Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2~r~2t~
Bac~ground of Invention
The aftertreatment of alloy steel grades in bottomblowing converters is carried out with oxygen as the process
gas, and with nitrogen and argon as the treatment gas~ Such
secondary steel-refining processes are known by the abbreviations
MRP (Metal Refining Process), AOD (Argon-Oxygen Decarburization),
UBD (Under Bottom Blowing Decarburization) and ASM (Argon Secondary
Metallurgy). They serve to refine low-alloy up to high-alloy
steel grades in converter ~ypes of the same name having bottom-bath
nozzles, whereby the steel grades are smelted in an arc furnace.
Non-alloy types of steel are not usually produced in such convert-
ers. However, in order to achieve high quality, there are manu-
facturers who, despite higher costs, refine non-alloy steel types
in such converters even though refining in an arc furnace would
be less expensive.
In this context, it is known from West German patent
no. DE-PS 2,430,975 to partially replace the nitrogen and the
argon by mixing them with CO2, West German patent no. DE-PS
934,772 shows a process for the production of non-alloy steel
in a Bessemer-Thomas converter, which is low in toxic gases. In
this process, CO2 is admitted into the bath either as a gas or
by adding limestone alone or else mixed with oxygen.
Summary of the Invention
It is common practice in secondary steel refining to
first smelt the steel melt in a smelting furnace and then to
transfer it to a fresh tank, in other words, a converter. The
melt is treated in this converter in that the process and treatment
gases are blown into the melt through the bottom of the converter.
For this purpose, metallic jacket gas nozzles are usually employed,
with which the process gas is admitted through the middle nozzle,
s
and the treatment gases are admitted through the ring nozzle.
The treatment gases admitted through the ring nozzle are inert
gases and they serve primarily to cool the metallic nozzles during
the blowing process and to blend the melt. In this case, the
inert gases are Ar and N2. By partially replacing these inert
gases with C02, it is possible to reduce the specific gas costs.
Treatment of the melt in the converter is carried out
in three process phases, namely, decarburizing, heating and mixing.
Desulfurizing and alloying are done concurrently with the heating
step. These three phases are followed by sampling, temperature
measurement and the addition of metallic and non-metallic solids,
and they are carried out at different times.
The Drawin~s
Figures 1-2 schematically show the process se~uence
for treatment with N2 and Ar for the alloy grade and steel grade
42 CrMo 4, respectively, in accordance with this invention.
Detailed Description
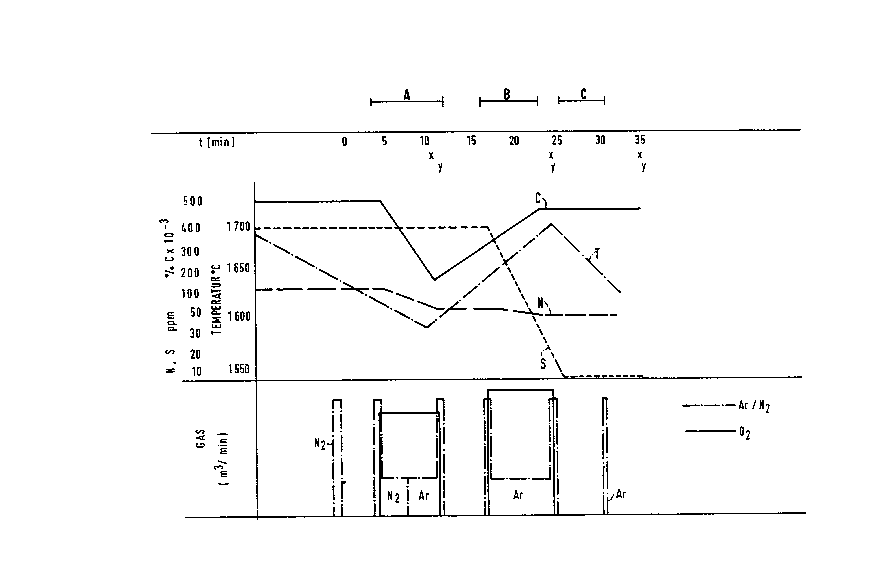
Figure 1 schematically shows a process sequence for
treatment with the inert gases N2 and Ar for the alloy steel
grade 42 CrMo 4. This sequence encompasses the decarburization
by means of area A, heating by means of area B and mixing by
means of area C. The measured points x for the temperature
measurements and y for the sampling are given below the line
which depicts the time course in minutes. Beneath that, the
concentration curves of nitrogen and sulfur as well as carbon
(N, S, C), and the temperature curve T are given. The use of
the process gas oxygen and of the inert and treatment gases argon
and nitrogen with respect to time and amounts are presented in
the lower section of Figure 1.
2~ SS
The invention is based on the task of further reducing
the gas-related costs within the scope of the secondary steel
refining of alloy steel grades.
The process according to the invention stems from the
surprising observation that the inert gases N2 and Ar can be
replaced by C02 not only partially but completely, as a result
of which the gas-related costs in secondary refining of steel
can be drastically reduced. The volume of C02 admitted to the
melt per time unit has to ~e such that sufficient mixing energy
is applied to the melt. Then it is possible for all of the reac-
tions to take place under conditions of equilibrium. In the
process according to the invention, N2 and Ar can be completely
replaced by C02 in all three process phases of the steel treatment,
that is, during decarburization, heating and mixing.
The schematic sequence of the process according to
the invention is shown in Figure 2, likewise for steel grade
42 CrMo 4 as in Figure 1. This clearly shows that essentially
the same treatment result is obtained.
The C02 has differing effects in the individual process
phases. This is described below.
When the converter is moved from the lying or horizontal
position to the upright, blowing position at the beginning of
the treatment process, the nozzles must receive inert gas in
order to prevent the melt from penetrating them. For the sake
of safety, it is only possible to admit C02 when the blowing
position has been reached. Corresponding measures must be taken
when the converter is tipped back to its lying position. The
volumes of gas admitted to the nozzles during such changes of
the position of the converter are called safety volumes.
--4--
2~3 4~,5
During decarbur~zation of the melt with oxygen, the
C2 makes up the safety gas volume when the converter is placed
in the blowing position. Subsequently, oxygen is blown in through
the middle nozzle, and the ring nozzle is continuously cooled
by means of C02. By admitting oxygen and C02 together, the partial
pressure of the N2 and H2 is reduced during the decarburization
phase. This leads to degasing of the melt. At the same time,
a charging of the melt with the gases N2 and H2 is prevented,
so that, for the most part, steel types low in N2 and ~2 are
obtained.
With the reaction of C02 + C = 2 CO, C02 is additionally
employed to decarburize the melt, that is to say, C02 is an addi-
tional oxygen carrier in the decarburization phase.
During the subsequent heating phase, the use of C02
for desulfurization and alloying has a different effect. In
this context, the melt is heated up to the desired temperature
by means of the exothermic reaction of oxygen with the aluminum,
silicon or aluminum-silicon mixture added. Up until now, in
the treatment phase, only argon has been used as the treatment
gas since nitrogen would dissolve in the melt, thus giving rise
to an undesired charging of the melt with nitrogen.
When replacing argon with C02, the following reactions
must be taken into consideration:
3 C2 + 4 Al = 2 A1203 + 3 C (I)
3C02 + 2 Al = A1203 + 3CO (2)
or
C02~Si=SiO2+C (3)
C2 ~ Si = SiO + CO (4)
Both reactions take place during the heating phase,
as a function of the concentration of aluminum in the melt.
2~t~, ~
Analogous to equation (1) or (3), the melt is carburized during
the heating phase, the C02 is completely reduced by the aluminum
and a carbon atom is released. At the same time, reaction ~2)
or (4), that is to say, the partial reduction of C02 takes place,
and these reactions do not result in the carburization of the
melt. For each melt, it is possible to calculate the carburization
of the melt in advance during the heating phase and then to take
this into consideration by means of more thorough decarburization
during the decarburization step.
As can be seen in Figures 1 and 2, a carburization
of the melt takes place during the heating phase. This carburiza-
tion can be calculated on the basis of the following calculation:
536x Qx Cf
dC =
dC - carburization rate in ppm C/min
Q - flow volume of C02-inert gas m3/min
Cf - carburization factor 0.3 to 0.5
G - melt weight in tons
In a 10-ton converter with the carburization factor
Cf ~ 0.5, at an inert gas volume of 2 m3, the carburization rate is
dC = 536 x 2 x 0.5/10 = 53.6 ppm C/min.
2~3 ~
Tables 1 and 2 below present the effect of the use
of C2 according to the invention in the decarburization and
heating phase for several steel grades. Table 1, which shows
the degasing of the melt measured according to the content of
nitrogen, also shows the operational results of the commonly
used process with nitrogen and argon, as well as the results
of the process according to the invention.
Table 1: Degasing of the melt, measured aocording to the nitrogen content
gas oonsumpdon gas content
melt 2 N2 Ar C02 nitroge~
steelgrade m3/t m31tm31t m31t start end
20 Mn 5 12.9 3.4 5.9 - 99 43
17 CrMo 55 12,1 3.7 6.0 - 122 75
42 CrMo 4 11.0 3.5 2.4 - 125 107
17 CrMoV S. l l 16.6 2.4 8.0 - 105 77
10 MnMo 74 16.1 - - 9.8 96 69
17 CrMo 5.11 17.1 - - 10.9 110 76
42 CrMo 4 12.6 - - 7.9 104 62
34 NiCrMo 14 18.6 - - 11.7 106 73
Table 2: Decarburization of the melt during the heating step
Steel grade carbon content gas consumption heating
start end 2 C2 with Al
% % m3/min m3/t m3/mlnm3/n kg/t
10 MnMo 74 0.04 0.08 9.0 6.0 2.4 3.0 10
17 CrMoV S.ll 0.12 0.16 3.0 9.0 2.3 3.0 10
42 CrMo 4 0.27 0.30 9.0 5.6 2.4 2.7 9
35 CrNiMo 14 0.27 0.32 9.0 6.6 2.4 3.5 11
A crucial factor for the effectiveness of the process
according to the invention, particularly during the heating phase,
is the purity of the C02. After all, the reduction of the melt
by means of aluminum brings about a higher degree of solubility
2~4~
of nitrogen in the steel. For this reason, the nitrogen and
hydrogen impurities in the C02 are absorbed by the melt and can
no longer be removed. In order to prevent this" for the metallurgi-
cal treatment of steel according to the invention, technically
pure C02 with a maximum of 500 vpm of N2 and 50 vpm of H20 must
be used. This degree of purity is preferably obtained by evaporat-
ing the C02 from the liquid phase.
In the mixed phase, according to the invention, C02
also completely replaced the argon. According to the state of
the art, shortly before tapping, the melt is mixed with argon
for 1 to 2 minutes so that temperature equilibrium can be achieved.
When argon is replaced by C02, an oxidation of the melt takes
places directly before tapping after the reactions mentioned
during the description of the heating phase. By means of the
stoichiometric addition of approximately 1.0 kg of Al/M3 of C02,
this change of the analysis is compensated for. The simultaneous
carburization can be ignored, since it only amounts to 50 ppm
and thus falls within the analysis tolerance limits.