Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~~C~~~~~
FIELD OF THE INVENTION
This invention relates to sheets or films of
transition metal dichalcogenides, particularly molybdenum
disulfide.
BACKGROUND OF THE INVENTION
It has recently been found that singular
molecular layers of layer-type transition metal
dichalcogenides, such as MoS2, TaS2 and WS2, can be prepared
by intercalating such compounds with lithium and then
reacting the intercalated compound with water. This gives
rise to a suspension of single molecular layers of the
transition metal dichalcogenides in water.
Attempts have been made in the past to produce
sheet-like forms of metal dichalcogenides as revealed, for
example, in United States Patent No. 4,299,892 to Dines and
Chianelli. Here, an amorphous transition metal
dichalcogenide product is prepared by low temperature,
non-aqueous precipitation of the compound from mixtures of
the metal salt. The amorphous products are converted into
sheets of metal dichalcogenides referred to in the patent as
having a "rag-like" structure by controlled heating at
temperatures between 250° C and 400° C: Ho~rever, neither the
end product, nor the intermediate product are oriented
films or sheets, that is films or sheets wherein the
crystalline c-axes of single layers of the metal
dichalcogenide are aligned.
~~3~a~3~~
- 2 -
United States Patent No. 4,647,386 to Jamieson
discloses an intercalated transition metal based solid
lubricating composition. A transition metal dichalcogenide
is intercalated with a metal, preferably a coinage metal.
SUMMARY OF THE INVENTION
The invention provides a process for forming
sheet-like compositions of the formula MX2:Y, wherein MX2
is a layer-type transition metal dichalcogenide, M is a
metal group consisting of niobium, tantalum, molybdenum
and tungsten, X is a chalcogen selected from sulfur and
selenium and Y is a material located between layers of
MX2. The process includes the steps of forming a
suspension of the MX2 in water and adding a liquid which
is immiscible with water to the suspension to form a
mixture. The mixture is agitated to form a temporary
emulsion. The emulsion is allowed to rest until the water
and the liquid separate with an interface therebetween. A
sheet-like composition of MXZ:Y forms at the interface,
MX2 has a crystalline structure with c-axes aligned in a
direction perpendicular to the plane of the layers.,
Preferably, the MX2 suspension comprises
~~~D~~~~
- 3 -
liquid, water molecules or molecules of a foreign
substance dissolved in the liquid.
The invention also relates to compositions
prepared according to the processes described above. The
compositions may be used to coat objects.
The invention provides thin. aligned sheets or
films and coatings of transition metal dichalcogenides by
a relatively expeditious process which can be readily
scaled to provide large area films and coatings. These
coatings or films have unique qualities. For example, such
thin, oriented films of molybdenum disulfide have unique
optical qualities and can be used as selective filters.
These films are believed to have advantageous lubrication
qualities when compared with non-oriented films. Because
thin, highly oriented films of MoS2 can be deposited on
curved surfaces, the invention may be used to make
relatively inexpensive, large area energy selective X-ray
focusing devices. The invention also provides unique
layered compositions which may be utilized for the
composition of the transition'metal dichalcogenide, for
the properties of other substances between layers of the
metal dichalcogenides or combinations of the two. For
example, the optical properties of MoS2 in combination
with the other organic molecules between MoS2 layers can
be used as selective optical filters.
~~.iC) ~L3~~
- 4 -
BRIEF DESCRIPTION OF THE DRAWINGS
In the drawings:
Figure la is a diagrammatic representation of a
process for making a thin, oriented film of
a transition metal dichalcogenide according
to an embodiment of the invention and
employing a water immiscible liquid having
a specific gravity less than 1;
.. Figure lb is a view similar to Figure la. showing the
process using a water immiscible liquid
with a specific gravity greater than 1;
Figure 2 is an enlarged view of the portion within
the circle in Figure lb.;
Figure 3a is a diagrammatic representation of the
coating of an object with a film produced
by the process of Figure la.;
Figure 3b is a diagrammatic representation of the
process of coating of a wet glass slide
employing the.film produced by the process
of Figure lb:;
~~~D ~~~f~
- 5 -
Figure 4 is an enlarged, diagrammatic representation
of a portion of an object coated with a
film of transition metal dichalcogenide
incorporating water immiscible liquid and
water molecules produced by the processes
Figures 3a or 3b;
Figure 5 is a view similar to Figure 4 showing the
object and film after heating;
Figure 6 is a diagrammatic side view of the metal
w dichalcogenide film shown after drying;
Figure 7 is an enlarged diagrammatic view of the
portion within a circle of Figure 6 showing
ferrocene between the layers of metal
dichalcogenide;
Figure 8 is an enlarged diagrammatic view of an
object coated with a film of transition
metal di.chalcogenide incorporating
ferrocene shown after baking;
Figure 9 i5 a diagrammatic view of a method for.
coating a hydrophobic substrate with
molybdenum disulfide film;
Figure 10 is a diagrammatic view of a prpcess for
transferring a molybdenum disulfide film
from one substrate to another;
_, ~4~~~~~~
- 6 -
Figure lla is a proposed model of single layers of
molybdenum disulfide in water; and
Figure llb is a view similar to Figure lla and showing
the layers as modified by the presence of a
water immiscible liquid.
1~ DESCRIPTION OF THE PREFERRED EMBODIMENTS
A known process for preparing single layers of
molybdenum disulfide in water involves exfoliating a
lithium intercalated layered transition metal
dichalcogenide, such as MoS2, TaS2, NbS2 or WS2 by
immersion in water. Selenium may replace the sulfur as
the dichalcogen. ,_,
In one example molybdenum disulfide powder was
soaked in a solution of n-butyl lithium in hexane for
about 48 hours in a dry box containing an argon
atmosphere. Once the molybdenum disulfide was fully
intercalated with lithium ions between the layers of
molybdenum disulfide, the product was removed and washed
repeatedly in hexane, dried and sealed in a vial while
still in the dry box under argon atmosphere. The vial was
then removed from the dry box, immersed in water, and the
cap removed from the vial. Upon contact of the contents
of the vial with liquid water,'copious'gas evolution
followed and the molybdenum disulfide powder formed a
':
~~Cl~~~,~i
highly opaque suspension in the water. The suspension was
agitated, in this case ultrasonicated, during the reaction
to assist in the exfoliation. The hydrogen gas produced
by the reaction between the lithium and the water pushed
the layers of molybdenum disulfide apart until they were
completely separated. The suspension was repeatedly
centrifuged and washed with distilled water. A final
concentration of molybdenum disulfide of about 5 mg/cc of
water was produced.
The formation of films or sheet-like forms of
transition metal dichalcogenides according to this
invention begins with the single layer suspension of
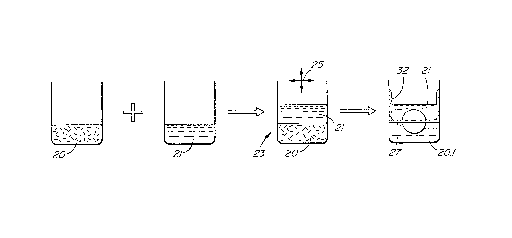
molybdenum disulfide in water obtained above. Referring
Fig, la,, a liquid 21 is immiscible with water and, in this .'
case, has a density less than water. Various organic
solvents were tested including alkanes, alkenes and butyl
alcohol (n-butyl and iso-butyl). The liquid was added to
the suspension 21 of molybdenum disulfide in water to form
a two-phase liquid 23. The suspended molybdenum disulfide
stays in the water.
The mixture was then shaken as indicated by
arrows 25 and formed an unstable emulsion containing
globules of water in the liquid. The molybdenum disulfide
layers placed themselves at the surfaces of the resulting
globules of water. When the shaking ceased, these globules
_ ~~~i~~~~~o
_8_
gradually migrated towards the water/liquid interface 27
where they coalesced with each other. The molybdenum
disulfide molecular layers formed a film at the horizontal
interface 27 between the two liquids. With moderate
shaking, both the water and the water immiscible liquid
became clear. Therefore. all of the molybdenum disulfide
was in the interface film 32 as shown to the right of Fig.
la. Clear water 20.1 remained below the film.
It was found, qualitatively, that the lower the
pH of the water, the more rapid the accumulation of the
molybdenum disulfide at the interface. The accumulation of
molybdenum disulfide at the interface occurred faster with
alcohol than with alkanes or alkenes. After the interface
is formed, it is possible to further transfer the
molybdenum disulfide layers to the alcohol but not the
alkanes or alkenes. This was done by lowering the pH of
the water. The transfer occurred at a pH between 2 and 3
in the case of n-butyl alcohol when dilute nitric acid was
gradually added while shaking. With alkanes and alkenes, '
attempts at such transfers resulted only in flocculation
of molybdenum disulfide at the water/liquid interface.
The following are organic solvents with a
specific gravity less than l were tested:
alkanes (n = 5 to 12)
hexene
benzene cyclohexane
n-butyl alcohol styrene
iso - butyl alcohol tertiary butyl benzene
1, 3-5, trimethylbenzene
g -
Fig. lb illustrates a variation of the process
of Fig. la wherein the specific gravity of the water
immiscible liquid is greater than 1. Suitable solvents
tested were 1,2-dichloroethane, carbon tetrachloride,
dimethoxybenzene, 1-chloronaphthelene, and iron
pentacarbonyl. However, it was found that mercury does not
work. In this process, the solvent 22 was mixed with the
molybdenum disulfide suspension 20 to form a two-phase
liquid 24 which is agitated as indicated by arrows 26.
Film accumulation 32 occurred both at water/liquid
interface 28 as well as at water/air interface 30.
Referring to Fig. lla, this shows one of the
single layers of molybdenum disulfide 32 contained within
the water suspension 20 of Figures la and lb. While we do
not wish to be bound to this theory, it is believed that
each of the single layers 20 possesses a net negative
charge due to surface hydration represented in Fig. lla by
the OH'ions.
Referring Fig. llb, this shows the result of
mixing an aqueous suspension of exfoliated molybdenum
disulfide with a liquid which is immiscible with water and
agitating the resulting mixture as described above.
As described above; the first configuration that
forms after agitating the two-phase mixture is that of
globules encased by molybdenum disulfide layers. These
~v~LD;~~~G
- to -
globules are inherently unstable and, in time, coalesce,
presumably because the free energy associated with the
globules is much higher (proportional to interfacial area
of the globules) than that of the final phase-separated
mixture with a single horizontal interface between the
water and the water immiscible liquid. With the
coalescence of globules, the modified single layers of
Fig. llb form a multi-layer membrane at the interface.
Referring to Fig. 2, this shows in diagramatic
form an enlarged section through the membrane. The layers
32 of molybdenum disulfide are stacked on each other with
water molecules 34, identified by while ovals, and liquid
molecules 36, identified by black ovals, trapped between
them. Based on the above discussion, it can be expected
that the immiscible liquid will be non-polar or weakly
polar.
2~,y. It was observed that the suspensions of
exfoliated molybdenum disulfide are completely cleared by
shaking the mixture, leaving no layers in the water phase.
This appears to indicate that all of the hydroxyl graups
on the basal planes are replaced by the liquid molecules:
If the hydroxyl groups were still present in any
significant amount, some degree of dispersion of
molybdenum disulfide in water would be expected after
shaking.
~~~CD~~~~
- 11 -
It has been found that the molybdenum disulfide
film at the interface has a tendency to spread. As shown
in Figures la and 1b, the film 32 tends to creep along the
walls of a glass container holding the mixture.
It has also been found that the thin film of
molybdenum disulfide spreads on a thin layer on wetted
surfaces. In particular, referring to Fig. 3b, a
pre-cleaned glass slide 38 was wetted after a brief wash
with dilute hydrofluoric acid and then dipped into the
phase separated mixture such that the lower end just
touched the accumulated molybdenum disulfide at the
interface of water 20.1 and solvent 21. This resulted in a
rapid spreading of a film 32 of molybdenum disulfide up
both sides of the slide as illustrated, covering the
entire wetted area. After the motion of the film stopped,
the slide was withdrawn from solution and was kept hanging
vertically in room air for drying. Although the film was
apparently dry after a few minutes, it was not completely
devoid of water at this stage as seen in Fig: 4 where
molecules 20.1 of water and molecules 21 of solvent are
trapped between layers of MoS2 32. However, the resulting
film was optically very uniform and highly oriented ds
determined by x-ray diffraction. The film is shown after
drying on slide 38 in Fig. 5.
Films of molybdenum disulfide were grown on
various hydrophilic substrates using this method including
- 12 -
glass, ceramics, oxidized copper, oxidized silicone,
tungsten trioxide, glass and even cardboard.
A slight variation of the above method is to dip
a dry hydrophilic substrate into the phase-separated
mixture past the accumulated molybdenum disulfide into the
water 20.1. The film was riot formed when inserting the
glass substrate through the interface 27 into the water
20.1, but a film of molybdenum disulfide was deposited
when the substrate, now wet. was withdrawn.
When a hydrophobic substrate. such as PTFE was
inserted, a film formed on it, but was restored to the
water/organic interface when the substrate was withdrawn.
Another method of depositing films tested was to
allow the water immiscible liquid to evaporate after the
process of Fig. lb., leaving the molybdenum disulfide film
at the air/water interface as shown in Fig. 9. The
resulting mufti-layer film 32 can be transferred to a
hydrophobic substrate 33 by simply touching the film with
the substrate oriented horizontally, alternatively from
above the film 32 disulfide interface and from below the
film 32.
Fig: 10 illustrates another method used for
coating hydrophobic surfaces with film. following
formation of the film using the spreading technique of
o~~~a~~~
- 13 -
Fig. 3b, there is a water layer between the film and the
substrate. When the substrate was reimmersed into
deionized (resistivity greater than 10 Mohm cm) water, the
film 52 disengaged itself from the glass slide and
re-spread on the air/water interface 54. The hydrophobic
substrate 56 was placed on a support 58 in a horizontal
position just under the air/water interface 54. The water
level was then lowered past the substrate and the film is
deposited on a non-polar substrate as shown to the right
of the figure. The proportion of the film transferred was
directly related to the fraction of the slide which was
-. immersed in water. The substrate 56 with the newly
transferred film of molybdenum disulfide 60 was then
removed from the container and let dry in room air.
0 ..
This method of transfer was used with such
substrates as PTFE, polystyrene, copper, titanium,
aluminum, brass and silicon. Relatively thin films
z5 (approximately 30 angstroms) as well as relatively thick
films (350 angstroms), have been transferred in this
manner. Dilute hydrofluoric acid (approximate 5 - 10
percent in water) can be used instead of the deionized
30 water as the intermediate medium when transferring films
of molybdenum disulfide onto silicon. Keeping the silicon
substrate in dilute hydrofluoric. acid ensures that the
native oxide of silicon is removed, thereby enabling
intimate contact between the molybdenum disulfide film and
the silicon. The fact that molybdenum disulfide does not
react with hydrofluoric acid is of importance here.
i~~(3 ~fi~~~
- 14 -
Fig. 3a illustrates a further method of
transferring films when the water immiscible liquid has a
specific gravity greater than 1 as previously used in the
process of Fig. la. In Fig. 3a, the film was deposited
directly on to a metal substrate. Freshly etched metals
including aluminum, copper or steel 64 were inserted into
the water/air interface 30. A coating of molybdenum
disulfide was deposited on them. This method could be
useful for preparing lubricant coatings on metal surfaces,
for example.
In one example styrene was used as the water
immiscible liquid to obtain styrene molecules between the
MoS2, layers giving a c-spacing of 11.5 angstroms. The
composition was subsequently heated to about 60°C in an
inert argon atmosphere to polymerize the styrene into
polystyrene. This may be of use in protecting the MoS2,
layers.
In another example a mixture of the water
immiscible liquid iron pentacarbonyl, Fe(CO)5, and a
suspension of single layer MoS2 in water was shaken to
produce a composition of MoS2 with iron pentecarbonyl
between the MoS2 layers with a c-spacing of 12.0
angstroms. The resulting composition was heated in an
inert atmosphere until the iron pentacarbonyl decomposed,
releasing carbon monoxide and forming iron between the
MoS2 layers. Other metal carbonyls can also be used as
the immiscible liquid:
_. o~~~ ~~a~s~a
- 15 -
X-ray diffraction measurements confirmed that
iron between the layers gave a c-spacing of 6.33 angstroms
as compared with 6.13 angstroms for the unexfoliated
MoS2. Novel compositions of Fex MX2, wherein M is Nb,
Mo,Ta or W and X is S or Se with varying x, can be
obtained using this method. This technique could
potentially be extended to obtain compositions of MX2,
with many other metals between the layers.
Instead of using merely a water immiscible
liquid as described above, it is possible to dissolve
other solutes in the liquid. For example,.referring to
Figs. 7 and 8, ferrocene was dissolved in benzene and,
alternatively in carbon tetrachloride, to produce films
that included ferrocene 76 between the molybdenum
disulfide layers 74.
To produce a powered material with ferrocene
included between the Mo52 layers, with reference to
Fig, la, the water 20.1 was decanted and the water
immiscible liquid 22 was pipetted. In the case of Fig.
lb., the liquid 21 was evaporated and the water 20.1
pipetted. The resultant composition was dried in room air.
The result was a powder 72 of molybdenum disulfide as
shown in Fig. 6. The enlarged view of Fig. 7 shows that
the powder includes a plurality of layers 74 of MoS2 with
molecules 76 of ferrocene, represented by x's, between the
layers. The resultant composition can be identified by the
~~C~;~~'~.'~
- 16 -
formula MX2:Y, where Y is the ferrocene. This was in
powder form. Fig. 9 shows a molybdenum disulfide film
incorporating ferrocene molecules coating a substrate 78
via the processes described above. It should be noted that
there is only a single layer of ferrocene molecules
between the layers of molybdenum disulfide instead of two
layers of liquid molecules as shown in Fig. 2.
The resulting films are highly oriented as shown
by x-ray diffraction. The orientation is much better than
obtained with organic solvent alone. Additionally, the
presence of ferrocene between the layers makes the
resultant films on substrates electrically more
conducting. The insertion of ferrocene into the interlayer
0 spacing of molybdenum disulfide is by itself novel and
cannot be done with conventional intercalating techniques.
Inclusions such as other metallocenes, dyes, monomers and
liquid crystals can be included in layered transition
metal dichalcogenide films by this technique.
zntercalcated complexes of NbS2 and TaS2 existed prior to
the invention, but not MoS2 and WS2 intercalated with
organic materials.
Besides ferrocene, other possible solutes are
copper phthalocyanine (CuPc), phthalocyanine (Pc),
stearamide. and chrysene. Below is a table of films
produced with molybdenum disulfide using a particular
solvent and a solute dissolved in the solvent. Large area
films can be produced using the above method.
e~~~:71~~~
- 17 -
Solvent Solute Properties and Uses
1. benzene, ferrocene Highly oriented, and
carbon better electrical
tetrachloride conductivity. Powder
may have applications
as a catalytic material.
2. benzene, PhthalocyanineBlue colour films,
hexene dyes, CuPc, possible gas sensing
Pc etc. ability.
1-chlorona-
Pthalene
3, benzene, chrysene whitish film,
fluorescent
hexene film, purple
fluorescence upon
exposure to
ultra-violet radiation.
4. hexene stearamide widely separated I~oS2
layers.
. .,.