Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
20~B96
~A492
-1-
METHOD OF TREATING HYPERTENSION
USING A THRO~OXANE A2 R~3CEPTOR ANTAGONIST
AND NEW COMBINAT I O
The present invention relates to a me~lod
for treating hypertension by administering a
thromboxane A2 receptor antagonist, which
preferably is a 7-oxabicycloheptane prostaglandin
analog, and to new combinations containi~g a
thromboxane A2 receptor antagonist.
In accordance with the present invention, a
method is provided for treating hypertension
wherein a therapeutically effective amou~t of a
` thromboxane A2 receptor antagonist is systemically
administered, such as orally or parenterally, to
reduce blood pressure in mammals, including humans,
during the period of treatment.
In addition, in accordance with the present
invention, new combinations are provided, for
treating h~pertension, containing a thromboxane A2
receptor antagonist and an angiotensin converting
.~ 25 enzyme inhibitor, a beta blocker, a diuretic, an
endopeptidase inhibitor anCVor human ANF 99-126.
The term "thromboxane A2 receptor
~ antagonist" as employed herein includes ompounds :
. which are so called ~hromboxane A2 receptor
30 antagonists, thromboxane A2 antagonists, ~ :
.~ "' .,
-
HA492
--2--
thromboxane A2/prostaglandin encloperoxide
;~ antagonists, TP-receptor antagonists, or
thromboxane antagonists except insofar as the
compound is solely an inhibitor of thromboxane
synth~sis.
Thromboxane A2 antagonists which may be
employed herein are specific inhibitors of the
actions of thromboxane A2 and therefore produce
t~e desired ef~ect of throm~oxa~e A2 inhibition
without causing other non-specific effects that
` may be undesirable.
The thromboxane A2 receptor antagonist
employed herein will preferably be a 7-oxabicyclo-
heptane prostaglandin analog and will include
7-oxabicycloheptane substituted diamide prosta-
glandin analogs as disclosed in U.S. Patent No.
4,663,336, 7-oxabicycloheptane substitutecl amino
prostaglandin analogs as disclosed in U.S. Patent
No. 4,416,896 a~d 7-oxabicycloheptane prostaglandin
analogs as di~closed in U.S. Patent No. 4,537,981.
; Other 7-oxabicycloheptane prostaglandin analogs may
be included as will be apparent to one skilled in
the art.
The 7-oxabicycloheptane substituted diamide
prostaglandin analogs suitable for use herein, as
disclosed in U.S. Patent No. 4,663,336, have the
. formula
(CH2)m~A~(CH2)n Q
< 1*/
(CH2~p-N - C-(c~2)q-N ~C-R3
O R O R O
:; :
:'
~,'.
, ,~ '
'1. ' " ' , ' '' ,
. .'~ ~' ' . ' , . :
~1 ' . . '
'.':, ~ ' ' , ' '
,
HA~92
--3--
including all stereoisomers thereof, wherein m is 0
to 4; A is -CH-CH- or -C~2-CH~-; n is 1 ~o 5; Q is
~CH=CH-, -CH2-,
IH H~alo Halo~ ~Halo
-C~ CH-, -C-
.
or a single bond; R is CO2H, CO2alkyl, CO2 alkali
metal, C02polyhydroxyamine salt, -CH20H,
: ~N -N
~ ~ or ~NR4R5
' ~'
; wherein R4 and R5 are the same or different and
are H, lower alkyl, hydroxy, lower alkoxy or aryl
at least one of R4 and R5 being other than hydroxy .
a~d lower alkoxy; p is 1 to 4; Rl is H or lo~er
20 alkyl; g is 1 to 12; R2 is H or lower alkyl; and
R3 is H, lower alkyl, lower alkenyl, lower
: alkynyl, aryl, arylalkyl, lower alkoxy, ~-
arylalkyloxy, aryloxy, amino, alkylamino,
arylalkylamino, arylamino, :
(Il)n ()n' (Il)n :~
lowex alkyl-S-, aryl-S-, arylalkyl-S-,
()n (,)n (I~n
aryl-S-alkyl-, alkyl-S-alkyl-, arylalkyl-i~-alkyl
(wherein n' is 0, 1 or 2), alkylaminoalkyl,
arylaminoalkyl, arylalkylaminoalkyl, alkoxyalkyl,
aryloxyalkyl or arylalkoxyalkyl.
:
. . .
2~7~396
HA492
_4_
; The 7-oxabicycloheptane subs~itu~ed amino
prostaglandin analogs suitable for use herein, as
disclosed in U.S. Patent No. 4,416,896, have the
formula
~H2-A- ( C'}~2 )ml CRa
*~
~ H2 )rl ~~H~Ra
0
and including all stereoisomers thereof, wherlein
A is C~=CH or (CH2)2; m1 is 1 to 8; n1 is 0
,! to 5, Ra is H or lower alkyl, and Ra is lower
alkyl, aryl, aralkyl, lower alkoxy, aralkoxy or
O
a
20 wherein R2a is lower alkyl, aryl, aralkyl, alkoxy,
~` aryloxy, aralkoxy, alkylamino, arylamino or
aralkylamino.
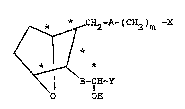
:~ The 7-oxabicycloheptane prostaglandin
analogs suitable for use herein, as disclosed in
25 U.S. Patent No. 4,537,981, have the formula
~2-A-(C~2)m -X
B-CH~Y
o OH
.
.,~
,.`~
.
, . ~ . .
., . :. . ' ' ' ' ' ' ' ' ' ' ' ' ' . . " ', .;`.~': . . , : - '
. ,, , ~ : ,. .: .. . . . :; ;:
.
~ 2t~ 7~96 ~:
HA492
_5_
and including all stereoisomers th~reof, wherein A
and B may be the same or differe~nt and A is
cH=CH or (c~2)2; B is CH=CH, c--c or
(~H2)2; m1 is 1 to 8;
X is OH;
N~-N
c~ il
N--N
: 10 CO2Ra wherein Ra is H or lower alkyl; or
' O :
:: It
CNH-Z
. wherein Z is H, lower alkyl, aryl, SO2-Q~
; (with Q1 being lower alkyl or aryl), -
I~ .
C-Q ,
" 1 ,~ .
or O~ wherein ~ is H, and Y is alkyl, ..
;. substituted alkyl; aryl-lower alkyl; alkenyl; , ~;
alkynyl, aryl; pyridyl; substituted pyridyl; ~-
20 pyridyl-lower alkyl; thienyl, substituted thienyl; :~.
thienyl-lower alkyl; cycloalkyl; cycloalkylalkyl;
substituted cycloalkylalkyl; or phenoxymethyl.
. Preferred exc~mples of thromboxane A2
receptor antagonists which may be employed herein
25 include the 7-oxabicycloheptane compounds disclosed ~ :
in U.S. Patsnt No. 4,537,981, especially, [lS-
[la,2a(5Z),3a(1E,3R,~S~,4a]]-7-~3-(3-hydroxy-4-
phenyl-l-pentenyl)-7-oxabicyclo-[2.2.1]hept-2-yl]-
5-heptenoic acid; the 7-oxabicycloheptane :~
substituted amino-prostaglandin analog~ disclosed
in U.S. Patent No. 4,416,896, especially, ~lS- :~
: [ la, 2~,(5Z),3~, 4a ~ ] - 7- [ 3 - ~ [ 2-(phenyl~mino)carbonyl]
hydrazino]methyl]-7-oxabicyclo[2.2.1]hept-2-yl]-5-
. ~'.
.,~. - . .
. . .
.. . . . ,, .. . , . ~ .,. . . ,. ., :. . . . , .~- .. .
HA492
-6-
heptenoic acid; the 7-oxabicycloheptane substituted
diamide prostaglandin analogs di~closed in U.S. ~.
Patent No. 4,663,336, especially, [1S-[1~,2al5Z),
3a,4~]]-7-[3-[[[[(1 oxoheptyl)amino~acetyl]amino]
; 5 methyl]-7-oxabicyclo[2.2.1]hept-.2-yl~-5-heptenoic
acid and the corresponding tetrazole, and [lS-
[la,2a(Z),3a,4~]]-7-[3-[[[[(4-cyclohexyl-1-
; oxobutyl)amino]acetyl]amino~methyl]-7-oxabicyclo~
[2.2.1Jhept-2-yl]-5-heptenoic acid.
Examples of other such thromboxane A2
antagonists suitable for use herein include but are
not limited to the phenoxyalkyl carboxylic aci.ds
disclosed in U. S. Patent No. 4,258,058 to Witte et
al, especially 4-[2-(benzenesulfamido)ethyl]phenoxy-
: 15 acetic acid, (BM 13,177 - Boehringer Mannheim),
the sulphonamidophenyl carboxylic acids disclosed
in U. S. Patent No. 4,443,~77 to Witte et al,
especially 4-[2-(4-chlorobenzenesulfonamido)-,
ethyl]phenylacetic acid, ~BM 13,505, Boehringer
Mannheim) the arylthioalkylphenyl carboxylic acids
disclosed in U. S. Patent No. 4,752,676 espscially
4-(3-((4-chlorophenyl)sulfonyl)propyl)benzeneacetic
acid, (E)-5-[~[(pyridinyl)[3-(trifluoro-
methyl)phenyl]methylene]amino]oxy]pentanoic acid
also referred to as R68,070 - Janssen Research
Laboratories, 3-[1-(4-chlorophenylmethyl)-5-
fluoro-3-~ethylindol-2-yl]-2,2-dimethylpropanoic
acid [(L-655240 Merck-Frosst) Eur. J. Pharmacol.
135(2):193, 17 Mar. 87], 5(Z)-7-([2,4,5-cis]-4-
(2-hydroxyphenylj-2-trifluoromethyl-1,3-dioxan-
5-yljheptenoic acid (ICI 185282, Brit. J.
:: Pharmacol 90 (Proc. Suppl):228 P-Abs., Mar. 87),
5(Z)-7 [2,2-dimethyl-4-phenyl-1,3-dioxan-cis-5-yl3-
, . . .
. ~ , . . ~
.' ' ' ',: . : " , :
' ., ' ' .,
',:. . ' . ' ' ;
.... ~
. . . . .. .
HA~92
--7--
heptenoic acid (ICI 159995~ Brit. J. Pharmacol. 86
(Proc. suppl):808 P-Abs., Dec. 85~,
N,N'-bis~7-(3-chlorobenzeneaminosulfonyl)-1,2,3,4- -
tetrahydro-isoquinolyl]disulfonylimide (SKF 88046,
~ 5 Pharmacologist 25(3):116 Abs, 117 Abs, Aug. 83),
; [la(Z)-2~,5a]-(+~-7-[5-[[(1,1'-biphenyl)-4-yl]-
methoxy]-2-(4-morpholinyl)-3-oxocyclopentyl]-4-
heptenoic acid (A~ 23848 - Glaxo, Circulation
72(6):1208, Dec. 85, levallorphan allyl bromide
(CN 32,191, Sanofi, Life Sci. 31 (20-21):2261, 15 ,~
; ~ov. 82), (Z,2-endo-3-oxo)-7-(3 acetyl-2-bicyclo-
[2.2.1]heptyl-5-hepta-3Z-enoic acid,
4-phenylthiosemicarbazone (EP092 - Univ.
Edinburgh, Brit, J. Pharmacol. 84(3):595, Mar. 85).
The disclosures of the above-mentioned
patents are incorporated herein by reference.
In carrying out the method of the present
invention, the thromboxane A2 receptor antagonist
may be administered systemically, such as orally
or parenterally, to mammalian species, such as
monkeys, dogs, cats, rats, humans, etc.
The thromboxane A2 receptor antagonist may
be incorporated in a conventional dosage form,
such as a tablet, capsule, elixir or injectable.
The above dosage forms will also include the
necessary carrier material, excipient, lubricant,
bufer, antibacterial, bulking agent (such as
mannitol), anti-oxidants (ascorbic acid or sodium
bisulfite) or the like. Oral dosage forms are
preferred, although parenteral forms are quite
satisfactory as well.
With regard to such systemic formulations,
single or divided doses of rom about 0.5 to about
. ' .
: .
2~37B96
~A492
2500 mg, preferably from about 5 to 500 mg one to
six times daily, may be administered in systemic
dosage forms as described above for a prolonged
period, that is, for as long as hypertension
continues. Sustained release forms of such
; formulations which may provide such amounts
biweekly, weekly, monthly and the lik~ may also be
employed.
The thromboxane antagonist can al90 be
formulated with a diuretic for the treatment of
hyper~ension. A combination product comprising
thromboxane antagonist and a diuretic can be
a~ministered in an effective amount which
comprises a totally daily dosage of about 30 to
600 mg, preferably about 30 to 330 mg of
thromboxane antagonist, and about 15 to 300 mg
preferably about 15 to 200 of the diuretic, to a
mammalian species in need thereof. Exemplary of
the diuretics contemplated for use in combination
with a compound of this invention are the thiazide
diuretics, e.g., chlorothiazide,
hydrochlorothiazide, flumethiazide,
hydroflumethiazide, bendroflumethiazide,
methylclo~hiazide, trichloromethiazide,
polythiazide or benzthiazide as well as ethacrynic
acid, tricrynafen, chlorothalidone, furosamide,
musolimine, bumetanide, triamterene, amiloride and
spironolactone and salts of such compounds.
In treating hyperten~ion, the thromboxane
receptor antagonists may be administered in
combination with an angiotensin-converting enzyme
(ACE) inhibitor such as captopril, zofenopril,
- fosinopril, enalapril, lisinopril, (S)~1-[6-amino-
~ .
- :," ~, " , . ,:
, , : ; , .
2~ 396
HA492
_ 9~
2-[[hydroxy(4-phenylbutyl)phosphinyl]oxy]-1-
oxohexyl]-L-proline (SQ 29,852) etc. employing the
ACE inhibitor in an amount as indicated in the
Physicians Desk Reference (PDR), for example,
from about 5 to about 350 mg per day. Such
combination would be at a weight ratio of
thromboxane antago~ist to ACE i~libitor of from
about 1:1~ t~ about 10:1.
; The thromboxane receptor antagonists can
; 10 also be administered in combination with a neutral
endopeptidase inhibitor or with human ANF 99 -
126 in treating hypertension. Such combination
would contain the ~hromboxane antagonist at from
about 1 to about 500 mg per day and an endopepti-
dase inhibitor of from about 1 to about 100 mg per
kg of body weight or the human ANF 99 - 126 at from
about 0.001 to about 0.1 mg per kg of body weight.
In addition, in treating hypertension, the
thromboxane receptor antagonists may be employed
in combination with a beta blocker such as
propranolol, ~adolol, timolol maleate, metoprolol
tartrate, labetalol hydrochloride and the like
employing ~he beta blocker in amounts as indicated
in the PDR such as in an amount of from about 5 to
about 350 mg per day. Such a combination would be
at a weight ratio of thromboxane antagonist to
beta blocker of from about l:lO to about 10:1.
The thromboxane receptor antagonist, in
treating hypertension, may also be used in
combination with a calcium channel blocker such as
dihydropyridines such as niedipine, nitrendipine,
nimodipine, phenylalkylamines such as verapamil,
benzothiazepines such as diltiazem and
., .
.. , '
''
~ . . . ., ;. . : . .. ..
'~ , . . ., , . ., ,, ' ,;. j, , . ~ ,: ,. .
2~:37B96
~A492
--10~
benzazepines and the like employing the calcium
channel blockex in amounts as indicated i~ the PDR
such as in an amount of from about 1 to about 1000
mg per day. Such a combination would be at a
weight ratio of thromboxane antagonist to calcium
channel blocker of from about 1:10 to about 10:1.
- The thrombo~ane receptor antagonist may
also be employed in combination with direct
vasodilators such as hydrala~ine or minoxidil.
` ~Q~g6 :
~A492
~he following ~xamples represent preferred
embodiment~ of the pr~sent invention.
Example 1
An injectable solution of ~hromboxane A2
receptor antagonist for intravenous use in treating
hypertension is produced as follows:
[lS;[la,2a(5Z),3a,4a]]-7-[3~[[2-
~phenylamino)carbonyllhydra7.ino]-
methyl]-7-oxabicyclo[2.~.l]hept-2-yl~-
5-heptenoic acid (SQ 29,548)2500 mg
Methyl paraben S mg
- Propyl paraben l mg
15 Sodium chloride 25 g
Water for injection gs. 5 1.
: The thromboxane A2 receptor antagonist,preservatives and sodium chloride are dissolved in
3 liters of water for injection and then the volume
is brought up to 5 liters. The solution is
filtered through a sterile filter and aseptically
filled into pre~terilized vials which are then
closed with presterilized rubber cloaures. Each
vial contains a concentration of 75 mg of active
ingredient per 150 ml of solution.
Exam~le 2
An injectable for use in treating
hypertension is prepared as described in Example 1
except that the thromboxane A~ receptor antagonist
employed is [lS-[la,2a(5Z),3a(1E,3R,4S),4a]] 7-[3-
.~ ' .
. ~ .. . . . . .
. : . . . ~ , ; -
.. . .. .. , . ~ . , . ;
~37l~6
HA492
-12-
(3-hydroxy-4-phenyl-1-pentenyl)-7-oxabicyclo-
[2.2.1]hept-2-yl]-5-heptenoic acid (SQ 28,668).
ExamPle 3
An injectable solution of thromboxane A2
receptor antagonist for use in treating
hypertension containing [lS-[1~, 2a ( 5Z ), 3a, 4a ] ] -7-
[3-[[[[(1-oxoheptyl)amino]acetyl]amino]methyl]-
7-oxabicyclo[2.2.1]-hept-2-yl]-5-heptenoic ac:id (SQ
30,741) as the thromboxane A2 receptor antagonist
is prepared as described in Example 1.
Example 4
An injectable for use in treating
hyperten~ion is prepared as described in E,xample 1
except that the thromboxane A2 receptor antagonist
employed is [lS-[la,2~(Z),3a,4a]]-7-[3-[[[[(4-
cyclohexyl-1-oxobutyl)amino]acetyl]amino]methyl]-7-
oxabicyclo[2.2.1]hept-2-yl]-5-heptenoic acid.
Example 5
, A thromboxane A2 antagonist formulation
suitable ~or oral administration for use in
;; treating hypertension is set out below.
1000 tablets each containing 400 mg of
thromboxane A2 recepkor antagonist are produced
from the following ingredie~ts.
. . . .
[lS-[la,2a(5Z),3a,4~]]~7-[3-[[[[(1-
Oxoh~ptyl)amino]acetyl]amino]methyl]-
7-oxabicyclo[2.2.1]hept-2-yl]-5-
heptenoic acid (SQ 30,741) 400 g
Corn Starch 50 g
Gelatin 7.5 g
:'
.
HA492
-13-
Avicel (microcrystalline cellulose) 2S g
Magnesium stearate 2.5 g
The thromboxane A2 receptor antagonist and
corn starch are admi~ed with an aqueous solution of
the gelatin. The mixture is dried and ground to a
fine powder. The Avicel and then the magnesium
stearate axe admixed with the granulation. This
is then compressed in a tablet to form 1000 tablets
each containing 400 mg of active ingredient.
Example 6
A thromboxane A2 antagonist tablet
: formulation for use in treating hypertension is
prepared as described in Example 5 except that SQ
29,548 is employed as ~he thromboxane A2 r.eceptor
antagonist in place of SQ 30,741.
.~ ,
Exam~e 7
A thromboxane A2 antagonist tablet
; formulation for use in treating hypertension is
prepared as described in Example 5 except that SQ
28,668 is employed in place of SQ 30,741.
It will also be appreciated that the above
thro~boxane A2 antago~ist formulations may also
include a beta blocker such a~ propranolol or
atenolol, an ACE i~libitor such a~ enalapril,
lisinopril, zofenopril, fosinopril or SQ 29,852,
an endopeptidase inhibitor or human ANF 99-126,
a diuretic or a vasodilator.
~.
.. . .. .
'',.,' ' ,,., '" '' , : ,
` 2~7139Ç~
HA492
-14-
Example 8
A thromboxane receptor A2 antagonist-
calcium channel blocker formulat:Lon suitable for
oral administration for treating h~pertension is
set out below.
1000 tablets each contain:Lng 400 mg of
thrombo~ane A2 antagonist and 100 mg diltiazem are
produced from the following ingredients.
[lS-[la,2a(5Z),3a,4a]]-7-[3-~[[[(1-
Oxoheptyl)amino]acetyl]amino~methyl]-
7-oxabicyclo[2.2.1] hept-2-yl]-5-
heptenoic acid (SQ 30,741) 400 g
Diltiazem 100 g
15 Corn starch 50 g
Gelatin 7.5 g
Avicel (microcrystalline cellulose) 25 g
Magnesium stearate 2.5 g
The thromboxane antagonist, diltiazem and
corn starch are admi~ed with an aqueous solution of
; the gelatin. The mixture is dried and ground to a
fine powder. The ~vicel and then the magnesium
stearate are admixed with the granulation. This is
then compressed in a tablet to form 1000 tablets
each containing 500 mg of active ingredients.
Example 9
An injectable solution for use in
administexing calcium channel blocker and
thromboxane A2 receptor antagonist is produced as
follows:
.' .
~ ,
.
` 2~7~6
HA492
-15-
[lS-[1~2~(5Z),3a,4~]]-7-[3-[[2-
(phenylamino)carbonyl]hydrazino]-
methyl]-7-oxabicyclo[2.2.1]hept-2-yl]-
5-heptenoic acid (SQ 29,548)
5 or SQ30,741 2500 mg
Nifedipine or diltiazem2500 mg
: Methyl paraben 5 mg
Propyl paraben 1 mg
Sodium chloride 25 g
:. 10 Water for injection qs. 5 1.
The calcium channel blocker, thromboxane A~
antagonist, preservatives and sodium chloride are
:dissolved in 3 liters of water for injection and
then the volume is brought up to 5 liters. The
solution is filtered through a sterile filter and
aseptically filled into presterilized vials which
are then closed with presterilized rubber closures.
Each vial contains a concentration of 75 mg of
active ingredient per 150 ml of solution.
ExamPle 10
~ n injectable for use in treating
hypertension is prepared as described in Example 9
25 except that the thromboxane ~2 antagonist employed
. i~ the phenoxyalkyl carboxylic acid 4-[2-(benzene-
sulf~mido)ethyl]phenoxyacetic acid, disclosed in
U. S. Patent No. 4,258,058.
Example 11
An injectable for use in trPating
hypertension is prepared as described in Example 9
. .
.. .
: :
,' ' '`'~' '' .' ' ': '. . 1'.'.'. .'. . '. . ' ' ` ''
' "'"' ;' .
. . . :'
- \~
Z~78~6
EA~92
-16-
except that the thromboxan2 A2 antagonist employed
is [lS-[la,2~(Z),3~,4~]~-7-[3-[[[[(4-cyclohe~yl~
l-oxobutyl)amino]acetyl]amino]methyl]-7-oxabicyclo-
[2.2.1]hept-2-yl]~5-heptenolc acid.
i Example 11 ~ -
1000 tablets each containing 200 mg of
SQ 32,324 and 400 mg SQ 30,741 are produced from
the following ingredients:
1 0
(d-cls)-3-(acetyloxy)-1,3,4,5-
tetrahydro-4-(4-methoxyphenyl)-1-
[2-(methylamino)ethyl]-6-(trifluoro-
methyl)-2H-1-benzazepin-2-one,
monohydrochloride salt (SQ 32,324) 200g
SQ 30,741 400g
Lactose lOOg
Avicel 150g
Corn starch 50g
20 Magnesium stearate 5g
.
SQ 32,324, SQ 30,741, lactose and Avicel are
admixed, then blended with the corn starch.
Magnesium stearate is added. The dry mixture is
compressed in a tablet press to form 1000 505 mg
tablets each containing 200 mg of active ingredient.
The tablets are coated with a solution of Methocel
E 15 (methyl cellulose) including as a color a lake
,; containing yellow #~. The resulting tablets are
useful in treating hypertension.
~' :
, '
.
.'' ,
:` .
, .,',~, -.. ,
7~ 6
HA492
-17-
Example 12
Two piece #l gelatin capsules each
containing 50 mg of captopril an~l 250 mg of SQ
30,741 are filled with a mixture of the following
ingredients:
SQ 30,7`41250 mg
Captopril 50 mg
Magnesium stearate 7 mg
10 UsP lactose 193 mg.
The resulting capsules are useful in
treating hypertension.
lS Example 13
An inject~ble solution for u~e in treating
hypertension is produced as follows:
(d-cis3~3-(acetyloxy)-1-[2-dimethyl-
amino)ethyl]-1,3,4,5-tetrahydro-4-
(4-methoxyphenyl) 6-~txifluoromethyl)-
2H-1-benzazepin-2-one, monohydro-
chloride (SQ 31,765) 500 mg
SQ 30,741 25Q mg
2~ Me~hyl paraben 5 mg
Propyl paraben 1 mg.
Sodium chloride 25 g
Water for injection qs. 5 1.
SQ 31,765, SQ 30,74~, preservatives and
sodium chloride are dissolved in 3 liters of water
.,
.,
: , ,:
Z0~7B96
H~492 -~
-18-
for injection and then the volumle is brought ~p to
5 liters. The solu~ion is filt~rled through a
sterile filter and aseptically filled into
presterilized vials which are th,_n closed with
.. 5 presterilized rubber closures. Each vial contains
5 ml of solutio~ in a c:oncentration of 100 mg of
active ingredient per ml of solution for injection.
Example 14
A thromboxane antagonist-beta blocker ::
formulation suitable for oral administration for
treating hypertension prepared as described in
Example 1, is set out below.
1000 tablets each containing 400 mg of
; 15 thromboxane antagonist and 40 mg nadolol are
produced from the following ingredients.
SQ 30,741 400 g
Nadolol 40 g
20 Corn star~h 50 g
Gelatin 7-5g
'. Avicel (microcrystalline cellulose) 25 g
Magnesium stearate 2.5 g
, :
Examle 15
` The following experiment demonstrates that
a thromboxane receptor antagonist decreases blood
pressure.
A thromboxane receptor antagonist
; 30 [lS-[la,2a(5Z),3~,4~]]-7-[3-~[[[(1-oxoheptyl)- :
~ amino]acetyl]amino]methyl~-7-oxabicyclo-
: '
: ' ~
~, .
:.'.
.- . . . .
~C~
HA492
--19 - .
[2.2.1~hept-2-yl] 5-heptenoic acid (SQ),
or placebo (PLA) was given to 3 groups of 12
healthy human subjects (8 per group received SQ and
4 PLA). SQ was given as a single 50 mg IV bolus
followed by an infusion of 3, 6 or 12 mg/hr for 48
hours. ~ean pharmacodynamic (PD) parameters at 48
hours were:
% Decrease
Max. % ~% IncreasePlatelet
Bleeding Aggregation
Dose Diastolic BPTime low hiqh
PLA -14~3 22~15 -9+11 -7~6
3 -20~3 156$55* 98~4* 89~17*
6 -18+3 111~19~ 100+0* 76i7*
12 ~28+~* 122i34~ lOOiO~ 88il6*
(*p<O.05 vs. PLA)
SQ specifically inhibited TXA2-mediated
platelet aggregation ex vivo at low and high
agonist (U46619) concentrations. Mean platelet
TXA2 receptor (R) affinity and density measured
ex vivo were not changed. SQ increased template
bleeding time when R occupancy was ~99% but not
when R occupancy was ~70%.
From the above, it is seen that the
thromboxane receptor antagonist reduced diastolic
blood prescure.
:
,,
.:
~..