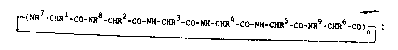Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
26474-192
MercX Patent Gesellschaft
mit beschr~nkter Haftung
6100 D a r m ~ t a d t
Cyclopeptides
S The invention relates to no~l cyclopeptides of
the formula I
. .
~(NR -CHRl-co-NR8-cHR2-co-NN-cxR3-co-NH-cHR4-co-NH-cHRS-co-NR9-CHR6-C0)~ I
__
in whi~h
n is 1 or 2,
Rl And R7 in o~ch c~-e ~re H, A, ~lk nyl or alkynyl in
each c~se h~ving up to 4 C atoms, Ar-~lkyl,
Het-~lkyl, cyclo~lkyl h~ving 3-7 C atom~, or
cyclo~lkyl~lkyl having 4-11 C ato~,
Rl and R7 together ~re ~180 alkylQne or alkenylQne in
each c~se h~ving 2-6 C ~tom~, which ~re unsub-
stituted or ~ubstltuted by A, Ar, Ar-~lkyl, OH,
OA or Het-~lkyl,
;~ R2 is A,
:~ R~ i- A, A-8(0).-alkyl, A-O-~lkyl, Ar-alkyl or
carb~oyl-~lkyl,
R~ l- H, A,~4N-alkyl, HAN-alkyl, A~N-al~yl or, lf
ir dlff r-nt fro H ~nd~or ~t lea~t on of
th radlcal- FP, R~, R~ aDd~or R~ h~s the R-
conflgur~lon, 1~ al~o carba~oyl-aikyl,
R l- ar-a~kyl or H t-alkyl,
R~ 1~ A or Ar-alkyI,
R' and R~ ar~ in each ca~e H or A,
A is al~yl havlng 1-8 C ato~,
Ar i- phenyl whlch lc unJub-tltuted, monosub~tl-
tuted or poly~ub-tltut~d by A, aA, ~al, CF~, OH
and~or NH2, or un~ubstltuted naphthyl,
Het i- a saturated or unsaturated 5- or 6-mffmbered
heterocycllc radlcal havlng 1-4 N, O and/or S
Atoes, which can be fused to a benzene ring or
20~3~
-- 26474-192
- 2 -
~ pyridine ring and/or mono~ub~tituted or
polysubstituted by A, OA, Hal, CF3, OH, NO2,
carbonyl oxygen, NH2, NHA, NA2, NHAc, SA, SO-A,
SO2-A, COOA, CN, CONH2, CONHA, CONA2, CONHAr,
CONH-alkyl-Ar, SO2NHl, NHSO~A, Ar, As-al~yl, Ar-
alkenyl, hydroxy-alkyl snd/or amino-alkyl in
aach case having 1-8 C atoms ~nd/or whose N
and/or S heteroatom~ can al80 be ox~dized,
-alkyl- 18 an alkyl~ne chnin having 1-4 C atoms,
-alkenyl- is an alkenylene chain having 2-4 C atoms,
Hal is F, Cl, Br or I,
m 18 0, 1 or 2 and
Ac iJ H-CO-, A-CO-, Ar-CO-, A-NH-CO- or Ar-NH-CO-,
and their salts.
Sim$1ar compounds ~re known fro~ Phar~azie 40
(8), 532-5~ (1985).
The ob~ct of the lnventlon was t.o find novel
compound~ h~ving usoful properties, ~n partlcul~r those
wh~ch can be used for the preparntion of m~dica~ents.
It ha~ b~en found th~t th~ compounds of the
formul~ I ~nd the$r s~lt~ have very u~eful properties. In
p~r*$cular, they h~ve A tachykln$n ~gonist (e.g. va~o-
dll~t$ng) and/or t~chykinin ant~gonist (e.g. an~lge~$c)
effect. The~e eff-¢t~ c~n be detected, Q.g. ~y the
m-thod~ ~hlch ~r- gi~en ln U8-A~ 72,305, the ~odll~t-
lng -ffect .g. al~o ~y the ~ethod of F. L~r~ec~ et al.,
81Oche~. R~. Co~e. ~Q~, 1318-1321 (1981), and the
analgeJ$c ffe¢t e.g. in the wr~thing te-t on r~tJ or
iq--~for ~ethod ¢onpare C. Vander Nende and S. Hargolin,
Fed. Pro¢. ~ 9~ ff. (1956)t ~. Sieg und et al., Proc.
Soc. e~p. 8iol. (NY) ~, 729-731 (1957)s L.L. Hendqr~hot
and J. Fors~lth, J. Phar a¢ol, e~p. Ther. 125, 237-240
(1959)). In the ca~e of the agoni8t~, ~xclt~tlon~ of the
motor sy~tQ~ and blood pre-~ure redu¢tion~ further~ore
o¢cur, and in the ca~e of th~ antagoni-t~, ~ntl-
infla~matory and/or spasmolytic effectJ. Furthermore,
stimulating effects on l~crimal secretlon, in particular
on local ~ministr~tion, are shown in the c~se of the
compounds of the formul~ I. The compounds of the
~1';.'; ~ ~
IA~` `
20~.!33~3
-- 3 --
formula I are additionally dist~nguished by high selec-
tivity and a good stability to proteases.
The compounds can be employed as pharmaceutical
active compounds in human and veterinary med~cine, in
particular for the prophylaxis and the treatment of
cardiovascular disorders, spastic disorders, states of
pain, inflammations, di~orders of the central nervous
system and/or the circulation, and/or for ~timulating
lacrimal secretion.
The abbreviations of amino acid radicals shown
above and below ~tand for the radicals -NR-CR'R''-C0- (in
which R, R~ and R'' have the specific meaning~ known for
each amino acid) of the following amino acidss
Ala alanine
Gln glutamine
Gly glycine
His histidine
Leu leucine
Net methionin~
~et(0) methionine-S-oxide
Net(02) methionine-S,S-dioxide
N-Me-Phe N-methyl-phenyl~lanine
Phe phenyl~l~nine
Pro proline
Trp tryptoph~n
V~l v~lln~.
In ~ddit~on~ the following h~ve the ~e~nings belOWt
BOC tert.-buto yc~rbony
Ba~ b n~yloxy~ethyl
30 i~i-Bo~ bensyloxy~ethyl ln the- 1-po~ltlon of the
imid~zole ring
C~8. benzyloxyc~rbonyl
DCCI dicyclohexylc~rbodlim$de
D~F dimethylfor 4 ide
DNP 2,4-dlnltrophenyl
i~i-DNP 2,4-dinltrophenyl ln the l-positlon of the
imid~zole ring
EDCI N-ethyl-N'-(}-di~ethyl~minopropyl)-carbodi-
imidehydrochloride
: .
: ~.:, .:,
i: '' ! ' . . ' . ~ : ' ' ~ "
-- 4 --
FMOC 9-fluorenylmethoxycarbonyl
HOBt l-hydroxybenzotriazole
Me methyl
OMe methyl ester
S OEt ethyl e~ter
POA phenoxyacetyl
TFA . trifluoroAcetic acid
If the amino acids mentioned above can occur in
3everal enantiomeric ~orms, then all these form~ and also
their mixtures (e.g. the DL-forms) are included above and
below, e.g. a~ constituents of the compounds of the
formula I. The L-forms are preferred. If individual
compounds are mentioned below, then the abbreviations of
these amino acid~ in each ca-e rel~te to the L-form, if
not expressly stated otherwise.
The invention further relates to ~ process for
the preparation of ~ cyclopeptide of the formula I,
char~cterized in th~t it i~ liberAted from one of its
function~l deriv~tives by tre~ting ~lth ~ solvolyzing or
hydrogenolyzing agent, or in th~t a peptide of the
formula II
( )n ~ . II
in which
n i- 1 or 2 ~nd
25~ NR7-C~YI-cC-NR3-C~-Co-NH-CHR3-Co-NH-cHR~-Co-NH
,~ CHR~-Co-NR9-CHR~-Co-,
. . . -N~3-C}F~-Co-iC-C8R3 ~ NH-CER~-oo-NH-CHR~Co-NR~-CHR3-
CO-NR7~1Rl-CO-,
; ~ -NII~IR3~_~NR~3~NR~IRl
Co-NR3-cHR2-co-~
-N}l-CIIR~-CO-NII-C~R~-CO-NR~-CHR~-CO-NR7-CHRl-CO-NR3-
CHR~-Co-NH-CHR3-Co-,
-Nll-C~lR~-CO-NR~-CHR3-CO-NR7-CHRl-CO-NR3-CHR~-CO-NH-
CHR3-CO-NH-CHR~-CO-
or
-NR~-CHR3-Co-NR7-CHRl-Co-NR3-CHR2-Co-NH-CHR3-Co-NH
CHR~-CO-NH-CHR~-CO-,
,~; ~ .
i . . .
; ' ~ . . .
s
or a reactive derivative of such a peptide i~ treated
with a cycliz~ng agent, and in that, if appropriate in a
compound of the formula I, a thioether group i~ oxidized
to a sulfoxide group or to a sulfone group and/or a 8ul-
foxide group i8 reduced to a thioether group and/or a
compound of the formula I i8 converted into one of it3
8alt8 by tr~ating with an acid
The radicals and parameter~ n, Rl to R~, A, Ar,
Het, Hal, m, Ac and Z above and below have the meanings
10 given in the formulae I or II, if not expressly stated
otherwise
In the above formulae, A has 1 - 8, preferably 1,
2, 3 or 4 C atoms A is preferably methyl, furthermore
ethyl, propyl, isopropyl, butyl, isobutyl, sec -butyl or
15 tert -butyl, and in ~ddition also pentyl, 1-, 2- or 3-
methylbutyl, 1,1-, 1,2- or 2,2-dimethylpropyl, l-ethyl-
propyl, hexyl, 1-, 2-, 3- or 4-methylpentyl, 1,1-, 1,2-,
1,3-, 2,2-, 2,3- or 3,3-dimethylbutyl, 1- or 2-ethyl-
butyl, l-ethyl-l-methylpropyl, l-ethyl-2-methylpropyl,
20 1,1,2- or 1,2,2-trimethylpropyl, heptyl or octyl
Cyclo~lkyl i8 preferably cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl or cycloheptyl, but al80 e g 1-,
2- or 3-methylcyclopentyl, 1-, 2-, 3- or 4-methylcyclo-
he y l
Corre-pon~gly, cycloalkyl-~l~yl i~ prefer~bly
cyclopropylmothyl, 2-cyclopropyl thyl, cyclobutyl ethyl,
2-cyclobutyl-thyl,cyclop~ntyl~e~hrl,2-cyclopentyl-thyl,
cyclohexyloethyl, 2-cyclohe yl'ethyl, but al~o e g 1-,'2-
' or 3-methylcycIopentyl~ethyl, or 1-, 2-, 3- or ''4-
30 '~ethylcyclohe y l~ethyl
H~ preferably F, Cl or Br, but ~180 I
AC i8 preferably ~-C0- or A-C0- such ~8 acetyl,
propionyl or butyryl, Ar-COL ~uch ~8 benroyl, o-, m- or
p-methoxybenroyl or 3,4-d~etho~yben~oyl, A-NH-C0- such
35 a~ N-methyl- or N-ethylc~rb~moyl or Ar-NH-C0- such as N-
phenylcar~amoyl '
Ar i8 preferably phenyl, ~nd ~n ~ddition prefer~
ably o-, m- or p-tolyl, o-, m- or p-ethylphenyl, o-, m-
or p-methoxyphenyl, o-, m- or p-fluorophenyl, o-, m- or
. . .
;;a~
-- 6 --
p-chlorophenyl, o-, m- or p-bromophenyl, o-, m- or
p-iodophenyl, o-, m- or p-trifluoromethylphenyl, o-, m-
or p-hydroxyphenyl, 2,3-, 2,4-, 2,5-, 2,6-, 3,4- sr 3,5-
dLmethoxyphenyl, 3,4,5-trimethoxyphenyl, o-, m- or p-
S aminophenyl, or 1- or 2-naphthyl
Correspondingly, Ar-alkyl i8 preferably benzyl,
1- or 2-phenylethyl, o-, m- or p-methylbenzyl, 1- or
2-o-, -m- or -p-tolylethyl, o-, m- or p-ethylbenzyl, 1-
or 2-o-, -m- or -p-et~ylphenyl~thyl, o-, m- or p-m~thoxy-
benzyl, 1- or 2-o-, -m- or -p-methoxyphenylethyl, o-, m-
or p-fluorobenzyl, 1- or 2-o-, -m- or -p-fluorophenyl-
ethyl, o-, m- or p-chlorobenzyl, 1- or 2-o-, -m- or -p-
chlorophenylethyl, o-, m- or p-bromobenzyl, 1- or 2-o-,
-m- or -p-bromophenylethyl, o-, m- or p-iodobenzyl, 1- or
lS 2-o-, -m- or -p-iodophenylethyl, o-, m- or p-trifluoro-
methylbenzyl, o-, m- or p-hydroxybenzyl, 2,3-, 2,4-,
2,5-, 2,6-, 3,4- or 3,5-dimethoxybenzyl, 3,4,5-trime-
thoxybenzyl, o-, m- or p_a~- nobenzyl, or 1- or
2-naphthylmethyl
Het i8 preferably 2- or 3-furyl, 2- or 3-thienyl,
1-, 2- or 3-pyrrolyl, 1-, 2-, 4- or 5-imid~zolyl, 1-, 3-,
4- or 5-pyrazolyl, 2-, 4- or 5-oxazolyl, 3-, 4- or 5-
isoxazolyl, 2-, 4- or 5-thiazolyl, 3-, 4- or 5-i~othi~zo-
lyl, 2-, 3- or ~-pyridyl, 2-, ~-, 5- or 6-pyrimidyl, and
further or pr ferably 1,2,3-tria-ol-1-, -4- or -5-yl,
1,2,~-triazol-1-, -3- or -5-yl, 1- or 5-tetrazolyl,
1,2,3-oxadiazol-4- or -5-yl, 1,2,4-ox~diazol-3- or -S-yl,
1,3,~-thiadiazol-2- or -5-yl, 1,2,~-thiadiazol-3- or -5-
yl, 2,1,5-thi~dlazol-3- or -4-yl, 2-, 3-, 4-, 5- ~r 6-2~-
thiopyranylj, ?-, 3- or 4-~H-thiopyr~nyl, 3- or 4-pyrida-
~inyl, pyr~zinyl, 2-, 3-, 4-, S-, 6- or 7-benzofuryl, 2-,
3-, 4-, 5-, 6- or 7-benzothienyl, 1-, 2-, 3-, 4-, 5-, 6-
or 7-lndolyl, 1-, 2-, 3-, 4-, 5-, 6- or 7-i~oindolyl, 1-,
2-, 4- or 5-bens~-~dazolyl, 1-, 3-, 4-, 5-, 6- or 7-
b nzopyr~zolyl, 2-, 4-, 5-, 6- or 7-benzo~azolyl, 3-, 4-,
5-, 6- or 7-benzi~oxazolyl, 2-, 4-, 5-, 6- or 7-benso-
thlazolyl, 2-, 4-, 5-, 6- or 7-benzisothiazolyl, 4-, 5-,
6- or 7-benz-2,1,3-oxadi~zolyl, 2-, 3-, 4-, 5-, 6-, 7- or
8-quinolyl, 1-, 3-, 4-, 5-, 6-, 7- or 8-i~oquinolyl, 1-,
.~
201~rj
-- 7 --
2-, 3-, 4- or 9-carbazolyl, 1-, 2-, 3-, 4-, 5-, 6-, 7-,
8- or 9-acridinyl, ~-, 4-, 5-, 6-, 7- or 8-cinnolyl, or
2-, 4-, 5-, 6-, 7- or 8-quinazolyl
The heterocyclic radicals can also be partially
S or completely hydrogenated Het can thus also be e g
2,3-dihydro-2-, -3-, -4- or -5-fury~, 2,5-dihydro-2-,
-3-, -4- or 5-furyl, tetrahydro-2- or -3-furyl, tetra-
hydro-2- or -3-thienyl, 2,3-dihydro~ 2-, -3-, -4- or
-5-pyrryl, 2,5-dihydro-1-, -2-, -3-, -4- or -5-pyrryl,
1-, 2- or ~-pyrrolidinyl, tetrahydro-l-, -2- or -4-imida-
zolyl, 2,3-dihydro-1-, -2-, -3-, -4- or 5-pyrazolyl, 2,5-
dihydro-l-, -2-, -3-, -4 or 5-pyrazolyl, tetrahydro-l-,
-3- or -4-pyrazolyl, 1,4-dihydro-1-, -2-, -3- or -4-
pyridyl, 1,2,3,4-tetr~hydro-1-, -2-, -3-, -4-, -5- or -6-
pyridyl, 1,2,3,6-tetr~hydro-1-, -2-, -3-, -4-, -5- or -6-
pyridyl, 1-, 2-, 3- or 4-piperidinyl, 2-, 3- or 4-morpho-
linyl, tetrahydro-2-, -3- or -4-pyranyl, 1,4-dioxanyl,
1,3-dioxan-2-, -4- or -S-yl, hexahydro-l-, -3- or -4-
pyrid~zinyl, hex~hydro-l-, -2-, -4- or -5-pyrimidyl, 1-,
2- or 3-piper~inyl, 1,2,3,4-tetr~hydro-1-, -2-, -3-,
-4-, -5-, -6-, -7- or -8-quinolyl, 1,2,3,4-tetrahydro-1-
, -2-, -3-, -4-, -5-, -6-, -7- or 8-i~oquinolyl ~~he heterocyclic r~dical~ c~n ~l~o be substitut~d ~ -
~ st~ted Het c~n thuz ~l~o prefer~bly be~ 2-~ ino-4-
thiarolyl, 4-~arbo y-2-thi~zol~ -c~rb~oyl-2~ o-
lyl, ~-(2-a-~nethyl)-2-thi~zolyl, 2-~ ino-5,6-di~ethyl-
3-pyr~zinyl, 4-carb~ooylplperidino, ~nd in ~dd1t~on e g
3~ or 5-m~thyl-2-furyl, 2-j ~- or 5-methyl-3-furyl,
2,4-d~ethyl-3-furyl! 5-nitro-2-furyl, 5-~tyryl-2-furyl,
3-, 4- or 5- -thyl-2-thlenyl, 2-, ~- or 5-~ethyl-3-
thienyl, 3-~ethyl-5-tort -butyl-2-thlenyl, 5-chloro-2-
thienyl, 5-phenyl-2- or -3-thienyl, 1-, 3-, 4- or 5-
~ethyl-2-pyrrolyl, 1-~ethyl-4- or -5-nltro-2-pyrrolyl,
3,5-dimethyi-4-ethyl-2-pyrrolyl,4-methyl-5-pyr~zolyl,4-
or 5-methyl-2-thi~zolyl, 2- or 5-methyl-4-thi~zolyl, 2-
or 4-methyl-5-thi~zolyl, 2,4-dimethyl-5-thi~zolyl, 3-,
4-, 5- or 6-methyl-2-pyrldyl, 2-, 4-, 5- or 6-methyl-3- ~`~
pyridyl, 2- or 3-methyl-4-pyridyl, 3-, 4-,~ 5- or 6-
chloro-2-pyridyl, 2-, 4-, 5- or 6-chloro-3-pyridyl, 2- or
:~:
26474-192
-- 8 --
3-chloro-4-pyridyl, 2,6-d~chloropyridyl, 2-hydroxy-3-,
-4-, -5- or -6-pyridyl (~ lH-2-pyridon-3-, -4-, -5- or
-6-yl),5-phenyl-lH-2-pyridon-3-yl,5-p-mathoxyph~nyl-1~-
2-pyridon-3-yl, 2-methyl-3-hydroxy-4-hydroxymethyl-5-
pyridyl, 2-hydroxy-4-amino-6-methyl-3-pyridyl, 3-N~-
methylureido-lH-4-pyridon-5-yl, 5- or 6-methyl-4-pyr~mid-
yl, ?,6-dihydroxy-4-pyrimidyl, 5-chloro-2-methyl-4-
pyrimidyl, 2-methyl-4-amino-5-pyrimidyl, 3-methyl-2-
benzofuryl, 2-ethyl 3-benzofuryl, 7-methyl-2-benzothi-
enyl, 1-, 2-, 4-, 5-, 6- or 7-methyl-3-indolyl, l-methyl-
5- or -6-benzimldazo}yl, 1-ethyl-5- or -6-benzimid~Azolyl
or 3-, 4-, 5-, 6-, 7- or 8-hydroxy-2-quinolyl.
Rl and R7 are preferably in each case H or A or,
together, an alkylene chain having 2-6, in particular 3
C atoms.
Ra is preferably propyl, isobutyl or sec.-butyl.
R3 is preferably A-S(0),-alkyl, in part~cular
CH3-S(0),-CH2CH2-, where m is preferably 0.
R ~ is preferably H or ~if Rl ia different from H
and/or at least one of the radicals R2 to R~ has the R-
configur~Ation) H2N-C0-CH2CH2-.
R~ is preferably Ar-alkyl or Het-alkyl, in par-
ticul~r b~n~yl or 3-indolyl- ethyl, and in addition p-
hydroxyben7yl or p- ethoxyben~yl.
R~ 1~ prefer~bly Ar-~lkyl, in particular ben~yl,
and in ~dditlon p-hydroxyben~yl or p-xethoxyben~yl.
-NR7-CHRl-co- i- preferablys Gly, Pro or D-Pro~
-NR~-CHR~-C0- i~ pr f-r~blys I~u, ~nd ~n addltion D-Leu,
, Ile or D-Ile;
: . . .
30 -NH-CHR~-C0- i~ preferablys Net, D-Het, Met~0), D-
Met(0), ~et~02) or D-
Met(02), and in addition Leu
or D-~eus
-NH-CHR~-C0-- i~ preferablys Gly or Gln, and in addition
3S Ala or D-Ala;
-NH-CHR5-Co-s 1~ preferablys Phe or Trp;
-NR~-CHR~-C0-s i~ preferablys Phe, D-Phe, N-Ne-Phe or D-
N-Ne-Phe, and in addition
Val or D-Val.
. '
~ ~ .
:,
- 9 -
The parameter n is preferably 1
Accordingly, the invention in particular relates
to those compound~ of the formula I in which at least one
of the said radicals and/or parameters has one of the
S preferred meanings given above Some preferred groups of
compounds can be expressed by the part formula Ia, which
otherwise corresponds to the formula I, but in which
R1 is H, or, together with R7, -(CH2)3-,
RZ i8 isobutyl,
R3 i8 CH3-S(O)~-CR2CH2-~
R~ is H or, if Rl i8 different from H and/or at least
one of the radicals R2, R3, R', ~5 and/or R6 have the
R-configuration, is also H2N-CO-CR2CH2-,
R5 i8 benzyl or 3-indolyl-methyl,
RB i8 benzyl,
R7 i8 H or, together with R1, -(CH2) 3-,
Ra i8 H and
R is H or Me
Particul~rly preferred compounds of the
formulae I and Ia are those in wh$ch n i8 additionally 1
The compounds of the formula I and also the
starting material~ for the$r preparation ~re oth~rwise
prepared by thod- which are known per ~e, a8 are
- de-cribed $n the liter~ture (e g in the standard works
such ~- Hbub n-Weyl, ~ethoden der org~ni-chen Che~$e,
(~ethod~ o~ Orga~ic Che~i~try) Gsorg-Th$ _ -Verlag,
Stuttg~rt), $n particular under reaction cond~tions which ~ i
ar known and ~uitable for the sa$d reaction~ In this
cont xt, uJ- can al-o be ~ade of variant~ which are known
per ~e and ~re not entloned in ~ore detall here
The ~tart~ng sub-tancs- can al~o be formed in -~
situ, if de~ired, uch that they ~r~ not i~olated from
th reaction lxture, but i~ edi~tely reacted further to
give the co~pound- of the ormula I
The co~pounds of the for~ula I can be obtained by
liberatlng thQm from thelr functlonal derlvatives by
solvolysi~, in particular hydrolysis, or by
hydrogenolysis
Preferred starting materials for the solvolysis
: ., ' : '- .. i ... . '. . .
-- 10 --
or hydrogenoly~is are those which contain appropriate
protected amino and/or hydroxyl groups instead of one or
more free amino and/or hydroxyl group~, preferably those
which carry an amino protecting group instead of an H
S atom which i9 bonded to an N atom, e g those which
correspond to the formula I, but contain an N(im)-R~-His
group (in which R~ i8 an amino protecting group, e g BOM
or DNP) instead of an His group
In addition, starting materials are preferred
which carry a hydroxyl protecting group instead of the H
atom of a hydroxyl group, e g those which correspond to
the formula I, but contain an R''O-phenyl group (in which
R~ is a hydroxyl protecting group) instead-of a hydroxy-
phenyl group
Several - identical or different - protected
amino and/or hydroxyl groups can be pre~ent in the
molecule of the starting material If the protective
groups present are different from one another, in many
cases they can be removed selectively
The expression ~amino protecting group~ i8
generally known and relates to groups which are suitable
for protecting (or blocking) an am$no group from ch d -
cal reactlon , but which are eas~ly removab~e, after the
de-ired ch d cal reaction ha- b~en carrled out on other
po-ltion~ of the ~ol-cule Typical group~ of this typo
are, in particular, w ub tituted or sub~tltuted acyl,
~ryl (- g DNP), ar~lkoxy ethyl (e g BQ~) or aralkyl
group- (e g b n~yl, ~-nitroben~yl or triphenylmethyl)
A~ the a ino protecting group are re~oved a~ter the
de-ired reaction (or react$on -equence), their n~ture and
si~e is otherwi-e not critical~ but those having 1-20, in
partlcular 1-8, C ato~ are preferr~d~ The expre~-lon
~cyl group~ $- to be taken in its w$de-t sen~e $n
connection with the pre-ent process It include~ acyl
groups derived fro~ al$phat$c, araliphat$c, ~romat$c or
heterocyclic carboxylic acids or sulfonic ac$ds ~nd in
part$cul~r alkoxycarbonyl, ~ryloxycarbonyl and, above
all, aralkoxycarbonyl groups 2x~mples of acyl groups of
th$s type are alkanoyl such as acetyl, propionyl or
.
.' . ~
. . . :
.... . .
,. ,-: , .. . .. ..
3 ~ ~
butyryl; aralkanoyl such a3 phenylacetyl; aroyl ~uch a~
benzoyl or toluyl; aryloxyalkanoyl such as POA; alkoxy-
carbonyl ~uch as methoxycarbonyl, ethoxycarbonyl, 2,2,2-
trichloroethoxycarbonyl, BOC, 2-iodoethoxycarbonyl;
S aralkylo~ycarbonyl sueh as CBZ ("carbobenzoxy~), 4-
methoxybenzyloxycarbonyl and FMOC. Preferred amino
protecting groups are DNP and BOM, and in addition CBZ,
FMOC, benzyl and aeetyl.
The expression "hydroxy protecting qroup~ i~ also
generally known and relates to groups whieh are suitable
for proteeting a hydroxyl group from ehemieal reaetions,
but whieh are easily removable, after the desired ehemi-
eal reaetion has been earried out on other positions of
the moleeule. Typie~l groups of this type are the ~bove-
mentioned unsubstituted or substituted aryl, aralkyl or
aeyl groups, and in ~ddition also alkyl groups. The
nature and size of the hydroxy proteeting groups is not
eritieal, as they are removed ag~in after the desired
ehemie~l re~etion or re~etion sequeneQ; preferred groups
are those h~Ying 1-20, in p~rtieular 1-10 C ato~s.
~x~mples of hydroxyl proteeting groups are, inter ali~
benzyl, p-nitroben~oyl, p-toluenesulfonyl and ~cetyl,
benzyl ~nd ~cetyl being p~rticul~rly preferred.
The functional deriv~t$ves of the compounds of
the for ul~ I to b u-ed ~ tartlng w~t~ri~l~ c~n be
prep~red by cu~to~ry ~ethod~ of a~lno ~c$d and peptide
ynthe~$~, uch ~- ~re de~cribe~ e.g. in the st~nd~rd
wor~ ~nd p~t nt ~pplic~t$on- mentioned, ~nd e.g. ~l~o by
the ~errifield ol~d ph~e ~ethod. ,
The liber~tion of the co~pounds of the ~oruul~
fro~ their function~l deriv~tlve~ $8 c~rried out -
depending on the protecting group u~ed - e.g. with strong
~cids, prefer~bly with TFA or perchloric ~cid, but ~l~o
with other ~trong inorg~nic ~clds such ~8 hydrochloria
~cid or sulfuric ~cid, or ~trong org~nic c~rboxylic ~c$ds
such ~8 trichloro~cetic ~cid or sulfon~c ~cids ~uch ~8
ben~ene- or p-toluenesulfonic ~cid. The presence of ~n
addition~l inert solvent is pos~ible, but not ~lw~ys
necess~ry. Suit~ble inert solvents are prefer~bly
2 ~
- 12 -
organic, for example carboxylic acids such as acetic
acid, ether~ such as tetrahydrofuran or dioxane, amides
such as DMF, halogenated hydrocarbon~ such as dichloro-
methane, and in addition also alcohols such as methanol,
ethanol or isopropanol and also water.
In addition, mixtures of the abovementioned
solvents are suitable. TFA is preferably used in an
excess without addition of a further solvent, perchloric
acid in the form of~a mixture of acetic acid and 70 %
perchloric acid in the ratio 9:1. The reaction temper-
atures for the cleavage are preferably betwe~n about 0
and about 50, preferably between 15 and 30- (room
temperature).
The BOC group c~n be removed e.g. preferably
using 40 % TFA ~n dichloromethane or with about 3 to 5 N
HCl in dioxane at 15-30-,-the F W group using an about
5- to 20 % solution of dimethylamine, diethylamine or
piperidine in DMF at 15-30-. ~emoval of the DNP group is
carried out e.g. al80 using an about 3 to 10 % ~olution
1 20 of 2-mercaptoethanol in DNF/w~ter st 15-30-.
Protecting groups which can ba removed by hydro-
genolys~s (e.g. B0~, CB~ or ben~yl) can be removed, e.g.
by treating with hydrogon in the presence of ~ catalyst
(e.g. a noble ~etal catalyst ~uch as palladium, proferab-
ly on ~ c~rri r uch a- carbon). Suit~ble ~olventJ in
thi~ ca~e ar tho~e entioned abov-, in p~rticular e.g.
alcohol~ uch a- oth~nol or ethanol or amide~ ~uch as
D~F. Tho hydrogenoly~ carried ~ut, as a rule, at
t~perature~ botw~en about 0 and 100- ~nd pre~ures
botwe~n about 1 and 200 b~r, preferably at 20-30- ~nd 1-
10 bar. Hydrogenoly~l- of the CBS group i~ ea~ily carrled
out e.g. on 5 to 10 ~ Pd-C in meth~nol or uslng a~onlum
form~te (iN te~d of H~) on Pd-C in methanol/DN~ ~t 20-30-.
Co~pounds of the formul~ I ean ~180 be obtalned
by eyeli~atlon of eo~pounds of the for ula II under the
eondition~ of a p~ptide synthesi~. In this e~se, the
re w tion $J preferably earried out by custom~ry methods
of peptide synthesls, ~8 are deserlb~d e.g. ln Houben-
Weyl, loe cit. volume 15/II, p~ges 1 to 806 (1974).
2`'i . ~ ~
.'` ~' "' :
'`.: :.. ., :~
2~ 3~
26474-192
- 13 -
The reaction i8 preferably carried out in the
presence of ~ dehydrating agent, e.g. a carbodiimide such
as DCCI or EDCI, and in addition propanepho~phonic
anhydride (compare Angew. Chem. 92, 129 (1980)), diphen-
s ylpho~phoryl azide or 2-ethoxy-N-ethoxycarbonyl-1,2-di-
hydroquinoline, in an inert solvent, e.g. ~ halogen~ted
hydrocarbon such as dichloromethane, an ether such as
tetr~hydrofuran or dioxane, an ~mide such as DMF or
dimethyl~cet~ide, a nitrile such ~8 acetonitrile, or in
mixtures of these solvent~, at temperatures between about
-10 and 40, prefer&bly between O and 30-. In order to
promote intramolecular cycliz~tion before intermolecul~r
peptide bonding, it is preferable to woxk in dilute
solut~ons (dilution prlnciple).
Inste~d of II, suit~ble react~ve deriv~tives of
these substances can also be employed in the reactlon,
e.g. those in which re~ctive groups ~re ~ntermedl~tely
blocked by protect$ng groups. The ~mlno ~cid deriv-
atives II c~n be used e.g. ln the form of their ~ctiv~ted
1 20 esters which ~re prefer~bly formed in situ, e.g. by
~ddition of HOBt or N-hydroxysuccinimide.
Tha at~rting m~teri~lo of the for~ul~ II are, ~
~ rule, novel. They c~n be prep~red by ~nown ~ethods,
e.g. the ~bove~entioned ~ethoda of peptide ynthesis ~nd
of remov~l of`prot ctive group~
A~ ~ rul-, prot~¢ted hex~peptid 0-t ra of the
for~ul~ R'-Z-OR'', e.g. BOC-~-ONe or BOC-g-OBt ~re ini-
ti~lly oynthe-i~ed, which ~re firat hydroly~ed to gl~e
~¢idJ of th formul~ R'-S-OH, -.g. BOC-Z-OH~ the protec-
ti~o group R' ia remo~ed fro these, by ~e~n~ of which
the free peptidea of the formula H-Z-OR (II; n ~ re
obt~ined. The dodecapeptide~ of the for ula H-(Z)~-OH are
prof-rably pr p~red by re~ction of acida of the formula
R'-Z-OH with a~ino e~ter~ of the for~ula H-~-OR'' to give
protected dodecapeptidea of the formula R'(Z)~-O-R'' and
subsequent re~oval of the protecting group~ R' and R''.
It is al80 possible to oxidize a thioether group
to a sulfoxide group or sulfone group, ~n particular a
group -NH-CHR3-Co- - ~et to a group -NH-CHR3-Co-
~.~ . . . . .
~ J~
,
- 14 -
= Met(0) or ~et(oz)~ e.g. by passing air into a solution
of the compound of the formula I (-NH-CHR3-Co- = Met) in
acetonitxile/water at temperatures between 0 and 30-. The
thioether can also be oxidized with H202. The sulfoxide is
thus predominantly obtained at 20~ in methanol using the
calculated amount of oxidizing agent, and the sulfone is
predominantly obtained at 50-, on the other hand, using
an excess of the oxidizing agent.
Conversely, a sulfoxide group (e.g. in I,
-NH-CHR3-Co- = Met(0)) can be reduced to give a thioether
group (e.g. in I, -NH-CHR3-Co- = Met), e.g. usin~ NH,I in
aqueous ~A at temperatures between -10 and 25-.
A base of the formula I can be converted into the
appropriate acid addition salt using an acid. Suitable
acids for this reaction are in particul~r those which
yield physiologically accep Wble salts. Inorganic acids
can thus be used, e.g. sulfuric acid, nitric acid,
hydrohalic acids such as hydrochloric acid or hydrobromic
acid, phosphoric acids such a8 orthophosphoric acid and
sulfamic acid, and in addition organic acids, in par-
ticular aliphatic, alicyelic, araliphstic, aro~tic or
heterocycllc mono- or polybasie carbo ylie, sulfonie or
sulfurie aeld~, e.g. for ie aeid, acetie aeld, propionle
aeid, pivallc aoid, diethrlacetic acid, malonic acid,
succinic ~cid, pin~lic acid, fum~ric ~cid, ~aleic acid,
lactic-acid, t~rt~ric acid, ~alic acid, benzoic acid,
salicylic ~cid, 2- or 3-phffnylpropionic acld, citr~c
aeid, glueonie aeid, a~eorbie aeid, nieotinie ~eid,
i~onieotinie aeid, methane- or eth~ne-ulfonic acid,
eth~nedlsulfonic aeid, 2-hydroxyeth~nesulfonlc acid,
benzenesulfonie aeid, p-toluene~ulfonie aeid, naphthal-
ene-mono- and -di~ulfoni~ aeid~, and laurylsulfurie aeid.
Salt~ with phy~iologieally unaeceptable acid~, e.g.
picrates, can be u~ed for the i~olation and/or purifica-
tion of the eompounds of the formul~ I.
The novel eompounds of the formula I and theirphysiologic~lly accept~ble salt~ can be used for the
production of ph~roaceuticAl preparations by bringing
them into a suitable dosage form together with at least
~ ~ ,~ ,... . .
... . . ~ .
. . . ... . .
2 ~
- 15 -
one excipient or auxiliary and, if de~ired, together w$th
one or more other active compound(~). The preparations
thus obtained can be employed as pharmaceuticals in human
or veterinary medicine. Suitable excipient ~ubstance~ are
organic or inorganic substances which are suitable for
enteral (e.g. oral or rectal), parenteral or local (e.g.
topicAl) adminiEtration or for administration in the form
of an inhalant spray and which do not react with the
novel compounds, foF example water, lower alcohols,
vegetable oils, benzyl alcohols, polyethylene glycols,
glycerol triacetate and other fatty acid glycerides,
gelatin, soya lecithin, carbohydrates ~uch as lactose or
starch, magnesium stearate, talc, cellulose and vaseline.
Tablet~, coated tablets, capsules, syrups, liquids or
drops, in particular, are used for oral ad~inistration;
film tablets and capsules having gastric ~uice-resistant
coatings or capsule shells are especially of interest.
Suppositor$es are used for rectal ~dministration, and
solutions, prefer~bly oily or agueous solutions, and in
addition suspensions, emulsions or implants, are used for
parenter~l admini~tration. Solutions, e.g., which can be
used in the for~ of eye drop~, and in addltion, e.g.
suspension~, e~ul~ion~, creams, oint~ent~ or co~presses
are ~uitabl- for topical appllcation. Sprays c~n be u~ed
which cont in th~ activ co~pound e$ther dl~olved or
su-pended in a propellant ga~ ~tUrQ (e.g. chlorofluoro-
hydrocarbonJ~ for ad lni~trat$o~ a~ inhalant spr~ys. The
activ- co pound here i~ prefer~bly usQd in micronized
for~, ~t being po -ible for one or re ~dditlonal
physlologically tolerable solvents to be present, e.g.
; ethanol. Inhalant ~olution~ can be adminl~ter~d with the
aid of customary inh~ler~. The novel co pounds can also
be lyophyli~ed and the lyophyli~ates obtained used e.g.
for the production of in~ection preparations. The prepar-
ations indicated can be sterilized ~nd/or can contain
au~iliarie~ such a8 preservati~e~, stabilizers and/or
wetting agents, e~ulsifiers, salts for influencing
osmotic pressure, buffer substances, colorants and/or
fl~vourings. If desired, they can al80 cont~in one or
.. ... . .. ~.. . .. "-.. ",.. ,~... .
.: - , .
2 ~
- 16 -
more other active compounds, e.g. one or more vitam$ns.
The substances according to the invention are as
a rule administered in analogy to other known commercial-
ly available peptides, but in particular in analogy to
the compounds described in US-A-4,472,305, preferably in
dosages between about 0.05 and 500, in particular between
0.5 a~d 100 mg per dosage unit. The daily dose i8 prefer-
ably between about 0.01 and 2 mg~kg of body weight. ~he
specific dose for each intended patient depends, however,
on many different factors, for example the activity of
the specific compound employed, the age, body weight,
general state of health, sex, the cost, the time and
route of administration, and the rate of excretion,
pharmaceutical comb~nation and severity of the part$cular
disorder to which the therapy applies. Parenteral admini-
stration i8 preferred.
All te~per~tures above ~fid below are st~ted in
C. In the following ex~mples, ~customary working up~
mean8 5 water i8 ~dded, if necessary, the mixture i8
neutralized and e~tr~ted with ether or dichlorometh~ne,
the organic ph~se is separ~ted off, dr$ed over sodium
sulf~te, filtered ~nd ev~por~ted ~d the re~idue is
purified by chrom~togr~phy on ilic~ gel ~nd/or cryst~l-
lir~tion. RT - ret ntion ti~e (~inute~) for ~PLC on ~n
RP 18 250-~ colu-n, if not t~t d otherwi~e; eluent~
w~ter, b - 0.3 % TFA in water, c ~ ~cetonitrile.
Rf - Rf v~lue by thin-layer chro~togr~phy on ~ilic~ gel;
eluent~ chlorofor~eth~nol/~cetic ~cid 85-10-5. FAB-NS -
- ~f~t ~to~ bo b-rdm-nt~ m~- spectrum.
Example 1
A ixture of 900 mg of cyclot-glycyl-L-phenyl-
~l~nyl-L-phenyl~l~nyl-L-~N-imi-2,4-dinitrophenyl-his-
tidyl)-L-leucyl-L- ethionine-l tcyclo(Gly-phe-phe-(~m~-
DNP-Hi~)-Leu-Met-); obt~in~ble by cycli~tion of H-Gly-
Phe-Phe-(imi-DNP-Hls)-Leu-Het-0~ ~n~logously to
~mple 3, see below], 2 g of 2-merc~ptoeth~nol, 20 ml of
DMF ~nd 20 ml of w~ter is ~d~usted to pH 8 with ~queous
N~2C03 ~olution while stirring ~t 20- and is stirred for
:,','`: .' ' '
.. . . . .
, . . . . .
!. ~ . . '
- 17 -
2 hour~ at 20 Cu~tomary working up give~ cyclo(-glycyl-
L-phenylalanyl-L-phenylalanyl-L-histidyl-L-leucyl-L-
methionine-)tcyclo(-Gly-Phe-Phe-Hi~-Leu-Met-)]
Example 2
1 g ofcyclo[-Gly-phe-phe-(imi-BoM-His)-Leu-Met-]
[obtainable from H-Gly-phe-phe-(imi-BoM-Hi8)-Leu-Met-oH
analogously to Example 3, see below] i8 dissolved in
25 ml of methanol, hy~rogenated for 3 hours on 0 5 % Pd-C
at 20 and 1 bar, and the mixture is filtered and evapor-
ated and, after customary working up, gives cyclo(-Gly~
Phe-Phe-His-Leu-Met-)
Example 3
1 8 g (2 mmol) of BOC-Leu-D-Met-Gln-Trp-Phe-Gly-
OMe are dissolved in 50 ml of methanol, 1 5 ml of 2 N
aqueous sodium hydroxide solution are added and the
mixture is ~tirred for 3 hours ~t 20 It i~ evaporated,
~nd the residue i~ taken up in water, acidified to pH 3
with HCl ~nd e~tr~cted with ethyl acetate The extr~ct is
dried over N~SO~ ~nd ev~por~ted The BOC-Leu-D-Met-Gln-
Trp-Phe-Gly-OH thu~ obt~ined i8 ~tirred ~t 20 for 2
hours with 20 ~1 of 2 N HCl in diox~ne The mlxture i~
ev~por~ted, the H-Leu-D-~et-Gln-Trp-Phe-Gly-OH obt~lned
i- di--ol~ d in a ~xtur of 1800 ~1 of dichloro ethane
~nd 200 ~1 of Dh~ ~n~ ¢ool~d to 0-! Q 5 g of DCCI, 0 3 g
of BOPt nd 0~23 d of N _ thyl~orphollne ~re ~dded
~ucc--~ively with ~tirring, and the mixture .i8 stirred
for 2~ hour~ at 0 and 48 hour ~t 20 The olution i~
concentrated and ~tirred wlth a ~ixed bed lon e~ch~nger
to re~o~e ~lt- ~hi~ filtered off, the -olution i~
ev~por~ted ~nd the re~idue i8 purified by chro ~togr~phy
Cyclo(-Gln-Trp-Phe-Gly-Leu-D-~t-) i~ obt~inediS RT 11 37
(b/c 6s4)
The following ~re obt~ined ~n~lo~ously from the
corresponding linear hex~peptlde~
Cyclo(-Al~-Phe-Phe-Pro-Leu-Met-), m p 134 - 140
Cyclol-Ala-Phe-Phe-Pro-Leu-Met(O)-1
Cyclo~-Ala-Phe-Phe-Pro-Leu-Met( 2 ) - ]
- 18- 20~ pr,,~
Cyclo(-Ala-Phe-Phe-Pro-Leu-D-Met-), m.p. 133 - 138
Cyclo(-Gly-Phe-N-Me-Phe-Gly-Leu-Met-),
RT 15 09 (a/c 6 4)
Cyclo~-Gly-Phe-N-Me-Phe-Gly-Leu-Met(0)-),
Cyclo(-Gly-Phe-N-Me-Phe-Gly-L~ -Net(02)-),
Cyclo(-Gly-Phe-D-N-Me-Phe-Gly-Leu-Met-),
RT 12 30 (a/c 6 4)
Cyclot-Gly-Phe-D-N-Me-Phe-Gly-Leu-Met(0)-]
Cyclo[-Gly-Phe-~-N-Me-Ph~-Gly-LQu-Met( 2 ) - ]
I Cyclo(-Gly-Phe-Phe-Pro-Leu-Leu-)
Cyclot-Gly-Phe-Phe-Pro-Leu-~et-), FAB-NSs (M+H)~ 694
Cyclo[-Gly-Phe-Phe-Pro-Leu-Met(0)-], FAB-MSs (M+H)' 710
Cyclot-Gly-Phe-Phe-Pro-Leu-Met( 2 ) - ], m p 157-162
Cyclo(-Gly-Phe-D-Phe-Pro-Leu-Met-)
Cyclot-Gly-Phe-D-Phe-Pro-Leu-Met(0)-]
Cyclot-Gly-Phe-D-Phe-Pro-Leu-M~t(02)-], m.p. 128 - 135
Cyclol-Gly-Phe-Phe-D-Pro-Leu-Met-), RF O 63
Cyclot-Gly-Phe-Phe-D-Pro-Leu-Met(0)-~, RF ~ 42
Cyclot-Gly-Phe-Phe-D-Pro-Leu-Nbt( 2) - ]
cyclot-Gly-phe-ph~-pro-Leu-D-~et-)~ m p 13~ - 140
Cyclol-Gly-Phe-Phe-Pro-Leu-D-NQt(0)-]
Cyclo[-Gly-Phe-Phe-Pro-Leu-D-Met (2) - 1
Cyclo(-Gly-Phe-V~l-Gly-Leu-Net-), RF 051
Cyclol-6ly-Phe-V~l-Gly-Leu-~t(O)-]
Cyclot-Gly-Phe-V~l-Gly-~eu-~bt(0~)-]
Cy~lo(-Gly-Trp-Ph -Pro-L~u-~t-)
Cyclol-Gly-Trp-Ph -Pro-Leu-~t(O)-]
CycloI-Gly- ~ Ph -Pro-Leu-15-t(0~)-]
Cyclol-GlyTrp-Ph -D-Pro-Leu-~et-)
Cyciot-Gly- ~ -Phe-D-Pro-leu-~et(O)- I
Cyclol--Glr-Trp-Phe-D--P~o-Leu-Nbt(2 ) - ] .
Esample 4
Cycio(-Gly-Phe-Phe-Pro-Leu-Net-)2, m.p. 216-218-
is obt~ined ~nalogou~ly to Es~mple 3 by cyclizatlon of H-
(Gly-Phe-Phe-Pro-Leu-~et)2-OH tobtainable by reaction of
BOC-Gly-Phe-Phe-Pro_Leu-Met_O~ w~th H-Gly-Phe-Phe-Pro-
Leu-~et-OM~ to give BOC-~Gly-Phe-Phe-Pro-Leu-~et)2-O~e
and subsequent remov~l of the protecting groupl.
The following are obtained ~nalogously from the
,, ~ . . .~
... . ~
-- 19 --
corre~ponding linear dodecapeptides:
Cyclo-(Ala-Phe-Phe-Pro-Leu-Met-)2, m.p. 137 - 144
Cyclo-(Ala-Phe-Phe-Pro-Leu-D-Met)21 RF 0.74
Cyclo[-Gly-Phe-Phe-Pro-Leu-Met(O)-] 2
S Cyclo[-Gly-Phe-Phe-Pro-Leu-Met( 2 ) - ] 2 ~ :
Cyclo[-Gly-Trp-Phe-Pro-Leu-Met-) 2
Cyclo[-Gly-Trp-Phe-Pro-Leu-Met(0)-~2
Cyclo[-Gly-Trp-Phe-Pro-Leu-Met-( 2 ) - ] 2
Cyclo[-Gly-Trp-Phe-D-Pro-Leu-Met-) 2
Cyclo[-Gly-Trp-Phe-D-Pro-Leu-Met-(O)-] 2
Cyclo[-Gly-Trp-Phe-D-Pro-Leu-Met-( 2 ) - ] 2 .
Exampl~ 5
1.7 g (2 mmol) of BOC-Gly-Phe-Phe-Pro-Leu-Met-OMe
(m.p. 92-99-) ~re dissolved in 50 ml of methanol, 2 ml of
hydrazine hydrate are added, and the mixture i8 stirred
for 16 hours and evaporated. The BOC-Gly-Phe-Phe-Pro-Leu-
Met-NHNH2 thus obtained i~ stirred at 20- for 2 hours with
20 ml of 2 N RCl in dioxane. The mixture is evaporated,
the H-Gly-Phe-Phe-Pro-Leu-Met-NHNH2 dihydrochloride
obtained i8 dissolved in 50 ml of DHF and cooled to -lS-
wlth ~tirring, and 0.83 ~1 of 35 % hydrochloric acld and
1.5 ml of ~ 1~ % aqueou~ NaNO2 solutlon are ~dded succes-
slvoly. The ~l~ture 1- stirr~d ~t -15- for 1 hour and
poured lnto 2 1 of cooled D~F. 1.1 ml of N- thylmor-
phollne ~r~ add d, and th ~ixture 18 ~tirred for 24
hour at--lS- ~nd ~llo~sd to stand for 2 d~y~ at 20-. The
: solution i- concentrated, a Jixed b~d ion exchAnger is
~dded to r~ ove ~alt~, the m~xture is filtered, the
fiitrate 1~ vapor~ted, ~nd the re~ldue 18 purif~ed by
chromatography to give cyclo(-Gly-Phe-Phe-Pro-Leu-~et-);
FAB-MS (M+H)~ 694.
Example 6
A ~olution of 100 mg of cyclo(-Gly-Phe-Phe-Pro-
.Leu-Met-) and 1 equivalent of 30 % aqueous H2O2 in SO ml
of meth~nol is stirred ~t 20- for 2 hours. ~vaporation
and custom~ry worklng up gives cyclol-Gly-Phe-Phe-Pro-
Leu-Met(O)-]; FAB-~Ss (~+H)~ 710.
Example 7
A solution of 100 mg of cyclo(-Gly-Phe-Phe-Pro-
~ .. , ~ , :
.. . .
. .
~Q~ ~13~j
-
- 20 -
Leu-Met-) and 10 equiv~lents of 30 % aqueous Hz02 in 50 ml
of methanol is warmed to 50 for 2 hours Evaporation and,
cu~tomary working up gives cyclot-Gly-Phe-Phe-Pro-LQu-
Met( 2)-]; m p 157-162
Example 8
~ir i8 pa~sed through a solution of 1 g of
cyclo[-Gly-Phe-Phe-Pro-Leu-Met-) in 50 ml of acetonitrile
a~d 50 ml of w~ter until reaction is complete Customary
working up gives the corresponding sulfoxide cyclot-Gly-
Phe-Phe-Pro-Leu-Met(0)-]; FAB-MSs (M+H)~ 710
Example 9
20 ~1 of a 2 M NH~I solu~ion is added at 0 to a
solution of 1 g of cyclot-Gly-Phe-Phe-Pro-Leu-Met(O)-I in
50 ml of TFA After stirring at 0 for 1 hour, the
resulting iodine i8 reduced by adding thio~lycolic acid,
and the mixture i8 worked up as is customary to give
cyclot-Gly-Phe-Phe-Pro-Leu-Met-); FAB-MS~ (~+H)~ 694
The ex~ples below relate to pharmaceutical
preparation-
~x~ple At In~ectlon ~$al-
A olutlon of 100 g of cyclot-Gly-Phe-Phe-Pro-
Leu-~et-) ~nd 5 g of d$-od$um hydrogenpho~phate $n 31 of
doubly d$-till d wat-r i- ad~u-ted to p~ 6 5 with 2 N
hydrochloric acid, terllo filtered, filled into in~ec-
t~o~ vi~ nd lyophili~ed under terile con~ition~, and
the vial~ ~re clos~sd in a terilo manner ~,ach in~ection
'~ vlal contain- 5 mg of active compound
E~4mple B~ Su,ppo~itorles
A DiXture of 20 g of cyclot-Gln-~rp-Phe-Gly-Leu-
D-Met-) i~ fuJed with 100 g of oya lecithin and 1400 g
, of cocoa butter, and the mi~ture is poured into ~oulds
and allowed to cool Each suppository contain~ 20 mg of
active compound
~;, " ~
3 ~
-~ - 21 -
Example C: Solution
~r- A solution of 1 g of cyclot-Gly-Phe-Phe-Pro-Leu-
Met(O)-], 9.38 g of NaH2PO~.2H20, 28.48 g of Na2HPO~.12H20
and 0.1 g of benzalkonium chloride i8 prepared in 940 ml
of doubly distilled water. The solution is ad~usted to
pH 6.8, made up to 1 1 and sterilized by irradiation.
This solution can be used in the form of eye drops.
Example Ds Ointment
500 mg of cyclol-Gly-Phe-Phe-Pro-Leu-Met-)2 i8
mixed with 99.5 g of petroleum ~elly under asoptic
conditions.
' ~