Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
7~
PCT-10160
~ALT HANDLING ~PP~RA'~S FOR ~ HY~OC~LOROUS ACIP REACTQ~
This invention relates generally to the
solids discharge handling apparatus associated with a
reactor vessel system and, more specifically to that
apparatus associated with a reactor vessel for the
production of hypohalo~enated acid by the mixing and
reaction therein of an alkali metal hydroxide and a
gaseous halogen. A preferred product acid is
hypochlorous acid.
1~ Hypochlorous acid is used extensively in the
preparation o chlorohydrin and chlorarnines.
Chloroisocyanurates are typical examples. Hypochlorous
acid has been produced by several processes or
techniques. The use of dilute hypochlorous acid and
large quantities of halogen to produce hypohalites,
such as sodium hypochlorite, is recent.
One technique employs the process in which
chlorine, steam and air are bubbled through ~n aqueous
solution of an alkali earth metal hypochlorite, such as
2~ calcium hypochlorite, to remove the resultiny
hypochlorous acid in vapor form. The hypochlorous acid
-1- ~.'
,
æ()~703~
is then condensed and stored for use. This process,
however, produces a large volume of undesirable
by-product, calcium chloride.
Another process uses a low concentration of
aqueous caustic solution to scrub chlorine gas.
However, the solution has an available chlorine content
of about only 5% and, because of the chloride ion
content, the hypochlorous acid that is formed quickly
decomposes, most preferably to chloric acid.
1~ Another related process prepares a solid
mixture of alkali metal hypochlorite and alkali metal
chloride by reacting chlorine gas with a spray of
alkali metal hydroxide, while drying with a gas the
reactants and product. Some cooling of the reacting
chemicals and the drying gas may be done. The primary
products of this process have very limited utility.
A more recent process, which produces
hypochlorous acid vapor, sprays aqueous alkali metal
hydroxide in droplet form or solid alkali metal
2~ hydroxide particles into gaseous chlorine. This
approach attempts to utilize droplet sizes to attain
the ma~imum surface to volume ratio possible. Droplets
having an average diameter of less than about 1000
rnicrons are employed.
These previous processes, and the apparatus
employed to produce these processes, have suffered from
not achieving substantially complete reactions between
the chlorine and the alkali metal hydroxide. A
critical factor in determining the complete reaction is
3~ the droplet size of the alkali metal hydroxide. It is
also desirable that any hypochlorous acid produced and
any water present be readily vaporizable. The salt
particles produced as by-products in any process should
be dry to facilitate handling and be continuously
20Q~7034
removable from the reaction while maintaining a seal to
the atmosphere to prevent the gaseous product from
escaping to the surrounding area and to prevent
atmospheric gases from mi~ing with the gaseous
product. The increased concentration of water and
inert gases such as oxygen and nitrogen, in the reactor
system when atmospheric gases intrude reduces the
operating efficiency of the system. The salt particles
should be sized so that they readily separate from the
1~ gaseous product mixture of hypochlorous acid.
Prior processes have produced oversized
alkali metal hydroxide droplets that result in the
undesired reaction of hypochlorous acid and the
oversized particles to produce significant alkali metal
chlorates. These oversized particles then retain
excessive moisture so that caking results and the caked
mass adheres to the reactor surfaces. These oversized
particles can hamper the byproduct salt removal
additionally. The presence of such alkali metal
2~ chlorates reflect reduced yields of the desired
hypochlorous acid, while increasing the raw material
and operatiny costs. Lastly, the presence of halogen
gas in the porous solid by-product salt can cause the
salt to clump or bind together, thereby plugging the
salt handling apparatus.
These problems are solved in the design of
the present invention wherein by-product salt handling
apparatus is provided for a reactor vessel for the
production of a hypohalogenated acid in which the
3~ mixing and reaction of alkali metal hydroxide and a
gaseous halogen occurs.
It is an object of the present invention to
provide a solids discharge system for use within a
system employing a reactor vessel within which a gas
phase controlled reaction occurs to produce a
, hypohalogenated acid.
; A
J
703~
It is another object of the present invention
to provide a solid by-product salt handling apparatus
for a reactor vessel in which both a liquid-gas
reaction and drying occur to produce a gaseous product
and the solid by-product.
It is a feature of the present invention that
a gas seal is provided in the salt handliny apparatus
from both the atmosphere and the system components to
preclude the escape of gaseous product into the
1~ surrounding atmosphere and the intrusion of atmospheric
gases into the system.
It is another feature of the present
invention that the salt handling apparatus connected to
reactor vessel has a valving arrangement to control the
release of the by-product salt from the salt handling
apparatus and minimize the amount of halogen gas and
stripping air leakage.
It is still another feature of the present
invention that a halogen stripper is located in the
2~ salt handling apparatus to remove any trapped gaseous
halogen frorn the porous solid by product salt by the
injection of air.
It is yet another feature of the present
invention that the solid by-product salt can be
continuously removed in dry form from the reactor
vessel and the salt handling apparatus.
It is still another feature of the present
invention that the gas seal is providsd by a bed or leg
of salt wlthin the salt handling apparatus.
3~ It is an advantage of the present invention
that the production of oversized alkali metal hydro~ide
droplets are avoided and that undesirable secondary
reactions are minimized, while permitting the solid
by-product salt to be removed rom the system.
U34
It is another advantage of the present
invention that ~he removal of the solid by-product salt
in dry form from the system provides inexpensive
flexibility in the desired end use of such a product.
These and other objects, features and
advantages are provided in solid discharge handling
system including by-product salt handling apparatus
associated with a reactor vessel for the production of
a hypohalogenated acid from the mixing and reaction of
1~ an alkali metal hydroxide and gaseous halogen in the
reactor vessel. The salt handling apparatus permits
the continuous removal of the solids discharge from the
system and specifically permits salt to be removed in
dry form.
The advantages of this invention will become
apparent upon consideration of the following detailed
disclosure of the invention, especially when it is
taken in conjunction with the drawings wherein:
FIGURE l is a side elevational view of the
2~ reactor vessel;
FIGURE 2 is a bottom perspective view of the
ellipsoid inlet for the exhaust duct.
FIGURE 3 is side elevational view of the salt
handling apparatus.
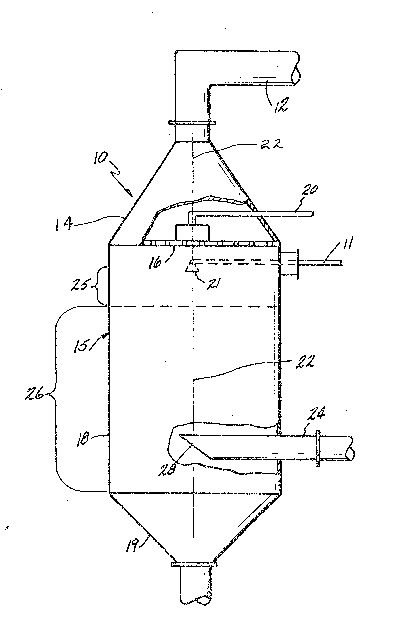
FIGURE l shows the reactor, indicated
generally by the numeral 10, which reacts th~ uid
alkali metal hydroxide, such as caustic, supplied by
feed line 11 with the gaseous halogen, such as
chlorine, to produce the solid salt crystals and the
3~ gaseous product, such as HOCl. Although the reactor
will be discussed in terms of producing hypochlorous
acid, it is to be understood that any halogen could be
employed to produce hypohalogenated acid, for example,
hypobromous or hypofluorous acid. Ths HOCl is
-5- .
2(:~7~)3~
condensed to produce liquid hypochlorous acid which,
for example, can be mixed with a lime slurry to produce
calcium hypochlorite. Gaseous chlorine, along with
some chlorine monoxide in the recycle system, is fed
into reactor 10 via gas infeed 12 in the top 14.
Top 14 is in the shape of an inverted funn~l, that can
be constructad of a suitable corrosion resistant
material, such as titanium; coated titanium; an alloy
of nickel, chrome, molybdenum, iron and other
1~ materials; tantalum; and lined carbon steel or lined
fiberglass reinforced plastic. The lining can be a
suitable polyfluoro-
polymer.
Reactor vessel 15 has a perforated plate 16
at the top between the reactor top 14 and the vessel
15. The plate 16 is also made of a suitable corrosion
resistant material, such as polytetrafluoroethylene or
one of the above mentioned materials with respect to
top 14, and serves to create a straight cocurrent flow
2~ path for the ch1orine gas flowing down from the
top 14. Ethylene chlorotrifluoroethylene has also been
used as a construction material for reactor vessel 15.
Vessel 15, similarly can be made from any suitable
corrosion resistant material, such as carbon steel with
a liner or coating of a suitable perfluoropolymer, such
as that sold under the tradenarne TEFLON(R) PFA.
Reactor vessel 15 has a generally elongated
cylindrical central section 18 which tapers to a
conically shaped funnel bottom 19 to permit solid
3~ alkali metal halide salt, such as NaCl, product to
discharge out through a standpipe, not shown, for
further processing. Vessel 15 has a caustic feed line
11 that enters through its side and provides the
caustic to an atomizer nozzle 21. Nozzle 21 is mounted
--6-- -
, ~
~01~7034
along the center line 22 of the vessel 15 about six
(06~ inches below the top of ve8sel 15. Nozzle 21
creates caustic droplets o~ a desired size between
about 50 to 200 microns which are of sufficient size to
absorb virtually all of the gaseous chlorine feed while
the chlorine and caustic react fast to produce the
gaseous and solid products as shown in the equation:
NaOH + C12 ? HOCl + NaCl
l~\
The reaction occurs at a pH of about 4 to
about 6 with a stochiometric ratio of about 30 to 1
chlorine to caustic. The gaseous HOCl is condensed
between about 0 to about 10C ater e~iting the
reactor to recover a concentrated HOCl solution.
Recycled gases, such as chlorine and chlorine
monoxide, are exhausted from the vessel 15 through
exhaust duct 24 and are fed back into reactor 10 via a
recirculation loop, after passing through a heat
2~ exchanger (not shown) to achieve the necessary heat,
when combined with the heat of reaction to evaporate
the hypohalogenated acid, such as hypochlorous acid,
and water phase to leave a dry sodium chloride or salt
solid by-product. The desired reaction temperature
ranges from about 80 to about 100 centigrade.
The recycled gases are also used as reactant gases in
the production of the hypohalogenated acid.
The recycled gases, for example chlorine and
chlorine monoxide, enter the reactor vessel lS,
3~ disperse outwardly in the inverted funnel top 14 and
pass through the flow directing means or perforated
plate 16 to enter the reactor vessel 15 in a generally
vertical flow orientation. Fresh halogen gas, for
example chlorine, is fed in through chlorine feed line
20 through the reactor top 14 and is directed down over
~ the nozzle or atomizer 21.
D7~
Nozzle 21 may be a single fluid atomizer, a
two fluid nozzle or a wheel atomizer dep~ndent upon the
viscosity and density of the alkali metal hydroxide
being atomized and the amount of pressure to which the
liquid is subjected. The materials of construction of
the nozzle must be capable of withstanding the
harshness ~f the environment within the reactor.
The vessel 15 has an outlet or exhaust duct
24 at the bottom of the dryiny zone 26 just above the
1~ funnel or conically shaped bottom 19 to remove the
product gas, the unreacted halogen gas and some
by-product into the recirculation loop as previously
described. Outlet or exhaust duct 24 exits through the
side of vessel 15 generally horizontally and has an
inlet 28 that is undercut such that the top overhangs
or overlies and covers the bottom to preclude solid
alkali metal chloride by-product, for example sodium
chloride, from falling directly into it. The preferred
shape of the inlet 28 is an undercut ellipsoid, as seen
2~ in FIGURE 2.
The vessel 15 has its central section 18
preferably cylindrically shaped, but it could also be
polygonal, as appropriate. The cylindrical lesign has
a desired diameter and length. The length extends from
the top at the perforated plate 16 to the bottorn oE the
drying zone 26, just above the fl~nnel bottom 19. The
dimensions of the length and the diameter can be
selected so that the length to diameter ratio, l/d, can
range from about 1 to 1 to about 1 to 5.
3~ In operation the halogen gas, for e~ample
chlorine, is fed into the reactor 10 through feed line
20 and is directed generally vertically downward over
nozzle 21. Recycled yases are fed in from the
recirculation system via gas infeed 12 into the reactor
3~
top 14 and are directionalized by perforated plate 16
down into reactor vessel 15. Vessel 15 has an elongate
cylindrical section 18 which has a spraying and drying
zone 25 adjacent the top surrounding nozzle 21 and a
drying zone 26 therebelow.
The reacted gases e~it the reactor 10 through
outlet or exhaust duct 24 for processing and
recirculation, as appropxiate. The solid by-product
alkali metal halide, such as sodium chloride, e~its the
1~ vessel 15 through the conically shaped funnel bottom 19
for processing. Bottom 19 is connected by conventional
flanging to connecting pipes (not shown).
The solid by-product alkali metal halogen is
dried as it passes down through the drying zone 26.
The overhanging top portion of exhaust duct 24 prevents
substantial quantities of the solid by-product from
being drawn out through the undercut ellipsoid inlet 28
with the product HOCl gas and the recycle gases.
Figure 3 shows the solids discharge handling
2~ apparatus preferably for granular solids indicated
generally by the numeral 45, connected to the bottom 19
of reactor vessel 15 via connecting pipe 20. Pipe 20
is connected to the salt handling standpipe 29 by
flanges 44.
Solid salt by-product is retained in the
standpipe 29 by valve means 41 that selectively
discharges the dry by-product while ensuring sufficient
quantity of solid salt by-product remains in the solids
discharge handling apparatus 45 to maintain a seal to
3~ the atmosphere, as previously described. Apparatus 45
initially can be seeded with the required amount of
solid by-product to establish the seal. Valve means 41
can be any appropriate system that is halogen
impervious and suitably sealable to prevent the escape
200t7~3g~
of the gaseous product to the surrounding atmosphere
and the intrusion of atmospheric gases into the
system. A double valve airlock system has been
employed using, for example, a disk valve and a knife
valve.
The desired by-product salt level within the
standpipe 29 is shown by numeral 31. This is at the
level or height of level controller 30 that has a
feed-forward connection to the valve means 41 to signal
when the valve means 41 should be opened for
discharge. Level controller 30 can be a microwave
level transmitter~sensor, as well as a point level
probe. One method of employing the feed forward system
has employed a solenoid valve as a pneumatic control
for the opening or closiny of valve means 41.
A stripping section, indicated generally by
the numeral 33, is connected to standpipe 29 by a
standpipe annulus 32. Annulus 32 consists of a
standpipe jacket 35 that is appropriately fastened to
2~ standpipe 29, such as by welding, and four exhaust
ports 34 90 apart in jacket 35 for carrying chlorine
gas and air to an exhaust manifold and scrubber (both
not shown). Stripper pipe 36 inserts within the lower
outwardly tapered portion of standpipe jacket 3S to
thereby form an annular chamber 37 from which the
chlorine and gas mixture is drawn off as a result of a
negative pressure system through the scrubber.
Stripping section 33 includes vertically
extending stripper pipe 36, stripper jacket 38 that
3~ extends about the periphery of pipe 36, stripper air
inlet 39 and perforated pipe sidewall section 40.
Stripping air is forced into the jac~et 38 under
pressure and is distributed into the solid salt
by-product by the perforati~ns in the sidewall of pipe
--10-- ,
:.,
Z1~7~3~
36 within the jacket 38. A 2-5 inch water pressure
drop across the bed of salt in standpipes 29 and 36
ensures 9OOa stripping of gases from the solid
by-product salt. Flow and pressure meters can be
employed to measure this pressure drop to verify that
successful stripping is occurring. The stripping air
ideally is introduced to the jacket 38 through a
dessicant to avoid the introduction of moisture into
salt. The porous solid salt by-product must be
1~ stripped of halogen, such as chlorine, because of the
tendency of the halogenated salt to clump together and
clog the standpipe and the handling apparatus.
Stripper pipe 36 is fastened with a gas tight
seal via flange 42 to valve means 41.
The solids discharge handling apparatus could
equally well be connected to a cyclone separator ~not
shown) employed within the process system to separate
out solids.
While the preferred structure in which the
2~ principles of the present invention have been
incorporated is shown and described above, it is to be
understood that the invention is not to be limited to
the particular details thus presented, but, in fact,
widely different means may be employed in the practice
of the broader aspects of this invention. The scope of
the appended claims is intended to encompass all
obvious changes in the details, materials, and
arrangement of parts which will occur to one of skill
in the art upon a reading of the disclosure.
,,
.,