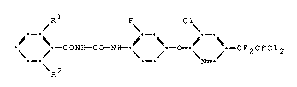Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2~6~7
- 1
PS/5-17578/=
Benzovlphenvlureas
The present invention relates to novel substituted N-benzoyl-N'-[2-fluoro-4-(pyridin-2-yl-
oxy)-phenyl]-ureas having insecticidal and acaricidal activity, processes for tbeir
preparation, and their use in the control of pests, preferably in agriculture. -~
The novel N-benzoyl-N'-[4-(pyridin-2-yloxy)-phenyl]-ureas correspond to formula I
R 1 FC 1~
~ ~---CON~CO--Ni~-~ ~---O---~ ~---CF2CFC12 (I),
R 2
wherein
Rl and R2 are both fluorine or
is chlorine and R2 is hydrogen.
The invention is represented by the ~wo compounds N-(2,6-difluorobenzoyl)-N'-{2-fluoro-4-[3-chloro-5-(2,2-dichloro-1,1,2-trifluoroethyl)-pyridin-2-yloxy]-phenyl]-urea and
N-(2-chlorobenzoyl)-N'-(2-fluoro-4-[3-chloro-5-(2,2-dichloro- 1,1,2-trifluoroethyl)-
pyridin-2-yloxy]-phenyl}-urea.
The compounds of formula I can be prepared analogously to processes known ~ se (see,
for example, German Offenlegungsschrift No. 2 123 236, 2 601 760 or 3 240 975).
For example, the compounds of formula I can be obtained by reacting the 2-fluoro-4-[3-
chloro-5-(2,2-dichloro-1,1,2-trifluoroethyl)-pyridin-2-yloxy]-aniline of formula II
F~ C l~ :
H 2 N~ / ---C F 2 C F C 1 2
c- N~e- ,,
2~3~
preferably in an inert organic solvent, with an equimolar amount of a benzoyl isocyanate
of formula III
Rl -
_ / .
--C O--N= C= O , ( I I I ) .
\R2
The compounds of formula I can also be obtained by reacting the isocyanate of formula IV
F~ C I~
/ ---C F 2 C F C 1 2 ( I V )
.=- N=-
preferably in an inert organic solvent, with an equivalent amount of a benzamide or
urethane of formula V
Rl
--C ON HR 3 ( V ) .
\R2
In the above formulae II to V, Rl and R2 are both fluorine or Rl is chlorine and R2 is
hydrogen; R3 is hydrogen or a radical -CooR4 in which R4 is Cl-C6aLlcyl, phenyl or
benzyl.
, i ,.
The mentioned processes are preferably carried out under normal pressure in the presence
of an organic solvent or diluent. Suitable solvents or diluents are, for example, ethers and
ethereal compounds, such as diethyl ether, dipropyl ether, dibutyl ether, dioxane,
dimethoxyethane and tetrahydrofuran; N,N-dialkylated carboxylic acid amides; aliphatic,
aromatic and halogenated hydrocarbons, especially benzene, toluene, xylene, chloroform,
methylene chloride, carbon tetrachloride and chlorobenzene; nitriles, such as acetonitrile
or propionitrile; dimethyl sulfoxide and ketones, for exarnple acetone, methyl ethyl
,, . : ,, - : . :
ketone, methyl isopropyl ketone and methyl isobutyl ketone. The processes are generally
carried out at a temperature of -10 to +200C, preferably from 0 to 100C, for example at
room temperature. The former process is optionally performed in the presence of an
organic base, for example triethylamine.
The starting materials of formulae III and V are known or can be prepared analogously to
known processes. The compound of formula II can be prepared in a manner known ~ se
by condensing 2-fluoro-4-hydroxyaniline in an inert organic solvent, in the presence of at
least an equimolar amount of a base, with 2,3-dichloro-5-(2,2-dichloro-1,1,2-trifluoro-
ethyl)-pyridine in accordance with the scheme:
F Cl F Cl
H 2 N--~ O H + C l_ - ~ ~ ---C F 2 C F C 1 2 ~ H 2 N--- \ / ---~ ---C F 2 C F C 1 2
(II)
The 2-fluoro-4-[3-chloro-5-(2,2-dichloro-1,1,2-trifluoroethyl)-pyridin-2-yloxy]phenyl
isocyanate of formula IV can be obtained by treating the aniline of formula II with
phosgene in accordance with a generally customary process. The aniline of formula II and
the isocyanate of formula IV are novel compounds to which the invention also relates. The
benzamides of formula V also to be used as starting materials are known or can be
produced according to known processes (see, for example, Beilstein "Handbuch derorganischen Chemie" Vol. 9, p. 336).
According to the invention, the novel compounds of formula I also include the salts
thereof, which are distinguished not only by a high level of insecticidal activity but also by
good solubility in solvents and diluents, especially in organic solvents, and by improved
formulation properties.
Attention should be drawn to the metal salts of the compounds of formula I according to
the invention, especially their alkali metal and alkaline earth metal salts, preferably the
sodium salts and potassium salts. These salts are prepared in a manner known ~ se, for
example by reacting a compound of formula I with a metal aL~canolate, for example
sodium ethanolate or potassium methanolate. With a given salt, desired salts of other
metals can be obtained by exchanging the metal cation.
. `. : `: - ~
2016~7
- 4 -
The salts of compounds of formula I with organic bases that are essentially characterised
by the presence of a quaternary nitrogen atom are of particular importance. These salts
correspond to formula Ia
1l F\ Cl\
/ \--CO-N-CO-NH--~ 0~ - CF2 CFC12 X (Ia),
~2
wherein R1 and R2 are as defined above and X~3 is the cation of an organic base. X~3 is
preferably one of the following organic cations:
(CH3)4 N , ~C2H5)4 N , ~n-C3H7)4 N , ~i C3H7)4 N ,
4H9)4 N ~ --CH2) ~CH3)3 N ~
.\ /-_) ~CH3)3 N ~ !~ , (C4Hg)3 NH
N~
H
! i ! und [ CH3-(CH2) - ] N-CH3
~ / \N~ 3
wherein _ is a number from 8 to 12. Salts according to formula Ia) shall also beunderstood as including mixtures of those salts wi~ different cations. The salts of formula
Ia can be prepared in a manner known ~ se, for example by reacting a compound offormula I with corresponding ammonium hydroxides of formula X/33 (OH)~ wherein X~
is as defined above.
In addition to the substituted N-benzoyl-N'-(4-pyridyloxyphenoxy)ureas having
insecticidal and fungicidal activity according to published Japanese Patent Applications
:., . . ~,
':.,, :: ~ :
,. . ~, ,.
JA-A 62 195 395 and JA-A 63 122 661, N-benzoyl-N'-2,5-dichlorophenylureas having a
haloalkoxy substituent in the 4-position of the phenyl ring are also already known as
insecticides (see US Patent Specification No. 4,518,804 and German Offenlegungsschrift
No. 2848794). In addition, US Patent Specification No. 4,162,330 describes insecticidally
active N-halobenzoyl-N'-(3,5-dichloro-4-haloalkenyloxyphenyl)-ureas, and published
Japanese Patent Application JA-A 63 270 662 describes the N-2,6-difluorobenzoyl-N'-[2-
fluoro-4-(3-chloro-5-trifluoromethylpyrid-2-yloxy)-phenyl]-urea having acaricidal
activity. In contrast to the compounds described in those literature references, the novel
benzoylphenylureas of formula I according to the invention have, as an essentialdistinguishing structural feature, a 3-chloro-5-(2,2-dichloro-1,1,2-trifluoroethyl)-pyridin-
2-yloxy group in the 4-position of the phenyl ring.
Surprisingly it has now been found that the present compounds of formula I and salts
thereof have excellent properties as pesticides while being weil tolerated by plants and
having a low toxicity to warrn-blooded animals. They are particularly suitable for
controlling insects and representatives of the order Acarina that attack plants and animals.
The pronounced leaf-penetration action and properties of the compounds according to the
invention are worthy of note.
In particular, the compounds of formula I are suitable for controlling insects of the orders:
Lepidoptera, Coleoptera, Homoptera, Heteroptera, Diptera, Thysanoptera, Orthoptera,
Anoplura, Siphonaptera, Mallophaga, Thysanura, Isoptera, Psocoptera and Hymenoptera,
as well as representatives of the order Acarina of the families: Ixodidae, Argasidae,
Tetranychidae and Dermanyssidae.
In addition to their action against flies, for example Musca domestica, and mosquito
larvae, the compounds of formula I are also suitable for controlling plant-destructive
feeding insects in ornamentals and crops of useful plants, especially in cotton (for example
against Spodoptera littoralis and Heliothis virescens) and in fruit and vegetables (for
example against Laspeyresia pomonella, Leptinotarsa decernlineata and Epilachna
varivestis). The compounds of formula I are distinguished by a pronounced ovicidal and,
in particular, larvicidal action against insects, especially against larvae of noxious feeding
insects. If the compounds of formula I are ingested by adult insect stages with their feed, - -
then a diminished oviposition and/or a reduced hatching rate is observed in many insects,
especially in Coleoptera, for example Anthonomus grandis.
;' ` :`,
.: ': . ., ~' .
2 ~ 7
- 6 -
The compounds of formula I are also advantageously suitable for controlling plant-
destructive spider mites, for example species of mite that attack the following crcps of
fruit and vegetables: Tetranychus urticae, Tetranychus cinnabarinus, Panonychus ulmi,
Broybia rubrioculus, Panonychus citri, Eriophyes piri, Eriophyes ribis, Eriophyes vitis,
Tarsonemus pallidus, Phyllocoptes vitis and Phyllocoptruta oleivora.
The compounds of formula I can also be used for controlling ectoparasites, such as
Lucilia sericata, in domestic animals and productive livestock, for example by treating the
animals, livestock buildings and pastures.
The benzoylureas of formula I according to the invention also possess properties that
enable them to be used to control snails and slugs. The repellent and feed-inhibiting
action of these compounds is often difficult to recognise in laboratory tests. In the open,
however, good activity is observed in agricultural and horticultural plant crops, even at
very low rates of application. In particular, sensitive salad, vegetable and fruit crops (such
as strawberry crops) and crops of ornamentals and flowers are protected against being
eaten by snails and slugs. The activity of the compounds of formula I according to the
invention extends to all slugs and snails, the majority of which occur as polyphagous pests
in crops of agricultural, horticultural and ornamental plants. Both feed-inhibiting and
killing action may occur. The land snails and slugs include some especially important
pests, for example the slugs Arion rufus (large red slug); Arion ater and other Arionidae,
Limax species and the field slugs, e.g. Deroceras reticulatum and D. agreste from the
family Limacidae, and species of the family Milacidae. In addition, the snails e.g. of the
genera Bradybaena, Cepaea, Cochlodina, Discus, Euomphalia, Galba, Helicigona, Helix,
Helicella, Helicodiscus, Lymnaea, Opeas, Vallonia and Zonitoides also damage useful
plants in the agricultural and horticultural sectors.
The good pesticidal, especially insecticidal, activity of the compounds of formula I
according to the invention corresponds to a mortality of at least 50-60 % of the above
pests.
The activity of the compounds of the invention and of the compositions containing them
can be substandally broadened and adapted to prevailing circumstances by the addidon of
other insecticides and/or acaricides. Examples of suitable additives include represent-
atives of the following classes of active ingredient: organophosphorus compounds, nitro-
phenols and derivatives thereof, formamidines, ureas, carbamates, pyrethroids, chlorinated
.,........... . - ~ :
.
:i . ;
:; : .. .
. .
- 7 -
hydrocarbons and Bacillus thuringiensis preparations.
The compounds of formula I are used in unmodified form or preferably together with the
adjuvants conventionally employed in the art of formulation, and can therefore be
formulated in known manner, e.g. into emulsifiable concentrates, directly sprayable or
dilutable solutions, dilute emulsions, wettable powders, soluble powders, dusts,granulates, and also encapsulations in e.g. polymer substances. As with the compositions,
the methods of application, such as spraying, atomising, dusting, scattering or pouring, are
chosen in accordance with the intended objectives and the prevailing circumstances.
The formulations, i.e. the compositions, preparations or rnixtures containing the compound
(active ingredient) of formula I, or combinations of those compounds with other
insecticides or acaricides, and, where appropriate, a solid or liquid adjuvant, are prepared
in known manner, e.g. by homogeneously mixing and~or grinding the active in~redients
with extenders, e.g. solvents, solid carriers and, where appropriate, surface-active
compounds (surfactants).
Suitable solvents are: aromatic hydrocarbons, preferably the fractions containing 8 to 12
carbon atoms, e.g. xylene mixtures or substituted naphtnalenes, phthala~es such as dibutyl
phthalate or dioctyl phthalate, aliphatic hydrocarbons such as cyclohexane or paraffins,
alcohols and glycols and their ethers and esters, such as ethanol, ethylene glycol, ethylene
glycol monomethyl or monoethyl ether, ketones such as cyclohexanone, strongly polar
solvents such as N-methyl-2-pyrrolidone, dimethyl sulfoxide or dimethylformamide, as
well as vegetable oils or epoxidised vegetable oils, such as epoxidised coconut oil or
soybean oil; or water.
The solid carriers used e.g. for dusts and dispersible powders, are normally natural mineral ~ - -
fillers such as calcite, talcum, kaolin, montmorillonite or attapulgite. In order to improve
the physical properties it is also possible to add highly dispersed silicic acids or highly
dispersed absorbent polymers. Suitable granulated adsorptive carriers are porous types,
for example pumice, broken brick, sepiolite or bentonite; and suitable nonsorbent carriers
are, for example, calcite or sand. In addition, a great number of granulated materials of
inorganic or organic nature can be used, e.g. especially dolomite or pulverised plant
residues.
Depending on the nature of the compound of formula I to be formulated or on the nature
, .. : ~ . , ~ ,
of the combinations of these compounds with other insecticides or acaricides, suitable
surface-active compounds are nonionic, cationic andVor anionic surfactants having good
emulsifying, dispersing and wetting properties. The term "surfactants" will also be
understood as comprising rnixtures of surfactants.
Both so-called water-soluble soaps and also water-soluble synthetic surface-active
compounds are suitable anionic surfactants.
Suitable soaps are the alkali metal salts, alkaline earth metal salts or unsubstituted or
substituted ammonium salts of higher fatty acids (C1O-C22), e.g. the sodium or potassium
salts of oleic or stearic acid or of natural fatty acid mixtures which can be obtained e.g.
from coconut oil or tall oil. Fatty acid methyltaurin salts and also modified and
unmodified phospholipids may also be mentioned as surfactants.
More frequently, however, so-called synthetic surfactants are used, especially fatty
sulfonates, fatty sulfates, sulfonated benzimidazole derivatives or alkylarylsulfonates.
The fatty sulfonates or sulfates are usually in the form of aL~calii metal salts, aL~caline earth
metal salts or unsubstituted or substituted arnmonium salts and generally contain a
C8-C22alkyl radical which also includes the alkyl moiety of acyl radicals, e.g. the sodium
or calcium salt of lignosulfonic acid, of dodecylsulfate or of a mixture of fatty alcohol
sulfates obtained from natural fatty acids. These compounds also comprise the salts of
sulfated and sulfonated fatty alcohoVethylene oxide adducts. The sulfonated benz-
imidazole derivatives preferably contain 2 sulfonic acid groups and one fatty acid radical
containing approximately 8 to 22 carbon atoms. Examples of alkylaryl-sulfonates are the
sodium, calcium or triethanolamine salts of dodecylbenzenesulfonic acid, dibutyl-
naphthalenesulfonic acid, or of a condensate of naphthalenesulfonic acid and form-
aldehyde. Also suitable are corresponding phosphates, e.g. salts of the phosph~ic acid
ester of an adduct of p-nonylphenol with 4 to 14 moles of ethylene oxide.
Non-ionic surfactants are preferably polyglycol ether derivatives of aliphatic or
cycloaliphatic alcohols, saturated or unsatuMted fatty acids and aL~cylphenols, said
derivatives containing 3 to 30 glycol ether groups and 8 to 20 carbon atoms in the
(aliphatic) hydrocarbon moiety and 6 to 18 carbon atoms in the alkyl moiety of the
alkylphenols. Further suitable non-ionic surfactants are the water-soluble adducts of
polyethylene oxide with polypropylene glycol, ethylenediarninopolypropylene glycol and
:, : :
,:, :; ~ . ,. : ,
g
alkylpolypropylene glycol containing 1 to 10 carbon atoms in the alkyl chain, which
adducts contain 20 to 250 ethylene glycol ether groups and 10 to 100 propylene glycol
ether groups. These compounds usually contain 1 to 5 ethylene glycol units per propylene
glycol unit.
Representative examples of non-ionic surfactants are nonylphenolpolyethoxyethanols,
castor oil polyglycol ethers, polypropylene/polyethylene oxide adducts, tributylphenoxy-
polyethoxyethanol, polyethylene glycol and octylphenoxy-polyethoxyethanol. Fatty acid
esters of polyoxyethylene sorbitan, e.g. polyoxyethylene sorbitan trioleate, are also
suitable.
Cationic surfactants are preferably quaternary ammonium salts which contain, as
N-substituent, at least one C8-C22aL~cyl radical and, as further substituents, unsubstituted or
halogenated lower alkyl, benzyl or hydroxy-lower allcyl radicals. The salts are preferably
in the form of halides, methylsulfates or ethylsulfates, e.g. stearyltrimethyl-ammonium
chloride or benzyldi(2-chloroethyl)ethylammonium bromide.
The surfactants customarily employed in the art of formulation are described e.g. in the
following publications:
"Mc Cutcheon's Detergents and Emulsifiers Annual" MC Publishing Corp.,
Ridgewood, New Jersey, 1979; Dr. Helmut Stache "Tensid Taschenbuch",
Carl Hanser Verlag Munich/Vienna 1981.
The pesticidal compositions usually contain 0.1 to 99 %, especially 0.1 to 95 %, of the
compound of formula I or combinations thereof with other insecticides or acaricides,
1 to 99.9 % of a solid or liquid adjuvant and 0 to 25 %, especially 0.1 to 20 %, of a
surfactant. Whereas commercial products are preferably formulated as concentrates, the
end user will normally employ dilute formulations having substantially lower
concentrations of active ingredient. The rate of application of the active ingredients of
formula I according to the invention, especially in the case of agricultural crop areas, is
usually 0.025 to 1.0 kglha, preferably 0.1 to 0.5 kg/ha, e.g. 0.1 to 0.25 kglha.
The compositions may also contain further ingredients such as stabilisers, antifoams,
viscosity regulators, binders, tackifiers as well as fertilisers or other active ingredients for
obtaining special effects.
.i.. ~- .. . .
, . ,
~ O ~ ! 7
- 10-
The following Examples illustrate the preparation of the compounds of formula I
according to the invention and the aniline of formula II required as intermediate. The
temperatures are glven in degrees Celsius.
Example 1: Preparation of N-(2,6-difluorobenzoyl)-N'- {2-fluoro-4-[3-chloro-5-(2,2-di-
chloro- 1,1 ,2-trifluoroethyl)-pyridin-2-yloxy]-phenyl } -urea
~ F F~ C I~
f ~---CH N~CO--N}~-~ 'f ~---CF CFCl
2-- \ / \ / 2 2
F
2.19 g of 2,6-difluorobenzyloxy isocyanate are added dropwise, with stirring, to a solution
of 4.67 g of 2-fluoro-4-[3-chloro-5-(2,2-dichloro-1,1,2-trifluoroethyl)-pyridin-2-yloxy]-
aniline in 20 ml of toluene. The reaction mixture is then stirred for 5 hours at room
temperature and filtered, and the filter residue is washed with toluene and dried to give the
title product in the form of colourless crystals which melt at 196 - 2û0C.
N-(2-chlorobenzoyl)-N'-l2-fluoro-4-[3-chloro-5-(2,2-dichloro- 1,1,2-trifluoroethyl)-
pyridin-2-yloxy]-phenyl)-urea is prepared in an analogous manner (m.p. 190 - 191C).
Example 2: Preparation of 2-fluoro-4-~3-chloro-5-(2.2-dichloro-1.1.2-trifluoroethYl)-
Pvridin-2-YloxYl-aniline (intermediate)
F~ C 1~
H 2 N~ C F 2 C F C 1 2 ( I I )
=. ~=-
5.8 g of pulverised 85 % potassium hydroxide are added to a solution of 10.17 g of2-fluoro-4-hydroxyaniline in 25 ml of dimethyl sulfoxide and the batch is stirred for 20
minutes. 23.9 g of 2,2-dichloro-5-(2,2-dichloro-1,1,2-trifluoroethyl)-pyridine are then
added dropwise thereto and the batch is stirred for 10 hours at room temperature. The
reaction mixture is then diluted with water and extracted with methylene chloride. The
organic phase is washed with water and dried and the solvent is distilled off. The oil which
2 ~ r~ 7
remains is purified by chromatography on a column filled with silica gel (Merck 60) using
methylene chloride as the eluant. The solvent is evaporated to give the title compound in
the form of colourless crystals having a melting point of 61 - 62C.
Example 3: Formulation examples for active ingredients of formula I according toExample 1 or combinatlons of those active in~redients with other insecticides oracaricides (throu~hout. percenta ,es are bv weight):
1. Wettable powders a) b) c)
compound or combination 25 %50 % 75 %
sodium lignosulfonate 5 % 5 %
sodium laurylsulfate 3 % - 5 %
sodium diisobutylnaphthalene-
sulfonate - 6 % 10 %
octylphenolpolyethylene glycol
ether (7-8 moles of ethylene oxide) - 2 %
~lighly dispersed silicic acid 5 %10 % 10 % ;
kaolin 62 %27 %
The active ingredient or combination is thoroughly mixed with the adjuvants and the
mixture is thoroughly ground in a suitable mill, affording wettable powders which can be
diluted with water to give suspensions of the desired concentration.
2. Emulsifiable concentrate
compound or combination 10 %
octylphenolpolyethylene glycol
ether (4-5 moles of ethylene oxide) 3 %
calcium dodecylbenzenesulfonate 3 %
castor oil polyglycol ether
(36 moles of ethylene oxide) 4 %
cyclohexanone 30 %
xylene mixture 50 % '
Emulsions of any required concentration can be obtained from this concentrate by dilution
with water.
i~}~
~ I
;' ~ .
3.Dusts a) b)
compound or combination 5 % 8 %
talcum 95 %
kaolin - 92 %
Ready-for-use dusts are obtained by mixing the active ingredient or combination with the
carrier, and grinding the mixture in a suitable mill.
4. Extruder ranulate
compound or combination 10 %
sodium lignosulfonate 2 %
carboxymethylcellulose 1 %
kaolin 87 %
The active ingredient or combination is mixed and ground with the adjuvants, and the
mixture is subsequently moistened with water. The mixture is extruded and granulated and
then dried in a stream of air.
5. Coated ranulate
compound or combination 3 %
polyethylene glycol (mol. wt. 200) 3 %
kaolin 94 %
The finely ground active ingredient or combination is uniformly applied, in a mixer, to the
kaolin moistened with polyethylene glycol. Non-dusty coated granulates are obtained in
this manner.
6. Suspension concentrate
compound or combination 40 %
ethylene glycol 10 %
nonylphenolpolyethylene glycol
ether (15 moles of ethylene oxide) 6 %
sodium lignosulfonate 10 %
carboxymethylcellulose 1 %
37% aqueous formaldehyde solution 0.2 %
:
2 ~ 7
- 13-
silicone oil in the forrn of a 75%
aqueous emulsion 0.8 %
water 32 %
The finely ground active ingredient or combination is intimately mixed with the adjuvants,
giving a suspension concentrate from which suspensions of any desired
concentration can be obtained by dilutiorl with water.
Example 4: Action against Musca domestica:
50 g of freshly prepared nutrient substrate for maggots are charged into each of a number
of beakers. A specific amount of an acetonic solution containing 1 % by weight of the
respective test compound is pipetted onto the nutrient substrate present in the beakers to
give an active ingredient concentration of 800 ppm. The substrate is then thoroughly
mixed and the acetone subsequently allowed to evaporate over a period of at least 20
hours.
Then 25 one-day-old maggots of Musca domestica are put into each of the beakers
containing the treated nutrient substrate for testing with each active ingredient at the given
concentration. After the maggots have pupated, the pupae are separated from the substrate
by flushing them out with water and then deposited in containers closed with a perforated
top. Each batch of flushed out pupae is counted to determine the toxic effect of the test
compound on the maggot development. A count is made after 10 days of the number of
flies which have hatched out of the pupae.
Compounds of formula I according to Example 1 exhibit good activity in this test.
Example 5: Action against Lucilia sericata:
1 ml of an aqueous formulation containing 0.5 % by weight of test compound is added at
50C to 9 ml of a culture medium. Then about 30 freshly hatched Lucilia sericata larvae
are added to the culture medium, and the insecticidal action is determined after 48 and 96
hours by evaluadng the mortality rate.
The compounds of Example 1 exhibit good activity against Lucilia sericata in this test.
Example 6: Action a~eainst Aëdes ae~ypti:
A concentration of 800 ppm is obtiained by pipetting a specific amount of a 0.1 % by
' .". '
.- j,
... .
2~ ~3~7
- 14 -
weight solution of the test compound in acetone onto the surface of 150 ml of water in a
beaker.
After the acetone has evaporated, 30 to 40 2-day-old larvae of Aëdes are put into the
beaker. Mortality counts are made after 1, 2 and 5 days.
The compounds of Example 1 exhibit good activity against Aëdes aegypti in this test.
Example 7: Insecticidal stomach toxicant action:
Cotton plants (about 25 cm high) in pots are sprayed with aqueous emulsions which
contain the respective test compound in concentrations of 15 and 50 ppm.
After the spray coating has dried, the cotton plants are populated with Spodoptera littoralis
and Heliothis virescens larvae in the L~ stage. The test is carried out at 24C and 60 %
relative humidity. After 2 days, the percentage mortality of the laIvae is determined in
comparison with untreated control batches.
The compounds of Example 1 effect 80-100 % kill against Spodoptera larvae when used at
a concentration of 15 ppm and against Heliothis larvae when used at a concentration of
50 ppm.
Example 8: Action against Epilachna varivestis:
Phaseolus vulgaris plants (dwarf beans) about 15-20 cm in height are sprayed with
aqueous emulsion formulations of the test compound in a concentration of 800 ppm. After
the spray coating has dried, each plant is populated with 5 larvae of Epilachna varivestis
(Mexican bean beetle) in the L4 stage. A plastic cylinder is slipped over the infested plants
and covered with a copper gauze top. The test is carried out at 28C and 60 % relative
humidity.
The percentage mortality is determined after 2 and 3 days. Evaluation of any feeding
damage (anti-feeding effect), and of inhibition of development and shedding, is made by
observing the test insects for a further 3 days.
The compounds of Example 1 exhibit good activity in this test.
2~3~ ~
Example 9: Ovicidal action on Heliothis virescens
Corresponding amounts of a wettable powder formulation containing 25 % by weight of
the test compound are mixed with sufficient water to produce an aqueous emulsion with
an active ingredient concentration of 800 ppm.
One-day-old egg deposits of Heliothis on cellophane are immersed in these emulsions for
3 minutes and then collected by suction on round filters. The treated deposits are placed
in petri dishes and kept in the dark. The ha~ching rate in comparison with untreated
controls is determined after 6 to 8 days.
The compounds of Example 1 exhibit good activity in this test.
Example 10: Action on Laspeyresia pomonella (eg~s):
Egg deposits of Laspeyresia pomonella not more than 24 hours old are immersed for one
minute, on filter paper, in an aqueous acetone solution of the respective test compound in
a concentration of 800 ppm. After the test solution has dried, the eggs are placed in petri
dishes and left at a temperature of 28C. After 6 days, the percentage hatching rate from
the treated eggs is evaluated and the percentage mortality is determined.
The compounds of Example 1 exhibit good activity in this test.
Example 11: Influence on the reproduction of Anthonomus grandis:
Anthonomus grandis adults which are not more than 24 hours old after hatching are
transferred in groups of 25 to barred cages. The cages are then immersed for 5 to 10
seconds in an acetonic solution containing 400 ppm of the test compound. After the
beetles have dried, they are placed in covered dishes containing feed and left for
copulation and oviposition. Egg deposits are flushed out with running water twice to three
times weekly, counted, disinfected by putting them for 2 to 3 hours into an aqueous
disinfectant, and then placed in dishes containing a suitable larval feed. A count is made
after 7 days to deterrnine the percentage mortality of the eggs, i.e. how many larvae have
developed from the eggs.
The duration of the reproduction inhibiting effect of the test compounds is deterrnined by
monitoring the egg deposits of the beetles further, i.e. over a period of about 4 weeks.
Evaluation is made by assessing the reduction in the number of deposited eggs and larvae
hatched from them in comparison with untreated controls.
.. . i
.: ~
.; .
5'~
- 16-
The compounds of Example 1 exhibit good activity in this test.
Example 12: Action a~ainst Anthonomus ~randis tadults):
Two cotton plants in the 6-leaf stage, in pots, are each sprayed with a wettable aqueous
emulsion formulation containing 400 ppm of the test compound. After the spray coating
has dried (about l.S hours), each plant islpopulated with 10 adult beetles (Anthonomus
grandis). Plastic cylinders, covered at the top with gauze, are then slipped over the treated
plants populated with the test insects to prevent the beetles from migrating from the
plants. The treated plants are then kept at 25C and about 60 % relative humidity.
Evaluation is made after 2, 3, 4 and 5 days to determine the percentage mortality of the
beedes (percentage in dorsal position) as well as the anti-feeding action as compared with
untreated controls.
The compounds of Example 1 exhibit good activity in this test.
Example13: Insecticidalstomachtoxicantactiona ainstPlutellaxylostella:
Potted Chinese cabbage plants (pot size: 10 cm diameter) in the 4-leaf stage are sprayed
with aqueous emulsions which contain the test compound in a concentration of 0.8 ppm
and which dry on the plants.
After 2 days, each treated Chinese cabbage plant is populated with 11~ Plutella xylostella
larvae in the L2 stage. The test is carried out at 24C and 60 % relative humidity in dim
light. After 2 and 5 days evaluation is made to determine the percentage mortality of the
larvae.
The first compound of Example 1 effects 100 % kill in this test.
Example 14: Feed-inhibiting action againstslu~:
S red slugs (Arion rufus) are left for 17 hours under controlled test conditions in each of a
number of cages containing 4 fresh lettuce leaves. In these tests, each cage either contains
only untreated leaves or only leaves which have been treated by spray application. The
concentration of the test compound in the aqueous formulation applied is 0.5 % by weight.
The extent of the feeding damage is determined on the basis of weight differences,
photocopies of the feed picture and visible evaluation criteria in comparison with the
untreated controls.
2~63~7
- 17-
In addition, any dead test animals are counted to determine the mortality rate.
The compounds of Example 1 exhibit good activity in this test.
Example 15: Acaricidal contact action a~ainst Tetranychus urticae:
12 hours before the test for acaricidal action, Phaseolus vulgaris plants are infected with
an infested piece of leaf from a mass culture of Tetranychus urticae. The mobile stages
which have moved from the infested piece of leaf to the plants are sprayed with the
emulsified test preparations from a chromatography atomiser in such a manner that the
spray mixture cannot run off them. The respective emulsifiable formulations used have a
concentration of test compound of g00 ppm. After two and ten days, evaluation is made by
counting the number of living and dead larvae, adults and eggs under a stereoscopic
microscope; the result is expressed as a percentage.
During the "hold time", the treated plants are kept in greenhouse compartments at 25C.
The compounds of Example 1 exhibit good activity in this test.
Example 16: Ovicidal action against Tetranvchus urticae
Young bean plants are populated with females of Tetranychus urticae which are removed
again after 24 hours. The egg-infested plants are sprayed with an aqueous emulsion spray
rnixture containin~ 4~0 ppm of the test compound. The plants are then incubated for 6
days at 25C and evaluation is then made. The percentage reduction in the population
(% action) is determined by comparing the number of dead eggs, larvae and adults on the
treated plants with the number on the untreated control plants.
The compounds of formula I exhibit good activity against Tetranychus urticae in this test.
Example 17: Action a~ainst Panonvchus ulmi (OP and carb. resistant)
Apple seedlings are populated with adult females of Panonychus ulmi. After seven days,
the infested plants are sprayed to dlip point with an aqueous emulsion containing 400 ppm
of the test compound and cultivated in a greenhouse. After 14 days, evaluation is made.
The percentage Nduction in the population (% action) is deterrnined by comparing the
number of dead spider mites on the treated plants with the number of dead spider mites on
the untreated plants.
2~3~
- 18-
The compounds of formula I exhibit good activity against Panonychus ulmi in this test.
,, , ~,
..,:: -