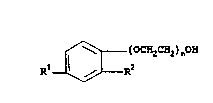Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2011~91
WETTING AGENT FOR AGRICULTURAL AND PLANT GROWTH MEDIA
The present invention relates generally to
compounds useful as wettinq agents for s~illess mixtures
to improve the water penetration, absorption and
retention properties thereof for use in agricultural and
horticultural industries. More specifically, the
invention relates to novel compounds characterized by
improved ability to wet soilless mixtures, improved
stability and by less toxicity to the plants which are
grown in the mixtures treated with these wetting agents.
Backaround of the Invention
Wetting agents, or surfactants have been used
to treat a variety of organic or synthetic plant growth
media, particularly growth media composed primarily of
aoilless materials, such as peat or bark. These
surfactants are employed to increase the ability of the
plant growth media to be uniformly penetrated by water
and absorb and retain moisture. See, e~g., U.S. Patent
2,867,944. Surfactants or wetting agents also enhance
the ability of plant growth media to drain excess water
~rom the media. Such wetting agents have recently been
employed in the maintenance of sports fields, turf and
golf courses, as well as for the traditional uses in
horticultural and agriculture.
20111~1
A variety of compounds selected from the class
of mono-, di- and trialkyl phenol ethylene oxide
condensates, have been used as wetting agents. For
example, Rainbow, U. S. Patent 4,404,013 describes a
plant growth medium comprising peat and a wetting agent
which is an alkylene oxide condensate of a tri
(alkyl)substituted phenol or a salt or an ester thereof.
Each alkyl substitution may be the ~ame or different and
is a straight or branched alkyl group of from 1 to 12
carbon atoms. The preferred alkyl group contains less
than 8 carbon atoms, especially 2-6 carbon atoms. There
are preferably 6-10 ethyoxylate groups. A tri (butyl)
substituted phenol condensate with ethylene oxide is
shown in the examples.
Wahlberg, U. S. Patent 3,231,365 refers to a
plant growth composition comprising a fertilizer and an
organic nonionic wetting agent and which may also include
peat or kieselguhr. The wetting agent may be a compound
of the formula: R-O-(CH2CH2O)820CH2CH2OH, where R is an
aralkyl group having a straight or branched chain
hydrocarbon group of 8 to 18 carbon atoms attached to the
aryl nucleus and attached to -O- through the aryl
nucleus.
201119~
British Patent 1,420,522 di6closes a soilless
plant growth medium containlng, as a wetting agent, an
alkyl phenol condensate with 8.5 mols of ethylene oxide,
the alkyl group containing 8-9 carbon atoms.
British Patent 1,375,829 discloses a soilless
plant growth medium containing a non-ionic wetting agent,
such as a condensate of nonyl phenol with 9 mols of
ethylene oxide.
Nunn et al, U. S. Patent 3,317,612 discloses
biodegradable surface active agents, including wetting
agents, obtained by the ethoxylation of the product
resulting from the reaction of phenol and an excess of
butene-2, which reaction product includes di-secondary
butylphenol, among other substituted phenols ethoxylated
with 9 mols of ethylene oxide.
Kistner, U. S. Patent 3,805,532 pertains to the
stabilization or consolidation of aggregate materials for
soilless planting media purposes and discloses the use of
a surfactant to insure the complete wetting of the
aggregate particles. Polyoxyethylene derivatives of
arylhydroxy compounds are among the surfactants shown.
Sterrett U. S. Patent 4,067,716 and Webb et al.
U. S. Patent 4,174,957 relate to manufactured soilless
growing media which may include wetting agents.
~11191
There remains a need in the art for additional
compositions and methods for improving the moisture
penetration, retention, and absorption of soilless
mixtures used to support the growth of plants and crops,
which provide less of a health hazard to the plant
itself.
Summary of the InventiQn
The present invention involves the unexpected
discovery that a class of compounds exist which
demonstrate surprising advantages as wetting agents for
~oil~ess mixtures of plant growth media in comparison to
known compounds for that use. The invention provides
condensates of dialkyl phenol with from 5 to 16 mols of
ethylene oxide, with each alkyl group on the phenol
containing from 5 to 7 carbon atoms. This group of
compounds, as a whole, provides significant advantages
over the compounds disclosed in the art, such as the
condensates of 9 mols of ethylene oxide with di-secondary
butylphenol; with nonyl phenol and with tributyl phenol
described in the references above.
2011~91
In one aspect, therefore, the present invention
provides a clas~ of compounds compri6ing ethoxylated diC5
7alkyl phenols, according to the following rormula:
~H3 ~ (ocHzcH2)noH
CH3 -~HCH2CH2 ~ CHzCH2CH-CH3
CH3
wherein the ethoxylate chain has between 5 and 16 mols
ethoxylate units. A desirable class of such agents
contains between 7 and 11 mols ethoxylate units. The
presently preferred material is a compound derived from
2,4-ditert-amyl phenol having an average of nine mols
ethoxylate units.
As another aspect, the invention provides a
wetting agent or surfactant composition comprising an
ethoxylated diCs7alkyl phenol, having 5 to 16 mols
ethoxylate units in association with a suitable carrier.
As 6till another aspect, the invention provides
a wetting agent or surfactant composition comprising a
mixture of two or more ethoxylated diC57alkyl phenols,
having an average of 5 to 16 mols ethoxylate units in a
suitable carrier.
A further aspect o~ the present invention
provides a method for treating soilless materials to
enhance the wettability thereof and provide substantially
2011191
improved safety to the growth of plants therein. The
method involves applying to a soilless mixture an
effective amount of a wetting composition described
above. An effective amount, for purposes of this process
comprises about one to about five ounces per cubic yard
of the soilless materials.
As a wetting agent or surfactant composition
for soilless plant growth media, the compounds of the
present invention are characterized by improved ability
to wet the growth media, providing to it enhanced
moisture distribution, penetration, and absorption. The
compositions of this invention are further characterized
by improved stability upon storage, in comparison with
known wetting agents. Additionally, the compounds of the
present invention demonstrate improved safety to growing
plant materials when used to wet plant growth media.
These compounds are less ~amaging and less toxic to the
plants grown in media treated with these compounds than
in media treated with other known wetting agents.
Other aspects and advantages of the present
invention are described further in the following detailed
description of preferred embodiments of the present
invention.
2011191
Brief ~escription of the Drawinqs
Figs. 1 through 6, described more specifically
below, are graphs which plot the wetting curves of an
illustrative compound of this invention, ACA0160, which
contains a 2,4-ditert-amyl phenol having an average of
nine ethoxylate substituents, compared to those of known
wetting agents, when tested in a compacted peat medium.
The ordinates of the graphs in Figs. 2-6 plot the Percent
of Control. As defined herein, the Percent of Control is
a value expressed as a percentage of the average time in
seconds re~uired to fully hydrate a measured volume of
compacted peat with a specific concentration of a wetting
agent as compared with the time required with water
containing no wetting agent. The abscissa of the graph
plots the concentration of the compound applied per cubic
yard. These graphs and figures are described further in
Example 2 below.
Fig. 1 illustrates a wetting curve of ACA0160
and AquaGro-L, plotting time in seconds vs.
concentration.
Fig. 2 illustrates three separate trials
~marked by a square, a cross, and a diamond) of ACA0160
and an average curve (marked by a triangle) generated by
tho~e trials.
2011191
Fig. 3 ~llustr~tes comparative wetting curves
of three compounds: ACA0160 (square), diamyl phenol with
8iX mols ethoxylate units (cross), and diamyl phenol with
12 mols ethoxylate units (diamond~.
Fig. 4 illu~trates comparative wetting curves
of four compound~: ACA0160 ~square), 2,4-ditert-butyl
phenol with 9 mols ethoxylate units (cross), disec-butyl
phenol with 9 mols ethoxylate units (diamond), and 2,6-
ditert-butyl phenol with 9 mols ethoxylate units
(triangle).
Fig. 5 illuctrates comparative wetting curves
of three compounds: ACA0160 (square), butyl phenol with 9
mols ethoxylate units (cross) and nonylphenol with 9 mols
ethoxylate units (diamond).
Fig. 6 illustrates ~ wetting curve of a second
~ource of nonylphenol with 9 mols ethoxylate units
(~quares).
~Detailed Description o~ the Invention
The compound~ of thi6 invention may be
de~cribed by reference to the formula appearing below:
~ ( OCH2CH2 ) nOH
R1~--R2
P 20111gl
in which n is an integer of between 5 to 16, R1 is the
same as R2 or a lower alkyl containing from 5 to 7 carbon
atoms, and R2 is a lower alkyl containing from 5 to 7
carbon atoms. For example, such compounds as 2,4-ditert-
amyl phenol, dihexyl phenol, diheptyl phenol, 2,4-diamyl
phenol, 2,4-disec-amyl phenol and 2,4-ditert-hexyl phenol
having ethoxylate groups of between 5 and 16 mols are
provided by this invention.
Preferred compounds according to this invention
are characterized by the presence of 7 to 11 mols
ethoxylate units. A presently most preferred compound is
characterized by 9 mols ethoxylate units. Preferred
compounds are also characterized by R1 and R2 being
identical amyl alkyl groups.
These compounds are prepared by the
conventional base-catalyzed epoxide reaction, as
described below in Example 1. The method of calculating
the mols of ethoxylate units on the desired compound is
the conventional method referred to as AOCS Method CD 13-
60. Thi6 method is known to one of skill in the chemical
arts and is described in detail in Example 1 below.
For use in a wetting agent or surfactant
composition, the compounds of the present invention may
be used singly or as a mixture of compounds, having
average ethoxylate units in the ranges described above.
2~
Whether used as a single compound or a mixture of
compounds as described above, the presently preferred
number of average mols ethoxylate units is between 7 and
11, and most preferably 9.
The wetting agent compositions of the present
invention can be prepared in either liquid or dry
formulations. For use in a liquid formulation, the pure
compound can be diluted to an acceptable concentration
with water. However, granular formulations of these
wetting agents may also be prepared by adsorbing the
compounds to a carrier, such as vermiculite or other
conventional dry agricultural carrier. The preparation
of a dry composition involves conventional techniques,
such as blending the compound with the carrier in a
blender so that the compound absorbs to the dry carrier.
This type of technique and other techniques for preparing
dry formulations of the wetting agent are known to those
of skill in the art.
For use in the liquid wetting agent
compositions, a preferred concentration of compound to
water i6 between approximately 300 to 3000 ppm. For
example, that range is acceptable for a preferred
compos$tion, ACA0160, a mixture of 2,4-ditert-amyl phenol
having an average of 9 mols ethoxylate units. This
wetting agent performs well when present in water at 10
~ 2~11191
to 3000 ppm. For a dry composition, the compounds or
mixtures thereof are desirably present in the dry carrier
at concentrations of between approximately 15 to 40%, and
more preferably at 40%. However, the concentrations of
the compounds in the selected liquid or dry carrier may
be easily determined by one of skill in the art for the
particular application. This invention is not limited to
any precise concentrations defined herein, but
encompasses all concentrations, which may vary based on
the type of soil or soilless material being wetted.
The wetting agent compositions according to the
present invention may be applied to the plant growth
media as liguids or solid granular form by conventional
means, ~uch as spraying, depending on the amount of media
to be wet. Generally, the concentration of wetting agent
according to this invention per size of media is
approximately 1 to 5 ounces per cubic yard, or
approximately 100 to 1500 ppm per cubic yard. This
application may also change based on the composition of
the growth media, e.g., peat, bark, turf, sandy soil, and
the like.
The compounds of the present invention are
characterized as surprisingly advantageous wetting agents
for use on soilless plant growth materials or in soils
substantially contaminated with sand or other non-soil
~ 2~11191
suhstances. In contrast to known wetting agents for
similar uses, the compounds of this invention demonstrate
a more efficient wetting time on such mixtures, such as
peat, peat-lite and bark mixes used for the production of
greenhouse crops and synthetic soil replacement
materials. They may alsQ be used in natural soils,
particularly turf soils as well as æoils used to grow
woody ornamental trees and shrubs. The Examples below
illustrate the improved "wettability" of illustrative
compositions of the present invention in comparison to
known wetting agent compounds.
An additional advantage of the present
invention lies in its improved safety upon exposure to
plants. The compounds of the present invention are shown
to cause very little damage to plants, as is illustrated
by root growth studies, and studies of the compound's
effect, or lack thereof, on other observable
morphological characteristics of plants grown in soilless
media treated with compounds of this invention, as
compared with other known wetting agent compounds.
Still a further advantage of the compounds
described herein is an enhanced stability of these
compounds in storage. This advantage relates primarily
to the ability to transport and store the compounds under
a variety of conditions without danger of the compounds
2~
becoming unstable and losing their advantageous
properties.
The compounds and wetting~surfactant
compositions containing these compounds may also find
application in other industries, such as asbestos
remo~al.
The examples below illustrate the preparation
of the compositions of this invention, and their
advantages.
E~mple 1 - Pre~aration of a Compound of this Invention
The presently preferred compound of the present
invention is 2,4-ditert amyl phenol having an average of
9 mols ethoxylate units on the phenol. This compound is
prepared by the conventional reaction of base catalyzed
lS cleavage of epoxides. Essentially, to prepare 10,000
pounds of the 2,4-ditert amyl phenol having an average of
9 mols ethoxylate units, 3,698 pounds of 2,4-ditert-amyl
phenol is introduced into a charging vessel. Into this
charging vessel is introduced approximately 6,258 pounds
o~ ethylene oxide. Potassium hydroxide is used as a
catalyst at a concentration of 0.1%. The reaction is
allowed to continue for approximately 4 to 8 hours at
approximately 140 to lS0C. One of s~ill in the art may
prepare larger or smaller amounts of the compounds of
?J01~191
this invention by reducing the amounts of reactants
proportionally.
After the reaction ceases, the resulting
average ethoxylation of the resulting compound is
established according to the American Official Chemical
Society (AOCS) Official Method Cd 13-60. This method is
a well known method to chemists and others of skill in
the art.
To produce similar compounds of the present
invention, having average ethoxylations of between 5 to
about 16 mols ethoxylate units, the above reaction is
carried out by varying the amounts of the reactants. One
of skill in the art following the above teachings could
produce such similar compounds.
ACA0160 is employed as the pure compound in all
of the remaining examples to illustrate the advantages of
compounds of this invention over known wetting agents.
Also employed in these comparative tests is the industry
standard wetting agent, called AquaGro-L, commercially
available from Aquatrols, Inc., Pennsauken, New Jersey.
This compound is not a dialkyl phenol, and is therefore
les6 similar to the compounds of this invention than the
other known wettlng agents. However, it is included in
the tests because it is a wetting agent of choice in the
industry for plant growth media. The following tests
2011~91
demonstrate the superior advantages attaching to the
compounds of this invention, even in contrast to the
industry ~tandard.
~xample 2 - Wettina Speed Test
To evaluate the Qpeed of wetting of various
wetting agents including an illustrative compound of the
present invention, each compound is prepared in the
following concentrations in water. For convenience in
charting the efficacy of these compounds in the wetting
test system described herein, the numbers along the
abscissa of the graphs in Fig. 2-6 were arbitrarily
asaigned to represent the following concentrations per
cubic yard. These numbers are charted in Table 1 below:
Table 1
NumberConcentration (~m)
9 1500
8 1333
7 1167
6 1000
833
4 667
3 500
2 333
1 167
O O
2~11191
16
Fifty milliliters o~ each solution is placed in
a standard petri dish containing a compressed peat disk,
commercially available as a Jiffy 7 ~Jiffy Products (NB),
Ltd, Canada3.
In this test the time in seconds required to
fully hydrate a measured volume sf compacted peat is
determined at various concentrations of wetting agent.
Results are shown in Fig. 1 which shows wetting curves
for ACA0160 and AquaGro-L [Aquatrol, Inc.] in a compacted
peat medium. The value for the control (no wetting
agent, not shown) is >900 seconds.
ACA0160 outperformed AquaGro-L at all rates
tested. Of particular note is the fact that the
inflection point (point at which the slope changes) of
- 15 the wetting curve for ACA0160 was lower than 1 ounce/yd3,
while that for AquaGro L was between 667 and 88~ ppm
~4.5)ounces/yd3. These results show that ACA0160 is not
only a much faster wetter than AquaGro-L, the industry
~tandard, but also that it is effective at much lower
concentrations.
Figs. 2-6 are results of identical tests with
other commercially available wetting agents, or with
compounds described in thé prior art. These figures
demonstrate that ACA0160 is a superior wetting agent.
20 111 91
Based on these teæts, Table 2 below rates the ability of
known surfactants to wet peat.
Table 2. Ability of Various Surfactants to Wet Peat
0 = best; 9 = worst; EO - ethoxylate unit
Fig./Curve Identi~y _ atina
-- Ditert Butyl Phenol (14 EO) 6
1 AquaGro-L 6
1-6 ACA0160 0-1
3 (cross) Diamyl Phenol (6 E0)
3 (diamond) Diamyl Phenol (12 E0) 2-3
4 (cross) 2,4-Ditert-Butyl Phenol (9 EO) 4-5
4 (diamond) Disec 8utyl Phenol (9 E0) 4-5
4 (triangle) 2,6-Ditert-Butyl Phenol (9 E0) 5
Example 3 - Plant/~rop Safety Evaluation
ACA0160 has been evaluated both foliarly and in
a wide variety of peat and bark mixes for its effects on
plant growth.
A. In the following test where ACA0160 was
compared with AquaGro-L for plug production. The rates
selected for AquaGro-L exceed the manufacturer's
recommendations.
~ 2011191
In this test plug mixes of commercial bark and
peat were treated with the level of AquaGro-L or ACA0160
indicated in Table 3 below and given to a commercial
grower for evaluation. Except for the differences in the
wetting agent, no other cultural practices were changed.
All mixes wet easily except for the control which was
heavily misted to insure a moisture content equivalent to
the treated mixes.
Of the five species evaluated in this test only
Impatiens and Begonia, which are known sen~itive plant
species, responded and then only at the 1500 ppm rate of
AquaGro-L [recommended rate is between 667-833 ppm
(approximately 4.5 ounces)/yd3 for plug production]. No
adverse effect on germination or seedling morphology
(including roots) was observed for ACA0160 at any
concentration tested. Thus, depending upon the rate
used, ACA0160 can be expected to have a 3 to 9 fold
safety factor for wetting agent sensitive species, such
as Impatiens or Begonias. Results are shown in Table 3.
~ 201~191
Table 3. Comparative Ef fect on Plant Growth of Two
Growth Media Wetting Agents used in Plug
Production
0 = no effect; 9 2 severely inhibited or dead
Treatments (ounces/Yd3)
SpeciesControl ~Ç~m ÇQ ~quaGro-L
(no wetting
agent~ ~ 9 3 9
Percent_Germination
Impatiens85 85 85 85 45
Marigold 95 90 95 90 90
Begonia 80 85 85 80 40
Tomato 95 95 95 95 95
Pepper 90 95 90 95 90
Phyto~oxicity
Impatiens 0 0 0 0 6
Marigold 0 0 0 0 0
Begonia 0 0 1 0 7
Tomato 0 0 0 0 0
Pepper 1 0 0 0 0
B. In a second phytotoxicity test, the
inherent phytotoxicity of ACA0160 was determined in
comparison with AquaGro-L. In this test, sheets of
filter paper are placed in petri dishes. Seven ml of
solution of the concentrations indicated in Table 4 are
added and ten seeds of Impatiens are sown on the
moietened ~ilter paper. The dishes are then covered. In
this test, the wetting agent remains in solution so that
the seed and seedling are in intimate contact with the
~ 201119~
free surfactant. The results of the test are described
in Table 4 which illustrates that Impatiens were less
sensitive to free ACA0160 than to free AquaGro-L.
Table 4. Effect of Free Wettinq Agent on Root Growth of
Impatiens Grown on Moistened Filter Paper
Concentration (pp~ Root Lenath (cm~
AquaGro-L ACA0160
1170 0 0
1000 0 5
833 0 5
667 5 10
500 13 17
333 12 23
166 16 20
0 (Control, no wetting agent) 22
C. ACA0160 was also eva~uated as a foliar
application in comparison with AquaGro-L. In this test
plants were sprayed at the rate indicated in Table 5 and
observed at 24 and 96 hours for signs of foliar damage.
ACA0196 tTricol 6972, Emery Chemical Co.] is a good
wetting agent which demonstrates phytotoxicity to plants,
like many surfactants. ACA0196 which had similar wetting
characteristics as ACA0160, was included in this test for
comparative purposes.
2011191
Neither AquaGro-L or ACA0160 had a significant
adverse effect on foliage. ACA0196 as the positive
control gave results typical of phytotoxic surfactants.
Table 5. Comparative Foliar Toxicity of Three Surfactants
0 = no effect; 9 = dead
Rate (ounces/1000 ft) Impatiens Pepper RyeGrass
10AquaGro-L
4 0 0 0
8 0 0 0
16 0
15ACA0160
4 o 0 0
8 o 0 0
16 1 0 0
20ACA 196
4 3 5 4
8 8 9 7
16 9 9 g
25Control 0 0 0
Example 4 - Evaluation Qf Storaae Stability in Soilless
Media
ACA0160 was evaluated for storage stability in
comparison with AquaGro-L. Peat moss treated with either
(3 ounces)/yd3 of ACA0160 or (9 ounces)/yd3 of AquaGro-L
were prepared and placed in Zip-Lock bags. Half of the
baga were left open to simulate an opened bale, or media
placed in growing containers but not in use. The closed
bags represented a sealed
201~19~
container as it might come from the manufacturer. At
weekly intervals an open and closed bag were selected for
each treatment and were evaluated for wettability by
percolation tests.
In the percolation test, 200 cc of peat or
media are placed in an inverted tube with a screen on the
bottom, and 200 ml of water are added. The milliliters
of water absorbed and the percent of peat or media wet
are then determined. Moisture loss was monitored
throughout the course of the experiment in both open and
closed bags. The open bags lost moisture quickly but did
not stabilize until week ll, after which little or no
more drying occurred. The closed bags also lost
moisture, but at a much slower rate. However, by week 29
- 15 both open and cloeed bags had the same moisture content
(12%). These results are illustrated in Table 6.
* 2011191
Table 6. ACA0160 and AquaGro-L Storage Stability Study
for Ease of Wetting in Open and Closed Bags
Storage Time %_Wet ~200/200 Percolation Test~
5~Weeks~
ACA0160 AquaGro-L3
(3 ounces/yd3~ Is ounçes~yd )
Open Closed Open Closed
100 100 100 100
2 100 lOO 9o 100
4 90 100 9~ 90
7 90 100 50 90
11 65 80 S0 50
13 40 95 5 70
19 60 60 5 50
29* 50 50 10 10
* At 29 weeks, moisture content of open or closed bags
was approximately equal.
This gradual decrease in moisture content
affected performance of both products. Generally,
rewettability is inversely proportional to wetting agent
rate and moisture content, which accounts for the gradual
decline in effectiveness of the two wetting agents with
time. ACA0160 did, however, demonstrate superior
rewetting characteristics even after severe dehydration
of the peat.
Numerous modifications and variations of the
pre~ent invention are included in the above-identified
specification and are expected to be obvious to one of
skill in the art. For example, the use of the compounds
2 o ~
24
of this invention for their advantageous properties as
wetting agents or surfactants in other than soilless
media may be expected. Additionally, these compounds may
prove useful for asbestos removal. Thus, other uses for
these compounds may become obvious upon a review of their
advantageous properties. Modifications and alterations
to the compositions and processes of the present
invention to suit such other uses are believed to be
encompassed in the scope of the claims appended hereto.