Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
USE OF MONOACYL FHOSPH05LYCERIDES TO ENHANCE
THE CORNEAL PENETR~TION OF OPHTHULMIC DRU~S
The present 1nventlon relates tQ the f1eld of ophthalmlc
drug del1very. More partlc~larly, th1s lnYQntton rel~t~s to
enhancement of th6 penetratlon oP Dphthalm1o drugs and oth~r
th~rapeutlc agents through the cornea of the ~y~.
In order for ~n ophthalmlc drug to be ther~peutic~lly
effectlve, lt 1s gonerally necesssry for the drug to pen~trate
the cornea ~nd bo taken up 1n tho ~queous humor, clllary
processes ~nd othQr tlssues 1n the eye. TherQ are notabl~
excepttons to thls general rule, such ~s drugs or drug products
wh1ch produca a th~r~poutlo effect by act1n~ on th~ ~xter10r
surf~cQ of the corn~a (e.g., drugs or dru~ products usQful ln
improvtng ocular comfort and/or tr~atlng dry or 1rrlt~ted eyes).
HowevQr, th~ tre~tm~nt of cond1tlons lnvolvlng phys1010glcal
mech~ntsms w1th1n tho ~ye (e.g., gl~ucoma, d1abetlc retlnopathy,
cat~r~cts, otc.) g~ner~llY doos requ1re tho movement of topically
appl1ed ophth~lmlc drugs through the cornoa,
In orter for a drug to pass throu~h tne corne~, 1t must
pen~trate threo l-yars of t1ssuQ, n~mely, th~ eplthQllum, stroma
and the ~ndothol1um. Except for h1ghly 11pophll1c drugs, the
eplthel1um ls the m~ln ~rr1~r to drug penetratlon of the cornea.
Pen~trat10n of the stro~a b~1cally lnYolvQs d~ffus10n of the
drug through a barrler wh1ch ls ~pproxlmat~ly 360 m1crons th1ck.
Thero ~re currontly no known.~othods of ~nhanctn~ drug,
penetrAt1on through th~ stro~ or ondothel1um. Howev~r, lt 1s
posslble to enhanc~ th~ p~netr~tlon of dru~s through tha
eplthellum, and theroby enhance the ov3rall absorptlon of drugs
appliet top1cally to the eyo. The prQs~nt 1nventlon 1s dlrectad
to such enhancement.
L-3~
Thero hava b~en prlor ~tt~mpts to enh~nce the
penetrat10n 9f drugs through the corneal ep1thellum, The goal of
such atto~pts h~s gon~rally been to enhance penetr~tlsn of drugs
throu~h the corno~l ep1th21ium to an optt~l po1nt ~t wh1ch the
stroma alona controls drug tr~nsport through the cornea The
prlor attempts have lncludod use of chem1c~1 agents to enh~nce
the penetrat10n of drugs through th~ eplthel1um It has been
reported ~h~t benzalkonium chlorlde (BAC), blte salts, dlmethyl
sulfox1de (DMS0), ethylen~dl~m1ne tetr~acctate (DT~) and 1-
dodecylazayl-cyclohaptan-2 one (A~ONER) enhance the corncal
penetratlon of certa1n dru~s Th~ follow1ng publicatlons may be
referrcd to for further background concernlng the use of such
agents to enhance cornea1 ponetratlon ~ 5~ 9,l~
Vol 53~ p 335 (19~5); ~,_p ~ , Yol 39, p I24 (1987);
Chem Abstracts, Vol 106, 125931t, p 402 (1987); ~Q~rn~l_Qf
Ph~rma~g~cal Sc1~n~, Vol ~7, No l (J~n ,1988); and
, Vol 29, No 2
~Feb ,198B) Notw1thstandlng such prlor attempts, th~ro
contlnues to be a neod for a moans of safely and e~fectlvcly
enhanc1n~ th~ penotratlon o~ drugs through tho corn~a
Su~murv o~ tho,Lny-n~ion
A pr1nc1p~1 obJoct1ve of tho present lnventlon ls to
provlde for a mQthod of ~nhancln~ thc ~b111ty of drugs and
ther~p~ut1c ~g~nts to ponQtr~tQ the cornes A further ob~octlve
of the prQ-ent 1nvent10n 1s to provido top1cal ophthalmlc
composltlons cont~1nlng ons or moro ag~nts for onhanc1ng th~
cornoal ponotr~t10n of the a~t1vo tngr-dlentls) cont~lnod
thcroln
The forogolng obJoctlves and other general obJoctlves of
th~ pr~sent lnventlon are satlsfled by the provlslon of a me4ns
of enhanc~ng corneal ponetrat10n by us1ng ~ class of compounds
~ ~v~
referr~d to hQrQln ~s "mono~cy) phosphoglycer1des~ to enhance the
p~nutr~tlon of ophthal~ic drugs throu~h the corneal op1th~11um
In ~dd1t10n, tha ob~sct1ves of the pr~sQnt lnvsntlon arR
furth~rod when v1scos1ty ~nhanc1ng polymors ~re used 1n
con~unct10n wlth thQ mono~cyl phosphoglycerides to ensur~ the
monoacyl phosphoglycerldes ar~ rcta1n4d 1n th~ oyo for a
rel~t1vsly longer per10d o~ ttm6, thus allow1ng th~ compoun~s
morQ ttme to f~c111tat~ drug transport through tha cornea
~ L~Yr~: I comp~res the amount of a drug, p~ra-am1no-
clontd1ne, found ln the aqueous humor of r~bb1ts wh1ch w~re
admln1stered th~ drug w1th and wlthout lysophosphAtldylchollne
~18 0 ~Lysopc)
Fl~ure Il comp~r~s th~ amount ~f a drug, p~rs-~m1no-
clonld1na, found 1n thQ aqu~ous humor of rabb1ts wh1ch were
admlnistered the drug wtth polyv1nyl ~lcohol ( W A), wlth lysopc
~nd PVA ~nd w1thout elth~r lysopc or PVA
D~t~ g~D~crtp~on or ~bfLll~o~
~ he proscnt lnvent10n ls b-sod on the dlscovery that
amph1pathtc ~ono~cyl phospho~lycerldos offoctlv~ly and safely
enh~nce th~ corn--l pon~trat10n of ophthslm1c drugs These
pen~tr~t10n onh~ncor~ c~n be usod ln compos1ttons compr1slng ~ny
ophth~lm1c ~rug ~hlch, to bc cff~ctlvo, must be subst~nt1ally
t~ken up by thc ~uoous humçr, clllsry processes snd oth~r
tlssuQs 1n tho ~yc upon top~csl ~dm1n1str~t10n Exsmplos of
cl~sses of ophth-lmlc drugs wlth ~hlch th~ mono~cyl
phosphoglycor1des of thQ pr~s~nt 1nvcnt10n c~n ba uscd, lnclude
sterolds, growth f~ctors, cycloplog1ts, m10t1cs, mydr/at1cs,
thorap~ut1c prot~lns and pdpt1des, ant1cx1d~nts, ldose roductas~
'rD '1,) r
lnh~bltors, nonsteroldal ~ntlln~lammatorles, 1mmuomodulatQrs,
ant1all~r~1cs, ant1m1croblals ~nd anti-gl~ucoma ag~nts.
~ he p~netr~t10n enhanc1ng mono~oyl phospho~lycer1des
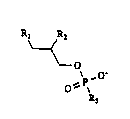
us~d ln thc present tnvent10n h~ve the follow~ng stru~tur~:
~ ,0'
0~ ~
R3
wher~1n one of Rl and R2 1s hydrogen, thlol, hydroxyl, amlno,
lower ~lky1, lo~er alkoxy (~S methyl, ethyl, methoxy or ethoxy)
or alkyl sulflde And the other ls an esterlfled, etherlf1~d or
amldtfl~d hydroph~btc group, and R3 ls a hydrophll1c group. The
pref~rred hydrophoblc sroups lnclude saturatsd ~nt unsaturatod
allphatlc hydroc~rbon groups whlch rang~ from 14 to 24 carbons in
length w1th zero to 5 doubl~ bonds. The allphat1c hydrocarbon
gro~ps c~n be str~1ght or br~nchQd chaln and msy be substltutQd
by one or mor~ aromat1c, cycloallph~tlc or hydrophilic (9.9.
hydroxyl, thlol, or am1no) groups. Examplos of sult~le hydro-
ph111c groups (~3) 1nclude O-inosltol, chollne, O~cholinQ,
O-c~rnlt1nel 0-(CH2)3-choltne. O-g~yc~rol and O-lysophosphatldyl-
glyc~rol.
Tho prbferr~d mono~cyl phosphoglycer1dos are
lysophospholtplds, such as lysophosph~tidylchollnQ, lysophos-
phattdyl1nos1tol, (lysolcclthln). lysocardiollpln,
lysodcsoxylip1ds, lysophosphoryllplds ~nd oC~lyso-r O-alkyl or o-
alkenyl phosphol1plds such ~s DL~o~-Lysolec1thln-r-0-hox~decyt
and DL-cC-Lysoloc1th1n-r-O-alkyl. The ~ost prcfcrrod mono~cyl
phosphoglyc~r1dQ 1s l-acyl lysophosph~tldylchol1na (Cl8:0, ClB:l,
C16:0 or Cl6:l). The l-acyl lysophosphat1dylsholine C18:0
(lyso~elth1n) wht~h ls most preferr~d h~s the follow1ng
structur~l fonmul~:
~ C~H~
/ ~ O~
L~
The monoaeyl phosphoglycer1d~s whlch ~re useful 1n the
pr~sent lnventlon may b~ descr1bed as be1ng ~mphlpathtc~, stnce
they 1ncludo both hydrophlllc ~nd hydrophoblc groups. ~h11e not
w1sh~ng to b~ bound by ony theory, 1t Is bellQved th~t
~mphlp~th1c monoacyl phosphoglycer1des onhance the corn~al
p~netrat10n of drugs by partltlon and lntorsctlon wlth protein,
glycoprotoln and llpld components pr~sent ln th~ membr~ne of the
cornQsl eplthel1um. Such lnter~ct10n ts bel1eved to ~ltsr the
t~gree of orter of th~ protelns and llpids ln the ~mbrane,
theroby modlfy1ng the functlon of th~ op1thellum as a barr1er to
drug pcnetr~tton. ~h~tov0r the moch~n1sm, th~ net result 1s ~hat
dru~ ponatr~t10n of th~ ep1th~11u~ ls enhancod.
_ .
The use of mono~cyl- phosphoglyeerld~ in ~ccord~nce wlth
the pros~nt ~nventlon to cnhAncc cornoal p~natr~tlon of drugs
s1gnlflcsntly lncre~s~s thQ ~mount o~ dru~ ~hlch 1s sblo tc
pon~trato the cornoa. Th~ dogree of ~nhanc~Fent wlll vtry wlth
dlfforQnt druos, but ln som~ CaSQS may be ~5 much as 3-fold or
f '~ J
more. Bec~u~ drugs con mors effectlvQly p~netrat~ the corn~,
less drug ls 105t due to flow down the punctum and th~refore less
drug n~ed b~ admlnlst~red to eff~etlvely tr~at a par~1cu1~r
1ndicatlon. Thls ls p~rt1cu~arly b~neflclal when 1t ls necesstry
to adminlstcr drugs whlch c~us~ severe systemlc slde effects.
The amount of monoacyl phosphogtycer1dQ requlred in
order to enh2nco corneal penetrat10n w111 depend on v~r10us
factors, suCh ~s thc solublttty, part~tlon csefflc1ent ~nd
molecular wRtght of th~ ophth~lmic druq or therapeutlc a~nt; ~he
excip10nts (surf~ct~nts, pr~s~rvat1ves, polymers) presQnt ln the
formul~tton; ~nd the part1cular mono~eyl phospho~lyccr1de be1ng
used. In gener~l, the mor0 11poph11ic the drug to be del1vered,
thQ less ~cnoacyl phosphoglyc~rlde 1s requ1red to enhanc~
pQnetr~tlon~ and the h19her the concentration of monoaeyl
phosphoylycerlde, thQ better the corneal pen~trat10n. Typically,
one or ~orc monoacyl ph~spho~lyc~rldes wlll b~ used 1n 2n ~mount
ef from ~bout O.OOlX to about O.5X (wet~ht/volumQ) prefar~bly
from about 0.01 to 0.1%.
The mono~cyl phospho~lycer1~es can b~ used 1n any
top1cal dru~ dellvery system ~her~1n ~n exclptent or v~hlcle wlll
not substantlally 1mp~1r or pr~vant the monoacyl
phosphoylyc~r1d~s fro~ functtontn~ as corn~al pen~tr~tlon
enhano~rs. For examp~o, th~ mono~cyl phosphoglycQrtdes can be
formulatod 1n composlt10ns which are solutlons, suspon~tons,
o1ntments, g~ls or ftlms. ~he tYp~ of composlt10n wlll dopend
on, among oth~r thlngs. the eh~mlcal and physlca1 prop~rtl~s of
thfl drug or therapeutlc ~nt--to be dellvered. ~heso propQrt~os
are well knom to a person of ord1nary sklll 1n the ~rt of drug
formulat10n and dellvQry.
In ~ preforrod 6mbodlm~nt, thQ pr~sent lnventlon further
comprlses the US9 0~ polymers ln tonJunctlon wtth the monoacyl
phosphoglycQrld~s to ~nh~nc~ ocul~r blo~v~llab111ty. The lon~er
a top1c~1 ophthalmtc formul~t~on ls 1n cont4ct wlth th~ ~ye the
better the ocul~r bio~vallablllty. Through the us~ of po7ymers
1n conjunction wlth th~ abov~ descr~bed monoacyl phospho-
glycer1des th~ compos1t~ons of the presont 1nv~nt10n ~re retained
on the corn~a longQr. As a result, the penetrdtlon ~nhanctng
components of the composlt10ns cbn more effectlvQly 1nteract w1th
the corn~al eplthel1um to enhance p~natrat10n of tho d~s1r~d
drugs or th~r~p~ut1c agents lnto thQ ~ye. It has b~en found that
the usc of polym~rs 1n conjunctlon wlth monoacyl
phosphoglyc~rld~s can provlde for up to ~bout a 9 to lO fold
lncr~se ln th~ ~ount of drug or thcrapeut1c a~ent made
av~lla~le to th~ ~ye. The ef~ectlv~n~ss of th~ mono~cyl
phospho~lycer1des Is lmproved when the vlscos1$y of the
co~pos1t10ns cont~1n~ng the mono~cyl phospho~lycer1des 1s
1ncr~ased up to ~bout I000 c~ntlpolse (cps), pref~rab1y bstween
~bout 50 cps. to 30G cps. Polym6rs aro ~dded to provlde for thls
dos1red Ytscos1ty 1ncro~ss.
Any synth~tlc or n~tural poly~er whlch ~lll 1ncrease
vlscoslty and ls compattble wlth tlssu~s of the ey~ and the
lngredtQnts of the monoacyl phosphoglycer1de composlttons.can be
used. Such polym4rs are refcrr~d to hcro1n as ~vlscoslty
onhancln~, ophth~lm1c~11y ~ccept~blo polym~rs.~ Exampl~s
1ncludc, but ~rs not 11~1tod to: natur~l polyt~ccharldes and
gums, such ~s: ~lgln~tes, carrag~on~n, guar, kar~ya, locust bean,
tragac~nth ~nd x~nth~n; ~nd synthotlc polymers, such ~s:
carbum~r, hydroxyethylcellul OSQ ~HEC), hydroxypropylc~llulose,
hydroxypropylmothylcellulosa-~HPMC), mQthylc~llulosQ, polyvlny1
alcohol (P~IA), polyv1nyl pyrrolldon~ carboxymothylcollulos~ and
agaros~. In ~ddltion protelns ~nd synthotlc polypeptldcs wh1ch
enhance vlscos1ty Jnd aro ophthalm1cally 4cceptab1~ c~n b~ used
to 1ncroase tho v1scos1ty of tho compos1t10ns to prov1de for
better b10~ billty. ~yplcally, protelns wh1ch c~n b~ used
include: gQlatln, coll~gen, album1n and c~sQln.
~ hR preferred v1scos1ty enhanclng a~ents ~re one or more
polymers select~d frDm: PVA, HPMC ~nd ~EC. Th~ most prefarrod
agQnt 1s HPMC. The v1scos1ty ~nhanc1ng ~g~nts are ~dded to
provlde for composlt10ns w1th a v1scoslty of betwQen about S0 and
300 cps.
~ hQ preferr~d m4thod for enh~ncing the pen~tratlon of a
drug or therap~utlc agent compr1sQs the use of lysophosphat1dyl-
chol~ne (C18:0~ ~t a concentrat10n of about O.OlX to 0.05X ln
comb1nat10n ~lth the polymÆr HPMC tn an amount suFflclent to
prov1de a composltion wlth a vlscos1ty of bout S0 to about 300
cps ~
The followtng ~x~mples furth~r lllustr~t~ the
compos1ttons whlch, ~ccordtn~ to the prcsont lnvcntlon, comprlse
monoac~l phospho~lrccrides, the cornaal pcnotrat10n enhanclng
propertlos of the monoacyl phosphoglycerld~s and the1r USQ to
enhance corneal pcnetr~t10n.
~L~
~ h~ following formulatlon is n ex~mpl~ of a top1cal
ophth~lmlc composlt10n whlGh can be USQ~ to tre~t glaucoma.
;3
~,,~
InarQdl~nts _ ~(we~gh~yol~L
P~ra-amlno~elon1dlne 0.293
Hydroxypropylmethyloellulose-E50L~,(HPMC)USP 3.3
Lysophosphat1dylcholine ~Cl8:0) ~.03
B~nzalkon1um chlorld~ 0.0l
Dlsodlum Edatate, USP ~.01
Sodlum phosph~te, monobas1c, USP 0.18
Sodlum phosphat~, d1bas1c, USP 0.12
M~nnltol, USP 3.3
~C1, NF ~nd~or NaOH, NF q.s. ph to 6.5+0.2
Purlf1~d ~ater, USP q.s. IOO
_
~Equiv~lent to Q.2SX + 2X excess para-am1no-clonldlne as fre~
base.
Prep~a~iQn
~ he formul~tlon 1s prep~red ln two parts. The
hydroxypropylmethy k ~llulose 1s f1rst d1ssolvQd ln purlflod water
to m~ke ~n ~pprox1~-tely IOX solut10n. Thls solutton ls then
cl~rlfled by f~ltr~tlon and st~rlllzed by autoclav1ng.
The othQr lngr~d1ents re n~xt dlssolved ln
approxlm~t~ly one-half of the purlfled wat~r. ~ho mlxturo ls
warmed to 40 ~ 6C for appro~lmatQly 30 mlnutos to omplete
dlssolutlon of th~ lysophosphatldylchollne. The pH of the
solutlon ls adJust~d to 6.S ~nd the solut10n ls sterlllzed by
sterlle f11tratlon.
Th~ t~o solutions Yre mtxed ~sept1cally, stirr~d, and
th~ r~1n1n~ purlft~d w~t#r ls u~ed to bring the solutton to
flnal volum4.
EXAMPL~ 2
FOr~ua~$1on
~~ ~
Potasslum chlorlde 0.02
Monobaslc potassium phosphate 0.02
Sodlum chlorlde 0.80
D1bas1c sodlum phosph~te 0~2l6
Lysophosphat1dylchol1n~ C18:0 (Lysopc) 0.03
Pdr~-am1no-clonld1n~ 0.25
W~ter q.s. to lOQ%
. . ~
Proc~du~ Prap~ratlQ~ Qf ~ormula~lon
Approx1m~tely 85X (8.5 ~1) of the batch ~olu~e of
purlf1Qd wator w~s add~d to ~ cont-lner. All of th~ tngredlents
were th~n ~ddsd to th~ contalner: 0.002g pot~sslum chlorld6;
0.0809 sod1um chlor1d~; 0.0021~ monobaslc potass1um phosphato;
O.Q216g d1b~slc sod1um ~hosphat0; 0.25~ p3ra~umlnoclontd1ne. The
1ngr~diQnts wQre m1xed w~ll. 0.003g lysopc was addod to the
conta1n~r and sonn1catod wlth he~t (30C) for 30 mlnu hs. Th~ pH
was 2dJust~d to pH 6.0 ~1th lH HCl (0.20 ~1). Th~ solutlon WAS
th2n f11t~red through ~ st~rll121n~ fllter lnto ~ st~rlla
roee1v1ng ves~sl. Pur1fl~d water (q.s. to 10 ml) was th~n pourQd
throu~h th~ st~rlllzlng f11ter and tho solutlon was mlxsd w311.
h.~X ~2~
Tw~lve New ~e21~nd albino r~bblts w~rQ sQ)ecte~ for
ev~luat10n o~ thQ pQn2tr~tlon throu~h the corn~a of ths p~ra-
amlno-clonld1ne formulat10n sst forth Above. All rabb1ts
rec01vad 30 ul of the 0.25% para-am~no-c70nldtne top1c~11y in
both ey~s. Four rabbtts wer~ sacrlf1ced ~t 20 mlnutes from
dosing ~nd aqu~ous humor was wlthdr~wn from th~1r oy~s. The
aqueous humor w~s ~ssaysd by 11qu~d sc1ntlllat10n count1ng to
det~r~1no tho mount of par~-~mtno-clon1dlne ln ths ~qu~ous
humor. The s~me procedur~ was done on ~ dlfferent rabb1ts at C0
mlnutes from dos1ng ~nd on ~nother 4 r~bb1ts, l20 mtnutes from
dos1ng. Twtlve control r~bblts reco1ved 0.25X par~-am1no-
clonld1ne as set forth 1n the formulatlon above wlthout 0.03%
lysopc. Aqucous humor W2S wlthdrawn and assayad ~s ~xplalnQd
abovc. The results are shown 1n the gr~ph dQplct~d ln F1gure I.
It can be seon from th~ gr~ph that the ~mount of p~r~-amlno-
clon1d1ne 1n tha aqU00~5 humsr ls gr~ter tn the r~bblts treated
wlth tho formulat10n contaln1nq 1YSDPC~ At 6~ mlnutes there 1s
almost ~ four fold lncreas~ tn the ~mount of par~-~mlno clonldtne
found 1n the aqueous humor of those rabblts wh1ch rec~ived the
drug 1n con~unctton wlth lysopc versus thoso who rece1vod the
drug ~lthout lysopc. ThereforQ, thQ rcsults lndkAte that lysopc
enhanced pRnetrat10n of p~ra-am1no-clonldtne through the cornea.
ID9L3~19D~ Co~n~ on X~w/v)
Monobastc potasslum phosph~te 0.067
D1baslc potass~um phosphat0 3.137
M~nnltol 2.~5
PVA 7~0
Lysophosphatldylcholine Cl8:0 (Lysopc) C.03
Par~-amlno-clonidlne 0,50
~t~r q.s. to ~oo%
11
Approximately B5% (8 5mt~ of the batth volume of
purlf1ed water was add~d to a cont~lner A11 of the lngr~dlents
w~re added to the conta1ner O OOt7g monob~s1c potasslum
phosph~te; 0 013~g d1b~slc potasslum phosphate; 0 2~5~ mann~tol;
0 7g P~A; 0 05g p~r~-~m1no-clonld1ne The 1ngredients w~re mlxed
well ~nd st1rrfld unt11 ~ll 1ngr~d1ants d1ssolved lnto ~ solutlon
0 0039 lysopc w~s ~dded to thc cont~1ner ~nd sonnic~t~d ~lth heat
(30C) for ~0 mlnutes Tho pH was adJusted to pH 6 5 wlth NaOH
Purlfl~d w~ter (q s to lOml) w~s th~n poured through a
sterll1zln~ filter ~nd the solutlon w~s m1xed well
Twelv~ New ~oaland ~lbtno r~bbtts were solected for
evaluat10n of tho p~nctr~t10n through the cornQa of the para-
~m1no-clon1dlne for~ulat10n set forth a~ove All rabblts
recelved 30 ~1 of the 0.50X para-am1no-clonld1ne top1cally ln
both eyes Four rabb1ts wore s~crlf1ced ~t 20 mlnutes from
dos1ng ~nd th~lr ~qu~ous humor w~s w1thdr~wn from tholr OyQS. The
aquoous humor w~s ~ss~yQd by liqu1d sclnt~llat10n count1ng to
d~terminc the ~mount of para ~m1no-clon1dlns ln thQ ~queou~
humor Th~ s~n4 proccdure w~s dono on four d1fferont rabb1ts at
60 mlnutos from dos1ng and on ~nother four rabblts, 120 minutes
from doslng Tw~lve control rabb1ts rece1v~d 0 50X p~ra~amlno-
clonldlno s SQt forth in the formulatlon abov~ wlthoùt 0 03X
lysopc ~nd ~.CX PVA Another 12 rabblts rocetved 0 50~ para-
~m1no~clon1d1no as s~t forth 1n tho formulat10n abov~ w1thout
0 03X l~sopc Aquoous humor w~s wlthdrawn ~nd ~ss~yod ~s
oxplalnod above Tho rosults are shown ln th~ or~ph dsp1ct~d ln
Flguro II It c~n bo so~n from the 9r-ph that tho amount of
par~amlno~clonld1no ln tho ~queous humor ts gro-t~r ln the
rabb~ts tr~ted wlth th~ ~ormul~tlon c~ntaln1n~ PVA ~nd PVA wlth
lysopc At 60 mtnutes thore 1s almost a 2 5 fold and lO fold
1ncr~ase ln th~ ~mount of pAra-~m1no~clonldlne found ln the
r~
~queous humor of those r~bbtts ~hlch rece1v~d th~ drug 1n
con~unctlon ~lth PVA or wlth PYA and lysopc, rospect1~ely, v~rsus
thos~ whlch recelY~d thQ drug without PVA or PYA ~nd lysopc. The
results 1nd1cate that lysopc h~s enhancod penetrat10n of para-
am1no~clon1dlno throu~h the corne~ over PYA alon~ snd p~ra-~m1no-
clonldlne ~lone.