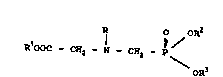Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2C)06270
- 1 - 09-21(2945)A
~ .
This in~ention relate~ to a p~oc-o~ for th~ prep~ration
of N-phosphonomethylglycine or it~ ~te~, and morQ
particularly to th~ pr-paration of N-phosphonomethylgly~ine
~r~m N-substituted glyc~ne derivatives
N-Phosphono~ethylglycine, known by it~ common name o~
glyphosate, i9 widely used around the world a~ a broa~-
spe¢trum herbicid~ to control th- gxowth o~ m~ny plant
specie8 Generally, it ~g u~ed in an aqueou~ solution ns
one of it~ salts for application to plant~ to control the
growth of woody plants, aquatic ~peci-~, qra~e~, ~nd the
like It i~ kno~n to be gen~rally non-toxio to ~umsn~ and
other mammals, and environment~lly ~- Millions of llter~ -
of the formulated product ar~ ~old each yea~ for such
purposes
It i~ kno~n that N-benzyl-N-phosphono~ethylglya$ne (or --
itR esters) unde~go~# hydrohal~c acid d-benzyl~tion to yield
benzyl halide and N-phosphono~ethylglyaine or it~ ester~
(see ~or example Briti~h Patent No 1 436 843) A large -
excess o~ very ~onc~ntrated (eg 48~) hydro~alic acid is
required, however, amounting to ~any mol-~ o~ ac~d ~or eacA ~-
mole o~ ~tarting co~pound Thls rendor~ ~h- proc-~ing ~nd
i~olation o~ the deslred glycin~ deriv~tive di~lcUlt,
mainly becau~e of th~ problem of r-moving thi~ large ~mount
o~ hydro~alia ac~d a~ter the reaction.
Attempts to lmprove the proce~- by the use o~ ~tarting
COmpOUnd# hav~ng other ~ubstitu~nts than b~n~yl on the
nitrogen atom, eg ts u~e N-alkyl-N-pho~phonomethylglya~ne~,
~0 have bccn ~tt~nded ~ith the sa~ proc~ing disAdvantag~
(se~ ~or exa~lQ US Patent ~o 3 g27 080) The lit~rature
contain~ no ~ugge~tiona as to the use o~ ~c~fl~ other than
th~ hydrohalla acldn to re~ove the ~lkyl group ~rom N-alkyl-
2~ 270
- - 2 - 09-21~2945)A
N-phosphonomethylglyainn ~o produc~ tho d~irad nd ~roduct
Now, t~e~e is provided nn ~A~y procQdure to dealkylato a
wide variety o~ N-alkyl-N-pho~phono~ethylglycine~ to provid~
N-phosphonomethylglycine in hi~h yield~ at an ~¢onomical
~o~
The~e and other advantage~ aro achiev-d by ~ proaes~
for the pr~p~ration of N-phosphonomQthylgly~ine which
compr~ S-8:
prepa~ing An N-alkyl-N-pho~phonomethylglycln- or it~
ester represented by the ~ormul~
R ~ oR2
RlOOC - CHz -- I -- CH2 P\
oR3
:: 15
wh~rein R i~ an alkyl group repr~ented by th~ formula
R~
R~ - C -
R5
and Rl, RZ and R3 ar~ independently select0d from the
group consisting of hydrog~n and alkyl hav~ng on~ ~o about
four carbon ato~s, and R4, ks and R6 ~re independ~ntly
~elected from ~ubstit~ted and un~u~titut~a ~lk~l ~ruu~
having ~rom one to about ~ix c~bon atom~ wh~rein any
sub~ti~u~ion on th~ alkyl group ha~ atron wlthdrawing
prope~es, and hydrogen, provided that R~, R5 and R6 cannot
~11 be hydrogen; and therea~ter
treating the N-Alkyl-N-pho~phonom~thylglycine with an
acid, oth~r than a hydrohalic aoid, having a pR~ value ~-low
a~out ~3 in the ~re~enc- o~ an o~ganic acid to proviao N-
pho~phono~ thylgl~clne
- 35
~0~2~o
_ 3 _ 09~ 2945)A
.,' _ ~_
We have found that, apart from known proces~e~ u~ing
hydrohalic ac~ds, cort~in ~-~lkyl-N-phos~honom~hylglycine~,
: and t~eir ester~, in which the N-~lkyl aub-tituent ~
suitably chosen, can bo dealkylat-d by treatment not only
with a hydrohalic aoid ~ut with any ~aid whose ~K. value i6
below ~3. Pref~rably, the acid u~ed is solected ~rom th~
group that con~i~ts o~ sulfurio ~aid, ~-toluene ~ul~onic
acid, methylsul~onic acid (subgroup ~) and trichl~r~a~tic
acid, phosphori~ acid and phosphorou~ acid (subgroup B).
The acids of ~ubgroup A are p~eferred, and sulfuria acid i~
e~pecially pre~erred.
In the case of sulfuric Aaid, an adoquate molar
proportion is le~ than about 10%, ba~ed on the ~OlQ~ 0~ -
alkyl substituted glycine derivati~e used in the p~oC~
OE th~ other ~c~d~ n~ed, molar proportion~ o~ ~bout 5% t~ ~
about 50% can be used, the ~refexred range being 10% to 20~, -
based on the moles o~ alXyl ~ub~titut-d glycin~ ~erivativ~
used.
Any nu~ber of organic acids known to those ~illed in
- the ~rt c~n be used in the tr~atm~nt of the N-alkyl-N- ---
pho~phonomethylglycine with th~ ~c~d having a pK, value of
le~ than about ~3. It ~ 8 only nece~ary that the o~anlc
acid i~ water 301uble, and low~r molecular w~ight orqAnic
acids are pre~err~d. Suitablo organic ac~ds inolude formic
acid, acetic acid, propionic acid, butanol~ a~id, and th~
lik~, for u~e in the rea¢tion medium. Aa~tlc ~cid i~
pre~erred.
m Q temperatures to be u~ed in the pXe~ant proces~ aan
vary w~th~n wid~ rango~ Temperaturo~ be~ween a~out 20~C
an~ about 100C pro~ide nati~actory ~esult~. Lower
~QmpQrn~ur~s can be u#ed, but tho reactlon ~- ~omewhat ~low.
TQmp~aturQ~ above 100C can b~ u~ed, but a~ ~7ill ocour to
,
~~iZ7()
,:
- 4 - 09-21~2g45)h
~hose s~illed in the art, the reaat~on v~8~1 may h~v~ to ~e
pres~urized at such higher tem~erature~. Temp~ratur-~
between about 40C and 100C ar- pre~err~d. When sulfuria
a¢$d i~ used, d~alkylation bQgin~ at a temper~ture of about
50C, and become~ rapid ~t about 80C, with aopious
evolution o~ the relevant alkene.
In a preferred ombod~m-nt o~ the proan~ of the
invention the dealkylation i~ carried out in the pre-enc- of
acetic acid a~ a ~olvent. This h~ b~en ~hown to impart
economie~ to the proc-ss, ~nae the target aompound
crystallizes directly from the Acetic acid in th- cour~ of
the dealkylat~on reaction.
In another pre~erred e~bodi~ent, which can bo co~bined
with any of the ombodimentoe de~aribed above, the proce~ of
the invention compri~e~ pr~paring th- alkyl d~rivative in a
~anner Xnown E~E_~ and thereafter dealkylating it
according to the invention a~ ~ot out above, without
previous i~olation, in a one-pot procedure. More
~pecifically, in the preferred embodiment, the N-alkyl-N-
phosphonomethylglycine or e~t-r u~-d i~ synthe~izQd f~om
ethyl chloroa¢etate and an appropriat~ alkylamin~, ~ollowed
;- by Q~te~ hydroly3~s, ~ollow~d by pho~phonomethylation of the
re~ulting N-alkyl glycine or ester, and that ent~re proce~s
~ performed without i~olation or purifi~ation of any
intermediat-. ~n the pre~erred e~bodiment
N-t-butyl-N-phosphonomethylglyaine may be pr~par~d by
pho~phonomethyla~ion of N-~-butylglycin~ whiah in turn may
be th- produc~ o~ the coupling o~ ~-butylnmin~ ana ~thyl
chloroac~tat~, ~ollow~d by e~ter hydroly~
Wh~n thQ mo#t pr~erred glycin~ d~rivativ-, namQly N-~-
bUtyl-N-pho~phono~ethylgly~in~ dealkylated in accordance
with th~ invQntion~ eB~ccially wh~n ac~tic acid ~ used as a
~olven~ utylene iB e~olved. If, how~v~r, th~r- l- a
6270
_ g _ 09-21(294S)A
~igni~icant amount of water pre~ent ln the reac~ion ~ixturo,
then in addition t~ ~Q-butylon~, ~-butanol i~ obtained a~ a
by-product, As will occur to tho~- ~killed in the art,
corre~ponding produats are ob~a~ned when o~her glycine
derivat~ve are u~ed
As previou~ly ~tat-d, thQ dealkylation of N-~-butyl-N-
phosphonometh~lglyoine proceed~ moro rapidly in ~cetic ncid
than it does ~n water; however, it is the b¦5~L~ 1l of N-
phosphonomethyl~lya~ne from tho AC~tiC nc~d m-diu~, with
minimal processing, that provid-~ ~ m~or technical
advantage in the proc~ss of the invention
A final product of ~urity exceeding 90% by w-ight is
obtainablQ by the proces~ of th~ invention in a routinely
reproduaible ~anner Typical reaction times ar~ 2 to 4
hours With cooling and filtration over g0% yield~ ar-
obtainable
The following example~ s~rve ~urth~r to illu~trate tha
invention
Exa~pl~
N-t-Butyl-N-phosphonomethylglycino (100 g, 96~ ~ure,
0 426 mol) wa~ m~xed with ac~tic ~¢id (500 ml) and 97~
sul~uria acid (4 0 g, 0 04 mol) On h-ating the mixture to
50C in a round-bottomed fla~k fitted wi~h a Liebig
condense~ Q-butylen- was det~t~d down~tream o~ th~
conden~er At 80C large guant~ti~s o~ ~Q-butylene were
ev~lved and tho rate o~ evolution of this off-ga~ in~rea~ed
with te~perature. After 3 hour~ ~t 100C th~re w~ no N-~-
butyl-N-phosphonomethylglycine detectable by ~PLC in the
reac~ion v~sel, and l~rge quantitie~ of ary~talline N-
pho~phon~methylyglycine wer~ pre~nt. Cooling to ambi~nt,
f~ ring ~n~ drying g~e N-pho~phono~ethylglycine (6g 0 g,
g5~ pure, 91 2~ yield) Analy~i~ o~ th- mother l~quor
~00~i270
- 6 - 09-21t2g~5)A
showed a further 3.7 g of N-pho~phonomethylglycin-, giving a
total c~emical yield for this dQal~ylation of 96.3~.
E~amplç I~
~-t-Butyl-N-pho~phonom~thylylyc~no (100 ~, 96~ ~ur~,
0.42 mol) was ~ixed with acetic ac~d ~500 m~s) ~nd ~-toluen~
~ulfoni¢ acid (14.6 g, 0.085 mol). HQating at 100C for 4
hour.~ co~pleted the react~on and tha che~ical yield of the
dealkylation was 94~.
~e~
Ethyl chloroacetate ~1~2.5 g 1.0 mole) wa~ reAotQd with
exces~ t-butylamin~ in trichloromethane and the ethyl
gly~inate ~eparated ~rom the t-butyla~in~ hydrochlorid~.
The ethyl ~lycinate was hydrolyz~d with hyd~o~hloric acid
and the N-~-butyl-N-pho~phono~e~hylqlycine (167.6 g a~
deter~ined by HP~C) was reacted with suluric ac~d (3.8 mle,
0.07 mol) a~ ambient temperatur- and the ~ixture wa~ th~n
; heated at 100C for 4 hour~. Cooling to 20C, filto~ng and
drying gave N-pho~phonomethylyglycine (118.5 g, 95.8
purity, gO.3% y~eld). The overall yi~ld ~rom ~thyl
chloroacetate was ~7.2%.
The invention i~ not li~ited by or to tho de~ail~ ~f ~ -
the speci.~ic embadiments describ~d, ~any o which c~n
undergo wide variation without departing ~or fro~ the ~ope
of the invention.