Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
200~3f~;
AUTOMATED SYSTEM FOR MICROPROPAGATION AND
CULTURING O~GANIC MATERIAL
Related AP~lication;
This application is a continuation-in-part of co-pending
U. S. Application Serial No. 207,405 filed June 14, 1988, which
is a continuation-in-part of U. S. Application Serial No. 021,408
filed March 4, 1987.
Background of the Invention.
The present invention relates generally to the field of
automated apparatu~ and processe~ for micropropagation and
culturing orqanic material. More particularly, the invention
relates to automated apparatus and processe~ for micropropagation
and tissue culturinq of plants. Still more particularly, the
invention relate~ to a new and automated ~ystem for performing
micropropagation and tissue culturing of horticultural and
agricultural plants using integuments.
MICROPROPAGATION AND TISSUE CULTURING
Micropropagation is the process of mass producing new
generation plants from a single tissue sample taken from a
carefully s~lected parent plant or cultivar. Microp~opagation
retains tho advantages common to all types of vegetative propa-
gation, i.e., identity of progeny and the ability to propagate
non-seed producing plants, while having the additional advantage
that only a small piece of tissue from the cultivar or parent
plant is required. Micropropagation thus eliminates the disad-
vantages associated with the other forms of vegetative propa-
gation.
Tissue culturing is the process of growing cells ln vitro
and is usel to grow both plant and animal cells. Tissue cultur-
ing techniques are commonly used in the early stages of the piant
micropropagation process where it is desirable to rapidly produce
plant cells.
Improvements in tissue culturing techniques also have
applications beyond the micropropagation of plants. Essentially
2'~0~3~
the same culturing proces~ is used to culture animal and even
human tissue, such tissue being used in the fields of animal
agriculture and human and veterinary medicine. Culturing of
organic material other than plant and animal cells and tissue,
such as bacteria, viruses and algea~, i9 also performed in vitro
for both research and commercial purpose~. Improvements in the
procedures and apparatu~ used to reproduce and maintain these
organisms would be beneficial, for example, to researchers and
industry who require a large or steady supply of such material.
Further, the automated system of the present invention can be
adapted for use with germinating seeds and growing plants
therefrom.
DEFICIENCIES IN PRIOR ART MICROPROPAGATION TECHNIQUES
The prior art micropropagation process i~ described in
detail in co-pending U. 5. Application Serial No. 021,408, page
5, line 3 through page 11, line 9, the entire disclosure of which
is hereby incorporated by reference. Despite the advantages
conventional micropropagation technique~ offer the commercial
grower, there are problems as~ociated with the prior art cultur-
ing apparatus and processes. One of the primary problems is
contamination. Any of a wide variety of microorganism~, includ-
ing viruse , bacteria, fungus, molds, yeast and single cell
algae, can ruin the cultures during any of the various stages of
micropropagation.
The prior art sterilized glass or plastic culture containe.s
such as test tubes, flasks or bottles have serious drawbacks.
For example, since plants require both carbon dio~ide and oxygen
to live and grow, these containers must provide a means for gas
exchange. The walls of these traditional glass and plastic
containers, however, do not ~ermir ~e required gaseous inter-
change. Thus, rubber stoppers having cotton packing or some
similar filter material, loosely fitting caps, or baffled plastic
caps have been employed to allow an adequate exchange of gas
3~5
between the tissue or plant and the ambient atmosphere and
environment. ~owever, such devices restrict the amount and rate
of gas which can be exchanged. E'urther, such caps and stoppers
do not totally protect the plant from contamination by microorga-
nisms such as viruses, bacteria and fungi. Thus, it has been of
paramount importance that the tissue culture room and laboratory
be maintained under aseptic conditions, i.e. kept extremely clean
and their atmosphere~ entirely filtered. Eurther, precise
temperature, humidity, and light conditions must also be main-
tained in th¢ culture room when using traditional micropropa-
gation techni~ues and apparatus. Gas exchange is also required
for culturing animal cells and for certain other microorganisms.
Traditional flask~, petrie dishes and the like, while allowing
for a certain degree of gas exchange, also allow contamination to
occur.
The original cost of the traditional glas~ or plastic
culture container~; the labor and equipment cost to maintain the
sterility of the containers; and the added cost of the facil-
ities, eguipment, and related conditions required to maintain a
sterile ~rowing environment, all represent major cost factors
associated with the use of such container~ in conventional
culturing processes.
A further significant disadvantage of the prior art micro-
propagatlon proces~ and apparatus is the fact that the conven-
tional culturing containers do not lend themselves to use in an
automated system. Currently, each step of the micropropagation
process must be performed by time consuming and laborious manual
operations. For example, when a tissue sample which has survived
stage one and has grown to a size that it is ready for multipli-
cation, the culture containeL, a gi~Y ~ u~, t~or example,
must be carried rom the culture room to the laboratory and
placed under a laminar flow hood. There, a technician sitting in
front of the hood, will typically spray the container with a
~0~)~3,4S
solution of alcohol to kill microorganisms which might be on or
near the entrance of the container and contaminate the culture
during the tissue manipulation. Next, the technician must grasp
the test tube in one hand, remove the cotton filled rubber
stopper (in this example), remove the tissue sample with ster-
ilized forceps and place it on a sterilized working surface. The
technician must then cut the tissue sample into a number of
individual samples each of which will then be placed in a sterile
container with fresh media.
The containers and media to be used in thi next stage will
themselves have already been manually prepared. Typically, a
measured amount of prepared media is placed in each test tube,
with the test tube~ being held vertically in a conventional test
tube rack. The racks o~ media-filled test tubes are th~n ster-
ilized and transferred to the laminar hood for the technician's
use in the next tissue manipulation. Similarly, culture contain-
er lid~ and stoppers must also be cleaned, sterilized and placed
under the laminar flow hood for the technician's use. Once
cooled, the technician will grasp a clean and sterilized test
tube in ona hand and will insert one portion of the newly divided
tissue sample into the sterilized media with the other hand, and
then place a cotton filled and sterilized stopper on the test
tube and replace the test tube in the rack. Oncé the tissue
manipulations are completed, the racks containin~ the new cul-
tures are then transported back to the culture room.
As can be appreciated, the number of cultures which can be
produced is directly related to the efforts and abilities of the
technicians and more particularly to the manual dexterity of the
technicians. Furthermore, the extensive manual operation and
human involvement in the process creates a tremendous potential
for contamination, even despite the precautions currently taken,
such as requiring the technicians to wear surgical gloves and
masks.
20~43~
Additionally, the remaining steps in the micropropagation
process must be carried out mam~ally. Test tube~ are manually
loaded and unloaded into washing apparatus and frequently require
a manual washing to completely remove media or residue from a
container which had a contaminated culture. Likewise, it is time
consuming to manually mix media~ and fill the test tubes or
culture containers with the prepared media in measured quan-
titie~. Culture vessels or containers are alRo manually loaded
into autoclaves for sterilization. As explained above, before
opening a culture container, its side~ are typically manually
sprayed with a solution of alcohol or chlorine solution to kill
microorganisms which might contaminate the culture once the
container is opened.
It is also currently left to technicians to visually inspect
the growing cultures for signs of contamination and growth and
take the appropriate action depending upon their obseruation.
For example, when tissue or plantlets have reached their desired
size, technicians must manually tranYfer the culture containers
from the culture room to the laboratory in order to perform the
next manipulation. When a culture is contaminated, it is also
manually removed from the culture room and transported to a
station for disposal and for container cleaning and ster-
ilization.
Current micropropagation techniques also lack the ability to
monitor inventory through automatic means. Instead, inventories
are controlled by maintaining physical separation between the
cultures of the various plants being grown and by simply counting
the number of culture containers and the culture~ contained
therein.
As can be appreciated, the coflventionaï micropropagation
process is extremely labor intensive and costly. In addition,
the level of production i4 limited by the number and abilities of
the technicians involved. A well qualified technician, using
X00~3~S
conventional culturing apparatus and procedure~ can establish
approximately 350 cultures per day. Using a laminar flow hood to
its maximum efficiency by employing three technicians, each
wor~ing eight hours in a 24 hour day, the maximum number of
cultures which can be established by well trained technicians in
a day i9 approximately 1050. Accordingly, there is a need in the
art for an automated system for performing micropropagation and
the culturing of organic material. It is desirable that ~uch a
system eliminate the time consuming and extremely expensive
manual steps currently employed, including tissue manipulation,
container cleaning and sterilization, culture transportation,
media preparation, and filling. In addition, it is desirable
that such a system have the capability of automatically detecting
culture containers which have been unfilled or underfilled with
media, cultures which have become contaminated, and tissue
samples of plantlets which are ready for the next stage of
micropropagation. An automated system also having the capability
of trackin~ a culture throughout the micropropagation process and
automatically computing the inventory of the variou~ plants or
materials b~ing cultured would also be a great advance over the
traditional culturing apparatus and processe~.
Other objects and advantages of the invention will appear
from the following description.
SummarY of the Invention.
The automated system for growing plant material includes a
length of membrane material having a plurality of open growing
chambers. The preferred membrane material is a high density
polyethylene sealed together at predetermined locations to form a
plurality of grow_ng chambers having an open end for the
insertion of media and plant material. A media preparation uni~
mixeC measur~d amounts of individual stock solutions to prepare 2
selected growing media for the plant material. A fill unit
dispenses the media into the open growing chambers of the length
XO~
of membrane material. A fill check scanner unit scans the
media-filled open growing chambers to insure that each of the
growing chambers has been filled with a predetermined amount of
media. The media-filled growing chambers then pass to a
sterilization unit for sterilization. A cooling and storage unit
cools and stores the media-filled open growing chambers until it
is time for the insertion of plant material.
Sealed growing chamber3, previously filled with media and
plant material, are housed in a plant culture room where the
plant material has been permitted to grow in another length of
membrane material. The length of plant-filled growing chambers
is periodically passed through a growth detection scanner unit to
scan the plant material to determine the extent of plant growth.
Upon the plant material having reached sufficient growth, the
length of plant-filled growing chambers is transported from the
culture room to a surface sterilization unit for surface
sterilizing the exterior of the growing chambers. A cutting unit
opens the plant-filled growing chambers in preparation for the
removal of the plant material. The plant material is removed
from th~ plant-filled growing chamber~ by the injection of
sterilized water into the closed end of the growing chamber to
wash the plant material out of the opposite open end of the
growing chamber which had been opened by the cutting unit. A
rotating tissue containment device receives the plant material
for transporting the plant material to the plant cutting unit.
The plant material is extracted from the tissue containment unit
and pushed against a reciprocating blade which cuts the plant
material into individual pieces. A planting unit inserts
individual pieces o the cut plant material into the media-filled
open growing chambers previously stored in the cooling and
storage unit. After the media-filled open growing chambers have
been planted with a piece of plant material, the open end of the
growing chambers is closed by heat sealing. The newly
20()''~3~S
plant-filled growing chambers are then transported back to the
culture room for new growth.
A tractor feed apparatus transport~ the lengths of growing
chambers throughout the automated system. A control system
synchronizes and controls the timing, se~uence and operation of
each of the units and tractor feed apparatu3. Bar coding units
uniquely identify each growing chamber to track each growing
chamber as it progresses through the operations of the automated
system.
Brief Description of the Drawings
For a detailed description of a preferred embodiment of the
invention, reference will now be made to the accompanying draw-
ings, wherein:
Figure 1 is a schematic of the automated system of the
present invention for automating the micropropagation and tissue
culturing of organic material;
Figure 2 is a perspective view of a roll of a continuous
length of cellules for the automated system of Figure l;
Figure 3 is a fragmented view of a portion of the continuous
length of cellule~ of Figure 2;
Figur- g is a cross-section of the continuous length through
a cellule at plane 4-4 in Figure 3;
Figure 5 is a plan view of the mechanism to manufacture the
continuous length of cellules from a film;
Figure 6 is a partial plan view of a portion of the tractor
feed apparatus for moving the continuou~ length of cellules
throughout the automated system of Figure l;
Figure 6A is a perspective view of a portion of the tractor
feed apparatus of Figure 6;
Figure 7 is a cross sec~ion of the tractor feed apparatus at
plane 7-7 of Figure 6;
Figure 8 i~ a schematic of the media preparation and media
fill units shown in Figure l;
~0~)43~
Figure 9 is an elevation view of the media fill apparatus of
Figure 8;
Figure 10 is a perspective view of a portion of the media
fill apparatus of Figure 9;
Figure ll is a top view of the fill check scanner of Figure
l; .
Figure 12 is an elevation view of the sterilization unit of
the automated system of Figure l;
Figure 13 is a sectional view of the sterilization unit
taken at plane 13-13 in Figure 12;
Figure 14 is a sectional view of the sterilization unit
taken at plane 14-14 in Figure 12;
Figure 15 is an enlarged view of the tractor feed apparatus
disposed within the sterilization unit shown in Figure 14;
Figure 16 is a perspective view, partly in section, of the
cooling and storage unit of the automated system of Figure l;
Figure 16A is a section view of the cooling and storage unit
taken at plane 16A-16A in Figure 16;
Figuro 17 i9 a sectional elevation view, partly
diagrammatical, of the surface sterilization unit of the
automated system of Figure l;
Figure 18 is a sectional view of the tractor feed apparatus
disposed within the surface sterilization unit taken at plane
18-18 of Figure 17;
Figure 18A is a sectional view of another portion of the
surface sterilization unit taken at plane 18A-18A of Figure 17;
Figure 19 is a perspective view of a portion of the tissue
manipulation unit of the automated system of Figure l;
Figure 20 is a front view of the tis~ue manipulation unit of
Figure l9;
Figure 20A is a front sectional view of the tissue manipula
tion unit o Figure 20 with the extraction mem~er in the staged
position;
_g_
~0~3~5
Figure 20B is a side sectional view of the tissue manipula~
tion unit of Eigure 20 with the extraction member in the extended
position;
Figure 21 iq a side sec:tional view of the tissue
manipulation unit of Figure 19;
Figure 21A i8 a side sectional view of the tissue manipula-
tion unit of Figure 21 with the cuttinq blade and stuffing
mechanism in the staged position;
Figure 21B is a side sectional view of the tissue manipula-
tion unit of Figure 21 with the cutting blade and stuffing
mechanism in the extended position;
Figure 22 is a perspective view, partially in section, of
the culture room of the automated system of Figure l; and
Figure 23 is a block diagram of a control system for the
automated system.
DESCRIPTION OF THE PREFERRED EMBODIMENT
System 10 Overview
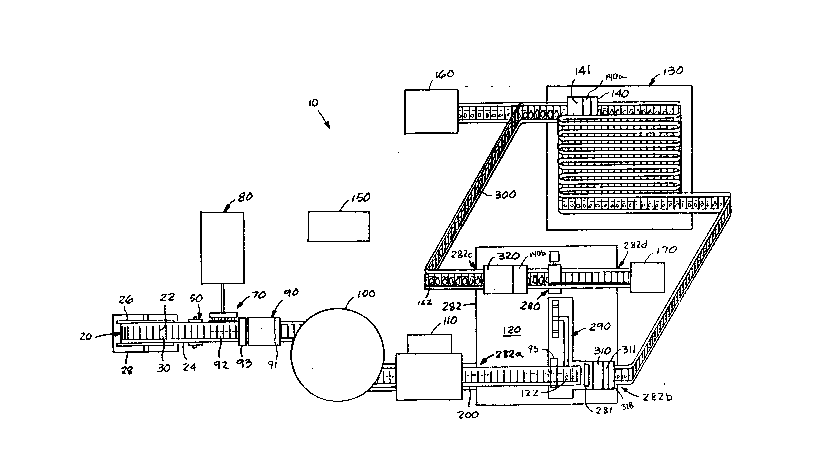
Referring initially to Figure 1, there i~ shown a schematic
illustration of the automated apparatuq 10 for performing micro-
propagation and tissue culturing of plant tissue. The apparatus
of the pre~ent invention i3 employed to automatically perform
micropropagation and tissue culturing procedures through the use
of the integument, a new pliable growing container described in
co-pending applications Serial No. 207,405 filed June 14, 1988
and Serial No. 021,408 filed March 4, 1987, both incorporated
herein by reference.
In general, an integument is a growing or culture container
formed from a translucent membrane that i8 liquid and contaminant
impermeable, but which allows necessary ga exchange and light
~ransmission between the living tissue being cultured and the
ambient environment. The membrane is formed into an envelope or
cellule for containing the tissue and growth medium. Once the
tissue and growth medium are placed in the cellule of the
-10-
20~ 3~
integument, the cellule is sealed and thus closed to the ambient
environment. As described in co-pending applications Serial No.
207,405 filed June 14, 1988 and Serial No. 021,408 filed March 4,
1987, an integument pack includes a number of individual cellules
which are pliant and collapsible such that they may be rolled.
For use with the apparatus and method of the present invention,
it is preferred that an integument roll 20, described in more
detail below, be employed. Integument roll 20 comprises a
plurality of integuments 22 attached at adjacent edges forming a
continuous ribbon-like sheet or length 24 of integument~ 22
loosely rolled onto a spool 26.
Referring again to Figure l, the integument roll 20 is
housed in an integument storage unit 28. In operation, cellules
30 of integuments 22 from integument roll 20 are transported as a
continuous length 24 by a tractor feed mechanism 50 throughout
the automated system 10. After leaving storage unit 28, the
cellules 30 move, first to the media fill apparatus 70. A media
preparation unit 80 automatically mixes the ingredients and
proportion~ thereof needed to form the growth medium used for
particular plants in the various stage~ o~ micropropagation.
Once the cHllules 30 are appropriately positioned within the
media fill apparatus 70, media fill apparatuq 70 injects media 92
from media preparation unit 80 into the individual cellules 30 of
the continuous length 24 of integuments 22 as it is unrolled from
the integument roll 20. Once filled witn the measured quantity
of the growth medium 92, a bar code indicating the type media is
placed on the outside surface of cellules 30 by bar coding means
93. Cellules 30 are then transported to a fill-check scanner 90
to insure the appropriate amount of growth medium 92 has been
inserted into thG cellulGs 3C. The cellule5 30 with growth
medium 92 are then transported to the sterilization unit 100
where they are heated under pressure to kill any microorganisms
in or on the cellules 30 or the prepared media 92. From the
!
i
~0~)4~5
sterilization unit 100, the sterilized cellules are transported
to the cooling and storage unit llO. The sterilized cellules
with media are stored in the cooling and storage unit 110 until
plant tissue growing in other cel].ules, a~ hereinafter described,
are ready for transplanting into the sterilized cellules stored
in unit 110. For transplanting, the cellules are transported
from unit 110 on to the tissue manipulation unit 120.
All the required tissue manipulation~ of the micropropaga-
tion process are carried out within the tissue manipulation unit
120. The sterilized cellules and growth media are therein
invested with tissue sample~ 122 and then closed by heat sealing
in sealing unit 310 to prevent contamination. The invested
cellules are then coded by bar coding means 311 with a bar code
indicating the type of plant and the date the culture was
established.
The coded cellules 30 with plant tissue are then transported
to and through the culture room 130 where they ara exposed to a
growing environment conducive to the particular variety of plant
being grown and th~ ~tage of micropropagation. After the culture
ha~ been in the culture room 130 for the appropriate time period
and grown to the desired stage of development, the cellules 30
containing the culture~ are transported through a growth
detection scanner 140, which detects the growth of the plant
material or tissue, and through a bar code reader 141. If the
culture is ready for the next stage of micropropagation, the
cellules containing the cultures are transported back into the
tissue manipulation unit 120 where the cellules are surface
sterilized and washed in surface sterilization unit 320 and
opened in cellule cutting unit 280. The tissue samples 122 are
then removed- and cut into smaller ~issue samples for
transplanting or investment in new sterile cellules with growth
media from cooling and storage unit llO in tissue planting unit
290. The cellules with new tissue samples are then sealed in
sealing unit 310 and transported back into the culture room 130.
~o~
Once the appropriate number of tissue multiplications have been
performed and the desired number of plantlets have been produced,
the cellules containing the plantlets are transported from the
culture room 130 to the packaging system 160 where the still
sealed cellules are bo~ed for shipp:ing.
The entire process is controlled and monitored by control
system 150.
Integument Roll 20
Referring now to Figure 2, there is shown an integument roll
20 generally comprising a continuou ribbon-like sheet or length
24 of individual cellules 30 wrapped loosely around a spool 26.
One portion of the continuous length 24 of cellules 30 is depict-
ed in Figure 3 and, as depicted, comprise individual cellules
30a, 30b, and 30c. As best shown in Figure 4, an individual
cellule 30 is formed by a front membrane 32 and back membrane 34
which are attached at their lower extremities by a wide
heat-sealed lower band 36. It is preferred that lower band 36 be
approximately one-half inch wide. While a narrower heat seal
will suffice to prevent contamination, the wider heat-sealed band
add~ an extra measure of protection against the introduction of
microorganisms and allows for lower tractor perforations 42 in
lower band 36 which, as described below, are used in conjunction
with the tractor feed apparatus 50 shown in Figure~ 6 and 7.
Referring still to Figures 3 and 4, front membrane 32 and
back membrane 34 are also heat sealed along lines perpendicular
to band 36 as shown at 38a, 38b, 38c, and 38d, thereby forming
individual cellules 30a, 30b, and 30c. Preferably, heat seals 38
do not extend the entire width of membranes 32 and 34, but
instead stop approximately one-half inch short of the upper edges
of membranes ~ and 34,` thereby`leaving upper front and back
bands 46 and 48 respectively, unattache~. In this configuration,
cellule 30 is defined by heat seals 38 and lower heat seal band
-13-
3~
36 lea~ing initially an open end 52 which serves as an entry port
into cellule 30 for receiving plant tissue and growth media.
Lower tractor perforations or apertures 42 are formed in lower
heat seal band 36 and upper tractor perforations 44, such as 44a
and 44b, are formed in upper bands 46 and 48 at uniform distances
along the entire continuous length 24 of integument roll 20.
Referring now to Figure 5, there is shown a manuacturing
process for the integument roll 20. Although it is anticipated
that the integuments 22 will be manufactured separately from the
micropropagation process, the manufacturing process may be a part
of the automated system 10. The integument roll 20 is man-
ufactured by the melt blowing of a polyolefin film such as
polyethylene. In manufacture, the film is blown into a large
bubble which is drawn upward to obtain the decired film thickness
and is then cooled. The blown film is then drawn between rollers
where a continuous double layer of film 54 is drawn from the film
making machine. In-line aperation~ can then be made on the
double layer of film 54. For example, two integument rolls 20a
and 20b ca~ be manufactured from the double layer of film 54;
As the double lay~r of film 54 is drawn from the film making
machine or roll 56, it may be drawn over a heat sealing roller 58
as shown in Figure 5. Heat sealing roller 58 includes raised
portions 60 and 62 used to simultaneously form heat seal band 36
and heat seals 38 respectively on two continuou lengths 24a and
24b which, at this point, are joined at their upper edges 64.
After passing over heat sealing roller 5&, the double layer of
film 54 may pass over perforation rollers 6~ which include
projections 68 formed about their circumference. Projections 68
engage recesse~ formed in a mating rollers (not shown~ which are
positioned above perfor~cior- r~ r~ 6~ the double layer of
film 54 is passed between these rollers, bands 36, 46 and 48,
best shown in Figure 4, are all perforated. The double layer of
film 54 is then cut into two separate continuous lengths 24a and
~14-
3~
24b as the film 54 is passed through a stationary knife blade 72.
The two lengths 24a and 24b are then wound on spools 26a and 26b.
The polymeric material for cellules 30 is critical to
providing the necessary environment for housing plant and animal
life. In particular, it is important to achieve optimum gas
exchange and light transmission to permit the necessary biochemi-
cal activity conducive to life. The material must readily pass
oxygen and carbon dioxide between the ambient atmosphere and tha
cellule 30 for use by the contained plant or animal life in their
metabolic processes to preserve the organic material and the like
in a living condition. Thus, the cellule 30 is made of a
semi-permeable and translucent material which permits gas trans-
fer therethrough. The ~referred material for cellule 30 is a
polyethylene film rom 1.0 to 2.0 mils. thick. It is preferred
that the material have thicknesa of 1.25 mils. If the membrane
material is thinner than 1.0 mil, handling the cellule 30, and
especially opening cellule 30, i~ made mor~ difficult because the
opposing sides 32, 34 of the material of cellule 30 tends to
adhere to each other when formed in such thin films les~ than 1.0
mil. Although a translucent low density polyethylene i8 suitable
and even allows greater gas permeability, a high density poly-
ethylene is preferred. The high density polyethylene can with-
stand greater extremes in temperature, such as is encountered in
an autoclave, where a low density polyethylene may tend to melt,
distend, or distort. Other polymeric materials may be used where
the gas and water vapor transmission rates are comparable to that
of the present invention.
The gas transmission rates of the material for cellule 30 is
of _he utmost importance. Eor practicing the invention described
herein, it is preferred that the membrane materiai have a per-
meability to CO2 of from 200 to 1190 cc/100 sq. in/24 hours at 1
atm. and a permeability to 2 of from 100 to 400 cc/100 sq. in/24
hours at 1 atm. Another important factor may be the moisture
-15-
2(:)(1143 ~
vapor transmission rate which is preferred from 0.2 to 0.684
gm/100 sq. in/24 hours at 1 atm. The preferred high density
polyethylene film exhibiting the above characteristics is high
density polyethylene material no. HiD-9650 manufactured by
Chevron Chemical Company of Orange, Texas. Upon sealing the
cellule 30, the organic material i~ completely enveloped and
enclosed from the ambient atmosphere and environment so as to
prevent any introduction of contaminants and permit the necessary
gas exchange between the organic material therein and the atmo-
sphere of the ambient environment. The material of cellule 30 is
also translucent to enable the organic material to receive the
necessary light for life and growth.
The published specifications for high density polyethylene
HiD-9650 are melt index of 0.3 (gms/10 min); density 0.950
(gms/cc); dart impact of 90 (gms/mil at 26 inches); tensile
strength at break of 7400 (psi); elongation of 4 and 60%;
Elmendorf tear md/td o 16/400 (gm~/mil); and a moisture vapor
transmission rate o~ 0.35 (gms/100 sq. in. 24 hr./mil).
Tractor Fe~d AD~aratus
The tractor feed apparatus 50 operates to transport the
continuouq length 24 of cellules 30 throughout and between each
of the apparatus which comprise~ the automated system 10.
Tractor feed mechanism 50 comprise~ a plurality o~ individual
tractor feed belts, belt guide channels, support~, rollers and
drive motors as described in more detail below. The description
of one segment of the tractor feed apparatu~ will typify the
remaining segments of the mechanism.
Referring now to Figures 6 and 7, there is shown one portion
of the tractor ieed apparatuq 50. ~epicted in Figure 6 is a
partial plan view of that portion of the tractor feed apparatus
50 which serves to draw the continuous length 24 of cellules 30
from roll 20 mounted in the integument storage unit 28 and to
200~3,45
transport the cellules 30 of integument roll 20 to the media fill
apparatus 70 shown ln Figure 1. As shown in Figures 6 and 7, the
tractor feed apparatus 50 generally comprises driver and receiver
support plates 74, 76, studded drive belts 78, receiving belts
82, driver and receiver belt guide channels 84, 86, drive and
receiver rollers 88, ag and tensioning rollers 94 respectively.
Belt guide channels 84, 86 are attached to support plates 74, 76
which are themselves anchored to a supporting base, not shown,
attached or resting on the floor or suspended from the ceiling or
other suitable support structure. It is preferred that guide
channels 84, 86 be bolted to support plates 74, 76. In this
manner, the distance between upper guide channelq 84a, 86a and
lower guide channels 84b, 86b can readily be changed in the event
that an integument roll 20 having a different dimension is later
used. Studded drive belts 78 and receiving beltq 82 are received
by and travel within the recesse~ of driver and receiver belt
guide channels 84, 86 respectively. It i9 preferred that driver
and receiver beltq be made of Teflon. Motion i~ imparted to the
belts 78, 82 by drive and receiver rollerq 88, 89, shown in
Figure 6, locatad at the ends of driver and receiver guide
channel 84, 86. As shown in Figure 7, drive and receiver rollers
88, 89 extend through slots 75 formed in support plate3 74, 76.
Referring to Figure 6A, driver and receiver rollers 88, 89
include teeth 98 which mesh with indentations 102 on the inner
surface 104 of drive and receiver belts 78, 82. The engagement
of teeth 98 with indentations 102 prevents slippage between
rollers 88, 8g and belts 78, 82. Upper and lower drive rollers
88a, 88b are mounted on a driver shaft 106 and upper and lower
receiver rollerY 89a, 89b are mounted on a receive: shaft 108.
Shafts 106, 108 are rotatably supported by journai bearings 118
and are driven by a common motor 112 through gears 107a, 107b.
It should be appreciated that it may only be necessary to drive
X~)V4~3~5
one of the shafts 106, 108 using one gear 107 driven by motor
112. Journal bearings 118 are mounted on belt guide channels 84,
86.
As rollers 88, 89 are rotated clockwise and counter-
clockwise respectively, as viewed in Figure 6, projections 114 on
studded drive belts 78 mate with indentation~ 116 formed in
receiving belts 82. The projections 114 and indentations 116 are
positioned along the belts 78 and 82 so as to coincide with the
dimensions between adjacent upper and lower tractor perforations
44, 42 formed in continuous length 24 as shown in Figure 3.
Thus, the lower band 36 and the upper bands 46, 48 of length 24
are captured and attached between driver and receiver belts 78,
82. The guide channels 84, 86 extend between each apparatus in
the automated system 10 as shown in Figure 1 and t.-ereby
transport the length 24 throughout the ystem 10. Tensioning
rollers 94 are positioned along guide channel~ 84, 86 to tension
and guide the belts 7a, 82. Although not shown, additional drive
and receiver rollers 88, 89 are strategically positioned between
apparatu~ located throughout the automated system 10 along guide
channels 84, 86 to propel the length 24 of cellules to each of
the apparatus in the system 10. It should be appreciated that
other mean-~ may be adapted for attaching the bands to a moving
track for transporting the length 24 throughout the automated
system 10.
As shown in Figure 7, continuous length 24 i transported by
its upper and lower edges by a total of four belts: upper drive
belt 78a; upper receiving belt 82a; lower drive belt 78b; and
lower receiving belt 82b. To ensure that the tractor feed
apparatus 50 functions propeLly and that continuous length 24 of
cellules 30 is not damaged from engagement with bel~s mû-v-i.,g a~ a
number of different velocities, it is important that drive
rollers 88a, 88b and receiver rollers 892, 89b be driven at the
identical velocity. As is evident, as rollers 88, 89 are rotated
clockwise and counterclockwise respectively as shown in Fisure 6,
-18-
20V~3~
the cellules 30 of continuous length 24 are transported along
with the moving belts 78, 82 at a uniform velocity. It should be
understood however that other continuous lengths 24 may be
transported at a different uniform velocity depending upon its
location in system 10.
Media Pre~aration Unit 80
As shown schematically in Figure 8, media preparation unit
80 comprises mix tank 124, stirrer 126, stirrer motor 128, heater
132, stock solution refrigeration unit 134, stock solution
containers 136 and metering pumps 138. A plurality of stock
solution containers 136 are refrigerated within refrigeration
unit 134 and maintained at a temperature of approximately two
degrees centigrade. The stock solution containers 136 each
contain a separate ingredient or nutrient used in the preparation
of the various media used in the micropropagation process. Each
medium used in the process is mixed in a batch mode within the
mix tanX 124, which is preferably made of stainless steel. When
a level switch 142 within mix tank 124 ~ignals controller 150
that another batch of media i8 required, controller 150 will
signal appropriate metering pumps 138, which are in fluid commu-
nication with fill lines 144, to inject a programmed amount of
stocX solution through individual fill lines 144 extending into
mix tank 124. It is preferred that metering pump 138 be a
parastolic pump such as Model No. 2P304 manufactured by the
Mec-0-Matic Co. Such pumps are reliable and extremely accurate.
Controller 150 also actuates solenoid valve 133 in sterile water
line 143 allowing the appropriate amount of sterile water to flow
into mix tank 124.
Once the appropriate stock solutions and sterile water have
been injec'.eu i,-..o t1le mix tank 124, the ingredients are heated
by heater 132 while the solution is stirred by stirrer 126.
Stirrer 126 include~ an impeller 146 mounted on the end of a
shaft 148 which is connected to and rotated by stirrer motor 128.
-19-
201)~3~
Both heater 132 and stirrer motor 128 are actuated by controller
150. The media is stirred and heated to a temperature of approx-
imately 100C in order to melt the agar or other gelling agent
which is used in the particular growth medium being prepared.
Optionally, the media may be supplemented with nutrients and
plant growth regulators. Once the growth medium iq prepared,
heater 132 and stirrer 124 are turned off and the growth medium
is then ready for injection into cellules 30.
Unused media and liquids used to clean and rinse mix tank
124 may be drained from mix tank 124 through drain 141 and pumped
to a disposal tank (not shown).
Media Fill Station 70
~ eferring now to Figures 8, 9 and 10, the media fill station
70 comprises fill lines 152, parastolic fill pumps 154, injection
nozzles 156, nozzle transport rack 158 and filling guide 162. In
general, measured amounts of growth medium from media preparation
unit 80 are simultaneously injected into a plurality of cellules
30 of continuous length 24 by media fill apparatus 70 as shown in
Figure 9.
Fiv~ f~ll line~ 152 are in fl~id communication with mix tank
124 as shown in Figure 8 and are comprised of a flexible plastic
tubing having an internal diameter of approximately 5/8 inches.
Injection nozzles 156 are connected to the ends of fill lines 152
and are positioned above filling guide 162 on nozzle transport
rack 158. Nozæles 156 are tapered and sized to be inserted
between upper bands 46, 48 of cellules 30 for entry through entry
port 52 into the chamber of cellule 30. Filling guide 162 is
supported by support arms 164. As can be seen in Figure 9, the
leading and trailing edges of filling guide 162 are formed with
wedge-shaped ends 166 to separate bands 4b, 48 or cellules 30.
In response to a signal from controller 150 after the unfilled
cellules 30 have been positioned below filling guide 162,
pneumatic cylinders 172 actuate and lower pistons 174, thereby
-20-
~00'~3~ ~
lowering nozzleq 156 into position for filling cellules 30 with
growth medium 92. An individual parastolic fill pump 154 is
dedicated to each fill line 152 and, like metering pump 138
described above, may be Mec-O-Matic Model No. 2P304. Upon
receipt of a signal from controller 150, fill pump 154 will pump
a predetermined measure of mixed growth medium 92 from mix tank
124 through fill lines 152, injection nozzle 156 and into
cellules 30.
In operation, the continuous length 24 of cellules 30 is
drawn by tractor feed apparatus 50 to a media fill station 70
where the upper bands 46, 48 of cellules 30 are separated by
filling guide 162 as continuous length 24 is drawn by tractor
feed apparatus 50 beneath nozzle transport rack 158. Controller
150 actuates drive motors 112 positioned along the tractor feed
apparatus 50, as previously described and shown in Figures 6 and
7, in timed intervals ~uch that five cellules 30 are positioned
and remain stationary underneath filling guido 162 for approxi-
mately three seconds while growth medium 92 is injected into the
cellules 30. Once in position, controller 150 signals the pair
of pneumatic cylinder~ 172 to lower nozzle transport rack 158 and
injection nozzles 156. In this manner, nozzles 156 are lowered
into the filling guide 162. Controller 150 then signals the
parastolic fill pumps 154 to inject the appropriate measure of
media 92 into each of the five cellules 30. Controller 150 then
actuates the pneumatic cylinders 172 to raise injection nozzles
156 back into position shown in Eigure 9 above filling guide 1~2
and then signals drive motors 112 operating tractor feed appara-
tus 50 to transport five new unfilled cellules 30 into position
underneath rack 158 for filling with growth media.
After a cellule 30 has been filled with media and passed
through the media fill ~tation, the cellule may be marked with a
bar code by a bar code printing system 93, such as a "~igimark"
variable information laser marker manufactured by Videojet
-21-
2~1~43~5
Systems International, Inc. of Elk Grove Village, Illinois. The
cellules may include a solid ink mark, portions of which are
vaporized by the laser printer of the bar code printing system 93
to indicate the type of media in the cellule. Other indicia may
also be coded on the cellule.
Fill Chec~ Scanner 90
Referring now to Figure 11, fill checX scanner 90 is used to
determine whether the cellules 30 have been injected with the
appropriate measure of growth medium 92. Fill check scanner 90
generally comprises enclosure 176, light source 178, polarized
panel 180 and photo receptor panel 182. As shown in Figure 11,
tractor feed apparatus 50, upon input from controller 150, draws
continuous length 24 through the interior of enclosure 176 so as
to transport the media-filled cellules 30 into the enclosure 176
for scanning. As described above with respect to the media
filling station 70, the cooperation of controller 150, with the
drive motors 112 of the tractor feed apparatu~ 50 (Figures 6 and
7), will transport cellules 30 for scanning in groups of five.
Positioned on one side of enclosure 176 i~ a light source 178
which may be, for example, quartz-halogen. Polarized panel 180
is affixed within enclosure 176 as shown in Figure 11 and divides
the interior of the enclosure into two compartments. Polarized
panel 180 is selected so that light waves passing in a direction
perpendicular to the panel 180 will be passed through the
polarized panel 180; however, light rays traveling in other
directions will not pass through polarized panel 180.
Light waves which pass through polarized panel 180 will
continue through the cellules 30 of continuous length 24 and will
contact photoreceptor panel 182 on the opposite side of the
enclosure 176. ehotoreceptor panel 182 comprise a surface con-
taining hundreds of photosensitive cell~ (not shown). Light
waves will pass through the membranes 32 and 34 of cellules 30
and activate the photosensitive cells on photoreceptor panel 182.
-22-
2004~2~
Light waves penetrating the areas of the cellules 30 filled with
growth media 92 will be defracted to a greater degree than those
which pass through the portion of cellule 30 containing no media.
Accordingly, the light intensity sensed by the portion of photo-
receptor panel 182 which is directly behind the media-filled
portions of the cellules 30 will be less than the intensity
sensed by remaining portions of panel 182. The photosensitive
cells on photoreceptor panel 182 are electrically connected to
the controller 150 by a plurality of signal wire~ 184. In this
manner, it can be determined which cellule~ 30 have been filled
and whether they have been filled with the appropriate volume of
growth medium 92.
An alternative embodiment of fill check scanner 90 is the
"Smarteye" photoelectric sensor manufactured by the Tri-Tronics
Company, Inc. of Tampa, Florida. The "Smarteye" photoelectric
sensor can sense size, texture, distance, opacity, depth and
color so as to havo the capability of determining whether an
appropriate measure of growth media 92 has been injected into a
particular cellule.
Upon fill check scanner 90 identifying a cellule which has
an inadequato amount of media, the inadequate cellule is marked
by an ink jet printer such as the "Excel" small character ink jet
printer 91 manufactured by Videojet Systems International, Inc.
of Elk Grove Village, Illinois. A print registration scanner 95,
such as the "Smarteye" color mark registration scanner
manufactured by Tri-Tronics Company, Inc. of Tampa, Florida, will
subsequently identify the inadequate cellule by scanning for the
ink mark prior to the insertion of plant material in tissue
planting unit 29J. The print registration scanner 95 will send a
signal to controller 150 which will in turn cause the tracto~
feed apparatus 50 to pass the inadequate cellule through the
tissue planting unit without inserting any plant tissue.
~0()43~5
Sterilization Unit 100
Referring now to Figures 12 and 13, there is shown sterili-
zation unit 100 generally comprising an autoclave 186 used to
sterilize the media-filled celluLes 30 before the cellules 30 are
invested with tissue. The autoclave 186 comprises a generally
cylindrical enclosure 188 mounted on a support structure 190.
The enclosure 188 comprises a pressure chamber 192 and a closure
194 coaxially aligned and attached by four pneumatic cylinders
196 used to open and close the closure 194. The pressure chamber
192 has attached to it~ interior entrance an inner lip 198 which
extend~ around the entire periphery of the interior entrance of
the pressure chamber 192 and cerves to guide the closure 194
during the closing of the autoclave 186 by pneumatic cylinders
196. Lip 198 also serves to protect an O-ring seal (not shown)
from the gases and extreme heat generated during the steriliza-
tion procedure. Pressure chamber 192, closure 194 and inner lip
198 are all manufactured from stainless steel, have an inner
jacket of monel and have a total thickness of less than 1/2 inch.
In operation, the leading end of continuous length 24 is
passed through a cutter 202 mounted on the side of autoclave 186
and in cooperation with the tractor feed apparatus 50. The
cutter 202 acts as a guide for the continuous length 24 of
cellules 30 as the cellules 30 are disposed within the au~oclave
186. Cutter 202 also includes a blade 204 which is activa~ed by
controller 150 after the autoclave 186 has been filled with a
strip 200 of cellule~ 30, strip 200 havin~ a length of as much as
several hundred feet and comprising many thousands of cellules
30. Referring to Figures 13, 14, and 15, integument strip 200 is
automaticall~ loaded into the auto~lave 186 for stexilization by
internal loader/unloader apparatus 210 which operates identically
to the tractor feed apparatus 50 previously described. As
described in greater detail below, the internal loader/unloader
-24-
20~ 3f-5
apparatu3 210 supports and transports the integument strip 200 by
use of a series of drive belts which cooperatively engage upper
apertures 44, 42 formed in the upper bands 46, 48 and lower band
36 of integument strip 200. The drive belts are supported in a
multi-level serpentine configuration within the autoclave 186 so
as to achieve the greatest density of cellules 30 as possible.
There is shown in Figure 13 a section view of the autoclave
186 which schematically illustrates the path of integument strip
200 as it is loaded in serpentine fashion into the autoclave 186.
Figure~ 14 and 15 depict how the integument strip 200 is
supported and transported within autoclave 186. Referring now to
Figures 14 and 15, perforated support plates 214 are rigidly
attached to the upper interior surface of pressure chamber 192.
Support plates 214 are perforated so aa to enable steam to
penetrate throughout enclosure 188. Attached to the perforated
platec 214 are belt guide channels 216. Retained within belt
guides channels 216 are the drive belts including studded drive
belt 218 and receiving belt 220. A3 de~cribed previou~ly with
respect to the tractor feed apparatus 50, the projections 222 on
studded dri~e belt 218 and the indentations 224 on receiving belt
220 are ~paced apart on belts 218 and 220 at a distance equal to
the the distance between adjacent apertures 42, 44 in the upper
bands 46, 48 and lower band 36 on integument strip 200. Still
referring to Figures 14 and 15, it should be understood that a
total of four belts are employed in the internal loader/unloader
apparatus 210: upper studded drive belt 218a; lower studded drive
belt 218b; upper receiving belt 220a; and lower receiving belt
220b. ~elts 218a, 218b, 220a and 220b serpentine through pres-
sure chamber 192, changing levels within the chamber 192 as
dlctated by ~h~ b~l~ yuidc ~h~n~ 2i~ which are inclined as the
path nears an end of pressure chamber 192.
-25-
~VO143~
In operation, tractor feed apparatus 50 transport.q the
leading end of integument strip 200 into and through the guide of
cutter 202 attached near the entrance of pressure chamber 192 of
autoclave 186. An external drive motor 226 has a sealed drive
shaft 228a extending into pressure chamber 192 and serves to
actuate rollers 232, 233 by meanY of gears 230a, 230b and
receiver ~haft 228b which are supported within pressure chamber
192 and form a portion of the internal loader~ unloader apparatus
210 for driving the belts 218, 220. The roller~ 232 and 233, in
turn, actuate and rotate drive belts 218 and 220 as previously
shown and described with refer~nce to the tractor feed apparatus
50. The external drive motor 226 will turn rollers 232, 233 and
thus transport belts 218, 220 at the same speed that tractor feed
apparatus 50 tran~port~ integument strip 200 into the guide of
cutter 202. Integument strip 200 will thus be loaded in serpen-
tine fashion into the autoclave 186. When the autoclave 186 is
loaded with integuments 22, the cutter knife 204 is actuated by
controller 150 to cut t~e strip 200 from the continuous length
24. After the traillng edge of integument strip 200 is loaded,
controller 150 will stop the external drive motor 226. It will
then actuate the pneumatic cylinders 196 to close the clo~ure 194
of autoclave 186 and initiate the ~terilization process. The
sterilization proces~ is accompliQhed through con~entional means
such as a steam generator 234. Water inlet valve~ 236 and drain
valves 238 are also provided as shown in Figure 14. Upon com-
pletion of the sterilization process, the external drive motor
226 is again actuated to unload the sterilized integument strip
200 from the autoclave 186 while simultaneously loading a new
unst~rilized integument -~trip as ju t described.
Becau~e the sterilization unit 100 is a batch operation, the
preceding operations at the media fill station 70 and fill check
scanner 90 mus~ be halted until the sterilization unit 100 is
emptied to receive a new batch of cellules 30. Means can be
-26-
~01~3~
provided to permit a continuous operation such as by rolling the
length 24 of cellules 30 passing from fill check scanner 90 onto
a spool or supporting the length 24 on an elongated tractor feed
track until the sterilization unit 100 is ready to accept a new
batch of cellules 30. A cutter, such as cutter 202, would be
used to cut a length of cellules 30 for later insertion into
sterilization unit 100. Such means would permit the continuous
filling of cellules 30 with media 92.
An alternative to the sterilization unit 100 includes the
use of a preYterilized length 24 of cellules 30 and filter
sterilized media 92. Using presterilized cellules and filter
sterilized media eliminates the need for a sterlilization unit
100 in the automated system 10. The elimination of the
sterilization unit 100 permits a continuous operation from the
fill scanner unit 90 to the cooling and storage unit 110. A
presterilized length 24 of cellules 30 may be produced since the
membrane for the cellules is aseptic at the time of manufacture.
The integuments 22 would then be produced a~ previously described
under aseptic conditionR. The filter sterilized media would be
prepared and sterilized by an inline filtration process.
Cooling and Storage Unit 110
Referring now to Figures 16 and 16A, there is depicted the
cooling and storage unit 110 which generally comprises cooling
chamber 240, an air filter assembly 242, and cooling system 244.
Air filter as~embly 242 includes a filter housing 246, a
prefilter 248, a blower motor 250 driving to a squirrel caqe
blower assembly 252, a flume 254 and a hepafilter 256. Air
filter assembly 242 is attached to and ~upported by the upper
surface 258 of the cooling chamber ?40. Filter housing 246 in-
cludes an air intake aperture 262 which is covered by prefilter
248 and attached ko the housing 246~ Prefilter 248 filters dust
and other large airborne particles and prevents them from being
drawn into the air filter assembly 242. Mounted within air
-27-
ZO~)4;3~
filter housing 246 is the squirrel cage blower assembly 252 which
is driven by a blower motor 250 mounted externally to the cooling
chamber 240. Blower assembly 252 draws air from the ambient
atmosphere through the prefilter 248 and injects the air through
1ume 254 into the hepafilter 256 which covers the aperture 264
formed in the upper surface 258 of the cooling chamber 240. The
hepafilter 256 removes 99.97% of all pollutants and airborne
contaminates from the air injected into the cooling chamber 240.
Cooling system 244 includes cooling coils 266 which are
supported near the top of cooling chamber 240 on a perforated
support plate 268 which is affixed to the sidewalls and endwalls
of the chamber 240 so as to be parallel with the upper surface
258 of the chamber 240. Through conventional means, coolant is
circulated through the cooling coils 266 so as to maintain a
constant temperature within the cooling cha~ber 240 of five
degrees centigrade.
The air drawn into the cooling chamber 240 is vented through
the entry port 272 and exit port 274 for integument strip 200. A
pos,itive pressure of 1.1 atmospheres is maintained within the
cooling chamber 240. The continuous flow of filtered air through
entry and exit ports 272 and 274 resulting from the positive air
pressure within cooling chamber 240 prevents contaminants, such
a~ air-borne microorganisms, from entering the cooling chamber
240 and contaminating the previously sterilized media. As shown
in Figure 16, cooling chamber 240 includes a housing extension
270 having a front face 273 in which entry port 272 is formed.
~ousing extension 270 extends from cooling chamber 240 to a
position in close proximity to sterilization unit 100 so as to
minimize the distance travelled by the sterilized cellules 30
before they enter the cooiing and ~t~ g~ un~ -110. After
leaving stèrilization unit 100 and before entering cooling and
storage unit 110, the sterilized cellules 30 are expo~ed to the
unfiltered air of the ambient environment. However, after
-2~-
20()4~5
undergoing the heat sterilization process, the heat radiating
from the sterilized cellules 30 creates air currents which, along
with gases generated by the hot media, combine to drive away
air-borne microorganisms which might otherwise contaminate the
media 92 or the surfaces of cellules 30 before they enter the
sterile environment of cooling and storage unit 110.
The tractor feed apparatus 50, previously described, is sup-
ported within the cooling chamber 240 and extends out~ide the
enclosure through entry and exit ports 272, 274. Because the
extremely high temperatures present in the sterilization unit 100
are not present in the cooling and storage unit 110, tractor feed
apparatus drive motors 112 may be located within the cooling
chamber 240; however, to allow as many cellules 30 as possible to
be contained within the cooling chamber 240, it i~ preferred that
tractor feed drive motor~ 112 be mounted outside cooling chamber
240. As previously described with reference to the sterilization
unit lO0, integument strip 200 i~ supported in serpentine ar-
rangement within coolinq chamber 240 by a series of perforated
support plates 276. In cooling chamber 240, the perforated
support plates 276 are rigidly attached perpendicularly to the
coil support plate 268. These support plates 276 in turn support
the guide belt channels 216 and drive belts 21~ and 220 in a
multi-level serpentine fashion ac described above and illustrated
in Figures 14-15 with regard to the sterilization unit lO0. ~n
operation, the leading edge of integument strip 200 is inserted
into entry port 272 to the cooling chamber 240 and is loaded
therein in serpentine fashion. The sterilized cellules 30 are
stored in cooling chamber 240 until the media 92 and cellules 30
are cooled. Then, as required, the sterile media 92 and cellules
30 of integument strip 200 are drawn into the tis~ue m.ani p~ ,ation
unit 120 described below. The steriliæation unit 100 can steril-
ize one integument strip 200 at a time. However, it is desirable
that cooling and storage unit llO have the capacity to cool and
-29-
2 0l0~ 3 ~3
store a plurality of such integument strips 200 simultaneously
and to house the sterile cellules 3~ until needed.
Tissue Manipulation Unit 120
Referring again to Figure 1, the tissue manipulation unit
120 generally houses a cellule cutting unit 280, a tissue plant-
ing unit 290, a sealing unit 310 and a surface sterilization unit
320. In the tissue manipulation unit 120, 'che sterilized
cellules 30 with growth media 9~ from the cooling and storage
unit llO are invested with a tissue sample 122. The tissue
sample may be meristematic tissue from a stock or parent plant or
more often is tissue from either a stage l initial culture or a
stage 2 multiplication culture grown in cellules of a previous
integument strip 300 transported from the culture room 130. The
tissue planting unit 290 will ordinarily receive plant material
for investing in media-filled cellules from cellules previously
housed in the culture room 130 and opened by cutting unit 280.
However, seeds or meristematic tissue may be manually fed into
tissue planting unit 290 for inserting into the media-filled
cellules. As depicted in schematic form in Figure 1 and for
purposes o~ the description below, it is assumed that the
cellules of sterilized integument strip 200, previously
described, are to be filled with plant tissue that has previously
been grown in culture room 130 in an integument strip 300
comprising a plurality of cellule~ 30 containing growing tissue
122. Integument strip 300 is transported from the culture room
130 into the tissue manipulation unit 120 where the cellules 30
with tissue 122 first undergo surface sterilization in surface
sterilization unit 320. The sterilized cellules 30 with tissue
are then opened by cellule cutting unit 280 and the grc~ing
tissue i22 contained therein is removed and cut into smaller
tissue samples which are then inserted into unused and sterilized
cellule~ 30 of integument strip 200 in the tissue planting unit
290. The newly planted cellules are then sealed by sealing unit
-30-
20()~3~5
310, are coded with a bar code by bar coding means 311 and
transported back to culture room 130.
Referring still to Figure 1, the tissue manipulation unit
120 includes a box-like enclosure 282 having an air filter
assembly like the one described above with regard to the cooling
and storage unit 110. The air filter assembly filters the air
that is used to pres urize the enclosure 282, such pressurization
precluding the entrance o airborne contaminates such as micro-
organisms which could contaminate the cultures 122 during any of
the tissue manipulations which take place within the tissue
manipulation unit 120. A positive pressure of approximately 1.1
atmospheres is maintained in enclosure 282. An entrance port
282a to the tissue manipulation enclosure 282 i~ formed in one
end and is sealingly attached to the exit port of the cooling and
storage unit 110 so that no airborne contaminants can enter the
enclosure 282. In this manner, cellules 30 making up integument
strip 200 transported from the cooling and storage unit 110 pass
directly into the tissue manipulation unit 120 and are continu-
ously exposed to filtered air. Air is exhausted from enclosure
282 through th~ entry and exit ports 282a, b for integument strip
200 and entry and exit ports 282c, d for integument strip 300.
Tractor feed apparatus S0 extends into and through enclosure
282 so as to transport sterile integument strip 200 from the
cooling and storage unit 110 into the tissue manipulation unit
120 and to transport to integument strip 200 to culture room 130
once tissue samples 122 have been placed in the cellules 30 from
tissue-filled integument strip 300 and once the cellules have
been sealed. Tractor feed apparatus 50 is also employed to
transport integument strips 300 conta_ning sealed cellules with
growing tissue therein from tne ~uii~re ro~m 130 to the tissue
manipulation unit 120, and to discharge used integument strips
300 from enclosure 282 to dispo~al unit 170 after tissue samples
122 have been removed from the cellules 30.
-31-
20{)~3~
Surface Sterilization Unit 320
Referring now to Figures 17 and 18, after being transported
from the culture room 130 to the tissue manipulation unit 120,
cellules 30 of integument strip 300 containing living plant
tissue 122 first enter the surface sterilization unit 320.
Surface sterili7ation unit 320 includes an enclosure 284 which is
divided into three compartments 286, 287, 288 that are separated
by flap-like closures 292a, 292b, 292c, 292d. Closures 292 span
the entire cross-section of enclosure 284 and serve to prevent
solution from being sprayed or splashed out of the compartments
286, 287, 288. Enclosure 284 is preferably made of acrylic
plastic, such as plexiglas~, approximately 1/2 inch thick. As
depicted in Figures 17 and 18, tractor feed apparatus 50 trans-
ports cellules 30 in integument strip 300 containing the living
tissue 122 through slit formed in closure 292a and between a pair
of sterilization spray bars 294 which are attached to lower
support plate 296 which serves as part of enclosure 284. Ster-
ilization spray bar~ 294 comprise plastic tubing approximately
1/2 inch in diameter having perforations 298 in the sides. The
lower ends of the spray bar~ 294 are connected to flexible tubing
302 through which a sterilizing solution o~ sodium hypochloride
is pumped by pump 304 from a storage tank 306. As the cellules
30 pass between the spray bars 294, the sterilization solution is
sprayed on the outside surfaceY of the cellules 30 through open
windows formed in support plate~ 74, 76. The sprayed solution
then runs down the sides of the cellules 30 and is collected in
drain basin 308 in fluid communication with holding tank 312 via
drain line 314.
After undergoing the urface sterilization in compartment
286, the celluleq 30 are then drawn thrGug}. slit ,orme& ln
closure 292b and into an identical compartment 287 where they
pass between a second set of spray bars 318 which are connected
to a source 322 of sterilized water. The sterilized water is
-32-
~20043~ ~
sprayed on the cellules 30 by pump 323 through open windows in
support plates 74, 76 to wash away remaining sterilization
solution. The resulting fluid is then collected in a second
drain basin 324 where it is drained via drain 326 to a second
holding tank 328.
Referring now to Figures 17 and 18A, the sterilized and
washed cellules 30 of integument strip 300 then pass through a
slit formed in closure 292c and are drawn into a drying chamber
330 in compartment 288. Filtered air within tissue manipulation
unit 120 is blown down and over the surface of cellules 30 by
squirrel cage blowerJ 331. The air is funneled over the surface
of cellules 30 by unperforated plates 332a, 332b. Solution which
drips off the surface of cellules 30 i collected in basin 324
and drained to holding tank 328 via drain line 315. Once the
cellules 30 have been sterilized and dried, they are transported
through slit formed in closure 292d and into cutting unit 280 as
shown in Figure 1 and 19.
Cutting Unit 280
Referring now to Figure~ 1 and 19, once in the cutting unit
280, the lower edge of integument strip 300 is drawn across
stationary cutting blade 334, best shown in Figure 20A, which
cuts open the bottom of the cellules 30 ju t above lower heat
seal band 36. Cutting blade 334 will be heated to destroy any
contamination which may be deposited on blade 334 due to a
contaminated plant in a cut open cellule. Lower heat seal band
36 and the attached lower portion of the cellules 30 is then
transported out of the manipulation unit enclosure 282 by the
lower drive and receiving belts 218b, 220b of the tractor feed
apparatus 50 shown in Figure 15 for disposal in disposal unit
1/0. The now open cellule~ 30 are next transported into the
tissue planting unit 290.
-33-
XOV43~5
Tissue Planting Unit 290
Referring now to Figures 19, 20A, and 21 tissue planting
unit 290 generally' includes a compartmentalized and rotatable
tissue containment device 336, a water injection means 338 and
pneumatic clamps 340.
Water and/or air injection means 338 is employed to pierce
the closed end of a cellule 30 whose other end has been opened by
cutting unit 280 and inject water such that the water pressure
and the force of gravity causes the tissue sample 122 contained
therein to pass out the bottom of the spen cellule 30 into the
tissue containment device 336. The water injection means 338
comprises a hypodermic-like needle 342 in fluid connection with a
sterile water source 344 and a pumping means 346. Best shown in
Figures 19 and 21, upon receipt of the appropriate signal from
the controller 150, a pneumatic clamp 340 closes and clamps an
open cellule 30 along heat seals 38 so as to maintain the
cellule'~ position directly above the tis~ue containment device
336. Controller 150 next si~nals water injection mean~ 338 such
that needle 342 i~ pneumatically lowered by cylinder 350 so as to
pierca th~ top of the opened cellule 30 as shown in Figures 19
and 20A. The pumping means 346 i~ then actuated and a stream of
sterile water is injected into the top of open cellule 30. As
shown in Figure 21, the injected water and gravity cooperate to
deposit the tissue sample 122 in one of the compartments 352 of
the tissue containment device 336 directly below the bottom of
opened cellule 30. It should be understood that pressurized air
may be used in place of the sterilized water. Once the cellule's
tissue sample 122 has been deposited in the tissue containment
device 336, the used cellules are transported out of the tissue
manipulation system enclosure 282 by thc upper drive and
receiving belts 218a, 220a of the tractor feed apparatus 50 for
disposal in disposal unit 170 as shown in Figure 19.
-34-
'20(~3~
Referring now to Figures 20A and 21, the compartmentalized
and rotatable tissue containment device 336 comprises a rotatable
inner hub 354 mounted on drive shaft 356 which is driven by a
stepping motor (not shown). Rotatable hub 354 includes a
plurality of flat bottom plates 355 disposed around the
circumference of the hub and forming a multi-sided polygon. On
each side of hub 354 are multi-sided polygon shaped end plates
363 which extend from shaft 356 to bottom plates 355. Shaft 356
passes through, but is not attached to, the circular end plates
358 and side plates 360. Identically dimen~ional rectangular
windows 362 are formed in end plates 358 and side plates 360 and
are coaxially aligned with push rod 376 and extraction member 378
as described below. Radiating from and attached to rotatable hub
354 are a plurality of pairs of flat spokes or divider plates
364. Each pair of spokes 364 is attached at it~ inner end to a
bottom plate 355 of hub 354 thereby forming a compartment 352.
The outer end is open to provide the openin~ to compartment 352.
Arcuate rim segment~ 366 connect the outer ends of adjacent
spokes 364, but do not extend over the openings to compartments
352. Bracing members 367 may be used to span the entrances to
compartment~ 352 and provide rigidity to the containment device
336. In this configuration, tissue containment device 336
comprises a compartmentalized, carousel-like, device having a
plurality of compartments 352 defined by outer surfaces or bottom
plates 355 of hub 354, inner surfaces of flat spokes 364, and the
inner surfaces of stationary end plates 358. The number and size
of compartments 352 may be varied by changing the diameter and
width of tissue containment device 336.
The outer surface 355 of hub 354, which forms the bottom of
COm~aFtmentS 352, is provided with perforations 370a to allow the
water and les~ Vi5CUS media 92, which were deposited along with
plant tissue 122 by water injection means 338, to drain from
-35-
ZO~)43~ ~
compartment3 352 and into hub 354. Such fluids may also drain
from compartments 352 by seaping between stationary end plates
358 and the edges of flat spokes 364 adjacent thereto.
Perforations 370b are also formed in side walls 357 of hub 354 to
allow collected liquids to drain therethrough and to seap between
hub 354 and end plates 358. Such liquids may also drain from hub
354 through perforation3 370a of the lower, empty, compartments
352. All such liquids drain into basin 368 where they then drain
to disposal tank 372 shown in Figure 21.
After a cellule 30 is opened and tissue sample 122 is
deposited in a compartment 352 of the tissue containment device
336, the controller 150 actuates the stepping motor to turn
tissue containment devico 336 a preset number of degrees and to
activate the tractor feed apparatus 50 moving the integument
strip 300 forward, so a~ to bring a newly opened cellule 30
directly above the next empty compartment 352 in the tissue
containment device 335. In thi~ step-like manner, a compartment
352 containing a tissue sample 122 ie~ positioned between the
aligned window3 362 in the circular end plate~ 358 and side
plate~ 360. Once 90 positioned, the tissue sample 122 may then
be removed from the compartment 352 and cut into a plurality of
tissue sample~ by the cutting mechanism 371 as hereinafter
described.
Cutting Mechanism 371
Referring now to Figure 20A, the cutting mechanism 371
comprises pneumatic cylinders 374, 384, pushrods 376, 382,
extraction member 378, and cutting blade 380. Extraction member
378 is a rectangular-shaped block of stainless steel having a
cross section identical in shape to the crose~ eection of a
compartment 3~ ot tissue containment d~evice 336 and rectangular
windows 362 in end plates 358 and side plates 360. Member 378 is
attached to pushrod 376 and is actua~ed by pneumatic cylinder
-36-
20~43~5
374. Extraction member 378 reciprocates in a cutting channel 379
having a cross-section which slidingly receives extraction member
378. The cutting channel 379 extends through windows 362 in end
plate 358a and side plate 360a, one of the compartments 352,
windows 362 in end plate 358b and side plate 360b, and exits into
planting conduit 398. Cutting blade 380 i3 sideably disposed
within a blade guide 381 at the exit of cutting channel 379 and
is attached to pushrod 382 which is actuated by pneumatic
cylinder 384.
When tissue sample 122 in compartment 352 i8 to be multi-
plied into a plurality of new samples, controller 150 will
actuate cylinder 374 and pushrod 376 so that extraction member
378 is extended through cutting channel 379 and the compartment
352 in tissue containment device 336 which is then aligned
between windows 362 in end plates and side plates 358, 360. As
pushrod 376 ic further extended as shown in Figure 20B, extrac-
tion member 378 puqhes the tissue sample 122 out of compartment
352 until a portion of the ti~sue sample 122 extends through the
exit of cutting channel 379 beneath cutting blade 380 in blade
guide 381. Pushrod 382 connected to cutting blade 380 is then
actuated by pneumatic cylinder 384 so that cutting blade 380 is
propelled downward and severs a portion of the-tissue sample 122,
the severed portion then resting on working surface 388 within
planting conduit 398. Referring now to Figure 213, with cutting
blade 380 still in its lowered position, stuffing mechanism 390
is actuated by controller 150, stuffing mechanism 390 including
pushrod 392, pneumatic cylinder 394 and stuffing member 396.
Stuffing member 396 reciprocates in a planting conduit 398 having
a cross-section which slidingl~ receives stuffing member 396.
Planting conduit 398 extend~ past the eYit o cutting channel 379
to the open end of cellule~ 30 at fill gate device 400. Pushrod
392, actu~ted by pneumatic cylinder 394, i3 extended so that
stuffing member 396, which is slideably engaged with working
-37-
20043~5
surface 388, pushe~ the severed tissue sample 122 through
planting conduit 398 and into a sterile media-filled cellule 30.
As shown in Figure~ 19 and 21A, a fill gate device 400 such as
that described previously with respect to the media fill station
70 is attached to the end of planting conduit 398 so as to
separate the opposing membrane surfaces 46, 48 of integument
strip 200 and thereby facilitate investment of the cellule 30
with the severed tissue sample 122. As the sterilized integument
strip 200 is transported from the cooling and storage unit 110
into the tissue planting unit 290, integument strip 200 i~
rotated 90 from its previou~ upright position so that the
cellules 30 in integument strip 200 can be invested with a tissue
sample 122 through fill guide 400.
The process described above is employed to multiply tissue
cultures already growing in cellules 30 of integument strip 300.
When first beginning the micropropagation process, before initial
cultures have been established in cellule 30 of integument strip
300, it is necessary to establish initial cultures for later
multiplication. Thi8 i5 accomplished by manually inserting
samples of meri~timatic ti.~sue from a selected parent plant or
cultivar into the tissue planting unit 290, investing the tissue
sample~ in cellule~ 30 of integument strip 200, sealing the
cellules and transporting them to culture room 310. According-
ly, forming a part of the enclosure 282 of tissue manipulation
unit 120 is a normally-sealed access door 281, as shown in
Figures 20 and 21. When initiating the micropropagation process,
an operator opens access door 281 and deposits a sample of
meristimatic tissue within each compartment 352 of tissue con-
tainment device 336 a it rotates in the counter-clockwise
direction as viewed in-~igure-2~ When 2 compartment containing
a manually-inserted tissue sample becomes aligned with windows
362 formed in end and side plates 35~, 360, the sample is
invested in cellules 30 of integument strip 200 in the same
X00~3~5
manner as described above. Alternatively, access door 281 may be
enlarged or repositioned, or another access door may be provided
in enclosure 282, so as to allow an operator to directly invest
tissue into cellules 30 of integument strip 200 prior to the
cellules being sealed by sealing unit 310, without employing
tissue planting unit 290. During this manual operation, the
operator will have manual ~Gntrol of the tissue manipulation unit
120.
Sealing Unit 310
Referring again to Figures 1 and 19, once the severed tissue
sample 122 has been invested into the sterile cellule 30, the
cellule 30 is drawn into sealing unit 310 comprising a pair of
roller heat sealers 404, thereby completely sealing the new
culture from the exterior environment. Once sealed, the cellules
30 are bar coded by a bar code printing system 311 such as the
Digimark variable information laser marker manufactured by
Videojet Systems International, Inc. of Elk Grove Village,
Illinois. The bar code indicates the type of plant material in
the cellule and the date the plant was invested in the cellule.
The cellulos are then transported into the culture room 130.
Culture Room 130
The culture room 130 compriseq a room or other enclosure
containing the tractor feed mechanism 50, a temperature control
system 404, and a lighting system 406. As described previously,
the tractor feed mechanism 50 will transport the integument
strips 200 in a multilevel serpentine fashion within the enclo-
sure 130. In this system, it is not necessary that the air be
filtered since the cultures have been sealed from the~ ambient
envir~lment by sealing unit 310 in the tissue manipulation unit
120 after pl.~t.ina T~ n.r~fQrred. tha~ t~ tract~ feed system
50 be supported by a gridwork of support brackets and channels
rather than by the perforated support plates previously described
with respect to the sterilization and cooling units 100, 110
-39-
200~3~
since, in this application, it is important that the light waves
generated by the lighting system are transmitted and reflected
throughout the entirety of the enclosure 130. The previously
described perforated support plates block too much of the light.
As shown in Figure 22, between each serpentined row of integu-
ments, there is a bank of fluorescent lights 40~ selected and
positioned so as to maintain approximately 1,000 foot-candles of
light throughout the unit 130. It is preferable that the system
allow the light intensity to be varied as the cultures are
transported throughout the unit 130 such that when desirable to
cease multiplication and grow finished plantlets at the comple-
tion of the growth period in the culture unit 130, the light
intensity can be increased to approximately 3,000 foot-candles so
as to harden the plant and ready it for shipment to the commer-
cial grower for planting in a soil medium in the greenhouse. To
enhance light transmission within culture room 130, the walls,
ceilin~ and floor are covered with a highly reflective surface
410 such as a mirrored acrylic sheet.
As depicted in Figure 1, individual lengths of plant-filled
cellules are periodically scanned in the culture room 130 by a
growth detection scanner 140 which detects the growth of the
plant or tissue. One type of growth detection scanner 40 is the
vision system, including the 2803-CM VIM module camera adapter
and 2802 line scan camera manufactured by Allen Bradley of
Milwaukee, Wisconsin. The vision system will detect, fill, size,
shape, contrast and multiple shades of gray whereby the system
can not only detect plant and tissue growth but also
contamination of the plant material within the cellule. Should
contamination be detected, an ink jet printer 140a, such as the
Excel small character ink jet prl~ LUL~e~Ui~d by videojet
System~ International, Inc. of Elk ~rove Village, Illinois, marks
the cellule containing the contaminated plant material to later
avoid removing the contaminated plant material from that cellule
-40-
X~ 3~5
at the cutting unit 280. A print registration scanner 140b, such
as the Smarteye color mark registration scanner manufactured by
Tri-Tronics Company, Inc. of Tampa, Florida, may be located at
cutting unit 280 to detect the re!ject mark so as to not remove
the contaminated plant material into tissua containment device
336.
A bar code reader 141 is stationed within culture room 130
near growth detection scanner 140 for identifying the plant
material, media, and date the plant was invested in the cellule.
Bar code reader 141 may be a bar code scanning system such as the
Skan-4100 moving beam laser scanner and Skan-D41 bar code decoder
manufactured by Skan-A-Matic of Elbridge, New York.
If the plant material is ready for the next stage of
micropropagation, the length of cellule~ are transported back
into the tissue manipulation unit 120. If the appropriate number
of tissue multiplications have been performed and the desired
number of plantlets have been produced, the cellules are
transported from the culture room to the packaging system 160
where the sealed cellules are boxed for shipment.
Control Svst~m
Figuro 23 depictQ a block diagram di~closing the basic
organization of the control system 150 for the automated system
for performing micropropagation and tissue culturing. The
control system 150 i9 centered around master control unit 500, a
programmable controller, and four local control units, 502, 504,
506 and 508. Local control units 502, 504 506 and 508, also
programmable controllers, are generally dedicated to controlling
and monitoring specific portions of automated system 10. More
specifically, local control unit 502 is generally dedicated to
the media preparation and fill units 70, 80, the fill c..eck
scanner 90, ink jet printer 91 and bar coding mean 93; local
control unit 504 i3 dedicated to monitoring or controlling
sterilization unit 100 and cooling and storage unit 110; local
-41-
2~0'~3~5
control unit 506 monitors and operates tissue manipulation unit
120; and local control unit 508 controls activities in culture
room 130. Each loc~l control unit also operates the drive motors
which form a part of the tractor feed apparatus 50 within its
region of control.
The master control unit 500 may be a dedicated controller
system where a programmable logic controller, such as the PLC-3
family of controllers having up to 4096 input/output channels
manufactured by Allen Bradley of Milwaukee, Wisconsin, may be
used to control all operations with individual units also having
keyboard input for manual operation whereby separate parts of the
automated syctem 10 can be operated separately.
Master control unit 500 and local control units 502, 504,
506 and 508 are configured in a master-slave arrangement such
that master control unit 500 can monitor all the conditions and
parameters sen~ed by th~ local control units and can coordinate
the operation of the entire system. Additionally, master control
unit 500 can assume the function of any local control unit when
it may become neces~ary to remove that local control unit from
the ~yste~ so as to reprogram certain steps or perform mainte-
nance on th~ local control unit.
The control provided by local con~rol units 502, 504, 506
and 508 will now be described in greater detail. Referring now
to Figures 8, 9 and 23, local control unit 502 monitors signal
from fill sensors 142 and actuates media mix system 510, com-
prising metering pumps 138, solenoid valve 133, stirrer motor 128
and heater 132. Local controller 502 also operate drive motors
112 (Figure 6) for transporting cellules 30 through media fill
apparatus 70 and fill check scanner 90. Local control unit 502
also hs responsibility .o~ cGntrGlling and actuating media
dispensing system 516 including fill pumps 154 and transport rack
158. Local control unit 502 also monitor~ signals received from
-42-
20043~S
fill check scanner 90 and controls bar coding means 93 and any
ink jet printer ~1 applying reject marks.
Referring now to Figures 12-14 and 23, local control unit
504 is shown to actuate motor drives 520 which transport cellules
30 within and through sterilization unit 100 and cooling and
storage unit 110. Also controlled by local control unit 504 is
the autoclave loading and unloading system 522, including cutter
202 used to cut the continuous length 24 of integument roll 20
and cylinders 196 employed to close autoclave 186. Local con-
troller 504 also monitor~ and actuateq the sterilization process
524, including the operation and monitoring of steam generator
234, and controls the cooling proces~ 526 in cooling and storage
chamber 110.
Referring to Figures 1 and 23, local controller 506 actuates
motor drives 528 and 530 which, respectively, transport
integument strip~ 300 and integument strips 200 into and out of
tissue manipulation unit 120. Local control unit 506 also
controls surface sterilization unit 320, cellule cutting unit
280, tissue planting unit 290, sealing unit 310 and bar coding
mean~ 311. Local control unit 506 also monitor~ disposal unit
170 and Qignals an operator when the unit if full. Local control
unit 506 also controls any print registration scanner 95 adjacent
tissue planting unit 290.
Referring now to Figures 1, 22 and 23, local control unit
508 actuate~ culture chamber motor control system 542, lighting
system 406 and temperature control system 404 located within
culture chamber 130. In addition, signals from the growth
detector 140 and bar code reader 544 are monitored by local
control unit 508. Local control unit 508 also controls any ink
jet printer 140a applying re~ect marks.
It is the function of the control system 150 to coordinate
and synchronize all operations throughout the automated system
10. Thus the control system 150 sets the timing, sequence, and
-43-
200~3~
speed of each operation by receiving input signals from the
apparatu~ located at each operation station and then sending
output signals to such apparatus.
While the preferred embodiment of this invention has been
shown and described, modifications thereof can be made by one
skilled in the art without departing from the spirit of the
invention. The embodiment described herein i8 exemplary only and
is not limiting. Many variations and modifications of the system
and apparatus are possible and are within the scope of the
invention. Accordingly, the scope of protection is not limited
by the above description, but is only limited by the claims which
follow, and that scope includes all equivalents of the subject
matter of the claims.
-44-