Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
20~212~
55-120.510
Benzimidazole compositions
The present invention relates to benzimidazole
compositions, in particular pharmaceutical compositions
containing a benzimidazole and a ~-blocker.
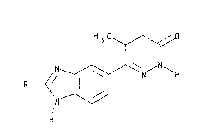
EP-B-8391 describes, inter alia, 5-(5-methyl-3-oxo- ~
4,5-dihydro-2H-6-pyridazinyl)-benzimidazoles substituted -
~0 in the 2-position by an alkyl, hydroxyphenyl or
methoxyphenyl group, that is compounds of formula I
H3C
H
:~'' '.
(wherein R represents a Cls alkyl, hydroxyphenyl or
methoxyphenyl group).
These compounds, their 3H-tautomers, optically
active antipodes and physiologically acceptable acid
addition salts thereof have valuable pharmacological
properties, such as antiviral, interferon-inducing and
anti-ulcerative activities, and, move particularly, a
cardiovascular activity, specifically cardiotonic,
hypotensive and/or antithrombotic activities.
The term "cardiovascular activity" indicates an ~ ~
activity affecting the heart and blood vessels, which in ~ -
the present instance occurs via an antithrombotic and
cardiotonic activity and an effect on blood pressure.
In the light of these pharmacological properties,
1. ':' . :. ' ' '. ., '.'.' . '.- ' ' . . ' ., ' '
201212~
possessed by the compounds of EP-B-8391, their 3H-
tautomers, optically active 3ntipodes and the
physiologically acceptable acid addition salts thereof
are suitable for treating chronic heart insufficiency or
angina pectoris and/or for the prevention of arterial
thromboembolism and arterial occlusive diseases, for the
treatment of ulcers and for fighting viruses and viral
diseases.
Their use as therapeutic agents in chronic heart
insufficiency is therefore based on the cardiotonic
activity and, in the case of arterial thromboembolism
and diseases of occlusion, on the antithrombotic
activity thereof, particularly the thrombocyte activity.
Furthermore, EP-A-330052, which is not a prior
publication, describes the combination of a
benzimidazole of formula I mentioned hereinbefore and a
~-blocker which has anti-ischaemic effects on the heart.
It is also known that ~-blockers have hitherto been
used predominantly for treating ischaemic heart diseases
in which the heart muscle is not yet diseased, but
recent attempts have also been made to use ~-blockers in
cases of chronic heart insufficiency in order to relieve
the strain on the heart and improve its pumping action.
In certain clinical cases which involve a reduced
contractile force of the heart, e.g. heart insufficiency
caused by various factors (see The American Journal of
Cardiology 55, 9A-14A (1985)), ~-blockers showed an
undesirable negative inotropic effect both in animal
experiments and also in clinical trials.
It has now been found that the benzimidazoles of
formula I in conjunction with ~-blockers not only negate
_ the negative inotropic effects of the latter but
actually lead to an improvement in heart function under
physical stress. This finding is surprising, for during
physical stress there is a physiological increase in
heart function via an increase in the activity of the
svmpathetic nervous system. This increased activity of
.. . .. . . ~ - . ~ . . , ~ . .
.; . . .. .
.. : - . . .: . : ..
2012~25
the sympathetic nervous system has no effect when ~-
blockers are present. A positive inotropic substance
may compensate for the negative inotropic activity of a
~-blocker under resting conditions. ~owever, it cannot
be predicted that the reflex adjustment of heart
function to physical stress during a ~-blockade will be
restored by a positive inotropic substance.
The present invention thus relates to new
pharmaceutical compositions with a synergistic activity.
Viewed from one aspect therefore the invention
provides a pharmaceutical composition having a positive
inotropic activity and comprising a benzimidazole of
formula I (as de~ined above) or a 3H-tautomer, optical
isomer or physiologically acceptable said addition salt
thereof together with a ~-blocker.
Viewed from a further aspect the invention provides
a process for the preparation of a pharmaceutical
composition having positive inotropic activity, said
process comprising combining, e.g. by admixture or by
incorporation unmixed into combined dosage units, a
benzimidazole of formula I (as defined above) or a 3H-
tautomer, optical isomer or physiologically acceptable
said addition salt thereof together with a ~-blocker.
Viewed from a still further aspect the invention
also provides the use of a ben~imidazole of formula I
(as defined above) or a 3H-tautomer, optical isomer or
physiologically acceptable said addition salt thereof
for the manufact~re of a pharmaceutical composition ;
having positive inotropic activity containing a
benzimidazole of formula I (as defined above) or a 3H-
tautomer, optical isomer or physiologically acceptable
-~ said addition salt thereof together with a ~-blocker for
use in the treatment of heart conditions.
Viewed from another aspect the invention provides a . -
method of treatment of heart conditions in the human or
- non-human animal body, said method comprising
2012~26
administering to said body a benzimidazole of formula I
(as defined above) or a 3H-tautomer, optical isomer or
physiologically acceptable said addition salt thereof
and a ~-blocker in concentrations sufficient to achieve
a positive inotropic effect.
The compositions of the invention preferably
contain benzimidazoles of formula I wherein
R represents a methyl, 2-pentyl, 4-methoxyphenyl or 4-
hydroxyphenyl group,
particularly preferred are a 4-methoxyphenyl or 4-
hydroxyphenyl group,
or a 3H-tautomer, optically active antipode or
physiologically acceptable acid addition salt thereof.
Examples of ~-blockers which may be used for the
preparation of the compositions of the invention include
a~enolol, metoprolol, pindolol, penbutolol, propanolol,
carazolol, alprenolol, bupranolol, bisoprolol,
mepindolol, metipranolol, betaxolol, acebutolol,
nadolol, sotalol, bunitrolol, timolol and oxprenolol.
Particularly preferred pharmaceutical combinations
are those wherein the two active substances have similar
half lives, especially preferably a combination
consisting of 2-t4-methoxyphenyl)-5-(5-methyl-3-oxo-4,5-
dihydro-2H-6-pyridazinyl)-benzimidazole (Compound A) and
a ~-blocker such as atenolol, betaxolol, metoprolol,
timolol, nadolol or propanolol or a combination
consisting of 2-t4-hydroxyphenyl)-5-(5-methyl-3-oxo-4,5-
dihydro-2H-6-pyridazinyl)-benzimidazole (Compound B) and
a ~-blocker such as metoprolol or pindolol. The first
of t~.ese combinations is particularly suitable for oral
_, administration and the second is particularly suitable
for intravenous use.
The single dose for adults is from 0.1 to 10.0 mg,
preferably from 1.0 to 2.5 mg, once or twice a day, for
a compound of formula I or isomer or salt thereof, in
. ~ . . . : . : . . .
: , , ., . . .. .. ,.. , ,,.. ,, :: " -: .:, . .. , , ,:...... .
-..~ : . . :. . , : : - :: : :
:' :: . .. . -. - :
:~ , .. . - . : .;, : .. ~. ~ . - :
: - : ~ . : : . ~:
20~212~
order to obtain the activity according to the invention.
In contrast, the single dose of the ~-blocker used
according to the invention may vary substantially
because of its varying potency. The single dosage will ~`
be, for example, 25 to 100 mg, preferably 50 mg per day
in the case of atenolol, 50 to 150 mg, preferably
100 mg, in the case of metoprolol, 5 to 15 mg,
preferably 5 to lo mg in the case of timolol, 25 to
80 mg, preferably 30 to 60 mg in the case of nadolol and
lo 50 to 100 mg, preferably S0 mg in the case of
propanolol, divided into one or two single doses.
The new combinations according to the invention
consisting of a ~-blocker and a benzimidazole of formula
I or isomer or salt thereof were investigated using the
combination of Compound A and atenolol as an example:
Influence on reflex adap~tation of heart function to
physical stress
Dogs (mongrel, both sexes, body weight 22 to 32 kg)
were premedicated with a combination of 0.~ ml/kg of ~ -
Polamivet~ and 0.05 ml/kg of Combelen~. After
endotracheal intubation the animals were respirated with
a mixture of 1% halothane, 24% 2 and 75% N20. Under
sterile conditions the thorax was opened up at the 5th
intercostal space on the left and a Konigsberg pressure
recorder was introduced into the left ventricle through
a rod incision at the apex. The pressure recorder was
fixed to measure the pressure in the left ventricle, the
cable was led out and the thorax closed. After the
operation the animals were given 2 months convalescence
in which they were familiarised with a treadmill.
Test procedure:
By the use of a treadmill (speed of treadmill:
- 0-2-4-6-8 km/h) the dogs were subjected to increasing
physical stress, each stress stage lasting 3 minutes.
- . , . . : - , . - . .
! . ' . . . . .
2012126
An initial run was used to determine the control values
whilst a second run was carried out following
intravenous administration of the test substances.
Throughout the experiment the pressure in the left
ventricle was measured, and the heart rate LV-dP/dtmaX
(the maximum rate of pressure change for the left
ventricle a measurement of the contractile force of the
heart) were recorded. The following Table sets forth
the values found:
Substance ~osage LV~dP/dtm~x (mmHg/s)
i.v. at rest under maximum
mg/kg stress during
experiment
(8 km/h)
Control --- 1.88 + 0.13 2.98 + 0.20
Compound A 0.3 3.40 + 0.18 4.77 + 0.18
Control --- 1.98 + 0.20 2.88 + 0.19
Atenolol 0.1 1.88 + 0.16 2.15 + 0.17
Control --- 1.95 + 0.15 3.28 + 0.21
Compound
A/Atenolol 0.3/0.1 2.57 + 0.14 4.18 + 0.20
~I .
The compounds used according to the invention are
well tolerated. Thus, for example, Compounds A and B
have the following acute toxicities:
._,
: . ., ~ , ~ : .
:: . . :.. ,: . .. . . ,, :
:, . ~ . - . :, ~ .
:: . , :
2012~26
Substance LD50
i.v. mg/kg p.o. mg/kg
- -
Compound A72 (rat) 600 (mouse)
Compound B>100 ~rat)
10 The toxicities of the ~-blockers used in the
combinations according to the invention are known from
the literature and are well tolerated in therapeutic
doses.
The combinations used according to the invention
are also well tolerated; thus, for example, at the doses
used for the combinations of Compound A and atenolol no
toxic side effects could be observed.
Eor pharmaceutical use the active substances
mentioned above may be formulated with one or more inert
conventional carriers and/or diluents, e.g. with corn
starch, lactose, glucose, microcrystalline cellulose,
magnesium stearate, polyvinylpyrrolidone, citric acid,
tartaric acid, water, water/ethanol, water/glycerol,
water/sorbitol, water/polyethyleneglycol,
propyleneglycol, cetylstearyl alcohol, carboxymethyl
cellulose or fatty substances such as hard fat, to
produce conventional galenic preparations such as plain
or coated tablets, capsules, powders, suspensions,
drops, ampoules, syrups or suppositories.
~0 The following non-limiting Examples are provided in
order further to illustrate the present invention:
,_,
2~2126
Exam~le 1
Tablets containing 1.0 mg of 2-(4-methoxyphenyl)-5-(5-
methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl)-benzimidazole
and 50.0 mq of atenolol
Composition:
Compound A 1.00 mg
10 Atenolol 50.00 mg
Lactose 47.00 mg
Corn starch 70.00 mg
Polyvinylpyrrolidone 8.00 mg
Aerosil 3.00 mg
15 Magnesium stearate 1.00 mq
180.00 mg
Compound A, atenolol, lactose and the corn starch
are mixed together, granulated with a solution of
polyvinylpyrrolidone in ethanol, dried, screened, mixed
with Aerosil and magnesium stearate and compressed to
form tablets 8 mm in diameter.
Example 2
Tablets containing 0.5 mg of 2-~4-methoxyphenyl)-5-(5-
methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl)-benzimidazole
and 50.0 mg of atenolol
., , . . . . ~ , .
. . -, . ,
- -- : : - - . ~ --
- . .
~'' . -: . : , , ~
~012125
Composition:
Compound A 0.50 mg
5 Atenolol 50.00 mg
Lactose 47.50 mg
Corn starch 70.00 mg
Polyvinylpyrrolidone 8 00 mg
Aerosil 3.00 mg
10 Magnesium stearate 1.00 mq
180.00 mg
Compound A, atenolol, lactose and the corn starch are
mixed together, granulated with a solution of
polyvinylpyrrolidone in ethanol, dried, screened, mixed
with Aerosil and magnesium stearate and compressed to
form tablets 8mm in diameter.
Example 3
2~
Tablets containing 1.0 mg of 2-(4-methoxyphenyl)-5-(5-
methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl)-benzimidazole
and 30.0 m~ of nadolol
: ~' .
Composition:
Compound A 1.00 mg
Nadolol 30.00 mg
Lactose 67.00 mg
30 Corn starch 70.00 mg
Polyvinylpyrrolidone8.00 mg
` ~ Aerosil 3.00 mg
Magnesium stearate l.00 mq
180.00 mg
Compound A, nadolol, lactose and the corn starch
are mixed together, granulated with a solution of ~ ~
.: ''
2~1212~
polyvinylpyrrolidone in ethanol, dried, screened, mixed
with Aerosil and magnesium stearate and compressed to
form tablets 8 mm in diameter.
Example_4
Tablets containing 1.0 mg of 2-(4-methoxyphenyl)-5-(5-
methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl)-benzimidazole
and 60.0 mq of nadolol
Composition:
Compound A l.00 mg
15 Nadolol 60.00 mg
Lactose 37.00 mg
Corn starch 70.00 mg
Polyvinylpyrrolidone8.00 mg
Aerosil 3.00 mg
20 Magnesium stearate 1.00 mq
180.00 mg
Compound A, nadolol, lactose and the corn starch
are mixed together, granulated with a solution of
polyvinylpyrrolidone in ethanol, dried, screened, mixed
with Aerosil and magnesium stearate and compressed to
form tablets 8 mm in diameter.
Example 5
Capsules containing l.0 mg of 2-(4-methoxyphenyl)-5-(5-
methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl)-benzimidazole
and 50.0 m~ of propanolol retard
50.00 mg of propanolol and 1.00 mg of Compound A are
dispersed in a melt ~90C) of l9.00 mg of carnauba wax
and 110.00 mg of stearyl alcohol.
. ~ . - . . ~ . ~ . ..
., . ~ : - , . . -
- ~ , ; ,
.. . .
- : : . ~ : .. ,
: - ' ' ' :~ .
: ; , ~ . ~ . '
2012~ 2~
The dispersion is sprayed in a suitable container.
The spray nozzle should be chosen so as to produce
droplets 300 to 800 ~m in diameter. The droplets are
moved in free fall counter to an air current cooled to
about 5 to 8C so that the droplets solidify. The spray
material thus obtained is mixed with 1 mg of magnesium
stearate and packed into hard gelatin capsules, size 3.
Example 6
Capsules containing 1.0 mg of 2-(4-methoxyphenyl)-5-(5-
methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl)-benzimidazole
and 100 0 mq of metoprolol retard
100 mg of metoprolol and 1.00 mg of Compound A-are
dispersed in a melt (90C) of 20.00 mg of carnauba wax
and 59.00 mg of stearyl alcohol.
The dispersion is sprayed in a suitable container.
The spray nozzle should be chosen so as to produce
droplets 300 to 800 ~m in diameter. The droplets are
moved in free fall counter to an air current cooled to
about 5 to 8C so that the droplets solidify. The spray
material thus obtained is mixed with 1 mg of magnesium
stearate and packed into hard gelatin capsules, size 3.
30 Example 7 ~
- _" Tablets containing 1.0 mg of 2-(4-methoxyphenyl)-5-(5- .
methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl)-benzimidazole
and 10.0 mg of timolol
--
.: : : ~ : . . . .. ~ . . . . . . .
~0~2~5~
Composition:
Compound A l.00 mg
Timolol 10.00 mg
5 Lactose 51.00 mg
Corn starch 50.00 mg
Polyvinylpyrrolidone 5.00 mg
Aerosil 2.00 mg
Magnesium stearate 1.00 mq
120.00 mg
Compound A, timolol, lactose and the corn starch
are mixed together, granulated with a solution of
polyvinylpyrrolidone in ethanol, dried, screened, mixed
with Aerosil and magnesium stearate and compressed to
form tablets 7 mm in diameter.
Example 8
Tablets containing 1.0 mg of 2-(4-methoxyphenyl)-5-(5-
methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl~-benzimidazole
and 10.0 mq of betaxolol
Composition:
Compound A 1.00 mg
Betaxolol lO.00 mg ~ :
Lactose 51.00 mg
Corn starch 50.00 mg
30 Polyvinylpyrrolidone 5.00 mg
Aerosil 2.00 mg
-~ Magnesium stearate 1.00 mq
. 120.00 mg
Compound A, betaxolol, lactose and the corn starch
are mixed together, granulated with a solution of
polyvinylpyrrolidone in ethanol, dried, screened, mixed
- . . ~ . . . .. . :
20~2126
13
with Aerosil and magnesium stearate and compressed to
form tablets 7 mm in diameter.
Example 9
Tablets containing 1.0 mg 2-(4-methoxyphenyl)-5-(5-
methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl)-benzimidazole
and 100.0 mg of metoprolol
1 0
Composition:
Compound A 1.00 mg
Metoprolol 100.00 mg
15 Lactose 27.00 mg
Corn starch 80.00 mg
Polyvinylpyrrolidone8.00 mg
Aerosil 3.00 mg
Magnesium stearate1.00 m~
220.00 mg
Compound A, metoprolol, lactose and the corn starch
are mixed together, granulated with a solution of
polyvinylpyrrolidone in ethanol, dried, screened, mixed
with Aerosil and magnesium stearate and compressed to
form tablets 9 mm in diameter.
Example ~0
Tablets containing 1.0 mg of 2-(4-methoxyphenyl)-5-(5- -
methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl)-benzimidazole
and 5.0 ma of timolol -
, :: - ', , . ~ :
~, . ....... . - .. -
-: .: ~: : . :. . .: , : . . - :.
::: . . : . . , -
2012~2g
14
composition:
Compound A 1.00 mg
Timolol 5.00 mg
5 Lactose 56.00 mg
Corn starch 50.00 mg
Polyvinylpyrrolidone5.00 mg
Aerosil 2.00 mg
Magnesium stearate l.00 mq
12~.00 mg
Compound A, timolol, lactose and the corn starch
are mixed together, granulated with a solution of
polyvinylpyrrolidone in ethanol, dried, screened, mixed
with Aerosil and magnesium stearate and compressed to
form tablets 7 mm in diameter.
Example ll
Ampoules containing 1.0 mg of 2-~4-hydroxyphenyl)-5-(5-
methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl)-
benzimidazole-hydrochloride and 5 ma of metoprolol
Composition:
Compound B 1.00 mg
Metoprolol 5.00 mg
Mannitol 100.00 mg
lN HCl ad pH 2.7 about2.4 ~1 -
30 Water for injections adj2.0 ml
:
Compound B, metoprolol and mannitol are dissolved
in water, adjusted to pH 2.7 with hydrochloric acid,
sterile filtered and transferred into 2 ml ampoules.
Sterilisation is effected for 20 minutes at 120C.
,. .. .
. .
- ; ~ ; . . , . , ~:, ,, . ,: .. -
. : ~ , .- . :: - .- . :
, .. :.: ~ . , : :
:; - - . : . I
2~2126
Example 12
Ampoules containing 1.0 mg of 2-(4-hydroxyphenyl)-5-(5-
methyl-3-oxo~4,5-dihydro-2H-6-pyridazinyl)-
benzimidazole-hYdrochloride and 0.4 mq pindolol _
Composition:
Compound B 1.00 mg
10 Pindolol 0.40 mg
Mannitol 100.00 mg
lN HCl ad pH 2.7 about .2.2 ~l
Water for injections ad 2.0 ml
Preparation:
Compound B, pindolol and mannitol are dissolved in
water, adjusted to pH 2.7 with hydrochloric acid,
sterile filtered and transferred into 2 ml ampoules.
Sterilisation is effected for 20 minutes at 120C.
'~;';.''~' `",~
`~
... - ,- ., . . .. : -:,: . , . : -
:.: . - : .:, . . . .
, . . . . .