Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
_ 1 _
RAN 4602/25
The present invention is concerned with the use of
mixed micelles as carriers for the parenteral administra-
tion of pharmaceutically active substances as well as
mixed micelle solutions which contain certain pharma-
ceutically active substances.
It is known to use mixed micelles as a vehicle for
pharmaceutically active substances. DE-PS 27 30 570
describes mixed micelle solutions for the parenteral
administration of fat-soluble active substances, i.e.
active substances which are not soluble in water or which
are difficultly soluble in water, especially benzo-
diazepines and vitamin K. EP-A2-133 258 describes mixed
micelle solutions for the parenteral administration of
vitamin E. Furthermore, mixed micelles have been proposed
for the production of aqueous protein solutions
(EP-A1-252004). In the use of mixed micelles for the
solubilization of non-steroidal antiinflammatories in
accordance with EP-A1-280 887 the problem lay in improving
the tolerance of the active substances in the case of
injections.
In the case of parenter'al, especially interstitial.
e.g. intramuscular, subcutaneous or intradermal.
administration of pharmaceutically active substances,
those having a molecular weight <1000 can be taken up
lymphatically~ while active substances whose molecular
weight amounts to about 16000 or above ate taken up to a
substantial extent from the lymphatic system.
It can, however, be desirable also to introduce
Grn/29.1.90
2~~1~~?
- 2 -
low-molecular active substances, which are normally not
transported to a substantial extent by the lymphatic
system. into this and thus to transport them to the
destination. This is especially true in the case of active
substances which influence the immune system. such as
immunosupressants and immunostimulants. It has
surprisingly been found that mixed micelles are
transformed in the case of interstitial applications into
liposomes which are taken up and transported by the
lymphatic system. In the case of certain hypotheses which
have been put forward, active substances contained in
mixed micelles are taken up by the liposomes formed in the
organism. Whether in each case such hypotheses are proved
can be ascertained experimentally by determining the
distribution coefficient of the active substance in
octanol/water. Active substances whose distribution
coefficient in the system octanol/water is greater than
about 104, preferably greater than about 106, fulfil
the hypothesis, i.e. ate taken up by the liposomes which
are formed.
Fat-soluble vitamins. e.g. vitamins A, E and K. have a
distribution coefficient of >106 in octanol/water. As
mentioned above. mixed micelle solutions of such vitamins
have become known. However, it has not become known that
these vitamins, after parenteral. especially interstitial,
administration as mixed micelle solutions, pass into the
lymphatic vessels in the form of liposomes.
The object of the invention is accordingly the use of
mixed micelles as carriers for the parenteral. especially
interstitial, therapeutic administration of low-molecular
active substances having a distribution coefficient in
octanol/ water of greater than about 104, with
fat-soluble vitamins being excluded.
'~~11~~~
- 3 -
As low-molecular active substances there are to be
understood in the present connection active substances
having a molecular weight below X0000. The low-molecular
active substances preferably have a molecular weight below
5000.
Examples of active substances in the case of whose
parenteral, especially interstitial, therapeutic
administration the use in accordance with the invention of
mixed micelles as carriers comes into consideration are
immunomodulators, e.g. immunosuppreseants such as (all-E)-
-3,7-dimethyl-9-(2-trifluoromethyl)-5-(nonyloxy)phenyl-
-2,4.6,8-nonatetraenoic acid and related compounds which
are described in EP-A1-169 571; cyclosporin or FK 506
(Immunology Today 10, 6-9 (1989)); as well as lipophilic
derivatives thereof, e.g. C1-20-alkanoyloxy derivatives;
immunostimulants such as N-acetylmuramyl-L-alanyl-D-
-isoglutamyl-L-alanyl-2-(1',2'-dipalmitoyl)phosphatidyl-
-ethanolamine (Cancer Immunol. Immunother. (1986) 22:
191-196); peptide antigens and oligosaccharide antigens or
lipophilic derivatives thereof, e.g. peptide-fatty acid
conjugates (EP-A2-290 246); contrast agents for lymphatic
scintillography; cytostatics, e.g, cis-Pt complexes such
as cis-bis-N-decylaminodiacetato-1,2-diaminocyclohexane-
-platinum; or anthra- cyclines such as doxorubicin or
methotrexate or lipophilic derivatives thereof such as the
fatty acid esters mentioned above, or lipophilic
derivatives of 5-fluoro-2'-deoxyuridine, e.g. palmitoate
(Microencapsulation 1988. Vol. 5, No. 1, p. 1-11) and
phosphate (Japan. Kokai 1091 195): lipophilic derivatives
of 1-l3-D-arabinofuranosyl-cytosine (Ara-C) such as oleate
and palmitate (Int. J. Cancer 37, 149-154 [1986]) and
cholesteryloxycarbonylglycyl-Ara-C (Chew. Pharm. gull
Vol. 36 (1988) 4060 et seq.); ph-osphatidylinositol and
lysophospholipids (DE-OS 3008082): and anti-infectives
such as amphotericin B, nystatin, gentamycin, chloroquin,
~~1~
- 4 -
penicillins such as ampicillin;and tetracyclines or
lipophilic derivatives thereof such as the fatty acid
esters mentioned above.
The previously named active substances can be
incorporated in mixed micelles using technologies known
pet se, fat example as described in DE-PS 27 30 570. In
comparison to Iiposomal solutions, mixed micelle solutions
are simpler to manufacture and have an improved stability.
The mixed micelle solutions manufacturable in accordance
with the invention therefore combine the advantage of the
stability of mixed micelle solutions with the improved
efficacy of lymphatically- and/or liposomally-applied
active substance.
In.the scope of the present invention there are to be
understood under mixed micelles or mixed micelle solutions
aqueous, homogeneous solutions of cholanic acid deriva-
tines, especially alkali salts thereof and lipids.
Examples of cholanic acid salts ate Na cholate, glycocho-
late, taurocholate, deoxycholate, glyco- and taurodeoxy-
cholate, chenodeoxycholate; and glyco- and taurocheno-
deoxycholate. Examples of lipids ate natural, semi-
_synthetic and wholly-synthetic lipids, especially
phosphatidylcholines, glycerol ether phosphatides.
phosphatidylethanolamine, phosphatidylinositol,
phosphatidylssrine, sphingomyelin, plasmalogens, cardio-
lipin, sulphatides and monoglycerides. The mixed micelles
can contain as additional components cholesterol (up to
about 30 mol% based on the amount of lipid) and lipids
having negatively- or positively-loaded groups such as
phosphatidic acids or stearylamine (up to about 10 mol%
based on the amount of lipid).
:;
The mixed micelle solutions can be manufactured by
simply mixing together the individual ingredients. It is.
~~~.~ ~6~
_ 5 _
however, advantageous to dissolve the lipid ingredient,
the micelle former and the active substance(s), which
is/are difficultly soluble in water or insoluble in water,
~ in an organic solvent, then to evaporate the organic
solvent and thereupon to add the water, the isotonizing
additives and, if desired. the additional ingredients,
whereby as a rule the isotonizing additives and for the
most part also the optional additional ingredients are
mixed with the water
prior to the addition to the
mentioned evaporation residue. As organic solvents there
come into consideration those in which the components to
be solubilized are sufficiently soluble, such as e.g.
lower alkanols, especially ethanol.
An especially preferred procedure comprises stirring
intensively approximately one molar part of micelle
former, agproximately one molar part of lipid ingredient
and approximately 0.3 to one molar part of water,
optionally in the presence of up to 23 of an organic
solvent such as ethanol, as well as the active
substances) which is/are difficultly soluble in water or
insoluble in water until the mixture appears homogeneous,
whereupon the water, the isotonizing additives and, if
desired, the additional ingredients are added until the
desired dilution or concentration has been achieved. It
is, how- ever. aleo possible to carry out the above
procedure with- out the active substance and only at the
end to solubilize the active substances) in the micelle
solution.
The ratio between the lipid ingredient and the
taicelle former lies in the order of 0.1:1 to 2:1. Mixture
ratios of 0.1:1 to 0.8:1 and 1.5:1 to 2:1 are preferred.
The mixture ratio 0.8:1 to 1.5:1 is quite especially
preferred.
The amount of lipoid ingredient plus micelle former in
- 6 -
the injection solution can vary over wide limits and can
amount to e.g. 2-200 mglml of injection solution.
S The amount of the phatmacon in the mixed micelle
solution can also vary over wide limits and can amount to
e.g. 0.1- 100 mgjml of injection solution.
Antioxidants such as tocopherols, ascorbic palmitate,
sodium asctobate, sodium hydrogen sulphite, sodium
pyrosulphite or sodium sulphite can be added to prevent
oxidation reactions of the active substance aad of the
carrier materials.
1S Additional substances of pH-adjustment, e.g.
phosphate, citrate or Tris buffer: for isotonization, e.g.
sodium chloride, mannitol, sorbitol or glucose, and for
preservation, e.g. methyl and propyl p-hydroxybenzoate,
benzyl alcohol or phenol, can also be added.
Where desired, the mixed micelle solutions can
be converted into dry preparations with the aid of con-
ventional lyophilization procedures.
2S The following Examples are intended to illustrate the
invention further.
Example 1
Manufacture of mixed micelle solutions:
Active substance (all-E)-3.7-dimethyl-9-(2-trifluoro-
methyl)-6-(nonyloxy)phenyl-2.4.6,8-nonatetraenoic acid (A)
30.8 mg of soya lecithin (Epikuron 200. Lucas Meyer.
Hamburg, Germany). 21.40 mg of sodium glycocholate and
0.4 mg of (all-E)-3.7-dimethyl-9-(2-trifluoromethyl)-6-
~0~1~6~
_ 7 _
-(nonyloxy)phenyl-2,4,6.8-nonatetraenoic acid are
dissolved in 5 ml of chloroform/methanol (l:l, vol/vol%)
in a round flask. The film which results after evaporation
of the organic solvent (rotary evaporator. 40°C) is
dispersed in 1 ml of 3.8% mannitol solution (wt/vol%). The
micelles obtained are adjusted to pH 6.0 t 0.1 with 1N
HC1, sterilized, filled into vials and lyophilized. The
mixed micelles ate manufactured with the exclusion of
1~ light and in an inert gas atmosphere, e.g. under nitrogen.
in order to prevent isomerization reactions of the active
substance as well as oxidation reactions of the active
substance and of the carrier materials, especially of the
phospholipids.
Example 2
Active substance 3',5'-di-O-palmitoyl-5-fluoro-2'-
-deoxuridine (B)
30.8 g of soya lecithin (Epikuron 200. Lucas Meyer,
Hamburg, Germany). 21.67 mg of sodium glycocholate and
1.0 mg of 3',5'-di-O-palmitoyl-5-fluoro-2'-deoxyuridine
are dissolved in 5 ml of chloroform/methanol (1:1.
vol/vol%) in a round flask. The film which results after
evaporation of the organic solvent (rotary evaporator.
40°C) is dispersed in I ml of 3.8% mannitol solution. The
micelles obtained are adjusted to pH 6.0 t 0.1 with 1N
HC1, sterilized and filled into ampoules. The manufacture
of the micelles is carried out in an inert gas atmosphere,
e.g. under nitrogen, in order to prevent oxidation
reactions of the active substance and of the carrier
materials, especially of the phospholipids.
The mixed micelle solutions were administered intra-
dermally to the right hind leg of experimental animals
(sheep) by means of a device described in EP-A-272530.
2~~.~.~~~
_8_
Blood was removed through a jugular vein catheter and
lymphatic fluid was removed through a cannula in the
branching lymph vessel at the right popliteal lymph node
(Pharm. Res. Vol. 5. 472-476 (1988)).
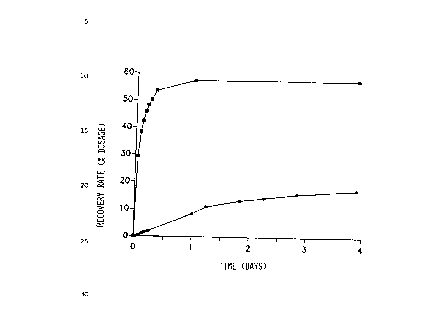
Figure 1 shows the amount of the administered dosage
found in the lymph. For comparison there are quoted the
values which were obtained with two low-molecular active
to substances whose distribution coefficient in octanol/water
is <1034.
Figure 2 shows the amounts of active substance A found
in the lymph after administration in mixed micelles and as
15 an oily formulation.
25