes 80 are secured to the member 78 with the lumens of
the tubes communicating with the upper ends of passageways
84 formed vertically through the member 78. orifices 86 are
formed in the walls of the tubes 80 for directing streams of
gas from the chamber 72 into the lumens of the tubes 80.
The inlets 66 and 70, the cone 68, the support~ 69 are
formed from a conventional corrosion and heat resistant
metal while the chamber 72, tubes 80 and member 78 are
formed from a conventional high temperature alloy or ~ ;~
refractory type material.
~ ' ,t,.J~
': . `' ' `~ ' ", "': ' ', . ~"'
'' ~' ~; ';., ''':, ~'
' ,''`~ ' ','
20042~
~ .: .. - ` . ,
The number of tubes 80, the internal diameter 90 -
(see Fig. 5) of the tubes 80, the size and number of the
orifices 86 in each tube are selected relative to the gas
input velocities and pressures through inlets 66 and 70 so
as to produce turbulent flow within the tubes 80 at a
velocity exceeding the flashback velocity of the mixture.
The minimum distance 92 of the orifices 86 from the bottom
end of the tube 80 at the opening into the diverging ~ ~:;n~:
passageways 84 is selected to be equal to or greater than `~ `'r'~
that required for providing substantially complete mixing of
the gas streams from chambers 68 and 72 under the conditions ;~
of turbulence therein. The size of the internal diameter 90
of the tubes 80 as well as the length 94 of the tubes iB ' .
designed to produce a sufficient pressure drop in the gas
passing from the chamber 68 to the reaction chamber so as to
provide for substantially uniform gas flow through the tubes
80 from the chamber 68. Likewise the size of the orifices
86 is selected to provide sufficient pressure drop between
the chamber 72 and the interior of the tubes 80 relative to
the velocity and pressures of the gas entering through ; `
inlets 70 so as to provide substantially uniform volumes of
gas flows through the orifices 86 into the tubes 80. ;
The diverging passaqeways 84 in the member 78 are
formed in a manner to provide for reduction of the velocity `
of the gas and to produce uniform gas distribution over the
inlet of the catalyst. The rate of increase of the
cross-section of the passageway 84 as it proceeds downward,
i.e., the angle 98 that the wall of the passageway 84 makes ~``
with the straight wall of the tubes 80, must generally be
equal to or less than about 15 and preferably equal to or -~
less than 7 in order to minimize or avoid creating vortices
within the passageways 84. This assures that the
essentially completely mixed gases, at a temperature near to
or exceeding the autoignition temperature, will pass into ~ -~
''' ''''~'`''`'' ' ~'~
20042~
.. - . - . .
- 20 ~
the catalyst bed in a time preferably less than autoignition
delay time. The configuration of the bottom end of the ;
passageways, as shown in Fig. 4, is circular, but other ~ '~
configuration such as hexagonal, square, etc. are possible.
The catalytic partial oxidation reaction is
preferably carried out in the catalytic reaction section 32 :~ :F
at a pressure greater than 100 psig (690 KPa), more -
preferably at a pressure greater than 250 psig (1720 KPa). ~ -
The catalytic partial oxidation reaction is carried out at a ~ ` ~
temperature between about 1400F (760C) and 2000F ~ `
(1090C).
The product gas exiting the outlet section 34 ~i ;
consists essentially of hydrogen, carbon oxides, i.e.
carbon monoxide and carbon dioxide, methane, water vapor and
any inert components (e.g. nitrogen or argon) introduced
with the feedstock. Trace amounts of C2- and higher ~ :
hydrocarbons may be present in the product gas. As used
herein "trace amounts" means less than about 0.1~ by weight.
Removal of Carbon Oxides
Since carbon oxides, i.e. carbon dioxide and
carbon monoxide, are present in the synthesis gas and they
are poisonous to the ammonia synthesis catalyst, they are
removed from the synthes$s gas prior to the ammonia
synthesis loop.
The synthesis gas exiting the catalytic partial
oxidation zone is cooled to a temperature from about 350F ~.
(175C) to about 750F (400C) using conventional heat
exchange methods, either by heating the hydrocarbon and ~ J'
steam feedstock, heating the oxidant stream, superheating
steam, raising steam in a boiler, preheating boiler
feedwater or a combination thereof. .
The first step in the removal of carbon oxides is
the conversion of carbon monoxide to carbon dioxide by the
'~'. ' '~,~ ', .
- 20042~
- 21 - -~
water gas shift reaction in which carbon monoxide is reacted ;
with water to produce carbon dioxide and hydrogen. The
water gas shift reaction is known, and suitable equipment
for carrying out the reaction is commercially available.
S The water gas shift reaction can be carried out in two
stages, i.e. a high temperature shift and a low temperature -~
shift. In this procedure, the synthesis gas is first ;
reacted with water vapor at a temperature from about 580F ~ 3
(300C) to 750F (400C) and a pressure from about 15 atm.
(1520 KPa) to 40 atm. (4050 KPa), followed by reaction at a
temperature from about 350F (175C) to 500F (260C) and a -~
pressure from about 15 atm. (1520 XPa) to 40 atm. (4050
KPa). Alternatively the water gas shift reaction can be
carried out in a single stage, low temperature tubular,
steam-raising reactor shift vessel. In this procedure, the
water vapor and synthesis gas are reacted at a temperature
from about 350F ~175C) to 500F (260C) and a pressure
from about 15 atm. (1520 XPa) to 40 atm. (4050 KPa). The
exit stream from the water gas shift reaction zones has a
carbon monoxlde content less than about 0.5 percent on a
volumetric basis.
Essentially all of the remaining carbon monoxide
can be converted to carbon dioxide by catalytic selective
oxidation. In this procedure, the exit stream from the
water gas shift reaction zone, after heat removal to reduce
its temperaturè to about 100F (35C) to 250F (120C), is
reacted with air in the presence of a catalyst that is
highly selective for the oxidation of carbon monoxide under
conditions in which little or no hydrogen is oxidized. The ;~
catalytic selective oxidation procedure is known in the art
and described by U.S. Patents No. 3,216,782, No. 3,216,783
and No. 3,631,073. Suitable process equipment for carrying
out is commercially available for example, under the ;~
trademark Selectoxo. ~ ;
';: "
~. ' ' ' ' ~' :
' ' ' :
'
2004~
. :.
- 22 -
Alternatively to the catalytic selective oxidation
procedure, remaining carbon monoxide can be methanated using
known procedures. However, since methanation reacts each
mole of carbon monoxide with three moles of hydrogen, this
procedure consumes hydrogen that would otherwise be useful
in the ammonia synthesis loop. Furthermore, although
methane is inert in the ammonia synthesis loop, increasing
the amount of methane in the feed to the ammonia synthesis ~ `~
loop increases the loop purge requirements.
Any of the other procedures known in the art for
removing carbon monoxide can be employed to remove traces of
carbon monoxide from the gas stream.
After conversion of carbon monoxide to carbon
dioxide, carbon dioxide is removed from the gas stream and
recovered ùsing known procedures such as, for example,
passing the gas through a countercurrent stream of a liquid `~
absorbent medium, such as potassium carbonate, which absorbs ; `i~ the carbon dioxide. Commercial processing units for carbon
dioxide removal are available for example, under the . `
trademarks Selexol, Amine Guard, and Benfield. These
processes absorb the carbon dioxide into a chemical or
physical absorption medium at relatively high pressure and ~ t4~r~
low temperature, sllowing other gases to pass through
essentially unchanged. The chemical or physical absorbent
is then regenerated by pressure let down into a lower
pressure vessel and, if a chemical absorbent is used, ~ `~
stripped of the carbon dioxide by a countercurrent stream of !",'.,.~,"'",.;~,',.
steam. The carbon dioxide gas is discharged from the top of
the regenerator and the absorbent returned to the absorber
to recover more carbon dioxide.
Adiustment of Nitroaen Content
Following removal of carbon dioxide from the gas
stream, the nitrogen content of the gas stream is ad~usted -
,"-''',':
'.'' ~" ' ', '~'
200~2~
- 23 - -~
to provide a hydrogen to nitrogen ratio suitable for ammonia
synthesis. Generally, the molar ratio of
hydrogen-to-nitrogen is ad~usted to between about 2:1 to 4:1
and preferably between from about 2.5:1 to 3.5:1 for ammonia
synthesis. Any suitable means of adjusting the nitrogen
content can be employed.
When air or oxygen-enriched air i8 used as the
oxidant in the catalytic partial oxidation step, the amount
of nitrogen present in the synthesis gas exiting the
catalytic partial oxidation zone is normally in molar excess
to the amount required for ammonia synthesis and therefore,
nitrogen must be removed from the gas stream. When oxygen
or an oxygen-rich (>70 mole.~) gas is employed as the
oxidant in the catalytic partial oxidation step, the
synthesis gas exiting the catalytic partial oxidation zone
normally requires the addition of nitrogen for ammonia
synthesis.
One method for ad~usting the nitrogen content of
the gas stream is by cryogenic separation and removal of
nitrogen. Cryogenic separation of gases is a known
procedure whereby gases are fractionated according to their
liquefaction temperatures. Commercially available cryogenic
separators can be employed to remove nitrogen from the gas
stream.
Alternatively, the nitrogen content of the gas
stream can be ad~usted by pressure swing adsorption.
Pressure swing adsorption involves the adsorption of -~
components to be removed at high pressure followed by their ;~ ~ ~
desorption at low pressure. The process operates on a 1',,~,.',!
repeated cycle having two basis steps, adsorption and
regeneration. Not all the hydrogen is recovered as some is -
lost in the waste gas during the regeneration stage. By
careful selection of the frequency and sequence of steps
within the cycle, however, the recovery of hydrogen can be
200~2~
~ .
- 24 -
maximized and the ratio of hydrogen to nitrogen iJl tl~e
product effluent ga~ can be strictly controlled to give tle
desired ratio.
negeneration of the adsorbetlt i8 carried out in
three basic steps: (a) Tl~e adsorber is depres3uri7ed to
the low pressure. Some of the waste components are dC?~OL'~e I
during this step. (~) The adsorbent is purged at low ~ -
pressure, with the product hydrogen removing the remainillg
waste componen~s. (c) The adsorber is repressurized to
adsorption pressure ready for service. The wasl;e ga~es
evolved during regeneration are collected in a waste ~as
surge drum and then used as fuel.
Pressure swing adsorption can also be used to
remove carbon dioxide, methane, water vapor and other trace
contaminants such as H2S. Accordingly, the pressure swing
adsorption unit can serve botll to remove carbon dioxide and
to ad~ust the nitrogen content of the gas stream. Cryogenic
separation can be employed to remove methane, however, water
vapor and final traces oE carbon dioxide must still be l~
removed by a separate procedure. A suitable method ~or
removing water vapor and carbon dioxide prior to ammonia
synthesis is by passing the gas stream over any of the ~ i
commercially available molecular sieve materials.
. . ~ , .. .:
nmonia SYnthesis
As previously indicated, methane removal is
optional because methane is inert in the ammonia synthe~
loop. It is "lowever, preferred to remove met!lane from
gas stream. After components other tllan hydrogen an(l
nitrogen have been removed and the hydrogen-to-nitroge
ratio has been ad~usted, the gas stream is,ready to enter -
the ammonia-synthesis loop. ~ny suitable procedure for ~ ~ -
reacting the hydrogen an3 nitrogen to obtain ammonia ~an l~e
employed. Advantageously, the basic ammonia synthesis
,.: . ,. , i
' '. . :',',""'~ ~'
200~2~
- 25 -
procedure employed is derive~ from the so-calle~
~laber-Bosch~ process. In ~hiB pr~cess, the ~nn 6t~enm
circulates under pressure in a loop wherein it ;8 pn~e~
into a heated reaction cham~r where it i8 reacted in
contact with an ammonia synt:~e~is catalyst. The gases
containing the product an~lonia tllell leave the reactio
chamber and ammonia is reco~sred by condensatioll. q~he
unreacted gases are recircul~ted by means of a compresso
and are admixed with feed gas prior to re-entering the
reactlon chamber. The ammonia synthesis reaction is carri~
out at a temperature from ab~ut 650F (340C) to 770P
(410C) and a pressure from about 80 atm (8100 KPa) to 15U
atm (15200 KPa).
The nitrogen and h~drogen are reacted in the
ammonia synthesls loop in ccntact wlth a conventional
ammonla synthesls catalyst. Suitable catalysts include, b
way of example, singly or dc~bly promoted iron catalysts.
Catalyst promoters include ~1~03, alone or in comblnat3.oll -
with K~O; ZrOz, alone or in _ombination wlth K20; or SiO~
alone or in combination with KO~
The varlous embodi~ents of the process o the
invention can be further ~ndarstood with reference to Figs.
6-10. , ~i~
Figs. 6, 7 and ~ illustrate schematically three
embodiments of the inventlor. which employ air or oxygen
enriched air to convert hydr~carbonaceous feedstock to
ammonia. -~
~ ccording to the ~rocess of Fig. 6, hydrocn
bonaceous feedstock is ~lrst optionally treated in
desulfurization ~tep 100 to remove sulfur from the
feedRtock. Sulfur removal can be effected by any su;ln~le
means, sucll as by absorp~ion on zinc oxide. Sullur ~ vn l
prior to catalytic partial cxldation is optlonal ln ench
embodiment ina~much as the catalytic partial oxldatlol~
' `,''"''''~'
, ~. .
- ~ - . . - ,: :
200~X~6
~ ''" :''`"".''`;
- 26 -
process is sulfur tolerant, downstream steps, such as acid
gas removal or pressure swing adsorptlon, can be used to
remove gaseous sulfur compoulld~
~fter desulfurizatioll, tlle feedstock enters ~ e
catalytic partial oxidation zone to~ether with air ancl -
steam. Catalytic part:ial oxi(ial:ion takes place at ~l.ep 10; .
The ef1uent gas, containing llydrogen, methane, carL~c)n
oxlde~ and nitrogen, exits the catalytic partlal oxi(lat:ion
zone at a temperature of about 1650F ~900C) and is l-asse(l
through heat excllanger(s) at step 104 to reduce its
temperature to between 350F (175C) and 750F (400C).
The gas is then passed to a water gas shif t
reactor where carbon monoxide is converted to carbon dioxi(le
at step 106 using the shift reaction previously described.
lS The exit qas from the shift reaction zone 18 passed to a -
heat exchanger 108, where the gas temperature is reduced to
between 100P (3aC) and 250F (120C). The gas is then
passed to a selective oxidation zone, where remaining carb(.
monoxide i8 converted to carbon dioxide at step 110 by the
previously described selective oxidation process. -;
The gas then undergoes removal of carbon dioxlde
by contactlng the gas stream Witll A countercurrent flow of ;
liquid wlllcll absorbs the carbon dioxide from the gas at step ~-
112. The carbon dlox~de can be recovered and ~old as a -
valuable article of commerce . The exit gas f rom the carb~ .n
dioxide absorber contains trace amoullts of water and carl
dloxide, whic)l are removed at step 116 by pas~3.ng tl~e ga6 '
stream through a molecular sieve.
Following removal of carbon oxides, the ni~l:ogel~
content of the gas is ad~usted to a hydrogen-to-nltr->gerl ~-
molar ratio between about 2:1 to 4:1, preferably frolll abo
2.5sl to 3.5:1. Since the embodiment lllustrated ln Fig.
employs air as an oxidant ln the catalytic parl;ial oxi(lati~-n
step, nitrogen is removed from the gas stream to obtaln the
: ' ''' : ''
: ., `.~ ' :'"
200~
- 2 7 - ~
~ ..
desired hydrogen-to-nitrogen ratio . ~rhe removal o n itroge
is achieved in a cryogenic separator at step 128.
Essentially, all of the methane in the gas stream 18 also
removed in this step. ;`~
~he gas stream is then compressed (not 8hOWII) to
between about 80 a~m (~100 Y~E~a) and 150 atm ~15200 Kl~a) ~n~
enters the ammonia synthesis loop 126 where the hydl.ogen a~ld
nitrogen are reacted under anmonia-producing conditions in
the presence of a catalyst to produce ammonia.
Fig. 7 schematically illustrates a variation of
the process of Fig. 6 wherein, following the conversion or
carbon monoxide to carbon dioxide at steps 106 and 110, tlle
gas stream is subjected to pressure swing adsorption at s~
118 to remove carbon dioxide, methane, water vapor and a
lS portion of the nitrogen. The exit stream from the pressure
swing adsorptlon unit is fed directly to the ammonia
synthesis loop 126. Because pressure swing adsorption is
capable of removing essentlally all of the carbon dioxlde
and water vapor from the ~as stream, it is unnecessary to
employ a carbon dioxide removal step 112 or a molecular
sieving step 116 in this embodlment of the invention.
Fig. 8 schematically illustrates an embodiment oi
the invention which employ~ oxygen-enriched air as tlle
oxidant in the catalytic partial oxidation step 102. By
properly ad~usting the ratio of oxygen to air in the oxida-
feed, and thereby the amount of nitrogen entering the
process, it is possible to ellminate the need for downstrea
ad~ustment of nitrogen. In order to obtain the desire(l
hydrogen-to-nitrogen ratio for ammonia production, the
air-to-oxygen molar ratio employed in the catalytic ~-artial
oxidation step of the process embodiment illustrated in Fig.
8 is between about 0.2 and 0.3. Since down~tream nitrogen
ad~ustment is not necessary in the embodiment of Flg. 8, ll
~8 preferred not to employ pressure swing adsorption, but
::
2O0L~2~
2 ~ - :
ratller, to remove carbon dioxid~ alld water vapor ~y tl~e ~n1ne
sequence of steps employed in the embodiment of Fig . 6, tlla t .
i8, by abfiorption of carbon dioxide in a coUn~ercUrrellt
absorber at step 112, f~llowed l~y molecular sieving at ste
116 to remove traces of carbon dioxide and water vapor.
Figs. 9 and 10 schematically illustrate the two
preferred embodiments of the invention that produce AllllllOn i.a . .
in processes tllat employ oxygell or an oxygen-ricll yas ns tlle
oxidant in the catalytic partial oxidation of hydrocar~
bonaceous feedstock. These embodiments are economically
advantageous because tlley reduce capital equipment costs
and/or energy requirements. As used herein, the term -s
~oxygen-rich gas-- refers to a gas having an oxygen contellt
of at least 70%, preferably at least 90%. Since the use oJ
oxygen or oxygen-rich gas in the catalytic partial oxidatie
process eliminates or greatly reduces the nltrogen load at
the front end of the process, the size of synthesis gas -~
generating equipment and downstream conditioning equipment
required is greatly reduced. Furthermore, energy
requirements to bring nitrogen to the catalytic partial
oxidation reactor conditions is reduced or eliminated.
Oxygen or oxygen-rich gas for use in the catalytic partial - ~;
oxidation process can be generated using known techniques ~ `
such as cryogenic fractionation of air.
Fig. 9 illustrates an embodlment of the invention - -
that incurs relatively low capital equipment costs. In tlli~
embodlment, sulfur is optionally removed from the ~ ~
hydrocarbonaceous feedstock at s~ep 100, after which tlle ~ -
feedstock, together with steam and oxygen or oxygen-ricl
gas, is fed to the catalytic partial oxidatlon zone as
previously described. Catalytic partial oxidation takes
place at step 102, thereby producing a synthesis ga~
containing hydrogen, carbon monoxide, carbon dioxide,
; ~`;-.,~
,~.'.'""',
200~2~6 `
. . .
~ '~
- 2 9 -
methane and little or no nitro~ell, i.e. less th.ln 30~ a
preferably less than 10~ nitrogen.
After heat exchange 104, sllift gas re;lction lU6
and heat exchange lOB as previollsly (lescribecl, the lI;troge
content of the gas stream is a~l justed to achieve a
hydrogen-l;o nilrogell molar ra~io rom aL)out 2:] to 4
preferably frolll about 2.5:1 to 3.5:1 by the ad(lition
n i trogen .
Impurities, including methane, carbon dioxi(~e,
carbon monoxide and 1125 are removed in a pressllre ~;wirlg
adsorption unit 118. r~ecovery of hydrogen in tlle pressllre
swing adsorption step 118 can be marginally improvecl by
depressurizing the adsorption beds and using nitrogell to
desorb the beds. 1~ portion o the stripping nitrogen is
carried forward with the product hydrogen.
The tailgas from the pressure swing adsorption
step 118, containing carbon monoxide, caxbon dioxide,
hydrogen, nltrogen, methane and water vapor, is fed to a
catalytic combustlon unit at step 122. The temperature o
the tai19as entering the catalytic combustion unit is from
about 570F ~300C) to 1100E' (590C). Catalyl;ic combustion
is effected at a combustion temperature from about 600F
(316C) to 1800F (980C) and a pressure from 1 atmospllere
(100 ~Pa) to 2 atm. (200 KPa). Space velocities rom abou~:
8000 hr~l to 500,000 hr-l can be achieved . The exit gas ` ~ `
from the catalytic combustion step 122 contain~ primarily -~
carbon dioxide and water, togel;her with minor amounts of
nitrogen, methane and any other inert materials, e.g. algo
Water is condensed from the exit gas by cooling the gns
through a heat exchanger at step 124. Carbon d~ox1de i8
thus recovered rom the gas stream and sold as a va lu~ql)le
item of commerce.
The product exit gas from the pressure swing
.
adsorption step llB is admixed with a sufficient amount ol
:: .: :,. :.
; ~, .
,.: . .: ` ' ~, ' :. '. '.
20042~6
- 30 - ~ -
: , '
nitrogen to bring the hydrogen-~o-nitrogen molar ratio to a .-
value from about 2:1 to ~:1, preferably from abou~ 2.5:1 to
3.5:1. The gas, consisting essentially of lIydrogen and ~-~
nitrogen, is compressed to a pressure be~ween about 8~ atm ~ ;
(8100 KPa) and lS0 atm (1520U KPa) and fed to the ammonia
synthesis loop 126, where the lIydrogen and nitrogerI are ~ -
reacted under ammonia-producing conditions, as prev;oIlsly ~-
described, to produce ammonia.
Fig. 10 illustrates an embodiment of the inventio
in which hydrocarbonaceous feedstock is converted to ammoni;
by an embodiment of the invention that employs relatively
small amounts of energy.
Following optional desulfurization 100, the
hydrocarbonacoeus feedstock, together with steam and oxygen
or oxygen-rich gas, is fed to tlle catalytlc partial
oxidation zone 102 wllere they undergo catalytic partial
oxidation, as previously described, to produce synthesis ga.s
containlng hydrogen, carbon dioxide, carbon monoxide,
methane and little or no nitrogen. After heat exchange 104,
shift gas reaction 106 and heat exchange 108, as previously
described, carbon dloxide is removed at step 112 by means
such as absorption of carbon dioxlde by contact wlth a :
countercurrent stream of liquid absorbent, as prevlously
described in connection with Fig. 6. Carbon dioxide from
the carbon dioxide removal step 112 can be recovered and ~,' ~ :'.''~'''! . ~ ''
sold as a valuable item of commerce.
The product gas stream exiting the carbon dioxide
removal step 112 is fed to a pressure ~wing absorber wllere - ~.
impurities including methane, carbon dioxide, carbo
3~ monoxide and H~S are removed. ~n improved recovery oE~ `~
hydrogen in the pressure swing adsorption step 118 can be
achieved by depressurizing the adsorption beds aIld l~ g ,.~ ' ,"'.,
nitrogen to desorb ~he beds. ~ portion of the s~ripplIlg
nitrogen is carried forward witII the product hydrogeIl. ;
, .:: . .... ~ i:,
200~Z~ ~
:
- 31
In the embodiment of Fig. 10, a portion o the
waste (off-gas) from the pressure swing adsorption Ullit is
recycled to the catalytic partial oxidation ZOIII' where it
provides heat to the reactants. Preferably, I)et:ween al,ou~
5 50~ and 80~ of the waste gas is recycled from the pressure
swing adsorptlon unit and the remainder i8 used as fuel.
The product hydrogen exiting the pressure swing adsor~Lion
step 118 is admixed with sufficient nitrogen to bring tlle .; ;~
nitrogen-to-hydrogen molar ratio to between ~bout 2s1 ancl ~--
4:1, preferably between about 2.5:1 and 3.5:1. The gas is
then compressed and fed to the ammonia synthesis loop, wher~
hydrogen and nitrogen are reacted at step 126 under
ammonia-producing conditions, as previously descrlbed, to
produce ammonia.
lS An ammonia plant for producing ammonia from
natural gas is illustrated in Figs. 16 and 17. This system ~ ~
employs a process similar to that of ~ig. 9 and has the : ` .;
catalytic oxidatlon step 102, heat removal step 104, carbon `
monoxide shlft step 106, heat removal step 108, pressure
swing absorbtion step 118, combustion step 122, and ammonia
loop process 126 indicated qenerally therein. ~rhe natural
gas feedstock is received on line 200 and i8 passed througl
saturator 202 countercurrent to a heated water 1OW from `
line 204 fed into the top of the saturator. The saturator
202 is designed to saturate the natural gas wlth wal;er 8
to reduce the steam requirements for the process. The
saturated feed gas stream 206 lrom tlle saturator 202 is tl
further mixed witll steam from branch 208 of the outpu~ c~l a
high pressure steam drum 210 to produce the desired s~eam
and natural gas mixture. This mixture on line 212 is ~hen
fed through heating coils 213 in a fired heater 214 to `~
produce the desired input temperature for the catalytic
partial oxidation. The fired heater is operated, at least
partially, by waste fuel in line 216 produced by the
... ,, ,,, ~ .
~... . , , . ,, ... , , ' . -:
200~ L6
;
- 32 -
process. rhe fired heater 21q also heats inpu~ water in
line 218 which is fed to the steam drum 210, an~l heats steam
in coils 221 from branch 220 rrom the output Or drum 210 to ~ -
produce superheated steam in line 222 W}licll is utilized in
the process, for example to drive a turblne compreSSOl.
A heated hydlocarbon process ~tream 226 from tl~e
output of the fired heater 214 is then fed to ~he catalyti
partial oxidation reactor 2B, shown in Fig. 1, where it 1
mixed with oxygen fed through line 228 and fed to the
catalytic reaction zone of the reactor 2U to cal:alytically ~ ~-
partially oxidize the natural gas and produce syntllesis ga;.
The synthesis gas from the reactor 2U in line 230 is ~a~se.l
through a heat exchanger 232 which is cooled by a water
stream from the high pressure steam drum 210 to partially
....
cool the process stream. From the heat exchanger 232, the
procesff stream is passed through line 234 which is mixed
with additional steam applied through line 236 to form an
lnput 23U to a higll temperature shift reactor 240. From
high temperature shift reactor, the process stream is fed
l~ne 242 to heat exchanger 244, where it iB further cooled
by a water stream from the steam drum 210. The process
stream output 246 from heat exchanger 244 i~ further cooled ``"``~
by water spray from line 24U to form a process stream 250
which is fed to low temperature shift reactor 252. Tlle h;gl
temperature shift reactor 240 and low temperature shift
reactor 242 perform the water shift reaction step 106 to
convert carbon monoxide in the process ~tream into hydrog~
and carbon dioxide. Process gas stream 254 from tlle teac~
252 i~ then passed through heat exchanger 256, line 25U, ~ ~-
heat exchanger 262, line 263, heat exchanger 264 and line
266 to a knock-out drum 26U where water i8 removed from tlle
process stream. Purged gas stream 270 from the amlllol-3a 1.
i8 combined with the process stream in the drulll 26n. 'llle ~
re~ulting proce~s ~tream 272 from the drum 26U is ~llell ~ ;
.. :, ~.. .
- 200~2~6 : ~
- 33 -
applied to a pressure swing adsorption unit 274 where carbo
oxide~ and other impurities are removel from the pr cess -~
stream. Nitrogen on 1ine 276 is applied to tlle Iressure
swing adsorption unit 274 to ai l in desorption. ~180 l~rancl
278 of the nitrogell feed stream is combined with output
stream 280 of t) e pressure stream a(lsorption unLt to Corm
the amrnonia makeup gas stream 282 which is fed î.o tlle
ammonla loop 126 of Fig. 17.
Condensate on line 286 from the knock-out Irull 26~3
is fed througll branch 288 Eor use in other processes arld
through pump 290 to form portions 292 and 294. The portio~ ?
292 18 combined with a water recycle stream from the wal.er
output 296 of saturator 202 througll pump 295 to form the
cooling stream 298 through the heat exchanger 256. Tllis ;~
stream 298, heated by the l)eat exchanger 256 forms the
heated water input stream for the saturator 202. The --
remaining portlon of the output 296 of the saturator 202 is
passed through line 304 for offsite blowdown. Tlle
condensate portion 294 is combined with heated water stream -~
302 to form the water spray stream 248 whlch 18 used to `~
chill the lnput 250 of the low temperature shlft reactor.
In the ammonia process 126 shown ln Flg. 17 the
loop makeup gas input stream 284 passes throutJh compressor
310, f1ash drum 312, water cooled heat exchanger 314,
compressor 316, ~lash drum 318, water cooled heat excllanqe
320 and 11ne 322 whlch i8 combined w1th ammonla loop recycl ~? .
~tream 324 to form process stream 326. The proce~ sl:ream
326 is circulated by compressor 324 through line 330 and
heat exchanger 332 to line 334 connected to an internal he.nl I ``~6
30 exchanger input of ammonia converter 336. l~fter heatinq i
the converter 336, the process stream from is passed t~
line 338 and tlle ammonia converter 336 wherein llydroJoll ;~n~
nitrogen are reacted in the presence of a cataly~t L lo~m n
portlon of the product stream into ammonia. Tlle OUtpllt o~
,: ,: . . ~ .; :;.
2 ~ 0~
- 34 -
.: .
the ammonia converter on line 340 passes tllroutJIIlleat
exchanger 342 where it is cooled by heat exchallge with water
flow from drum 344. From heat exchanger 342 thr3 proce~s
stream passes through lirle 346 to the hea~ exchanger 332
where it heats the incoming st;realll 330. rhe process .stream
then passes through line 348, water cooled heat exchallger
350, line 352 and heat exchanger 354 which i8 cooled l~y the
recycle stream 356 from an ammonia condensation apparatus. ~-
From heat exchanger 354, the illcoming strean pa~st?~ lrr-lnJI~
line 35B and successive chiller sections 360 and 362 tt~ a
catch pot 364 where liquid ammoni~ is gathered. From ~l~e
catch pot 364, the non-condense~ overhead forms ~he r~tytlt~
stream 356. The purge gas stream 270 is taken Erom the
stream 356.
Liquid ammonia from the catch pot 364 i9 transfere~ ~
through line 366 to an ammonia receiver section 368, and ~ ` i`
from there ls wlthdrawn by pump 370 to ammonia product line
372. Sectlons 374 and 37G of the ammonia receiver provicle `
,, ~
ammonia streams Eor the respective chiller sections 360 and
362 to condense ammonia in the product stream. Gaseous
product from the ammonia receiver sections 36B, 376 and 374
are compressed by compressors 3B0, 3B2 and 384, and passed
through line 3~6 to a heat exchanger 3BB and a reErigeratior
loop receiver 390 from which liquid refrigerant over line
392 is fed back to ammonia receiver section 374. Flash ga~
condenser 394 receives a portion o the stream rom lille :3
and returns the further cooled portion througll llne 396 ~o
the receiver section 368. ~ turbine 385 drives the ; ;
compressors 3B0, 382 and 386.
When compared to present day commercial prol~?s~sen, - -
the catalytic partial oxidation process using, as oxi(la~
stream containing in excess of 70 mole percent o oxy~ell, as
described herein, the process oE ~he invention O~rers ~lle ` j~
following advantages.
, ~ , .,: ,. ~ , .
- 200~2~
- 35 -
(1) The higll cost s~ m reforming fulrl.~co ~lld
secondary refonner are eliminated whell compared
the conventional commercial process. ;~
(2) Low oxygen conslllllp~:ioll when comp-~red to '~
S conventional partial oxidation. ' ~
(3) Low water consumption when compare(i to steam ~' ';
reforming. ~,
(4) Low cost when compared to cataly~ic partial ~'
oxidation processes which use air or enriched ai
to produce a nitrogen rich or stoichiollletric , ;~
synthesis gas at the exit of the catalytic ~artial ; ~h ~-
oxidation reactor.
(5) ~educed area requirement when compared to tlu? '~
steam reforming route (particularly suitable for ,~`
offshore application).
(6) lligll efficiency when compared to convelltix~ll.~l ' ',,,'~ "'`'~
commercial ammonia production processes and whe~
compared to catalytic partial oxidation usi.ng air
or cnrict)ed air conl:aini~g less than 7U mole ;~.~;,,',,~',
percent of oxygen. `~ ' --,,''~
(7) Lower in capital cost then all pre~ent ~ ',' ~'
commercial processes. ;' ,~I;,',,
The following examples are intended to illustrate ,'`~
further the invention deecribed herein and are rlot in~ende-l ' , ;,,'
to limit the scope of the invention irl any way. ,~ ','' ,'C
.~.,,:
EXI~MPLE I
Natural gae is converted to syntheeis gae il~ a ,''"',',",~,i~''
catalytic partial oxidation reactor of the construct;ol~ '~'~- '"'""-
shown in Fig. 1. There are included nine catalyet,discs 5~, ","
each having a diameter of 30 inches (0S76m) and a thlckl)esi~
of 10 inches (0.25m). ~he discs are formed from a holleycollil- .,-',,,'~',",',',~','`
monolith of cordierite material witl- a geometric sul:C;~ce ,',,',~':`,',,~'area of approximately 25 cm'/cm3. A high eurface area - ~ ,'
i",,,.",.;,,. ,~,~.,.
~: ~ ,~' ,",.,',,
2004Z~6
~ ~ .
- 36 ~
alumina layer is deposited OII the cordierite to serve as a
support upon whicll finely ~ispersed catalytic m~
components are di~ten~ed. qI~e c.~t~ly~ic metal (:~n~ponenLs
are approximately 50~ by weigI~ platiIlum and 50~ by weiyl~t
5 palladium. Space velocity of ~he cat.~lyst is 97 000 hr.-
Natural gas (>95~ metIIane) is mixed with sleam aL ;~
various steam-to-carboIllnolar ratlos lleated and su~plied ~ ~ -
through 10-incII ~iame~er inlet 66 at a pressure oE 4()~ psi~
(2760 KPa). ~ir is heated and supplied through ~wo ~ ch
inlets 70 at a pressure of 410 psig (2830 KPa). The
diameter o~ t~e lower portioIl 76 of the cllamber 72 i; 27
inches (0.68m) with the diameter of the upper portion 74
belng 36 inches (0.9lm). There are 261 tubes B0 having 0.5
lnch (12.7mm) internal diameters and l)aving lengths of 20
inches (O.Slm). Six orifices 86 of 0.123-inch (3.2m)
diameter are formed in eacll tube with four of tlle orifices
evenly spaced around each tube at a distance of 4 lncl~es
(0.102m) above the lower end of the tube and with the
remalning two orifices formed opposlte each otller at a
distance 6 inches (0.152m) above the lower end of the tube.
The bottom member 78 has a thickness of 5 inches (0.127m)
and the passageway sections 84 are conical with upper ~ ;
diameters of 0.5 inches (12.7 mm) and lower diameters of ~ ;
1.75 inches (44.5 mm). Pressures within the chambers 68 an(I
72 are malntained at essentlally the inlet pressures. ~
The temperature of the mlxed reactant gases ls i j-
1 100F (590C). Fig. 11 shows oxygen consumption for the ~ ;~
catalytic partial oxldatlon process as a functlon of
steam-to-carbon molar ratlo for reactlon temperatures of
1 600F (870C) 1 750F (950C) and 1 900F (1040C) and ;n - .
operatlng pressure of 400 psig (2700 KPa). It can be seen
from the graph that oxygen consumptlon expressed as ; ~ --
.: ~ j .. ....
oxygen-to-carbon molar ratlo is relatively low for tlIe :
proces3 of the invention as compared with present comlllercl;lJ
',, ~",~
~.`' '' ''.,,', ',: .
20(~2~6
- 37 -
partial oxidatios~ processes. Tlle dashed line 25 in Fi~
represents the linear function oE minilllum tempera~uJ:es and
steam/carbon ratios requ;red to ~ evellt carbon ~ L~osits. ~-~
Fig. 12 shows the molar ratio of ~Iyd~:o~en, as 112,
to carbon monoxide in the product as a functioll o tlle
steam-to-carbon ratio for reac~ion temperatures of 1,600 F
~870C), 1,750F (Y50C) and 1,900F ~1040C).
Figs 13 and 14, respectively, show the amounts Or
methane and carbon dioxide, as volume %, in tlle producl as a ~ -
function of ~he steam-to-carbon ratio or reactior
temperatures of 1,600F (870C), 1,750F (950C) and 1,900l ;
(1040C).
Fig. 15 shows the effective Hz production of the ;~
process, expressed as total moles of 1~, and carbon monoxide
in the product divlded by total moles of ~12 and carbon in
the feedstock.
ExamPle II
The followlng example describes the production of
ammonia from a gaseous hydrocarbonceous feedstock using the iS?
process o~ the invention represented by Figs. 9 and 10. ` ~;;
Hydrocarbonceous feedstock is desulfurlzed u8ing ~ i
conventional methods depending on the quantity and type of
sulfur compounds, contained in the feedstock. ~` ;
Desulfurization may, for example, be conveniently carried
out by preheating the hydrocarbonceous feedstock at a
temperature between 250F (120C) and 750F ~400C) an
absorbing the sulfur compounds, into zinc oxide contained ~
one or more desulfurization vessels. .`' `~ r,.,'
Steam is added to the desulfurized feedstock ~o
give a steam-to-carbon ratio of between 1.0 and 1.7 ~.o 1.
The steam may be added either directly or by feed ~a~
saturation. The hot water for feed gas satura~ion is ;
. :.~ '..',:.,:'
," ,~ " ," " . .
.',.'~'.,:-:''
-- Z0042~
- 38 - ~ ;
conveniently provided by recovering heat from tlle syn~hesis ~-
gas at a point downstream of tlle shift reactors.
The mixed feedstock i~ prelleate~ to .~I~L)roxima~ely
1100F (590C) in a fired hea~er and passed to the ca~aly~
partial oxidation reactor where i~. is admixed with prehea~el
oxygen as oxygen containiny gas containing at least 7U mole
percent of oxygen ln a ratio of between 0.5 and 0.55 moles
of oxygen per atom of carbon contained in the feed~tock,
before passing to the partial oxidation catalyst where tl)e
above reactions (3) and (4) occur. The exit temperature ~ - }
from the partial oxidation reactor is about 1700F (930C). ;~
}leat is recovered from the effluent gas from the reac~o~
raising steam in a boiler, before additional steam is added
to the synthesis gas passed to the lligh temperature shift
reactor at a temperature of approximately 700F (370C) - ;`
where more of the carbon monoxide i8 reacted accordiny to ` `
the above equation (4). The synthesis gas emerges from ~l~e
high temperature shift reactor at about 850F (450C) and i~
cooled to about 650F (340C) in a second boiler which also
generate~ high pressure steam. ~ water quench is used to ; i~
reduce the synthesis gas temperature to 425F ~220C) at
which point it undergoes low temperature shift according to
the above equation (4) to reduce the carbon monoxide conten~
and increase the hydrogen content further; Heat is ; i~
recovered at the exit of the low temperature shift reactor
by preheating water, which is used to saturate the .
hydrocarbonceous feedstock Witll steam. Further heat is ?~
recovered by preheating demineralized water whicll is pas~e(J ~;
to a deaerator for use as boiler feedwater. Then the ;~
synthesis gas i8 cooled to approximately 100F (38C) w;~h
cooling water and condensed water is separated in a knock~
out drum. .'"''.'!.'.''''''~'~'''
Two options of the process route are inclu(le(l i
the processes of Figs. 9 and 10 dependlng on the rela~ive
2V0~2~>
- 39 ~
requirementi~ for minimizing capital cost and maxlmizlng
feedstock conversion efficiency. Fig. 9 8hows L~le min.imum
cap~tal cost option. In Fig. 9 the remaining c;~rbon
monoxide, methane, water vapor and carbon dioxide are
removed from the synthesis gas by pressure swing adsorptio
as described earlier, to ~-iel(l a high purity hydrogell
stream. Nitrogen, from the air separation plant is then ~:,!"`.'.. '.'`
added to produce a gas contairling hydrogen and nitrogell in a ---
- mole ratio of between 2.5 and 3.5 moles of hydrogen per moJe
of nitrogen. The waste gas from tlle pressure sWirlg
adsorption unit ~off-gas) is used either as fuel in the
fired heater or, if carbon dioxide is requlred, for exalllpl~
for the downstream production of urea, it may be burned
catalytically, wlth a portion of the oxidant stream, using `~
lS an oxidation catalyst. The methane, carbon monoxlde and
hydrogen are converted to carbon dloxlde and water and the
effluent stream from the catalyst combust10n tllerefore
conta1ns essentially only carbon dioxide, nitrogen and wat~r
vapor. Water ls condensed from th1s stream by reducing its
temperature and at the same time useful heat is recovered.
The water 1~ flnally separated in a knock-out drum to yield ~`
the product carbon dioxide stream. ~ ,
Fig. 10 shows tlle maximum efficiency optlon. In j j
Fiq. 10 carbon dioxide is recovered from the synthesis gas, ~ ~ -
in a conventional chemical or physical adsorption process ;
112, as previously described. ~rhe carbon dioxide may be -~
sold as a commercial product. Pressure swing adsorpl;io
is then used to produce a pure hydroyen stream. The
pressure sw1nq adsorption off-gas contains essentially o
hydrogen, carbon monoxide, nitrogen, methane and any
res1dual water vapor and carbon dioxlde. Part of Ihi~ gag
is used as fuel to the fired heater and the remainder is
recycled to be used as feedstock in the catalyti.c partial
oxldation reactor. Alternatively, it may be recycled to a
..., . '~
200~2~
~ r
point upstream of either the higIl temperature sh1f t reactor
or ~he low temperature shift reactor. Nitrogen rrom the air
separation plant is added to tlIe yurifled hydro-Jen stream i
a molar ratio of between 2 . S an(I 3 . 5 moles of hy dro~eI~ per
mole of nitrogen to produce a (~a!; sui ta~>~e for ;umnoIlia - `;
synthesis. ;Before it can be used for ammonia synl.lIes; 9 I:he
synthesls gas must be compressed to approximately l00 atm -
( 10130 KPa ) .
The make up gas is mixed with the circulatiI~(J ga~ `
ln the syn~:hesis loop at the suction of the circulato~. Th~
bulk of the gas leaving the circulator is preheated 1I~ tI~e I~ ;u
loop interchanger after which the gas splits into two \ `
streams. One stream is used as quench gas for moderating `~
the synthesis reaction temperature and 1s ln~ected in `~
between the first and the second beds of the ammonia
converter. The other stream is the converter feed gas and `i~ }~ r~
thls is preheated to reaction temperature, by heat exchange
w1th the effluent gas leaving the second bed, in a heat
exchanger located inside the ammonia converter. ;-
Ef f luent gas leaving the converter a t
approximately 766F (408C) 18 cooled to 7F (-13C) in a
boiler whlch raises high pressure steam, loop gas
lnterchanger, loop cooler, recycle gas interctIal-ger~ and th~?
loop chlller, and then passed into the catchpot. The
uncondensed gas is taken from the top of the catchpot, `
preheated in the recycle gas interchanger and then recyc~ed
to the suc:tion of the circulator. J~ small purge 18 take
from the recycle gas in order to avoid buildup of the inert ~
level in the ammonia synthesis loop. This purge i8 recycle I
to a point upstream of the pre~sure swing adsorpt.ioll ~uui. t
alternatlvely upstream of the cal:alytic pnrtial oxid;l~.lo
reactor .
., " , .
': `` :: `
200~$
- 4 ~
Liquid ammonia leaves the catchpot under 1evel
control and is reduced to atmosp11eric pressure ill tlle
ammonia receiver. ~mmonia and dissolved gases tlash oCf an~
are separated from the liquid amlllonia in the a~ lollia
receiver, and recompressed in ~he three stage refri~eration
compressor, to 240 psig (165~ Kl~a). From the discharye oC
the refrigeration compressor, the f1ash gases are coole~
with cooling water to condense the bulk of the ammonla,
which is separated in the refrigeration loop receiver. ~-
The ammonia content of the flash gases i8 rnl~ e.r
reduced by cooling the flash g~ses ~o -14F (-2GC) wilh
ammonia let down Erom the reErigeration loop receiver ani
returning to the atmospheric pressure section oE the ammoni.l
receiver. The ma~ority of the condensed ammonia from tlle
refrigeration loop receiver is let down to the higll pressur~
section of the ammonia receiver, operating at 49 psig (33B r .
KPa) and 33F (1C). The hlgh pressure ~ection oE the
ammonia receiver a1so acts as a Elash vessel for the primar~
section of the chi11er. F1ash gases from this section entel ~ .
the third step of compression of the refrigeration ~ ;
compressor whi1e the liquid is let down to the medium
pressure ~ection of the ammonia receiver operating at 16 ;
psig (110 KPa) and 0F (-17C). ~rhis section acts as a
f1ash vesse1 Eor tlle secon~ary section o~ the chil1er. Tlle ;~
2~ f1ash gases Erom the medium pressure section oE the ammonia
receiver enter the second stage of the refrigeration
compression whi1st the liquid pllase is let down to tlle low - ;~;
pressure section of the an~onia receiver operating at ~ ~`
atmospheric pressure and -28F (-33C). From het-e, ~ ?
product ammonia is pumped to atmospheric storage. ~ ~-
Exam~le III ~ :
The following T~BLES I, II and III contain
mo1es/hour, mo1e percent, and parameters of pressure,
temperature, water/steam flow, and heat transEer ~or an
: `, ',.,.,:'
`.::
~' . , .
200~
. .
. .
- 42
ammonia plant according to Figs. 16 and 17. The moles/hou
are lb moles/hollr (0.4536 kg moles~llollr) and ~IO ~-lanL :-~
produces 600 5hort ton Nll, I)er d.ly ($44 x 10~ K-JJday)~
~''.: "''."-`. ' `.''`
..: :. ., :.:.....
?
" ::' ~
` . ::`~-
': ~ ,'~ , "
',~;" '.'';'
..
:~ :: '': ~ . '
'. ~'. ..',~
....~ :
~' ' ',''~ `;'
~' ~. '': '' .'
: , ~ :.
.': ~ ':~:~
200~
- 4 3 ~
o~ e~eo n r~~ ~o r~ W ~ ~~n ~ ~n
O o ~ ~o ~ m o r~ ~ o
¢ ea ~o 0 e~ erl ~ ~ CO O ~D ~ ~ r~ r~ r
~ r~ ~ ~ tJ~ ~O r - er~ O ~ ri~ ~ ~ ea eo
o ea ~nt~ ef~ 0~ e~ e~ e~ 0tn r~ t~ ~ r~
E- r- ~ ~ eJ~ O ~O ~ r~ ~ ~O e~J e~ tn
ef ~
e~ ~ . . . ~ o
P~ r~ r I er~
Z r~_I r~ r~
t~ t~ O t~ t~l t~ e~l O .'~'~''~"'""'i''',`'''`'
~ e~l tJ~ ~O t~ Ut e~) : :: ',::-.,:'. .-:'
e~l e~ o t~
:11 e~l ~O trt ~ eJ '1 e~ t.~ .. . :. : . .::
~_t~ ~ Vl t.~ t.~l ~` ' -' ' ' ' - '
e~l e~l e~7 ~ ~ ' . : '' . . . :. :`'
ea r~ t` ` '``,"
W tO ~O W ~O tO O ~t~ ,, . ;. ' ',:. ~
ert e~
e~l ~ e~ J r-~ t~ W ~D e~
eO ef ef ef e~ ~O ~ t~ eJ~ e~
~i r l r~ ~i r~ ~n ~ ~o ~o ~ r ~
r-~ r-l ~0 tO r~ erl
r r l r-l ,, , " ,
O O
t ~n
: : :~
o o
totn ~n m ~n ~n~
r-l r-l r~ r-l r-l r~ r l ' ~'
r-l t~
ef~ ~ r~ r~ O ~ e~l ~ t~
O~ 01 t~ ~ 1~ 0 tn m
ea o ~ ~n ~ ~ r~ tn . .
O ~ N 0~ e m ~n eo ~ ~
~ O " ~: ' ''' ~ '"' "
o ~ o eo eo~n ~ e~
) t-i ~ O ~0 ~ r~ ~ O 0
e~- rl ~n In ~n
r~ r~ r-~ ~ r ~ r ~
~: . .: ",,' ', ',
t~ e~ n n N
~ tn e~
t~ ~n
U~ O U~ ~o eO N ~1~ O ~ t~ 0 0 p ~ 0 r~ ~ -
O tg O td t~ I e~ n ~ 0
~1 e~ e~l U~ NIJ e~l V ~ ~ 1 111 e~ 111 e~l O t~
~t r~ tu ~ tu 11~ U 10 (11 V IU ~ 11 t a~
~ ~ a V a d d rJ d ~ d r~ a ~ a d d a on a ~ d X a g r: V~ a
r~ .g r~ r~ O r~ n r~ It --~ -r~ . 1 t~ O r~ O .~ t~ ~
Z r-l ~0 r-~ r~ r-~ v r-l t~O r-~ r~ r-~ r-l O- _~ -I 0 r-~l U
~ o -- -- -- L~ ~ ~C ~ od ~ .,~ ~d o v~ O
a z m o u ~ ~ z P~
2 0 ~ L~
- 4 4
O O~ ~, o ~ ~ ~ ~D ' .. '. "
~ r~ o 0 1~ ~ ~ : :: ::.:: . ., :-~
P ~ O~ O O~ ,0 ~, ~ :.. ..... .
. : , .. ... ~
' " ',''=:~"'~"'.
' ' '; ' ' ':' ,:,
O
O
: . . ' ' :. ' .': .: ' . ' i. '
O ~ ~ ~ ~ , " """' '. "
Z ~, ~ ' " ',
to ~
Ul ~ ' ' .. ' .
: . - .
,, ~ O ~O ~ 0 .
:. ~, .,,:
o
Z r~ o O o ~ o o ~
:- ~.: :'
~ 1 ~ o ~1 ~ ~ o o ~ ~1)
1~ ~ d ~- d 0- d ~-1 d IJ- d
~ ~. ~ 3 ., ~
. ..
~:004X~
_45- ; .` `
0 ~ o
'` '~ ~ o ~' ~, ',,','-,- ,"
O O cn ~ o ~ o~ ~ ` ~
o o. o . ,. - .. ., ... ,.r.. :
. . . . . . . . . .-....... "; ~.. `, `.,,:
o o o o o o o ~
o ~ ~ ~ ~ o ~ "
o o o o o ~: ,i 0 ~ , .,: ".,.,~,~ ,
O O
o o
_ a o
O O
a~ . .
;~;
O ~ O 0 ~ ~
ul .J r~ r~ O ~ o
~. . .
O 1~ r~
~, :', '.~ . ' ' :
O ) r I rl O O O~ . . ..
N O r~ 0 0 r~
O ~:t ~i 0 0
Z Ul O ~ ~OCO ~ ~ ~ O ~ ~O 0~ 0 ~ ~ 10 ~ r - ' ', . .. .
O n~ O ~ 1~ IU 0 1~ 0 I GO rl d rl I . I :~
J d d .~lt v ~
.3 ~ .a v e J a a a a a a a a oo a v~ ~ 3 v
u~ v _ ~ h ~ O ~ _ ~ ~ C ~ a v ~ o .c
~ p ~n Z p, ~ U V~
200~23~6
- 46 - `~`````;.`
.,.- `~,. '." ., ., '` -", .
o ,~,
o .. ,.. ,` ", ~
'` .'~ .~' .` ,' ". ':
:z; . .
o o~ -
-
o
~ ~ .. ..
Z ~
' ' , ' :., '. '
~ O O u~ "; .. .
o , ~
;-.. ,~ '.. ' .. .
8 `~
V . ~ ` ,.
d ^ ~
O O O ~ O O ~I , .:
V
~ d 1- 3 ~ r~ ~ c ~ r d
g _ g `_ u ~ d
.. ., .: : . ' :: : : ,
- 2004Z~
-- 4 7 ~
' ~".' , ` ., :'~ "
-- t~ O ~ tt~t`l ~ ,.;" . .'
: ' , :' : .' .': ': ':
V ' ~ ~" ".'`'.'~ '
t~ t~ t ~o
O U) O ~D ~ ~ '' "''' -"' '
p . . . . . . '. ', : ,~""`,`" '
t`l ~ .",. ,~
o ~ ~O '' :~
K ~ ~ '~
t
t~
t~
' ,. ~" '~`'' ' ',
J~ ~0 ~ ~ 0 ~ 0t~ t'~ ~: O ~ ~ :? tr O~ 0 0
3 ~ ~~ ,, ,. t`~t~l ~ ~ t~ .0 t~ t~ t'~
';'. ~ :'
~' ~o ~o Vl 0V~ ~ O o O O 1~ 0 0 0 0 V~ ~ O ~ V~ vl Vl
'D rl 0 0o ~ o o o V~ 0 o v~ V~ ~ o 0 o
~~ v~ o 0 ~ ~ o ~ ::
o~ ~ ~ ~ ~ ~ ~ o a~ 0 co
~ ~ ~ o o v~ o o o o ~ v~ ~ v~
O u~ v~ o ~ ~ o
0 ~ , ,.,':: ~; .:.
: . : : ~ ~: :
~0 :t ~ o vl ~ ~ J
o ~ c~ ~d o~ 1`1 ~ v ~o v no r~ ~0 '.. ,~ . ". .
O ~-O U ~ ~J V O ~ ~ ~ vl ~ V,~
~v ~E~ o~ o~ v r~ o~ ~ ~ a o~ D. O- t~l 01 t~l 0- ~`I O
Z ul ~ -- d a~ a v ~ N d d :1 d o~ c~ d r~ lo r1 vo ~ ~0 ~ r
O ~ d~3 ~o ~1 V d 01 ~: 0 v d d ~ O al d ,~ O d d d d ~
~o~0~æ~ u a~ u~v~ 0~.v~u~ u~
v ~ o V ~ o ~ ~ ~ o ~ o 11 vo
d u~ W ~ u W .¢ ~ r I W u~ W ~ ~ W o W lo W (o r~
g J ~ d u ~ --I d v ~ oo v u ~ ~ ~ v u o~ v u v u v u
(n v V v ~ O ~ ~ ~ P.O :~ O ~ O ~ r~ O U ~ O d o ~ o
W d 1~1 d V V 1~ U :~ ~ tl d 1~- X ~ IU ~ V ~ ~ d ~ ~ ~
~ z ~ n ~ u~ O ~ a ~ ~ u~ a r. m P- ~
- 200
- 48
: ` ~, ..
n~ vl t~l O r ~ ~
~o ~ 0 ~!
3~
~ ~ '
E~ ~ - `:
V
m ~ ~. O
~ ~ O .--
V _ ~ ~ ~ O O~ ~ -' ' :
~ O U~
:1~ 0 U~ 0 ~ 1~ ~ ~ . : ' : ` - :
V ~ ~ ~ .. : ~: ,.. :
.
a . ~ , ,0, ~ ~ 0 0 ~0
.~ v o ~ ~D
W
J ~ v~ 0 w a~ D O~ ~1 ~ o> O ~D )
~9 ~ O O ~D O ~ ~a. n u~ ~ o D
""''`' '" ''" '''''
V~ Vl W ~ V) ~1 0 0 W I~
~ D ~D
IU ~ O O ~ O O Vl O ~D cn o . ~ ;
~`1 Vl
~, ,'' ' ."' '`,'~ " ~'-'
O r~l ~D ~ r.l
1~1 t~ O ~ ~D ~D ~ rr~ r~
0 ~ D o ~ r~ ~o 0 ~ 0 d d g O O 0 : .
O ~ 0 ~ O O ~ J
~D ~ ~ ~ 00 J bO 00 00 ~ D U U U
n d t~l 0 d ul u~ d 1l7 d ~ U u r~ u O :
d i~.!C d o ~ v~ ~ o o ~ ~ u ~ ~) du ~ ~ ~d ~ ~
ps o o ~ J ~ 0 ~ O~ r r~ d
~ ~ U u u ~ u :~ v u u J u ~ u
V) J ~d O d q JJ q -- v o O O 11l 0 d ~ o o ~ o d O~d~ J U U U
:: ::
:: :
200~2~6
~: . - .
. :
- ~,
. , ~ . .
- 49 ~
Since many variations, modifications and cllanges
in detail can be made to '~he above described embodimellts, Ll
is intended that ~he sub~ect de!;lri~ed al)(lve alld shown ln :: :~
the accompanying ~rawinys be il~tel:pre~e~ as illustra~ive alnl
not in a limiting sense.
'''~' '.`"~'`''~"."`.'';
' ~. :..
::: ., .::.:
..: ,.
~ - .
i':
' ~,
':, ' . ' :,
.: ~, :. : .::
~: . ~....-.
. ~
" , ~, " ,
", .
, ~,: ' '':
,.,,' ~' '
, :, .. .....
'`.',' , ' '" ~'',', ~
'~',' ':" "''. "','',',: '
:: . .. :::.:. ::,. .: