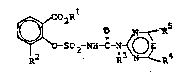Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
f~
HOECHST ARTIENGES~L~SCHAFT ~O~ 89/F 091 Dr. WE/rh
De~cription
Ph.enOyBUlfOnylllrea~ ba8~3!d on 3-substituted
~11~1 salicylates, pr~e~e~ for their preparation
S and their use a~; lherbicid~s andl plant gro~h ~egulators
It iB known that phenoxy~ulfony:Lureas containing hetero-
cyclic 8ubstituenl:6 ~ave herbicidal ~ nd plant growth-
regulating properties (EP-A-4 9 163, D~-A-3, 151, 4S0,
DE-A-3,725,939 (ZA-B8/5725~ and Berman Patent ~pplication
P-3,816,704.2 (~P-A-0/342,569, and ZA-89/3463))o
EP-A-4,163 thus describes, in~er alia, 2-methoxyph noxy-,
2-ehlorophenoxy- and 2-al~ylphenoxy as well as 2-carbo
methoxy-phenoxysulfonylureas having a herbicidal action.
Surprisingly, it has now been found that combination~ of
the carbomethoxy substituents with in each case one of
the other radicals mentioned leads to a considerable
improvement in the herbicidal properties.
The present invention thus relate~ to compounds of ~he
formula (I) or ~alts thereof
~ O. ~ ~ (I)
in which
Rl i8 I Cl-C,, ~ alkyl,
R2 i~ halogen, methoxy, ethyl or propyl,
R3 i~ hydrogen or methyl,
E i6 CH or ~ and
R4 and R~ independently of one anothex are halogen,
( Cl-C~ ) alkYl, ( C1-C4 ) alkoxy or ( Cl-C,~ ) alkylthio, t~e
abovementioned alkyl-containing radicals being
..
., , ~
- .
. . : . . ..
- 2 ~ 5
unsub~tituted or sub~titu~ed by one or more halogen
atoms or one or more (Cl-C~jalkoxy or (Cl-C4)al~ylthio
group~.
Halogen i8 fluorine, chlorine, ~romine and/or iodine,
S preferably fluorine, chlorine and/or bromine, in partic-
ular f luorine or chlorine.
(Cl-C~)alkyl and the corra~pond~.ng alkyl radical in tha
alkyl-containing radicals, such a~ alkoxy or nl~ylthio,
is methyl, ethyl, n-propyl, i~propyl, n-butyl, i-butyl,
~-butyl or 2-butyl.
The compounds of the formula I ~an ~orm salts in which
the hydrogen of the -SO2-NH group i~ replaced by a cation
suitable for agriculture. These ~alt~ are in ~eneral
métal ~alts, in particular alkali metal or alkaline earth
metal salts, ~nd if appropriate inorganic or org~nic
ammonium salts.
Preferred compounds of the formula I or 8alt8 thereof are
those in which Rl is methyl or ethyl, E i8 a group of the
formula CH and R~ and R5 independently of one another are
chlorine, bromine, ICl-C~)alkyl or (C,-C~)alkoxy, it being
possible for the abovementioned alkyl-containing radical~
to be substituted by one or ~ore fluorine or chlorine
atoms.
Particularly preferred compounds of the formula I ox
salts thereof are those in w~ich R1 i8 methyl or ethyl, E
is a group of the for~ula C~ ~nd R~ and R5 independently
of one another are ~hlorine, ~Cl-C2)alkyl or (C1-C2)alkoxy,
it being possible for the abov~mentioned al~yl-~ontaining
radicals to be substituted by one or more fluorine or
chlorin~ atoms.
The present invention furthermore relates to processe~
for the preparation of the compounds of the general
formula I or ~alt~ thereof, which comprise
.
- 3 ~ 5
a) reacting a compound of the formula ~II)
~ O -~CO (II)
with a ~ompound of the fo~mula ~III)
RS
~III)
or
b) rPacting a compound of the formula (IV)
~ ~1 (IV)
with a chlorosulfonylurea o~ the fo.rmula (V)
~,5
~ 02-N~-CY- ~ R (V)
or
c) reacting a compo~nd of the fon~la (VI)
2~
~2 ~2 ~2 ~YI)
.z~,
with a carbamate of the for~ula ~YII~
~3 ~ II3
. . - . ~-
,,` ' ~ - :
~ 2~
- 4
in which Z i~ phenyl or ~cl-C6)alkyl,
and if appropriate convertin~ ~.he re~ulting compounds of
thP formula ~I) into their ~alt~.
The reaction of the compound~ of the formulae ~ and
(II) is preferably carried out i.n ~nert apro~c ~olvent~,
6uch a5, for example, acetonitrile, methylene ~hloride,
toluene, chlorobenzene, ~e~rah1ydrofuran or dioxane; at
temperature~ between 0C and t:he boiling p~int of the
~olvent.
The phenoxysulfonyl isocyana~es of the formula (II) can
be prepared in a 3imple manner by proces~es which are
known in principle from the corre~ponding ~alicylic acid
esters of the formula ~IY) and chlorosulfonyl i30cyanate
(c.f. G. Lohaus, Chem. Ber. ~Q~, 2791 ~1972)).
The starting substances of the formula ~III) are known
or can be prepared by processes which are known in
principle, for example by cyclization of corresponding
guanidine derivative~ with corre~pondingly substituted
1,3-diketones, c.f., for example, ~The Chemistry of
Heterocyclic Compound~n, Vol. XVI (1962) and Suppl~mant I
(19~0), or by deri~atization of cyanuric chlori~e, c.f.,
for example, ~The Chemistry o~ Het~rocyclic Compounds",
L. Rapaport: "s-Triazines and Dsrivatives" (1959)~.
The reaction of the compounds of the formula (IV) with
the chlorosul~onylureas of the for~ula ~V~ i8 preferably
carried out in inert solvents, ~uch as, for example,
methylene chloride, tetrahydrofursn, dioxane or di-
methoxyethane, at temperatures ~etween 10C ~nd 80C in
the presence of a ba~e as the ~Cl-binding agent. Bases
which ~an be employed ~re alkali ~etal ~arbonates or
bicarbonates and alk~line earth metal carbonate~ or
bicarbonates, 6uch as, for example, R2CO3, N~C03 and
Na2CO3 or tertiiary amine~, uch as, for ex2mple, pyTidine
or triethylamine.
- 5 ~ S
The salicylic acid e~ters of ~he formula ~IV) are known
from the litera~re or can be prepared by processes which
are known from ~ha literature. ~he chlorosulfonyluxeas of
the formula (V) sre accessible rom the amines of the
formula (III) and chlorosul:Eonyl isocyana~e (~P A
141,1g9) .
The reaction of the compounds of the ormula (VI) with
the heterocyclic carbamate~ of the formula (VII) is
preferably carried out in the pre~ence of tertiary
organic bases ~uch as, for e~ampls, 1,8-diazabicyelo-
15.4.0]-undec-7-ene (DBU) in inert ~olvents, ~uch as
acetonitrile or dioxane, at temperatures between 20C and
the boiling point of ~he solvent (analogously to
EP-A 44,307).
The carbamates of th~ formula (VII) required for thi~
reaction are known from the literature or are preparsd by
known processes (EP-A 70,804~. The sulfamates of the
formula (VI) are prepared by known processe~ from the
salicylic acid esterE on which they are based (c.~., for
example, Synthesi~ 1978, 357; 2. Chem. 15, 270 ~1975);
and Chem. Ber. 105, ~791 (1972)).
~he salts of the compounds of the ~oxmula (I) are prefer-
ably prepared in inert ~olvent~, ~uch a~, for ex~mple,
water, methanol or acetone, at temperatur~ of 0-100C.
Example~ of ~uitable base~ for the preparation of the
salts according to the invention are alkali metal
carbonates, ~uch as pota6sium carbonat2, ~lkali metal and
alkaline earth metal hydroxides, ~onia or ethanolamine.
The comp~unds of the formula I according to the i~v2ntion
3D have an exce:Llent herbicidal activity against a broad
~pectrum of economically important mono- and dico~yledon
harmful plant . ~Y2n perennial weeds which ~re difficult
to ~ombat and ~hoot ~rom rhizome~, root~tock or other
permanen~ organs are re~dily affec~2d ~y the ac~ive
compounds. It i~ irrelevant here whe~her the sub~tance~
,
2~5
are applied by the pre-sowin~, pre-~mergence or pO8~-
emergence methodO Some repre~entatives of ~he mono- and
dicotyledon weed flora which can be controlled by the
compound6 according to the invention may be mentioned ~
examples, without the list representlng a limitation to
cer~ain ~pecies.
On the part of monocotyledon weed ~pecie~, exampl2s which
are readily a~fected &re Avena, ~olium, Alopecurus,
Phalaris, Ech~nochloa~ Digitaria, Setaria and the like,
as well a~ Cyperus ~pecies from ~he annual group, and on
the part of perennial species Agropyron~ ~nodon,
Imperata and Sorghum and the like, and also perennial
Cyperu~ species.
In the ca e of dicotyledon we~d specles, the action
spectrum extends to Galium, Violat Veronica, ~amium,
Stellari~, Amaranthu~, Sinapis, Ipomoea, Matricaria,
Abutilont Sid~ and the like on the annual ~ide, and
Convolvulus, Cirsium~ Rumex, Artemi6ia and the like
amongst the perennial weeds.
Weeds which occur under the ~pecific crop conditions in
rice, such as, for ~xample, Sagittaria, Alisma,
Eleocharis, Scirpu~, Cyperus and the like, are also
controlled outstandingly well by the a~tive compounds
according to the invention.
If the compounds according ~o the in~ention are applied
to the ~oil surface before germination, either emergence
of the weed seedlin~s is prevented completely, or the
weeds grow to the cotyledon ~tage, but then ~top growing
and finally ~ie completely after a period of three to
four weeks.
If the active compounds are applied to the green parts of
plants by th~ post-emergence process, a drastic ~top i~
growth likewise occurs very rapidly af er the treatment,
and the weed plants remain in the growth ~tage which
7 ~ 5
existed at the time of applica~.ion, or die moxe or 1~8~
rapidly after a certain period of ~ime, 80 that weed
competition which is damaging ~.o th~ cxop plants can in
this way be elLminated very early and in ~ lasting manner
S by using the novel agents accor~ding to ~he invention.
Although th~ compound~ according ~o ~he invantion have an
excellent herbicidal ac~ivi~y again~t m~no~ and di-
cotyledon weeds, crop plant~ of econ~mically important
crops, ~uch as, for example, whea~, barley, rye, rice,
maize, ~ugar beet, cotton and ~oya9 are hanmed only
insignificantly, if at all. ~or these reason~, ~he
present compounds are particularly ~uitable for selec-
tively controlling unde~irable plant ~rowth in agricul-
tural crop plantations.
The compounds according to the invention moreover e~hibit
growth-regulatory propertie6 in crop plants. They inter-
vene in a regulatin~ manner in the andogenous metabolism
of the plant and can therefore be employed for ~scilitat-
ing harvesting, such a~, for example, by inducing desic-
cation, absci~sion and grow~h compression. They aremoreover al80 suitable for the general control and
inhibition of undesirable vegetative growth, without at
the ~ame tLme killing the plants. An inhibition of
vegetative growth i8 of great Importance in many mono-
and dicotyledon crops, cince lodging can in this way bereduced or completely preventedO
The compounds of th~ fonmula (I) can be formulated in
various ways, according to the biological and/or ~hemico-
physical paraneter6 ~hich exist. ~xamples of ~uitable
formulation po~ibilities ~re: wettable po~der~ (WP),
emulsifiable concentration~ (EC) and aqueou ~olutions
(SL3; emulsions, sprayable solutions, oil- or water-based
dispersions (SC), suspoemulsions, dusting agents (DP),
dressing agents, granules (GR), such as soil or scatter-
35 ing granul~s (FG) or water-di pexsible granule~ (WG~, ULV
formulations, microcapsules or waxes.
I
- 8 ~
These individual types of formulations are known in
principle and are de~cribed, ~or example, in: Winnacker-
Ruchler, ~Chemische Technologie tChemical Technology)"
Vol. 7, C. Hauser Verlag Munich, 4th Edi~ion, 1986; van
FalenXenberg, "Pesticide~ Formulatlons n ~ ~rcel ~ekker,
N.Y., 2nd Edition, 1972-73; ~nd R. ~artens, ~Spray Drying
Handbook" 3rd Edition, lg79, G. Goodwin Ltd., London.
The formulation auxiliaries required, such a~ inert
materials, ~urfac~ants, ~olvents and other additives, are
like~ise known and are de~cribed, for ex~mple~ ins
~Handbook of Insecticide Dust Dil~ents ~nd Carriersn, 2nd
Edition, Darland Books, Cald~ell N.J.; H.~. Olphen,
"Introduction ~o Cl~y Colloid Chemi6try~; 2nd ~dition,
J. Wiley & Sons, N.Y~; Narschen, "Solvents Guide", 2n~
Edition, Interscience, ~.Y., 1950; McCutcheon~ "Deter-
gents and ~mulsifier~ Annual n ~ NC Publ. Corp.,
Ridgewood N.J.; Sisley and Wood, "~ncyclopedia of Surface
Active Agents~, Chem. Publ. Co. Inc., N.~., 1964;
Schonfeldt, n Gren~fl~chenaktive ~thylenoxidadduXte
(Surface-active ethylene oxide adducts) n t Wi8~ . Yerlag3-
gesell., Stuttgart 1976; Winnacker-Ruchler, ~Ch~mische
Technologie ~Chemical Technology) n ~ Volume 7, C. Hauser
Verlag Munich, 4th Edition, 1986.
~ettable powders are preparations which are uniformly
dispersible in wa~er and which, in addition to the ~ctive
compounds, and if appropriate in addition to a diluent or
inert substance, also contain wetting agent~, for ex~mple
polyoxyethylated alkylphenol~, polyo~yethylated fatty
alcohols and fatty zmine6, alkane sulfonate~ or alky~aryl
sulfonates, su~h as alkylbenzenesulfonate~, and di~per~-
ing agents, for example ~odium ligninsulfonate ~ sodium
2,2'-dinaphthylmethane-6,6'-di~ulfonate, sodium dibutyl-
naphthalenesulfonate or ~odium oleoyl-methyl-taurate.
They are prepared in the customary manner, for e~ample by
grinding and ~ixing the components.
Z ~
- 9 -
~mulsifiable concentrates can be prepared, for ~xample/
~y dissolving the active compound in an inert organic
solvent, for example butanol, cyclohe~anone, dimethylw
formamide, xylene or higher-boiling aroma~ics or hydro-
carbons, with $he addition of one or more emulsifiers. Ifthe actiqe compounds are ligu.id, all or ~ome of the
solvent content can also be dispen~ed with. ~xamples of
emul~ifier~ which can be u~ed are: calcium alkylaryl-
~ulfonates~ ~uch as Ca dodecylb2nzene~ulfona~e, or
nonionic emulsifier~, such as fatty acid polyglycol
esters, alkyl-aryl polyglycol ether~, fat~y alcohol
polyglycol ether~, propylene oxide-ethylene oxide conden-
sation products, fa~ty alcohol-propylene oxide-et~ylene
oxidP ~ondensation product~, alkyl polyglycol ethers~
sorbitan fatty acid esters, polyoxyethylene ~orbitan
fatty acid esters or polyo~yethylene sorbitol ester~.
Dusting agents can be obtained by grinding the active
compound with finely divided ~olid sub~tances, for
example talc or n tural clay~, such as kaolin, bentonite,
pyrophyllite or diatomaceous earth.
Granules can be prepared either by spraying the active
compound on to an absorbent granular inert material or by
applying active compound concentrates to ~he surfaca of
carriers, such as sand or kaolinites, or of granular
inert material by mean~ of binder~, for example polyvinyl
alcohol, sodium polyacrylate or mineral oil~ 5uitab1e
active compounds can also be granulated in the ~anner
customary for the preparation of fertilizer granule~ if
desired as a mi~ture with fertilizers.
The agrochemic:al formulation~ as cu6tomary contain 0~1 to
99% by weighl:, in particular 2 to 95% by weight, of
active compound of the formula (I). The concentrations of
the acti~e compound can vary here, depending on the type
of formulation.
In wettable powders, the active compound concentration
-- 10 --
is, for example, about ln ~o 90~ by weiyh~, the rQmainder
to make up to 100% by wPight consisting of customary
formulation constituent~. In eslulsifi~ble concentrate6/
the active compound concentration can be about 5 to 80%
5 by weight. Dust-like formulations u~ually c~ntain 5 to
20~ by weight, and sprayable ~olutions about 2 to 20g by
weigh~. In granule~, ~he ac~ive ~ompound content depends
in part on whether the ac i~e compound i8 in liguid or
solid form and on what granula~:ion auxiliaries, filler6
and the like are u~ed.
In a~dition, the active compound formulations men~ioned
contain, if appropriate, the particular customary
adhesives, wetting agents, dispersing agents, amulsi-
fiers, penetr tion agents, solvents~ fillers or carrier~
or mi~tures thereof.
The invention thus also relates to herbicidal and plant
growth-regulating agents which contain a ~ompound of the
formula ~I) or salts thereof and cu~tomary formulation
auxiliaries which are inert undex the storage conditions.
For use, the concentrates in the co~mercially available
form are diluted, if appropriate, in the customary
manner, for example by means of water in the cas~ of
wettable powders, emulsifiable concentrates, disper~ion&
and in some cases also microgranules. Dust-like and
granular formulations and sprayable ~olutions are u~ually
not diluted further with additional iner~ ~ubstances
before use.
The application amount required varie~ in accordance with
the external ~ondition , such as temperature, humidity
and the like. It can vary within wide limit~, for e~ample
between 0.005 an 10.0 kg~ha or more of active ~ubstance~
but is pre~er;~bly between 0.01 and 5 kg/ha.
Mixtures or mixed ~ormulations with other active
compounds, such as, for example, in~ecticides, acarici
des, herbicid~s, fertilizers, growth regulator~ or
fungicides, are also po~sible if appropriats.
The inYention is e~plained in more de~ail by the follow-
in~ Examples.
For~ul~tion ~ample~
A. ~ dusting agent i~ obtainec~ by mi~ing 10 par~s by
weight of active compound with 90 part~ by weight of
talc or a comparable inert ~ub~tance and c~minuting
the mixture in an impact mill.
B. A wettable powdPr which is readily di~persible in
water i8 obtained by mixing 2S parts by ~eight of
active compound, 64 parts by weight of kaolin-
containing guartz, as the inert ~ubstance, 10 part~
by weight of pota~sium ligninsul~onate and 1 part by
weight of sodium oleoyl-methyl-taurates, ~ the
wetting and dispersing agent, and grinding the
mixture in a pinned di~k mill.
C. A dispersion concentrate which i8 readily di0per~ible
in water is obtained by mixing 20 parts by weight of
active compound with 6 part~ by weight of alkylphenol
polyglycol e~her (~R)Triton X 207~, 3 part~ by weight
of isotridecanol polyglycol ether (8 mol of ethylene
oxide) and 71 parts by weight of paraffinic mineral
oil (boiling range, for example, about 255 to more
than 377 C), and grinding the mixtur~ ~o a finene~s
of le~s than 5 micron~ in a bead mill.
D. An emulsifiable concentrate i~ obtain~d from 15 part~
by weight of active compound, 75 parts by weight of
cyclohexanone, as the solvent, and 10 parts by weight
of oxyetllylated nonylphenol (10 mol of ethylene
oxide), a~i the emulsifier.
E. Water-disper~ible granules are obtained by mixing
75 parts by weight of a ~ompound of the formula (I),
.:
~Z~L~5
- 12 -
N of calci~ nin6ulfona~e,
ll of sodium lauryl~ulfate,
3 n of polyvinyl alcohol and
7 " of kaolin,
grinding the mi~ture on ~I pinned di~k mill and
granulating the powder in a:Eluidiz~d bed by ~praying
on wa~er as the granulatin~ liquid.
F. Water-dispers~ble granul~s are al80 obtained b~
homogenizing and precomminuting
25 parts by w~ight of a compound of the ~ormula (I),
" of ~odium 2,2'-dinaphthylmethyl-
6,6'-di~ul~onate
2 " of ~odium oleolyl-methyl-taurate,
1 " of polyvinyl alcohol,
1~ 17 ~ of calcium carbona~e and
" of water
on a colloid mill, ub~equently grindin~ the mixture
on a bead mill, atomi~ing the re~ulting suspension in
a ~pray tower by means of a one-componen~ ~t and
drying the product.
G. Extruded granules are obtained by mixing
20 part6 by wzight of active oompound,
3 n of sodium lignins~lfonate,
1 " of carboxymet~ylcellulose and
76 ~ of kaolin,
grinding the mixture and moi~tening it with water.
This mixture is extruded and then dried in a stream
of air.
Chemi~al ~s2m]ple~
~xample 1 (precur60r)
~ethyl-2-iso~ya~atos~lfonylo~y-3-~tho~y-be~zoate
3.4 g (0.024 mol) of chlorosulfonyl i~ocyanate are add d
dropwise to a solution of 3.6 g (O.02 mol) of methyl
3-methoxy-salicylate in 20 ml of xylene at 25C. When the
:' ~ ~ ,-. .
, ' ' ~ . -
- 13 -
dropwise addition ~as ended, the temperature is ~lowly
increased to 140C and the mixture i~ heated under reflux
for 2.5 hours. It i8 cooled and the ~olvent and excess
chlorosulfonyl isocyana~e are r~oved on a rotary evapor-
ator. The yellow oil which r1~main~ (5.4 g 100% of
theory) is u~ed withou~ further purification.
~a~ple 2
~et~yl ~-[3-(4,6-di~etho~ypyri~idin-2-yl)~reido-
sulfo~ylo~y]-3-~2~ho~y-be~oate (forDula I ~her~ Rl= C~3
R ~C~3, ~3 = ~, R4 = OC~ ~5 = OC~ d
A solution of 5.4 g (0.02 mol) of the product from
Example 1 in 10 ml of methylene chloride i~ added ~rop-
wise to 3.1 g (O.02 mol~ of 2-amino-4 r S-dimethoxy-
pyrimidine in S0 ml of methylene chloride ~t 0C. The
mixture i6 subseguently stirred at 25C for ~4 hour~ and
diluted with 50 ml of methylene chloride and ~he organic
phase i~ washed twice with S0 ml of 2~ hydrochloric acid
each time and once with 50 ml of water. After drying with
sodium sulfate and removing the ~olvent on a rotary
e~aporator, an oily product remains, ~hich crystalllzes
on trituration with diethylether. 6.8 g (77~ of theory)
of methyl 2-t3-(4,6-dimethoxypyrimidin-2-yl)-ureido-
~ulfonyloxy]-3-methoxy-benzoate of melting point
169-170C are obtained.
2i Example 3
~ethyl 2-[3-(4-~hloro-6-methylpyrimidi~-2~ ureido
sulfo~ylo~y~-3-~etho y-b~zoate (fo~mula I where R1 = C~
R2 = ~CH3 R3 ~ HJ R4 a C1 ~ R5 = CEI3 a~ 3~ = Q[)
A solution of 5.4 g (9.02 mol~ of the product irom
Example 1 in 10 ml of methylene chloxide i8 added drop-
wi~e to 2.9 ~ (0.02 mol) o 2-amino-4chloro-6~methyl-
pyrimidine in 50 ml of methylene chloride at oCC. The
mixture is ~uhsequently ~tirred at 25C for 24 hours and
diluted with 'iO ml of methylene chloride, and the organic
pha~e i8 washed twice with 50 ml of 2N hydrochloric acid
each tLme and once with water. ~fter drying with sodium
~ulfate and removing ~he ~olvent on a rotary evaporato~,
an oily produc~ remain~, which cry~talli es on
trituration with dii60propyle~her. 7.8 g (91~ of theory)
S of methyl 2~[3-(4-chloro-6-methylpyr.imidin 2 yl3-ureido~
sulfonyloxy]-3-m~thoxyb~nzoa~e of mel~ing point 140-143C
are obtained.
The compounds li~ted ~low in Table ~ ~an be prepared ~8
described in Examples 1 3.
O ~1
Table 1
O æ 3 =~
o
ExampleR~ R2 R3 R4 Rs ~~elting
No. Point ~C]
. ~
4 CH3 OCH~ H CH~ CH3 ~ 4~145
5 ~t n H ~CH3 CH3 CH 147 149
6 ., .. H CH3 ~H3 N
7 " " H OCH3 ~H3 N1~0-161
8 ~. .. H OCH3 oc~3 N
9 " " H OCH~ Cl CH130~133
" ~ H OCF2H ~H3 CH
N OCF2H ~2~ CH
12 " " H OCH3 Br CH
13 .. " H OCH3 OCaHs CH
14 ~ n H OCH3 SCH3 CH
~ N H OCH3 C2Hs N
16 " ~' H OCH3 OC3H7 CH
17 l~ n H OCH3 Cl N
18 " ~ H Cl ~C2Hs CH
19 ~ ~2~5 OC2~s ~H
~ . n H ~3~S OCH3 ~
F3 ~CH3 CH
22 " " H OC~2~F3 CH3 OH
23 ~ n H OCHaC~ ~CH3 CH
1 5
Example R1 R~ R3 R' R5 X Plelting
_ _ Point ~ C]
24 ~ OCH2cF3 ~CH2CF3 CH
2a ' H OCH2CF3 OC:H3 N 143-144
27 ~ H OCH3 CH~OCH3)2 CH
28 C2Hs ~ H OC~3 ~3 CH
29 " " H OCH3 CH3 CH
" ~ H CH3 ~H3 CH
31 H OCH3 Cl CH
32 n H OCHl O~H3 N
3 3 " " H OCH 3 CH 3 N
34 C3H7 H OCH3 OCH3 CH
" " H OCH3 CH3 CH
36 ~ ., H OCH3 ca3 N
37 ~4Hs " H OCH3 oc~3 CH 129
38 ~ .. H OCH3 ~B3 CH 113-119
39 " " H OCH3 ~H3 N
CH3 CH~ OCH3 OCH9 CH 144-145
41 " CH3 OCH3 C~3 CH
42 ~ ~ CH3 OCH3 C~3 N 100-103
43 " " CH3 OCH3 Cl CH
44 " CH3 OCF~H OCF2H CH
CH3 ~H3 CH3 CH
46 C2H5 " CH3 OCH3 OCN3 CH
47 ~ ~ CH3 OCH3 CH3 CH
48 CH3 Cl H C~3 CH3 CH
49 " " H OCH 3 CH3 CH 92- 95
aO H CH3 ~3 N (Dec~ositio~)
51 ~ H OCH3-- CH3
5~ " ~ H OCH3 OCH
;3 " " H OCH3 Cl CH
54 " " H OCF~H ~H3 CH
aa " ~ H OCF~ OCF2H CH
~ ~ OCH3 ~r CH
57 H OCH~ OC2H5 CH
5B " " H OCH3 SCH~ CH
59 " " H OCH3 C2~5 N
~ H OCH3 ~C3H? CH
61 " " H OCH3 Cl N
62 " ' H Cl C2~s CH
63 ~ H OC2H5 O~2H5 CH
64 H~2~5 OCH3 CH
~ 16 ~ L~
~xample Rl R2 R~ Ri R5 X ~elting
No. P~int [ C]
~ ~ ~ CF3 OCH~ CH
66 n ~ H O~2CF3 ~H3 ~
67 ~' n H OCH2CF3 OCH~ CH
68 n n H OCH2CF'~ OCH2C~ CH
69 ~ N H OCH2CF'~ ~C~3
~ ~ B OCH3 CH(OCH3~a C~
71 C~H5 ~ H OCH3 ~CH3 CH
72 n a H OCH3 CH3 CH
7 3 n ~ CH3 CH3 CH
,4 ~ ~ H OCH3 Cl CH
~ a n n H OCH3 OCH3 N
1 6 ~ n H OCH3 ~H3 N
77 C3H7 H OCH3 ~CH~ CH
78 ~ n H OCH3 CH3 CH
?9 ~ ~ H OCH3 CH3 N
C~Hg n H OCH3 OCH3 CH
81 n n ~ OCH3 CH3 CH
82 ~ n H OCH3 CH3 N
~3 CH3CHs OCH3 OCH3 CH
84 " CH3 ~CH 3 CH3 CH
"~ CH 3 OCH 3 CH3 N
86 "CH~ OCH3 Cl CH
87 ~~ CH3 ~CF2H OCF~H CH
88 CH3 C~3 ~H3 CH
89 C2H5" ~H3 OCH3 OCH3 CH
~n CH3 OC~3 CH3 CH
91 CH3 n H OCH~ O~H3 CH 134-135
92 " " H CH3 Cl CH
93CH3 C2H3 ~ ~3 CH~ CH
. H ~3 ~H3 CH 140-141
n n H CH3 ~3 N
96 ~ ~ H OCH3 ~H3 ~
97 ~ OCH3 OCH3 N
98 " u B ~C~3 Cl CH
99 ~ ~ H OCF2B CH3 CH
100 n ~ H ~CF2H OCF2H CH
101 ~ " H OCH3 Br - CH
102 ~ OC~3 OC2H5 CH
103 ~ ~ H OCH3 ~CH3 CH
104 n ~ H OCH, C2Hs N
- 17 ~
~xample Rl R2 R3 ~ ~5 ~ ~elting
N~. Point [C]
. . ~
1~5 ~ H OCH3 ~C3H7 CH
106 " ~ H OCH3 Cl N
107 n n H Cl ~aHs CH
108 ~ ~s OC2~s CH
109 .. ~ C2H5 OCH3 CH
110 " " H C~ OCH3 CH
~ H OCH2CFg ~Hs
112 " ~ ~ OCH2CF3 OCH3 CH
113 n ~ OCH2CF~ OCH~CF3 CH
114 ~ OCX~CF3 OCH3 N
1 1~ n ~OCH3 CH~OCH3)2 CH
116 C2Hs ~ H OCH3 oc~l3 CH
117 H 8CH3 ~Ha CH
118 n ~ ~ C~3 ~H3 CH
119 n n ~ ~CH3 Cl CH
120 ~ n H ~CH3 OCH~ N
121 " ~ H OCH3 ~H3 N
122 C~H7 ~ H OCH~ OCH3 CH
123 n n ~ OCH3 CH3 CH
124 ~' n ~ OCH3 CH3 N
125 C~Hg ~ H OCH3 OCH3 CH
126 " n H OCH3 ~H3 CH
127 H OCH3 CH3 N
128 CH3 nCB3 OCH3 OCH3 CH
129 ~n CH3 OCH3 CH3. CH
130 "CH3 OCH3 CH3 N
131 . ~ CH3 OCH3 Cl CH
132 . ~3 OCF2H OC~2B - CH
133 ~~ CH3 CH3 ~H3 CH
134 C~H5 ~ CH3 OC~3 ~H3 CH
135 n~ ~H3 OCH~ c~3 CH
136 CH3 H ~3 O~H3 CH 137/138
i37 ~ ~ H CH3 ; Cl C~
138 CH3 O3H7 H CH~ ~H3 CH
13 9 n ~ H OCH3 CH3 CH
140 ~ ~ H CH3 CH3 N
141 " ~ H OCH3 CH3 N
142 ~ n. ~ OCH3 ~CH3 N
143 " ~ ~ OCH~ Cl CH
144 " ~ H OCF~H CB3 CH
- 18 -
Rl R2~3 ~ ~g
145 n ~ H OCF~B OC~2H CH
146 " ~ H OCH3 Br CH
14? n ~ oc~3 ~C2~s CH
148 " . ~ H OCH3 SCH~ CH
149 ~ ~ H OCH~ C2~s N
150 ~ n H OCH3 OC~H7 CN
lal n ~ ncH~ Cl N
152 n ~ Cl ~2~s ~
153 ~ n ~ C2~5 C2Hs CH
154 " ~ ~ C2~s O~N3 CH
1~5 ~ H CF3 OCH3 CH
1~6 n ~ H OCH2CF3 CH3 CH
la7 ~ c. H OCH2C~3 OCH
la8 " " H OCH2GF3 O~H2CF3 CH
1~9 . n n H OCH2CF3 ~CH3 N
160 " ~ H OCH3 CH(OCH~)2 CH
161 C2Hs u H OCH3 OC~3 ~H
162 n H ~CH3 CH3 CH
163 .. ~ ~ C~3 CH3 CH
164 n H ~CH3 Cl CH
16a " n H OCH3 OCH3 N
166 ~ n H OCH3 ~H3
167 C3H7 n H OCH3 OCH~ CH
168 .~ ~ H GCH3 CH3 CH
169 ~ ~ H OCH3 CH3 ~
:l0 , C4Hg ~ H C~3 O~H3 CH
171 n n H OGH~ ~H3 CH
1 1 2 ~ n H OCH3 ~3 N
173 GH3 n CH3 OCH3 ~CH3 CH
174 ~ ~ GH3 OCH3 ~3 CH
17 ~ n ~ ~H3 0CH3 ~3
176 ~ ~ C~3 OCHs Cl CH
177 " ~ ~H3 OC~2H OC~28 CH
17B " CH3 CH~ CH3 CH
179 C2H5 n CH3 OCH3 OCH3 CH
180 ~ CH3 OCH3 CH3 ~
181 CH3 n H OCH3 OCH3 CH
182 ~ ~ B CH~ Cl CH
". ~
-. ~ .
,.
~3,;7o~
-- 19
~iological ~ ple~
The d~mage to weed plants and l~he cxop plant ~oleranca
were ra~ed in accordanc~ wi~h a code in which the
activity i~ e~pres~ed by numerical values from 0 ~o 5. In
~hi~ code:
0 = no ~ction
1 = O to 2C% action or damage
= 20 to 40% ~ction or damage
3 = 40 to ~0% action or damage
4 = 6D to B0% action or damage
5 - 80 to 100% action or damage
1. Action on ~ed~ by the pre~ rge~ce ~th~d
Seeds or pieces of rhizome of mo~o- and dicotyledon weed
plants were laid out in ~andy loam ~oil in pla~tic pOtfi
and covered with soil. The compounds according to the
invention, formulated in the form of wettable powders or
emulsion concentrates, wers then applied in various
dosages to the ~ur~ace of the covering 80il aB a~ueou
suspension or smulsion~ with a water application amount,
when con~erted, of 600 to 800 l/ha.
After the treatment, the pot~ were placed in ~ greenhouse
and kept under good grow~h conditi~ns for ~he ~*ed~
Vi6ual rating of the plants or the ~mergence damage was
performed after the ~mergence of the te~t plants after ~n
~5 experLmental period of 3 to 4 w~ek6 in compari~on ~ith
untreated con-trols. A~ the rating value~ in Table 2 8how,
the compound~i ~ccordiny to the inv~n~lon have a good
herbicidal pre-emergence ac~i~ity again~t a broad
6pectrum of gramineou~ weeds and broad-lea~ed weeds.
2. A~tion on ffeed~ ~y the p~st-~mer~i3~ce method
Seeds or pieces of rhizo~e of mono- and dicotyledon w~eds
were laid out in sandy loam 80il in pla~tic pot~, covered
- ~o ~-
with ~oil and grown in a gree~ouse under good growkh
conditions. Three week~ aftex ~owing, ~he test plant~
were treated in the ~hrae-leaf ~3~ge.
The compounds according to ~h8 inven ion, ormul~ted a6
wettable powders or as ~mul~ion conc~ntr~tes, ~re
sprayed in various dosages on to the ~r~e~ p~rt~ o~ the
plants with a water applica~ion ~moun~, ~hen ~onverked,
of 600 to 800 l~ha, and after ~he ~e~t plants had ~tood
in a greenhou~e under op~Lmum growkh ~onditions for ~bout
3 to 4 weeks, the action of ~he prepara~ion~ wa~ rated
visually in comparison wi~h untreated control6.
The agents according to the invention al80 exhibit a good
herbicidal ac ivi y against a broad ~pectrum of econo~ic-
ally Lmportant gr~mineou~ weeds and broad leaved weeds by
the post-emergence method (Table 3).
3. Crop plant tolerance
In further experimen~s in the greenhouse, ~eed~ of a
relatively large number of crop plant~ and weeds were
laid out in sandy loam ~oil and covered with soil.
Some of the pots were ~reated immediately a~ de~cribed
under 1., and the remainder were plac~d in a greenhou~e
until the plant~ had developed two to three proper
leaves, and were then sprayed witn the substances accord~
ing to the invention in ~arious dosagee, a~ described
under 2.
Four to five weeks a~ter the application and standing in
the greenhou~e, it was a6certained by m~ans of visual
rating that the compounds according to the invention left
dicotyledon crops, such as, for ex~mple, 80ya~ cotton,
rape, ~ugar beet and potatoe ~ undamaged when used by the
pre- and post-emergence method, even at high dosages of
active compound. Some ~ubstances moreo~er also ~pared
gramineous crops, ~uch a~, for examp~e/ barley, wheat,
rye, ~orghum, maize or ric:e. The compound~ of the
formula I ~hus ha~re a high se:Lectl~rity s~hen u~ed ~or
controlling unde~irabl~ plant grow~h in agricultural
;:rops .
5 Ta~le 2 s Pre emergence action
E~ampleDo e ~erbicidal action
No .kg of a. i . fha SIAI, CRS13 LOHU ISCC:R
~_ ",
0.3 5 5 2 5
3 0.3 ~ 5 5 S
4 0.3 5 5 ~ 5
0.3 5 5 5 5
7 0.3 5 5 5 5
9 0.3 5 5 2
4~ 0.3 5 5 5 5
136 0.3 5 5 3 5
94 0.3 5 5 5 5
~1 0.3 5 5 2 5
49 0.3 5 ~ 5 5
37 0 3 5 5 3
3~t 0.3 5 5 ~ _
0.3 5 5 2 4
-- 22 --
Tabl~ 3- Post-emergence actlon
ExampleDo~e He:rbicidal action
No .kg of a . i . ~ha SI~CI~DI~ L~ CCR
2 003 5 5 3 5
3 0.3 ~ 5 5
4 0.3 5 5 5
S 0.3 5 5 ~ 5
7 0.3 5 5 3
9 U.3 5 5 3 2
0.3 ~ 2 2
42 0.3 5 5 3 ~L
136 0.3 5 5 3 4
94 0.3 ~ 5 5 5
91 0 . ~ 5 5 3 5
49 ~.3 5 5 4 5
37 0.3 5 5 3 3
38 0.3 5 5 ~ 3
4ù 0.3 5 5 1 2
2 0 .abbreviatiorl~:
SIAL = Sinapis alba
CRSE = Chrysanthemum seget
LOMU = ~olium multiilorum
ECCR = Echinochloa cru~-galli
a. i . = active ingredient
- ~