Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
-` 20~3~8
BE~RINGWERKE AKTIENGESELLSCHAFT 89/B 018 - Ma 772/773
Dr. Ha/Sd
Description
Rhodomycin derivatives with cytostatic activity
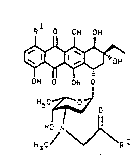
The invention relates to new anthracycline derivatives
with cytostatic activity and the formula I, and the
physiologically acceptable salts thereof, a process for
the preparation thereof and the use thereof as pharma-
ceuticals. -~.
R O OH OH
2 ~ I
OH O GH G
H"Cj~J o
H3C
In formula I:
Rl i~ hydrogen or a hydroxyl group and
R2 i8 NRA-(CHz)~-NR~*
with n = 0-12, preferably with n = O or 2-5, where Rn
and * can each be hydrogen, C~-C4-alkyl or benzyl, or
R2 i8 NRA-CHR3-CH2-R4,
where R~ has the said meaning, and R3 is hydrogen or ~-
a carboxyl group and R4 is the residue of an L-amino
acid or of a biogenic amine, preferably the residue
of an aromatic L-amino acid or of an aromatic biogenic
amine and, particularly preferably, the residue of the
biogenic amine tyramine, tryptamine, histamine,
dopamine or 5-hydroxydopamine, in which case the L-
amino acid i8 defined by R2 with R3 = carboxyl and the
biogenic amine i~ defined by R2 with R3 = hydrogen.
2~39~
-- 2 --
Currently about 7 to 10% of malignant tumors which are no
longer curable by surgical or radiotherapeutic measure~
can be cured by chemotherapy. ~n particular, rapidly
proliferating tumors such as, for example, acute lym-
phatic leukemia in children, Hodgkin~s disease, testicu-
lar tumors and Wilms~ tumors display high sensitivity to
chemotherapy with remission rates of 60 to 90%.
Furthermore, it is possible by chemotherapy to achieve a
slowing of tumor growth or partial or complete remission
of the oncoses in about 30~ of all tumor patients.
However, even when the chemotherapy is continued there
i8, after time intervals which vary in length, recurrence
of the tumor, which is then uaually resistant to the
initially success~ul chemotherapy. This so-called ~secon-
dary resistance occurring during chemotherapy thenlimits the possibility of a further influence on the
tumor by chemotherapy. This group of tumors include~
tumors of the breast and ovary, small-cell lung car-
cinomas and some lymphomas.
:
At present, the influence on many malignant diseases
possible by chemotherapy is, however, zero or only
marginal, so that it must be assumed in these cases that
there is a primary resistance of the tumor to the sub-
stances currently known. This group of tumors includes
non-small-cell lung tumors and many gastrointestinal
tumors. When this group of tumors with primary resistance
is considered in terms of their rate of proliferation, it
is ~een that the tumors which can be influenced by
chemotherapy with difficulty or not at all are precisely
the ones which have a particularly ~low proliferation
rate.
Thus, it is a matter of the utmost priority to look for
new substances which are effective precisely on human
tumors which proliferate slowly and have primary resist-
ance. Moreover, future chemotherapeutics should, wherepossible, have no cross-resi~tance with substances
~ .
:,1 , . .. . .
''~' ' : .'- . .- -, : ........ .: .
2Q~539~
already known, in order to be utilizable with prospects
of success even where there is secondary resistance to
already established substances.
In the anthracycline class, particularly doxorubicin
(adriamycin), epirubicin or daunomycin have therapeutic
efficacy on a large number of oncoses. However, repeated
treatment is followed by a distinct reduction in the
antitumor activity, as far as to ineffectiveness, caused
by the development of ~secondary resistance" of the tumor
cells to the anthracycline.
Another considerable problem in the use of these known
anthracyclines for tumor therapy is that, besides the
desired cytostatic activity, these compounds also have
undesired side effects such as, for example, hemato-
logical or cardiac toxicity.
~ 'The activity of daunomycin and adriamycin in tumor
therapy is mainly attributed to the ability of these
anthracyclines to intercalate into the DNA, which sup-
presses growth of the tumor cell and brings about death
of the cell.
Based on this state of the art, the ob~ect of the present
invention is to provide new anthracycline derivatives
which are, where possible, not cross-resistant with
adriamycin and which are distinguished by a new spectrum
of action and a lower toxicity than adriamycin, and thus
can be used advantageously in tumor therapy.
It has already been proposed for this purpose to synthe-
size rhodosaminylanthracyclinones of the formula I in
which R1 has the said meaning, and R2 is hydroxyl, Cl-C4-
alkoxy, branched or unbranched or ~ubstituted or unsub-
stituted, or NR~2 with R~ = hydrogen or C1-C4-alkyl.
The anthracycline derivatives of the formula I have now
been found, in which the ~olubility in water and/or
:",, ~ ", ~ .
4 20~398
reactivity and/or toxicity of the anthracycline is
altered from the anthracyclines of the state of the art
by changing the radical R2 in the manner indicated by the
definition of R2.
For example, it has been found that when R2 is NH-(CH2)n-
NH2 with n = 2 or 4, the anthracycline molecule has
amphoteric properties and it~ solubility in water is
higher than that of the anthracyclines of the state of
the art.
It has furthermore been found that when R2 is NH-CHR3C~2-R4,
in which R3 is hydrogen and R4 is the residue of the
biogenic amine histamine, tyramine or tryptamine, the
anthracycline i8 able - besides its ability to inter-
calate into DNA - also to interact with certain enzymes,
which alters its reactivity by comparison with the
anthracyclines of the state of the art. -~
Hence the invention relates to compounds of the formula
I with the stated meanings.
Preferred meanings:
2 0 R2 i8 NRa--( CH2) n--NRA*;
R2 is NR~-NR~Rb~ NR~- ( CH2)2-NR~Rb or NR~- ( CH2)4-NRH* ~ where
derivatives with R~ = Rb = hydrogen are preferred;
R2 is NRn-CHR3-CH2-R4~ where compounds in which
R3 is hydro~en are ~referred;
R4 iS the residue of an aromatic L-amino acid or of an
aromatic biogenic amine;
R4 is the residue of the biogenic amine tyramine, trypt-
amine, histamine, dopamine or 5-hydroxydopamine.
The process for the preparation of the new anthracycline
derivatives according to the invention which have cyto-
static activity and the formula I comprises reacting a
compound of the formula I in which R1 has the said mean-
ing, and R2 i8 Cl-C4-alkoxy, branched or unbranched, or
benzyloxy, with a compound of the formula HR~N-(CH2)n-
-; . . . . . . . . . : .
2 ~ 9 8
-- 5 --
NRaRb in which n, R~ and * have the said meaning, or with
a biogenic amine or an amino acid in a suitable solvent
such as, for example, dimethylformamide or ethanol, at a
temperature between, for example, ODC and the boiling
point of the solvent, and isolating and purifying the
compound of the formula I obtained in this way, in which
R1 has the said meaning, and R2 is NR~-(CH2)n-NRa* or NH-
C~R3-C-~R4 where n, R~, *, R3 and R4 have the said meaning.
The n0w anthracycline derivatives obtained by the process
according to the invention are distinguished by cyto-
static activity, and they can therefore be processed -
together with the customary pharmaceutical formulating
agents and/or diluents to give pharmaceuticals which are
used in cancer therapy. The mode of dosage and adminis-
tration thereof e~sentially corresponds to that for the
known substances adriamycin, daunomycin, aclacinomycin,
4'-epi-adriamycin, 4~-methoxyadriamycin or 4~-deoxyadria-
mycin.
The pharmaceuticals prepared in this way can additionally
also contain other active compounds as long as these do
not show any undesired side effects together with the
compounds according to the invention.
The cytostatic activity of the compounds according to the
invention was tested using L1210 mouse leukemia cells.
The formation of colonies of L1210 leukemia cells in agar
plates was used for this purpose. This method iB employed
to detect the effect of test 6ubstances on the growth
behavior of the cells over 1 hour or over several genera-
tions. At a cell cycle time of 10-12 hours, this entails
about 14 consecutive generations being observed in the 7
days the test lasts. In this test, the substances with
cytostatic activity according to the invention bring
about a reduction in the number of colonies to be
observed by comparison with an untreated control sample.
Details of the test method employed are evident from the
- 6 - 2~3 g8
following descriptions of the procedures for determining
the formation of colonies.
To explain the preparation process according to the
invention, Examples 1 to 5 in which preferred compound~
according to the invention were prepared by the claimed
process are detailed hereinafter.
Characterization of comPounds of the formula I
The progress of the reactions and the resulting compounds
were investigated by thin-layer chromatography or with
the HPLC technique. Thin-layer chromatography was carried
out, unless noted otherwise, on precoated silica gel
plates (Merck). Column chromatography was carried out on
silica gel 60 (Merck) of particle size 0.040-0.063 mm.
The yields are not optimized.
The following solvent mixtures were used for the thin-
layer and column chromatography (data in percent by
volume in each case):
Solvent mixtures A B C
Composition
` 20 Chloroform (%) 70 89 77
Methanol (%) 18 7.4 14
Acetic acid (%) 8.5 3 7
Water (%) 3.5 0.6 2
The ~tructures of the prepared compounds were determined
by lH-NMR and MS spectroscopy.
.
.
~ .;; , . , ~
2~a3~
EXAMPL~S
Preparation of the startina com~ounds:
Preparation of the startina com~ound of the formula II in
which R1 is hydroaen and R2 is ethoxy:
7-0-(3'-N-Ethoxycarbonylmethyl-3'-N-meth~l-u-L-daunos-
aminvl)-~-rhodomycinone
100 mg (0.189 mmol) of 7-0-(3~-N-methyl-~-L-daunosam-
inyl)-~-rhodomycinone and 75 ~1 (113 mg = 0.677 mmol =
3.58 equiv.) of ethyl bromoacetate were reacted in the
presence of 80 ~1 (58 mg = 0.574 mmol = 3.0 equiv.) of
triethylamine for 2 h. The ~-
product mixture after concentration was dissolved without
delay in a little chloroform, loaded onto a silica gel
column made up in ether (15 g of silica gel) and eluted
with about 100 ml of ether to remove the excess bromoace-
tate. The desired compound was then eluted in chloroform/
ethan~l (20/1) (RF 0.32).
Yield 70 mg (0.114 mmol) = 60%
MS-FAB (M+Ht) m/e = 616 `-
lH NMR (300 NHz, CDC13) ~ 1.12 (t, 3H, Jl314 = 7.4 Hz, Me-
14), 1.16 (t, 3H, Jbc = 7.1 Hz, Me-c), 1.40 (d, 3H, J5 6~
= 6.8 Hz, Me-6'), 1.7-19 (m, 4H, CH2-2' and CH2-13), 2.11
(dd, lH, J7~, = 4 Hz, J8.8b = 15 Hz, H-8a), 2.26 (d, lH,
J8. 6b = 15 Hz, H-8b), 2.38 (8~ 3H, N-CH3), 2.72 (d, lH,
J1o ~-1o = 4 Hz, OH-10), 2.82 (m, lH, H-3'), 3.14 (bs, lH,
OH-4'), 3.31 (d, lH, J,,. = 17 Hz, H-a), 3.33 (d, lH, J~
= 17 Hz, H-a'), 3.65 (bs, lH, H-4'), 4.03 (s, lH, OH-9),
4.08 (m, 3H, CH2-b and H-5'), 4-92 (d, lH~ J1oo~-1o = 4 Hz~
H-10), 5.15 (m, lH, H-7), 5.51 (bd, lH~ J1 2~ = 2-5 Hz, H-
1'), 7.33 (dd, lH, J13 = 1 Hz, J23 = 8.4 Hz, H-3), 7.73
(t~ lH~ J12 = J23 = 8-4 Hz, H-2), 7.90 (dd, lH, J12 = 8.4
Hz, J13 = 1 Hz, H-l), 12.16 (bs, lH, OH-4), 12.84 (bs,
lH, OH-6), 13.63 (bs, lH, OH-ll).
~,,
2 ~
,
Example 1:
Preparation of a compound of the formula I in which
R1 i8 H and R2 is NH-NH2
7-0-(3'-N-Hydrazinocarbonylmethyl-3'-N-methyl-~-L-daunos-
S aminyl)-~-rhodomycinone (compound 1)
A solution of7-0-(3~-N-ethoxycarbonylmethyl-3~-N-methyl-
~-L-daunosaminyl)-~-rhodomycinone (154 mg = 0.25 mmol) in
ethanol (38 ml) was mixed with 100% hydrazine hydrate
(17.5 ~1 = 18 mg = O.36 mmol = 1.44 equiv.) and stirred
at room temperature in the dark for 60 h. The reaction
mixture was then evaporated to dryness in a rotary
evaporator and chromatographed on 16 g of ~ilica gel in
solvent mixture A ( RF . 26). Water wa~ added to the
combined fractions to ~eparate the phases, the pH was
ad~usted to 7 with 10 percent (w/v) sodium hydroxide
solution and then ad~usted to pH 8 by addition of satura-
ted aqueous sodium bicarbonate solution. The phases were
then separated in a separating funnel, the aqueous phase
was extracted several times with chloroform, and the
combined organic phases were evaporated to dryness in a
rotary evaporator.
Yield 51 mg (0.085 mmol ) = 34%
MS-FAB (M~H+) m/e = 602
lH-NMR (300 MHz, CDC13)~D6-DMSO (6/1)) ~ 1-10 (t, 3H~ Jl3 14
= 7.4 Hz, Me-14), 1.35 (d, 3H, J5~ 6~ = 6.5 Hz, Me-6'),
1.6-2.0 (m, 4H, CH2-2'and CH2-13), 2.~ (bs, 2H, CH2-8),
2.26 (s, 3H, N-CH3), 2.52 (bd, lH, J2~ 3. = 10-5 Hz, H-3')~
3.06 (d, lH, J~ A~ = 16.6 Hz, H-a), 3.19 (d, lH, JA ~
16.6 Hz, H-a'), 3.70 (bs, lH, H-4'), 3.90 (bs, lH, OH-9),
4.06 (q, lH, J5~6~ = 6.6 Hz, H-5'), 4.84 (8, lH, H-10),
5.12 (m, lH, H-7), 5.48 (bd, lH, J1' 2. = 3.2 Hz, H-1'),
7.31 (dd, lH, Jl3 = 1 Hz, J2 3 = 8-4 Hz, H-3), 7-72 (t~
lH~ Jl2 = J23 = 8-3 Hz, H-2), 7.87 (dd, lH, Jl 2 = 7.7 Hz,
J13 = 1 Hz, H-l), 9.03 (8~ lH, hydrazide-NH).
- 9 -
Exam~le 2:
Preparation of the compound of the formula I in which
R1 is H and R2 is NH-(CHæl7-NH~:
7-0-(3'-N-Ethylenediaminocarbonvlmethyl-3'-N-methyl-~-L-
daunosaminvl~-~-rhodomvcinone (compound 2)
A solution of7-0-(3'-N-ethoxycarbonylmethyl-3'-N-methyl-
~-L-daunosaminyl)-~-rhodomycinone (150 mg = 0.24 mmol) in
dimethylformamide (20 ml) was mixed with 1 ml of ethyl-
enediamine and stirred at room temperature in the dark
for 3 h. The reaction mixture was then evaporated to
dryness in a rotary evaporator and chromatographed on
15 g of silica gel in solvent mixture A (Rp 0.15), and
the fractions were worked up in analogy to Example 1.
Yield: 120 mg ~0.19 mmol) = 79%
MS-FAB (M+H~) m/e = 630
1H-NMR (300 MHz, CDCl3/D6-DMSO) (4/1)) ~ 1.09 (t, 3H, Jl3 14
= 7.4 Hz, Me-14), 1.30 (d, 3H, J5 6. = 6.5 Hz, ~e-6'),
1.6-1.9 (m, 4H, CH2-2' and CH2-13), 2.19 (bs, 2H, CH2-8),
2.28 (s, 3H, N-CH3), 2.55 (m, lH, H-3'), 2.84 (m, 2H, CH2-
c), 3.01 (d, lH, J~ = 18 Hz, H-a), 3.15 (d, lH, J,~.
= 18 Hz, H-a'), 3.25 (m, lH, H-b), 3.38 (m, lH, H-b'),
3.62 (bs, lH, H-4'), 4.05 (q, lH, J56 = 6.6 Hz, H-5'),
4.82 (s, lH, H-10), 5.12 (m, lH, H-7), 5.48 (bd, lH,
Jl-2 = 3 Hz~ H-1'), 7-32 (dd, lH, J13 = 1 Hz, J23 = 8-4
Hz, H-3), 7.73 (t, lH, J12 s J23 = 8 Hz, H-2), 7.87 (dd,
lH, Jl2 = 7.5 Hz, J~3 = l Hz, H-l), 8.06 (t, lH, J~ b = 6
Hz, -CO-NH-).
ExamDle 3:
Preparation of a compound of the formula I in which
Rl is H and R2 is NH-(CH2~2-N(CH?)2
7-0-(3'-N-(Dimethylaminoethylaminocarbonyl)-3'-N-methyl-
~-L-daunosaminyl~-~-rhodomycinone (com~ound 3)
A solution of 7-0-(3'-N-ethoxycarbonylmethyl-3'-N-methyl-
~-L-daunosaminyl)-~-rhodomycinone (16 mg = 0.026 mmol) in
- 10 -
methanol (8 ml) was mixed with 100 ~1 (81 mg = 0.92 mmol)
of N,N-dimethylaminoethylamine and 6tirred at room
temperature in the dark for 16 h. The mixture was then
evaporated to dryness in a rotary evaporator and dried
under high vacuum. The reaction mixture was chromato-
graphed on 4 g of silica gel in solvent mixture A (Rp
0.14), and the fractions were worked up in analogy to
Example 1.
Yield 13 mg (0.020 mmol) = 77%
H-NNR (300 MHz, CDCl3) ~ 1-12 (t, 3H, Jl314 = 7-4 Hz, Me-
14), 1.30 (d, 3H, J5.6. = 6.4 Hz, Me-6'), 1.7-2.05 (m, 4H,
CH2-2' and CH2-13), 2.11 (dd, lH, J78~ = 4 Hz, J~b = 15
Hz, H-8a), 2.25 (d, lH, J~ ~b = 15 Hz, H-8b), 2.28 (8, 6H,
N(CH3)2), 2.31 (~l 3H, N-CH3), 2.51 (m, 3H, CH2-c and H-
3~)l 2.98 (d, lH, J~a = 18 Hz, H-a), 3.19 (m, lH, H-b),
3.20 (d, lH, J~ n~ = 18 Hz, H-a'), 3.68 (m, lHI H-b'), 5.04
(q, lH J5.6 = 6.5 Hz, H-5~), 4.10 (8l lH, OH-9), 4.91 (8l
lH, H-10)~ 5.15 (m, lH~ H-7), 5.52 (bd, lH, J1.2. = 3 Hz,
H-1~), 7-33 (d~ lH~ J23 = 8 Hz, H-3)~ 7.72 (t, lH, Jl2 =
J23 = 8 Hz, H-2), 7-89 (d, lH, Jl2 = 7 Hz, H-1), 8.14 (m,
lH, NH).
Example 4:
Pre~aration of a com~ound of the formula I in which
Rl is H and R2 i8 NH-(CH?)~
7-0-(3'-N-(4-Aminobutylaminocarbonyl)-3 ~ -N-methyl-~-L-
daunosaminvl)--~-rhodomycinone (com~ound 4):
A solution of7-0-(3'-N-ethoxycarbonylmethyl-3'-N-methyl-
~-L-daunosaminyl)-~-rhodomycinone (16 mg = 0.026 mmol) in
acetonitrile (3 ml) was mixed with 200 ~1 of 1,4-diamino-
butane and stirred at room temperature in the dark for 3
h. The reaction mixture was then evaporated to dryness in
a rotary evaporator, and chromatographed on 3 g of silica
gel in solvent mixture C (RF 0.16), and the fractions were
worked up in analogy to Example 1.
Yield: 11 mg (0.017 mmol) = 65%
MS-FAB (M+H+) m/e = 658
- 11 - 2~398
lH-NMR (300 MHz, CDCl3/D6-DMSO (4/1)) ~ 1.10 (~, 3H, J13,14
= 7.4 Hz, Me-14), 1.32 (d, 3H, J5~ 6~ = 6.5 Hz, Me-6'),
1.4-1.6 (m, 4H, CH2-c and CH2-d), 1.6-2.0 (m, 4H, CH2-2'
and CH2-13), 2.17 (bs, 2H, CH2-8), 2.29 (s, 3H, N-CH3),
2.55 (m, lH, H-3'), 2.69 (bt, 2H, Jd u = 6.5 Hz, CH2-e)~
3.06 (d, lH, J~a~ = 17 Hz, H-a), 3-08 (d, lH, Ja a' = 17
Hz, H-a'), 3.20 (bt, 2H, Jb c = 6.5 Hz, CH2-b), 3.71 (bs,
lH, H-4'), 4.06 (q, lH, J5~ 6~ = 6.6 Hz, H-5'), 4.82 (s,
lH, H-10), 5.12 (m, lH, H-7), 5.48 (bd, lH, Jl' 2~ = 3 Hz~
H-l'), 7-29 (dd, lH, Jl 3 = 1 Hz, J23 = 8.4 Hz, H-3), 7.70
(t~ lH~ Jl2 = J23 = 8 Hz, H-2), 7.85 (dd, lH, Jl2 = 7.5
Hz, H-l, Jl3 = 1 Hz, H-l).
Example 5:
7-0-(3'-N-Hi6taminocarbonylmethyl-3'-N-methvl-~-L-daunos-
aminyl)-~-rhodomycinone (compound 5)
A solution of 7-0-(3~-N-ethoxycarbonylmethyl-3'-N-methyl-
~-L-daunosaminyl)-~-rhodomycinone (25 mg = 0.04 mmol) in
dimethylformamide (7 ml) was mixed with 20 mg (0.18 mmol)
of histamine and stirred at 50C in the dark for 30 h.
The reaction mixture was then evaporated to dryness in a
rotary evaporator and chromatographed in solvent mixture
C (RF 0.14), and the fractions were worked up in analogy
to Example 1.
Yield: 21 mg (0.03 mmol) = 75
NS-FAB (M+Ht) m/e = 681
H-NMR (300 MHz, CDCl3) ~, 1.13 (t, 3H, Jl31~ = 7.4 Hz, Me-
14), 1.40 (d, 3H, J5. 6' = 6.5 Hz, Me-6'), 1.6-2.0 (m, 4H,
CHz-2' and CH2-13), 2-11 (dd, lH, J7Ba = 4 Hz, J8~Bb = 15
Hz, H-8a), 2.26 (d, lH, JôuBb = 15 Hz, H-8b), 2.29 (s, 3H,
N-CH3), 2.30 (bd, lH, J2.3. = 11 Hz, H-3'), 2.78 (bt, 2H,
Jbc = 5.6 Hz, CH2-c), 2.96 ~d, lH, Ja- = 17 Hz, H-a),
3.14 (d, lH, J,~. = 17 Hz, H-a'), 3.49 (m, 2H, CH2-b),
3.73 (8~ lH, H-4'), 4.10 (q, lH, J5 6~ = 6-3 Hz, H-5')~
4.92 (8~ lH, H-~0), 5.16 (m, lH, H-7), 5-51 (bd, lH, Jl.2.
= 3.5 Hz, H-l'), 6.81 (8, lH, H-d), 7.33 (dd, lH, Jl 3 =
1 Hz, J23 = 8.4 Hz, H-3), 7.56 (s, lH, H-e), 7.73 (t, lH,
I
` - 12 - 2~ 98
Jl,2 = J2,3 = 8 Hz, H-2), 7.89 (dd, lH, Jl 2 = 7.5 Hz, Jl 3 =
1 Hz, H-1), 8.57 (bs, lH, NH).
Example 6:
7-0-(3'-N-Methvl-3'-N-tyraminocarbonylmethyl-~-~-daunos-
aminvl)-B-rhodomycinone (compound 6)
The reaction was carried out in analogy to Example 5 with
20 mg (0.032 mmol) of startin~ compound and 20 mg
(0.15 mmol) of tyramine in dimethylformamide (3 ml), but
with stirring at 50-60C for 50 h. Purification was
carried out in solvent mixture C ( RF . 5)-
Yield 12 mg (0.017 mmol) = 53%
MS-FAB (M+H~) m/e = 707
lH-NMR (300 NHz, CDCl3/D6-DMSO (4/1)) ~ 1.10 (t, 3H, Jl314
= 7.4 Hz, Me-14), 1.32 (d, 3H, J5. ~. = 6.5 Hz, Me-6'),
1.6-2.0 (m, 4H, CH2-2' and CH2-13), 2.19 (bs, 2H, CH2-8),
2.20 (s, 3H, N-CH3), 2.54 (bd, lH, J2' 3~ = 11 Hz~ H-3')~
2.69 (bt, 2H, Jb c = 7 Hz, CH2-c), 3.03 (d~ lH~ J~ a~ = 17
H~, H-a), 3.05 (d, lH, J~ a~ = 17 Hz, H-a'), 3.40 (m, 2H,
CH2-b), 3.65 (bs, lH, H-4'), 3.89 (bs, lH, OH-9), 4.05
(q, lH, J5~ 6~ = 6.7 Hz, H-5'), 4.85 (s, lH, H-10), 5.12
(m, lH, H-7), 5.47 (bd, lH, Jl' 2~ = 3 Hz, H-l'), 6.74 (d,
2H, Jd ~ = 8.5 Hz, CH2-d), 6.97 (d, 2H, Jd ~ = 8.5 Hz, CH2-
e), 7.32 (dd, lH, Jl 3 = 1 Hz, J2 3 = 8-4 Hz~ H-3)~ 7-49
(bt, lH, J~,b = 6 Hz, NH), 7-72 t (lH, Jl,2 = J2.3 = 8 Hz~
H-2), 7.87 (dd, lH, Jl 2 = 7-5 Hz, Jl 3 = 1 Hz~ H-l)-
Example 7:
7-0-(3'-N-Methyl-3'-N-tryptaminocarbonylmethyl-~-L-
daunosaminyl~-g-rhodomycinone (compound 7)
The reaction was carried out in analogy to Example 5 with ~ --
20 mg (0.032 mmol) of starting compound and 20 mg
(0.12 mmol) of tryptamine in dimethylformamide (3 ml);
but with stirring at 50-60~C for 50 h. Purification was
~ '':''
~,-,~, . .. . . .
13 2~ ~3~8
carried out in solvent mixture C (RF 0.46).
Yield 17 mg (O.023 mmol) = 72%
Ms-FAs (M+H+) m/e = 730
lH-NMR (300 MHz, CDCl3) ~, 1.13 (t, 3H, Jl314 = 7-4 Hz, Me
S 14), 1.23 (d, 3H, J5~ 6' = 6.5 Hz, Me-6~), 1.5-2.0 ~m, 4H,
CH2-2' and CH2-13), 2.12 (dd, lH, J7,8n = 4 Hz, J8~,~b = 15
Hz, H-8a), 2.17 (8, 3H, N-CH3), 2.20 (d, lH, J8~,8b = 15
Hz, H-8a~), 2.48 (m, lH, H-3~), 2.96 (bt, 2H, Jb c = 6.5
Hz, CH2-c), 3.46 (bs, lH, H-4~), 3.59 (m, 2H, CH2-b), 3.96
(s~ lH, OH-9), 4.00 (q, lH, J5~ 6' = 6-3 Hz~ H-5')~ 4-93
(s, lH, H-10), 5.11 (m, lH, H-7), 5.41 (bs, lH, H-l'),
7.0 (d, lH, Jd~ = 2.3 Hz, H-d), 7.08 (dt, lH) and 7.17
(dt~ lH) (Ja,f = Jf,g = J8,h = 7 Hz~ J~,e; = J~ h = 1 Hz~ H--f
and H-g), 7.32 (dd, lH, Jl3 = 1 Hz, J2 3 = 8.4 Hz, H-3),
7.33 (d, lH, Je ~ = 8.0 Hz, H-e), 7.54 (d, lH, Jg h = 7.7
Hz, H-h), 7.72 (t, lH, Jl 2 = J~ 3 = 8 Hz, H-2), 7.88 (dd,
lH~ Jl,2 = 7-5 Hz~ Jl,3 = 1 Hz, H-l), 8.29 (bs, lH, CO-NH).
Cytotoxicitv of com~ound~ of the formula I for L1210
mouse leukemia cells in vitro
Procedure for determining the formation of colonie6 of
L1210 leukemia cell6 in soft agar
500 leukemia cells per plate were incubated with various
concentrations of the test substance at 37C for 1 hour.
The cells were then washed twice with McCoy5A medium and
finally, after addition of 0.3~ agar, poured into Petri
dishes. Controls were incubated only with fre~h medium.
In some cases, in place of the incubation for one hour,
various concentrations and test substances were mixed
with the upper agar layer in order thus to achieve
continuous exposure of the cells throughout the incuba-
tion time. After the agar had solidified, the plate6 were
incubated in an incubator at 37C for 7 days (5% by vol.
CO2, 95~ relative humidity). The number of resulting
colonies with a diameter of more than 60 ~m was then
counted. The results have been reported as the number of
colonies in treated agar plates as a percentage of the
~''~' "' ~' ' '' , . . .
~.` " ' '. ' ' . . ,, , ' .
- - 14 - 2~ 398
untreated control. The IC50 was determined as a measure of
the activity of the substance from the dose-effect plot
obtained in this way. The results for the compounds
described here are compiled in Table 1, comparing with
adriamycin.
- 20~5398
- 15 -
Table 1: Cytotoxicity of the prepared compound~ of the
formula I on L1210 leukemia cells in vi~ro
Substance Continuou~ 1 h
No. incubation incubation
(Example) Rl R2 IC50 (~g/ml) IC50 (~g/ml)
1 . R NH-NH2 0.32 0,21
b c
2 H NH-CH2-CH2-NH2 0~35 1.0
b c
3 H NH-CH2-CH2-N(CH3)2 0. 12 o . 2 6
b c d e
4 H NH-CH2-CH2-CH2-CH2-NH2 0~35 1~3
b c ~
H NH CH2 CH2 ~ ~ 0.31 1~0
b c ~
6 H NH CH2 2~H 0.41 1,3
7 H NH-CH2-CH2 ~ 0.36 1, 2
.~ . ,~,.:
~, . .. . . .