Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
This invention relates to a vapor pressure
measuring apparatus.
While the apparatus of the present in-~ention was
designed specifically for measuring the vapor pressure of
liquid hydrocarbons, it will be appreciated that the
apparatus can be used to measure the vapor pressure of a
wide variety of liquids, both hydrocarbon and non-
hydrocarbonO The analyzer may be used to determine liquid
Reid vapor pressure in "gage" or "absolute" units for
l.O hydrocarbon liquids as defined in procedure ANSI/ASTM D
1269-7~ and ~apor pres~ure of hydrocarbon liquid defined in
procedure ANSI/ASTM D 323-79, respectively. Depending on
inlet conditions such as pressure and temperature of a
sample, the analyzer will measure vapor pressure o~er a wide
range of temperatures thus determininy a ~apor pressure
curve for each sample. The analyzer is intended for
~; operation on a batch basis with time cycles typically in the
range of one to three minutes, and can produce repeatable
results within the accuracy specified by the above mentioned
: 20 ANSIJASTM pxocedures.
At present, the above mentioned ANSI/ASTM
laboratory procedures are commonly used to measure Rei.d
-~ vapor pressure of petroleum products. Both methods are
laboratory procedures which require the performance of
specific steps to produce predictably accurate results.
.~ ,
~ 1 --
.
'
æ~e
Both methods require careful handling of samples, pre-
conditioning of sample containers, heat bath systems, and
agitation o samples during an approximate thirty minute
time period to ensure mixing and vapor separation. The
ANSI/ASTM D 323-79 procedure requireæ an air chamber to be
preheated to lOO~F before sampling.
~ In the past, a variety of methods o~ measuring
; vapor pressures have been proposed, including the use of an
apparatus consisting of a temperatule control bath, a
continuous sampling device and two positive dlsplacement
pumps of dif~erent capacities to cauæe vapor separation.
Vapor pressure is measured in a chamber, the~temperature of
`: :
~hich is controlled by a b th at 100F. The device was
designed primarily for measuring the vapor pressure of crude
oil. In accordanoe with another method, the vapor pressure
of blended gasoline products has been determined by
measur1ng the~tempera~ture drop produced by the expansion of
a llquld sample rom a high to a low pressure under
substantially adiabatic conditions. In order to improve
~ ~ 20 accuracy, methods have been devised to remove a~cumulated
;; ~ixed gases ln the apparatu~ E'inally, vapor pressure has
~been measured using an apparatus including a temperature
control bath~and two;sample pumps of different capacities.
In essence, a sample is continuously vaporlzed across an
orifice plate into a chamber. The vapor pressure is
:
~ - 2 -
~: : :
.
,
,: .
,, :' ' ' - ' . ' :,
,
- ' ' '' ,' - ' ' -
' .: . ~ , ' . :
., ~ : .. .
"
measured in the chamber by a bellows-type pressure gage
employing a Wheatstone Bridge.
The above described methods are reasonably
accurate. However, they employ elaborate sampling
techniques such as time consuming manual laboratory
proced~res and the use of two pumps in series to collect
samples and ensure vapori2ation. The laboratory procedure
includes special sampling steps and agitation of the liquid
to ensure mixing. The continuous sampling device require~ a
liquid, temperature controlled bath which is difficult to
use in industrial applications without special
consideration. Bath temperature is difficult to control
within +/- 1.5F accuracy, The continuous device requires
varying levels of calibration to a standard before handling
liguids of significarltly different composition or physical
propertiesO Moreover, the prior art devices cannot provide
vapor pressure values over a wide range of temperatures
durin~ each sampling cy~le.
The ob~ect of the present invention is to overcome
the above-defined difficulties encounter~d with prior art
devices by providing an automated apparatus which is
relatively simple in terms of design, reliability and
; accuracy, and which can be used to determine the vapor
pressure o~ a wide variety of hydrocarbon and non-
hydrocarbon liquids.
-- 3
27~;
Another object of the invention is to provide an
apparatus which can be used for precise quality control of
liq~lids in industrial applications, and to provide an
apparatus which can be portable for use in remote locations
such as pipelines.
An additional object of the invention is to
provide an apparatus with a relatively short cycle for
providing data of temperature versus vapor pressure for a
given liquid.
Accordingly, the present invention relates to an
automated vapor pressure measuring apparatus, comprising:
casing means definin~ a first liquid sample chamber and a
second vapor decompression chamher; a first valve between
; said first and second chambers; first inlet means for
introducing a liquid sample into said first chamber; first
drain means for draining said first and second chambers;
heatin~ means for heating said casing means and consequently
the contents of said first and second chambers; first probe
means for measuring the temperature in said first chamber;
second probe means for measuring the temperature in said
second chamber~ pressure measuring means for measuring the
vapor pressure in said first and second chamber; and
program~able control means for controlling said first valve,
said f.irst inlet means, said first drain means and said
hea-ting means, and for monitoring said first an~ second
2~
probe means and said pressure measuring means, whereby
automatically to perform the steps of introducing a liquid
sample intQ said first chamber, depressuring liquid sample
into said second chamber, heating saicl sample in said
chambers to a predetermined temperature at which vapor
pressure is required, measuring the vapor pressure in said
first and second chamber~ and discharging the vapor-liquid
mixture from said chambers to automatically measure vapor
pressure of a liquid sample.
The invention further may include means to rotate
said ~asing means to effect mixing of the contents thereof,
i.e. the vapor-liquid mixture.
The invention will be described in greater detail
with reference to the accompanying drawing, which
~ 15 illustrates a preferred embodiment of the invention, and
; wherein the single figure is a schematic flow diagram of an
apparatus in accordance with the present inven-tion.
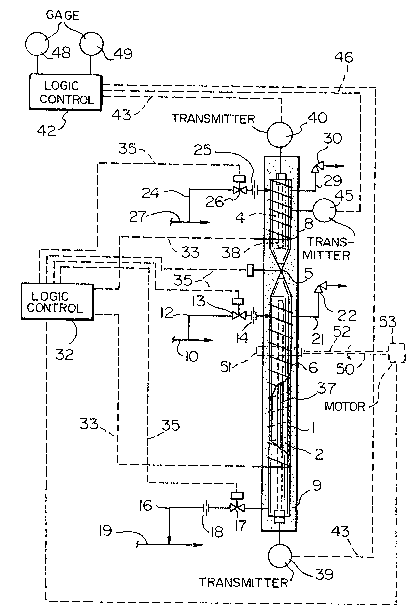
With reference to the drawing, the apparatus of the
present invention incl~des an elongated casing 1, defining a
2~ liquid sample chamber 2 and a vapor decompression chamber 4
separat~d by a valve 5. The casing 1 can, but not
necessarily, be formed of 1/2 - 3/4 inch stainless steel
tubing and is generally from one to two feet in tokal
lengthO A first hea-ting element 6 is enclosed around the
portion of the casing l defining the cham~er 2, and a second
5 --
7~
heating ele~ent 8 is enclosed around the portion of the
casing 1 defi.ni.ng the chamber 4. Means can be also
provided, as shown schematically at 50, to effectively
rotate casing 1, one or more times, to effect efficient
mixing of the liquid-vapor mixture contained within casing
1. A suitable structure could include a collar 51,
extension shaft 52 and electric motor 53. The sequence o~
rotation may be manually controlled by means of a switch
~not shown), or, as shQwn, by a programmable logic
controller 32. The heating elements 6 and 8 are
individually controlled, and the entire casing is wrapped in
an insulating materiai 9.
A liquid sample from a sample line 10 is introduced
into the ~.hamber 2 through an inlet duct 12, which contains
a valve 13 and a restricted orifice plate ~4. When sampling
liquids such as propaner the pressure drop across the valve
13 can result in low temperatures which can cause sealing
problems and leakiny at the valve. Allowing the pressure
drop to occur a-t the plate 14 eliminates valve sealing
probl~ms. The sample is drained to atmospheric pressure
from the cha~ber 2 through an outlet duct 16 containing a
valve 17 and an orifice plate 18 to a drain line l9.
Overpressure in the chamber 2 is relea~ed through a line 21
~ and a pr~ssure relief valve 22. Vapor from the cham~er 2
; 25 passes through the valve 5 into the chamber 4, and is
:~ - 6 -
:
- ~ :
discharged to atmosphere through a line 24 containing an
orifice plate 25 and a valve 26 to ven-t line 27. Any
overpressure in the chamber 4 is released through a line 29
and a pressure relie~ valve 30. Heating elements 6 and 8
are connected to a programmable logic controller 32 by li.nes
33, and the valves S, 13, 17 and 26 are connected to the
logic controller 32 by line 35.
The temperature in the chambers 2 and 4 is
measured using ~urface sensitive resistance thermal probes
37 and 38, respectively, which extend out of t.he casing 1 to
transmitters 39 and 40l respectively~ The probes 37 and 38
should be of the surface sensi.tive type and of maximum
length in order -to maintain optimum acouracy when measuring
the temperature of multi-component samples. The ratio of
; 15 temperature sensing surface area to the volume of the
chamber is also a factor in the accurate measurement of the
temperature of a mulki-component mixture. The transmitters
39 and 40 are connected to a logic controller 42 by lines
43~ The vapor pressure in the chamber 4 is measured and
transmi.tted to the logic controller 42 by a pressure
transmitter 45 and a line 46. When the valve 5 is opened,
and a sample is being heated to determine vapor pressure,
the pressure in both of the chambers 2 and 4 is measured by
the transmitter 45. Gages 48 and 49 are connected to the
logic controller 42 for providing a ~isual indication of
.
temperature and pressure respectively.
As mentioned above, the temperature in the
chambers 2 and 4, and the introduction and discharge of
fluid from such chambers are control].ed by logic controller
32, and the temperature and pressure in the cha~bers is
monitored by the logic controllex 42. The controller 32 is
programmed to ensure that the sampling process, timing,
valve operating, and casing ro~ational sequences are
properly controlled.
While the logic controllers 32 and 42 are shown as
separate devices, in fact, such controls are a single
device r i.e. a microprocessor.
The use of the apparatus will be described by
means of two specific examples, the first being a
determination of the absolute vapor pressure of volatile
crude oil and non-viscous petroleum products as defined in
procedure ANSI/ASTM D 323~79.
Assuming that sampling has already occurred, the
vent valve 26 from the chamber 4, the liquid sample valve 13
and the drain valve 17 are closed~ and the valve 5
; interconnecting the chambers 2 and 4 is open. In order to
drain ~oth ~hambers 2 and 4, the valves 1.7 and 26 a~e
opened, the valve 26 being open to atmosphere. The liquid
and vapor in both of -the chambers 2 and 4 are drained or
aspirat~d to a hydrocarbon drain line 19 at atmospheric
-- 8 ~
'.,~ ' :
2~
pressure, As the sample is drained, air is drawn into the
chambers 4 and 2~
After sufficien-t time has elapsed to permit the
sample to be drained and the chamber 4 to be filled with
air, and chambers 2 and 4 are both at atmospheric pressure,
-the drain valve 17 and the valve 5 are closed. Heat is
applied to the chamber 4 by means of the element 8 to
preheat the vapor decompressi.on chamber to a base
temperature at which a vapor pressure measurement is
required. Normally the base temperature is lOO~F for
determining Reid vapor pressure. The temperature of the
vapor decompression chamber 4 is measured by the probe 38
and the transmitter 40. When the temperature in the chamber
4 reaches the required level, heating is terminated and the
valve 26 is closed. Du~ing operation of the apparatus over
:~ an extended period of time, residual heat in the ca~iny 1
and the insulatio~ ~ may provide suffi.cient heat for the
preheat stage. Upon completion of preheating, the vapor
decompression chamber 4 is at the base temperature and
atmospheric pressure, which counteracts external
atmospheric pressure. The net result is that the
-transmitter 45 can provide an accurate measurement of vapor
pressure, equivalent to the absolute vapor pressure, to the
logic controller 42 for reading on the gage 49 in gage
. 25 pressure. The valve 13 is opened~ and a liquid sample is
_ g _
usecl to flush the chamber 2 for several seconds to ensure
that the previous sample is khoroughly washed from the
sample duct 12 through the outlet duct 16 to the drain line
19. When flushing has been completed, the drain valve 17 is
closed to liquid fill the sample chamber 2, and the valve 13
is closed. The liquid sample is isolated in -the chamber 2
at sample process conditions. The sample may cool slightly
due to pressure drop across the orifice plate 14. For the
analyzer to function, the liquid sample from the line 10
must be a-t a temperature below the required base
temperature. Otherwise sample conditioning will be
required.
The valve 5 is opened and the vapor in the chamber
4 and the liquid sample in the chamber 2 reach equilibrium
at a temperature below the base temperature, and at a
pressur~ below the required vapor pressure. Also sample
conditioning may be required if the sample is volume
compressible at sample conditions. If, when valve 5 is
opened, the liquid sample in chamb~r 2 expands under
decompression into chamber 4, error could be introduced
clepending on the compressibility of the liquid sample. With
the vent valve 26, the sa~ple valve 13 and the drain valve
17 closed and the valve 5 openr the heater 6 i~ actuated to
apply heat to -the casing 1 in the area of the chamber 2.
The rotating means 50 is actuatecl to rotate casing 1 by
.
-
180, one or more times to ensure complete mixing of theliquid-vapor mixture. As heat is appliecl to the liquicl
sample chamber 2, the temperature rise is monitored by the
probes 37 and 38, and pressure increase is monitorecl by -the
pressure transmitter 45. When -the temperature reaches the
base temperature, the pressure is measured and recorded by
the logic controller 42 to provide the liquicl sample
absolute vapor pressure in gage units at the base
temperat~re~
The process can then be repeated starting with the
draining step described ahove.
The same apparatus as described hereinbefore may be
used to determine the gage vapor pressure of liquified
petroleum gas products as definecl in procedure ANSI/ASTM D
1267-79. In this process, the line 24 and the valve 26 are
connected to the liquid sampling line 10~ and there is no
vent to atmosphere. During the sampling step immediately
preceding drainin~, the valves 13 and 26 and 17 are closed,
and the valve 5 is opened to interconnect the chambers 2 and
4. In order to drain the chambers, the valve 17 is opened
to a hydrocarbon drain llne l9. I,iquid and vapor in both
the chambers 2 an~ 4 are drained or aspirated to the
hydrocarbon clrain line 19 at atmospheric pressure.
The liquicl sample valves 13 and 26 are open so that
liquid samples can flush both chambers 2 and 4 for several
11 ~
- , ' '. , :
seconds to ensure that the previous sample is thoroughly
removed through the duct 16 and the line 19.
Following flushing of -the chambers 2 and 4, the
valves 13 and 26 are closed and both chambers 2 and 4 drain.
~pon completion of the flush and drain, the drain valve 17
is closed.
Both chambers are thus isola-ted in a vapor phase at
atmospheric pressure. The valve 5 is closed. The valve 13
is opened to introduce liquid sample into the chamber 2 at
liquid sample process conditions. The sample may cool
because of the pressure drop across the orifice plate 14.
In order for the analyzer to function, the liquid sample
must be at a temperature below the required base
temperature. Otherwise, sample conditioning will be
required.
The valve 5 is opened and the vapor in the chambex
4 reaches equilibrium with the liquid sample in chamber 2 at
a temperature below the base temperature and a pressure below
the required vapor pressure.
With the sample valves 13 and 26 and the drain
valve 17 closed) and the valve 5 open, heat is applied to
the chamber 2. The rotating means 50 is again activated,
following which, the temperature and pressure in the
apparatus is monitored, such tha-t when the temperature
reaches the base temperature, the pressure is measured and
- 12 -
. .: . : . .
,
: ' , , ' ' '
'' ,~
recorded by the logic controller 42 as the sample gage vapor
pressure at the required base temperature. The process can
then be repeated starting with the draining step.
The accuracy of the above described apparatus when
carrying out the above defined processes has been found to
be at least equal to the accuracy set out in procedures
ANSI/ASTM D 323-79 and ANSI/ASTM D 1267-79, The ~pparatus
has been tested with a wide variety of liquids over a wide
range of vapor pressures, and determined to be ~
accurate. Accuracy of the apparatus is at present dependent
upon the volurne ratio of the vapor decompression chamber 4
: to the liquid sample chamber 2, the length to diameter
dimension ratio o~ each chamber, and the rate of heat
input. Al 1 of these values are variable and dependent upon
the physical properties and composition o~ the liquid being
tested.
; Both o the processes described herein are carried
; out on a batch basis.
For industrial use, when the analyæer is -to be used
to measure vapor pressure o one or more liquids, the
apparatus can be permanently mounted in a heated, weather
; resistant cabinet. The logic con-trol can be mounted either
in the cabinet or remotely in a control room, For portable
use, the apparatus can be mounted in a metal case which
contains the analyzer and the logic controller, requiring a
power supply .for operation.
- 13