Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
20~9~
CYCLIC AMIDOCARBOXY SURFACTANTS, SYNTHESIS AND USE THEREOF
Field of the Invention
The invention relates to surfactants and somewhat more particularly, to improvedamidocarboxy compounds, compositions containing such compounds, methods of
preparing such compounds and compositions, as well as methods of using such
compounds or compos-~ions in a wide variety of technically useful fields.
8ackaround of the Invention
A wide variety of surface active (surfactant) compounds are known and widely
used. Further, certain relatively specific phthalamate derivatives are at least nominally
disclosed in academic and patent literature. Some specific phthalamate derivatives have
been su3gested as being useful in plant growth regulator formulations, insect repellent
formulations, bactericidal, fungicidal, herbicidal formulations, additives for improving low
temperature flow characteristics of petroleum distillate fuels, solvent extraction formulations
for certain heavy metal ions, catalyst systems for polyurethane foam formulations,
additives for thermal recording materials, thickeners for silicone grease and oil-based
drilling muds, additives for water-insens~ive coatings, plastickers, etc. For example,
U.S. Patent 2,101,323 provides a scant disclosure of certain monoamides of particular
dicarboxylic acids including cyclic amidocarboxy compounds and various phthalamate
derivatives, solvent-based synthesis routes and a recitation of potential uses therefore.
Somewhat similarly, U.S. Patent 2,467,192 provides a disclosure of cer~ain ammonium
salts of ~amic~ acids, including various phthalamate derivatives, synthesis thereof and a
use therefore as a plast~cizer which may involve forming water-insensitive surfaces or
films. Numerous other, relatively remote, disclosures invoh ing phthalamic acids or
2~5~
phthalamate derivatives include the followin3 U.S. Patents: 2,6~4,249 (additive for
insecticidal compositions); 2,582,670 (additive for vulcanization activators); 2,742,496
(additive for rust and corrosion inhibitor formulations); 3,09~,286 (additive to screen-
clogging prevention and rust inhibition formulations); 4,211,534 (additive for improving low
temperative flow characteristics of petroleum fuel oils); 4,478,958 (additive to catalyst
systems for polyurethane foam formulations); etc.
In addition, so-called fast-breaking personal-care compositions involving vinyl
- polymers are disclosed in European Patent Application Number 02 68 164 A2. Further,
an oil-in-water hair/skin conditioning composition containing certain water-insoluble,
o unctuous oleaginous materials and a water-dispersable hydrated polyvalent metal salt (i.e.
Al), adjusted to a relatively low pH so as to invert to a water-in-oil emulsion when rubbed
onto hair/skin and provide a moisturizing or protective barrier is disclosed in U.S. Patent
4,551,330.
Further, U.S. Patents 3,928,432 and 4,544,726 disclose certain monomeric latex
polymerization surfactants, which during polymerization are chemically incorporated into
the polymers so as to prevent migration thereof and thereby reduc~ water-sensitivity of
the resulting polymeric mass.
However, while various amidocarboxy compounds, including certain fatty
phthalamates may be suggested in certain arts, and some fast-breaking emulsions may
be suggested for personal-care products as well as some latex polymerization surfactants
capable of providing water:insensitive films or coatings may be known, the surfactant arts
have not heretofore recognked the specific materials, synthesis routes and uses disclosed
2~1~Sg~
herein and which exhibit superior surfactant/emulsification characteristics useful in a wide
variety of technical applications including surfactants per se, caustic-stabile surfactants,
chlorine-stabile surfactants, fast-break emulsifiers for personal-care products, emulsifiers
for agricultural chemicals, domestic fabric soReners, destructible latex polymerkation
surfactants, domestic detergent additives, emulsifiers for portland cement and concrete,
floatationlbenefication additives for various mineral ores, additives for electroplating
and/or surface finishing baths for metal goods, additives for plaster, gypsum and
miscellaneous building materials, additives for enhanced oil recovery formulations, wetting
and lubricating surfactants for textile processing, pulp digestive additives, surfactants for
o polyurethane/ isocyanurate foam formulation, pour-point depressant for transporting
viscous petroleum oils, industrial surfactants for emulsifying a wide variety of oily materials,
as suspending agents for various particular materials, etc.
2~1~58~
Summary of the Invention
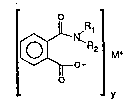
The invention provides surfactants of a general formula:
s ¦~ R2¦ M' (I)
wherein R~ and R2 are independently selected from the group consisting essentially of H
or C, - C~O linear or branched, substnuned or unsubstnuned alkyl, cycloalkyl, alkylene,
o alkaryl, aryl or R3-O-R~ groups, and mixtures thereof with R3 and R~ being independently
selected from the group consisting essentially of C, - C22 linear or branched, substituned
or unsubstituned alkyl, cycloalkyl, alkylene, alkaryl, and aryl groups; y is an integer of a
value satisfying the valency of M; and M is a cation; synthesis routes for producing such
surfactant at relatively high yields wnh little or no purification and methods of unilizing such
15 surfactants.
These surfactants are useful as surfactants per se, 8S caustic-stabile surfactants,
as chlorine-stabile surfactants, as cosmetic emulsifiers for personal care product, such as
skin creams, skin lotions, hair condnioners, etc, as fast-breaking skin-care product
emulsifiers; as emulsitiers for agrTcultural chemicais, as domestic fabric softeners; as
20 destructtble latex polymerizaUon surfactants in coaUng and/or adhesive systems, as
domestic detergent additives, such as in heavy-duty detergents, in light-duty detergents,
in dishwashing detergents, in various hard surface cleaners, etc.; as emulsifiers for
2~:19~8~
portland cement and concrete; as floatation/benefication additives for various mineral
ores; as additives for electroplating and/or surface finishing baths for metal goods; as
additives for plaster, gypsum and miscellaneous building materials; as enhanced oil
recovery additives; as wetting and lubricating surfactants for textile processing; as pulp
5 digestive additives; as surfactants for polyurethane/isocynaurate foam systems; as pour-
point depressants for transporting viscous petroleum oils; as industrial surfactants for
emulsifying a wide variety of oily materials; as low temperature stabilizers for fatty
alcohol/water emulsions; as suspending agents for various particulate material, such as
coal tar, sulfur or coal; etc.
Accordingly, it is an object of the invention to provide surfactants of the general
formula (I) above. In certain embodiments of this feature of the invention, Rl and R2 are
independently selected from the group consisting essentially of H or C~ - C30 linear or
branched, substituted or unsubstituted alkyl, cycloalkyl, alkylene, alkaryl, aryl or R3-O-R4
groups, with R3 and R~ being independently selected for the group consisting essentially
of Cl - Cæ linear or branched, substituted or unsubstituted, alkyl, cycloalkyl, alkylene,
alkaryl and aryl groups. In more preferred embodiments, Rl and R2 are independantly
selected from the group consisting essentially of H or Cl - C22 and more preferably are
selected from the group consisting of H or Cl - Cl8 alkyl groups. The cation, M, is
preferably selected from the group consisting essentially of Na, K, NH4 [including
(CH3CH2)3NH, (C~13CH2)2NH2, (HOCH2CH2)3NH, (HOCH2CH2)2NH2 and similar
ammonium derivatives], Ba, Ca, Mg, Al, Ti, Zr and mixtures thereof and even morepreferably is selected from the group consis~ing essentially of Na, K, NH4 [including
(CH3CH2)3NH, (CH3CH2)2NH2, (HOCH2CH2)3NH, (HOCH2CH2)2NH2 and similar
ammonium derivatives] and mixtures thereof. Specific preferred exemplary embodiments
of this feature of the invention include
sodium N-dodecylphthalamate,
5 sodium N-octadecylphthalamate,
sodium N-hexadecylphthalamate,
sodium N-tallowphthalamate,
sodium N-cocophthalamate,
- sodium N-isododecyloxypropylphthalamate,
sodium N-methyl-N-dodecylphthalamate,
sodium N-methyl-N-octadecylphthalamate,
sodium N-methyl-N-hexadecylphthalamate,
sodium N-methyl-N-tallowphthalamate,
sodium N-methyl-N-cocophthalamate,
mixtures thereof and their corresponding potassium, ammonium, triethylammonium and
triethanolammonium salts. Certain of the surfactants (I) above are caustic-stabile, i.e.,
function and do not break-down in relatively high pH environments, while certain of the
dialkyl surfactants are chlorine-stabile, i.e., function and do not break-down in
environments having a relatively high chlorine-ion concentration.
It is a further object o~ the invention to provide methods of producing surfactants
- of formula (I) above. In certain preferred embodiments of this feature of the invention,
molten phthalic anhydride may be brought into contact with at least a primary (R5NH2)
amine, wherein Rs is selected from the group consisting essentially of C, - C40 linear or
branched, substituted or unsubstituted alkyl, cycloalkyl, alkylene, alkaryl, aryl, or
~Q~8l~
R3-O-R4 groups, with R3 and R4 being independently selected from the group consisting
essentially of C1 - C22 linear or branched, substituted or unsubstituted alkyl, cycloalkyl,
alkylene, alkaryl and aryl groups, under reaction conditions conducive for formation of an
imide between the anhydride and the amine, and a nucleophilic source yielding a cation
5 selected from the group consisting essentially of Na, K, NH~ and mixtures thereof may be
added to the imide under reaction conditions conducive tc formation of the corresponding
alkali salts. In certain forms of this embodiment, a hydroxide of an alkaline-earth metal
may be added, after addition of the nucleophilic source, to form the corresponding
alkaline-earth salt. Further, in yet other forms of this embodiment, a mineral acid may be
o added after the formation of the alkali salt and thereafter a C1 - C4 alkoxide of a metal
selected from the group consisting essentially of Al, Ti, Zr and mixtures thereof or at least
one amine, may be added to form the corresponding salt. In other preferred
embodiments of this feature of the invention, molten phthalic anhydride may be brought
into contact with at least a secondary (R5R6NH) amine, wherein R5 and R6 are
15 independently selected from the group consisting essentially of C, - C40 linear or
branched, substituted or unsubstituted, alkyl, cycloalkyl, alkylene, alkaryl, aryl, or
R3-O-R4 groups with R3 and R~ being independently selected from the group consisting
essentially of Cl - C22 linear or branched, substituted or unsubstituted alkyl, cycloalkyl,
alkylene, alkaryl and aryl groups, under reaction conditions conducive for formation of
20 phthalamic acid between the anhydride and the amine, and a source yielding a cation
selected from the group consisting essentially of Na, K, NH~ [including (CH3CH2)3NH,
(CH3CH2)2NH2, ~HOCH2CH2)3NH, (HOCH2CH2)2NH2 and similar ammonium derivatives]
~01~4
Ba, Ca, Mg, Al, Ti, Zr and mixtures thereof may be added to the phthalamic acid so as
to form the corresponding salt. In yet other preferred embodiments of this feature of the
invention, phthalic anhydride may be brought into contact with a tertiary C~ - C40 amine
so as to obtain an admixture thereof and a primary or secondary (R5R6NH) amine,
s wherein R5 and R6 are independently selected from the group consisting essentially of H
or C~ - C40 linear or branched, substituted or unsubstituted alkyl, cycloalkyl, alkylene,
alkaryl, aryl, or R3-O-R~ groups, with R3 and R~ being independently selected from the
group consisting essentially of C, - C22 linear or branched, substituted or unsubstituted
alkyl, cycloalkyl, alkylene, alkaryl and aryl groups, may be added to the admixture under
10 reaction conditions conducive for formation of an alkyl phthalamate salt. The primary or
secondary (R5R6NH) amine may be added sequentially to or substantially simultaneously
with the tertiary amine.
It is a further object of the invention to provide a foodstuff emulsifier having the
general formula (I) above; as well as a foodstuff composition which may be comprised of
15 about 0.01 percent to about 5.0 percent by weight of the foodstuff emulsifier, and an
amount up to about 99.99 percent by weight of a food-grade material, as well as a
method of producing prepared foodstuff wherein select unprepared foodstuff ingredients
may be admixed with about 0.01 to about 5.0 percent actives of the foodstuff emulsifier
having the formula (I) above and subJecting the resulting admixture to preparation
20 conditions sufficient to produce a prepared foodstuff. In certain preferred embodiments
of this feature of the invention, the foodstuff emulsifier comprised of formula (I) above,
includes specie wherein R, and R2 are independently selected from the group consisting
essentially of H or Cl - C18 alkyl groups and mixtures thereof and the cation is sodium
or potassium.
2Q~9~84
It is yet a further object of the invention to provide an emulsifier for skin-care
products having the general formula (I) above; a solvent-tolerant skin-care composition
which may comprise about 0.01 percent to about 10.0 percent of a primary emulsifier
having the general formula (I) above, about 0.01 percent to about 10.0 percent of a
5 secondary emulsifier, and a sufficient amount of an emollient to attain a ratio of emollient
to emulsifier in the range of about 3:1 to about 30:1. In certain forms of this embodiment,
the skin-care compositions may be relatively salt-tolerant. In yet other embodiments of
this feature of this invention, the skin-care composition may exhibit a pH value in the range
of about 5 to about 12. In certain preferred forms of the emulsifier for skin-care products
lO having the formula ~I) above, R, and R2 are independently selected from the group
consisting essentially of H or C, - C,8 alkyl groups and mixtures thereof and M, the cation,
may selected from the group consisting essentially of Na, K, NH4 [including
(cH3cH2)3NH~ (CH3CH2)2NH2, (HOCH2CH2)3NH, (HOCH2CH2)2NH2 and similar
ammonium derivatives] Ba, Ca, Mg, Al, Ti, Zr and mixtures thereof and more preferably
15 may selected from the group consisting essentially of Na, K, NH~ [including
(CH3CH2)3NH, (CH3CH2)2NH2, (HOCH2CH2)3NH, (HOCH2CH2)2NH2 and similar- -
ammonium derivatives] and mixtures thereof.
It is yet a further obJect of the invention to provide a hair-care composition
comprising about 0.01 percent to about 10.0 percent of a conditioning surfactant having
20 the general formula (~) above, about 10 percent to about 25 percent of a hair-cleansing
material; and Q.S. 100 of an aqueous material. In certain preferred embodiments of this
conditioning surfactant, R~ and R2 are independently selected from the group consisting
201~
essentially of H or C, - C18 alkyl groups and mixtures thereof. Specific exemplary
embodiments of conditioning surfactants for hair-care products or compositions include
materials selected from the group consisting essentially of
sodium N-dodecylphthalamate,
5 sodium N-octadecylphthalamate,
sodium N-hexadecylphthalamate,
sodium N-tallowphthalamate,
sodium N-cocophthalamate, sodium N-isododecyloxypropylphthalamate,mixtures thereof
and their corresponding potassium, ammonium, triethylammonium and
10 triethanolammonium salts and mixtures thereof.
A particularly attractive object of the invention comprises applying to human skin
a relatively salt-tolerant, relatively solvent-tolerant, and relatively fast-breaking anionic oil-
in-water cosmetic composition having a pH in the range of about 5 to 12, with such
composition being comprised of about 0.01 percent to about 10.0 percent of a primary,
15 relatively high HLB emulsifier having the general formula (I) above; about 0.01 percent
to about 10.0 percent of a secondary, relatively low HLB emulsifier;-and a sufficient
amount of an emollient to attain a ratio of emollient to emulsifier in the range of about 3:1
: to about 30:1 at a select skin area; and rubbing the composition into the skin area for a
relatively brief period of time sufficient to break the composition and allow the emollient
20 to penetrate into the skin area. A somewhat similar embodiment of this feature of the
invention comprises conditioning or treating hair (to avoid tangles, snarls, etc.) by applying
to wet hair a shampoo composition comprised of about 0.05 percent to about 10 0
: percent of a water-dispersable anionic surfactant having the general formula (I) above;
11
about 10 percent to about 25 percent of a hair-cleansing material; and Q.S. 100 of
aqueous material; admixing such shampoo composition with the wet hair and rinsing the
resultant hair with water or aqueous solution so as to substantially remove any free
shampoo composition.
It is also an object of the invention to provide an emulsified oil-in-water composition
comprising about 0.01 to about 1G.0 percent of a relatively high HLB emulsifier having the
general formula (I) above; about 0.01 percent to about 10.0 percent of a relatively low
HLB emulsifier; up to about 80 percent of an oily material and Q.S. 100 water. The oily
material may be preferably selected from a group consisting essentially of crudeo petroleum oil, distilled petroleum oil, heavy paraffinic oil, asphaltene oil, linseed oil, tall oil,
soybean oil alkyd, linseed oil alkyd, mineral oil, petrolatum, isopropyl palmitate, isopropyl
myristate, caprylic/capric triglyceride, lanolin, acetylated lanolin alcohol, dimethicone,
hydrogenated vegetable oil, sesame oil, safflower oil, avocado oil, glycerine, propylene
glycol, sorbitol, C,2 - C,6 alcohol benzoates, cyclomethicone, dimethicone, cocoa butter,
vitamin E acetate, squalane, sodium pyrolidone carboxylic acid, methyl glucose ether,
panthenol, melanin, mixtures thereof, etc.
It is yet another obJect of the invention to provide an emulsifier having the general
formula (I) above, for agricultural chemicals and agricultural chemical compositions
comprising, in combination, an amount up to about 10 percent, by weight of the above
emulsifier and an effective amount of an active agricultural chemical in a suitable carrier,
such as an oil or an aqueous system. A feature of this embodiment of the invention
involves applying an active agricultural herbicide/pesticide to a growing crop so as to
12
2 ~
avoid post-application washoff and comprises producing a sprayable emulsion containing
an effective amount of an active agricultural herbicide/pesticide in a suitable carrier and
an amount of up to about 10 percent of a destructible surfactant having the general
formula (I) above; spraying such emulsions onto the growing crop; and subjecting the
sprayed emulsion to ambient temperature conditions over a period of time whereby the
surfactant chemically changes so that the resulting agricultural chemical composition
becomes substantially water-insensitive. Also, the desired chemical change may be
accomplished by subjecting the sprayed crop to a pH environment of less than about 5.
It is yet a further object of the invention to provide a fabric softener having the
o general formula (I) above, as well as a method of providing softness characteristics to
fabrics by adding an amount of such softener to fabrics undergoing cleansing in a
domestic washing machine, substantially unHormly dispersing the surfactant throughout
such fabrics and drying the fabrics. In certain preferred embodiments of this feature of
the invention, the surfactant may be added to the rinse cycle of the machine and in other
preferred embodiments of this feature, the surfactant may be added to the wash cycle of
domestic washing machines, along with a detergent composition. In certain preferred
embodiments of this feature of ~e invention, R, and R2 in formulation (I) above, may be
selected from the group consisting essentially of H or Cl - Cl8 alkyl groups and mixtures
thereof and the cation in the above formulation may be selected from a group consisting
essentially of Na, K, NH~ [including (CH3CH2)3NH, (CH3CH2)2NH2, (HOCH2CH2)3NH,
(HOCH2CH2)2NH2 and similar ammonium derivatives] and mixtures thereof.
13
`Q 4
A feature of this object of the invention is a fabric softener prepared by contacting
molten phthalic anhydride with a least a primary (R5NH2) amine wherein R5 is selected
from the group consisting essentially of C1 - C40 linear or branched, substituted or
unsubstituted alkyl, cycloalkyl, alkylene, alkaryl, or R3~-R4 groups, with R3 and R4 being
5 independently selected from a group consisting essentially of C1 - C22 linear or branched,
substituted or unsubstituted alkyl, cycloalkyl, alkylene, alkaryl, and aryl groups under
reaction conditions conducive for formation of an imide between the anhydride and the
. amine, and adding to the imide, a nucleophilic source yielding a cation selected from the
group consisting essentially of Na, K, NH4 and mixtures thereof to form a corresponding
10 alkali salt.
It is yet another object of the invention to provide a ~'destructible" latex emulsion
polymerization surfactant prepared by contacting phthalic anhydride with at least a tertiary
C, - C40 amine so as to obtain a mixture of the anhydride and the amine, and adding a
primary or a secondary (RsR6NH) amine to the admixture, with R5 and R6 being
` 15 independently selected from the group consisting essentially of H or C1 - C40 linear or
branched, substituted or unsubstituted alkyl, cycloalkyl, alkylene, alkaryl, aryl,~or
R3-O-R~ groups, with R3 and R~ being independently selected from a group consisting
`; essentially of C1 - C22 linear or branched, substituted or unsubstituted alkyl, cycloalkyl,
alkylene and aryl groups, under reaction conditions conducive for formation of an
"` 20 alkylphthalamate salt. In certain embodiments of this feature of the invention, a latex
polymerization surfactant is prepar0d by contacting molten phthalic anhydride with at least
~r a primary (RSNtl2) amine, wherein R5 is selected from the group consisting essentially
of C1 - C40 linear or branched, substituted or unsubstituted alkyl, cycloalkyl, alkylene,
20~9~
alkaryl, aryl, or R3-O-R4 groups, with R3 and R4 being independently selected from the
group consisting essentially of C, - C22 linear or branched, substituted or unsubstituted
alkyl, cycloalkyl, alkylene, alkaryl, and aryl groups under reaction conditions conducive
for formation of an imide between the anhydride and the amine, and adding to the imide
a source yielding a cation selected from the group consisting essentially cf Na, K, NH4
[including (CH3CH2)3NH, (CH3CH2)2NH2, (HOCH2CH2)3NH, (HOCH2CH2)2NH2 and
similar ammonium derivatives] and mixtures thereof to form a corresponding salt. In
certain preferred embodiments of this feature, Rs may be selected from the groupconsisting essentially of C, - C1a alkyl groups and mixtures thereof and the cation may be
o NH4 [including (CH3CH2)3NH, (CH3CH2)2NH2, (HOCH2CH2)3NH, (HOCH2CH2)2NH2 and
similar ammonium derivatives] and mixtures thereof. A feature of this embodiment of the
invention involves producing a stabile latex composition useful in various pigmented or
' unpigmented coatings or adhesive systems and which comprises preparing a substantially
uniform mixture of ethylenically unsaturated monomers, methacrylate esters, vinyl esters
and other known latex forming monomers, along w-nh initiators, base, water and a given
amount of a surfactant having the general formula (I) above; and subjecting such mixture
to an emulsion polymerization reaction so as to obtain a relatively stabile latex. Yet
another feature of this embodiment of the invention involves producing a latex polymeric
coating substantially devoid of water-sensitive ingredients and comprises the steps of
preparing a relatively stabile latex composition as set forth above, with or withoun desired
pigments, defoamers, antimicrobial agents, etc.; applying a coating of such latex to a
surface and
2 ~
subjecting the coated surface to drying conditions including a temperature in the range
of about 10 to about 300C for a period of time sufficient to chemically change the
surfactant within said coating to render such latex polymeric coating substantially water-
insensitive. A somewhat similar feature of this embodiment of the invention involves
5 producing an adhesive coating substantially devoid of water-sensitive ingredients by
prepering a relatively stabile latex composition as set forth above, with or without various
other known ingredients, such as alcoholic solvents, defoamers, antimicrobial agents, etc.,
applying a coating of the adhesive mass to a surface and subjecting the coated surface
to drying conditions including a temperature range of about 10 to about 300C for a
10 period of time sufficient to chemically change the surfactant within the coating to render
the adhesive coating substantially water-insensitive. In a particularly preferred
embodiment of this feature of the invention, a latex polymerization surfactant (which may
be utilized in protective coating systems, adhesive systems, etc.) may be prepared by
contacting phthalic anhydride with at least a tertiary C~ - C~0 amine with stirring so as to
15 obtain an admixture of the anhydride and the amine, and adding a primary or secondary
(R5R6NH) amine to the admixture, with Rs and R6 being independently selected from the
group consisting essentially of H or Cl - C~0 linear or branched, substituted or
unsubstituted alkyl, cycloalkyl, alkylene, alkaryl, aryl, or R3-O-R~ groups, with R3 and R4
being independently selected from the group consisting essentially of C, - C22 linear or
20 branched, substituted or unsubstituted alkyl, cycloalkyl, alkylene, alkaryl, aryl groups and
mixtures thereof, under reaction conditions conducivefor formation of an alkylphthalamate
salt. In certain preferred embodiments of this feature of the invention, R5 and R6 in the
16
8 ~
above amine may be independently selected from a group consisting essentially of H or
C1 - C,8 alkyl groups and mixtures thereof.
It is yet a further object of the invention to provide a method of transporting
relatively high viscosity crude petroleum oil which comprises forming a relatively low
5 viscosity emulsion from a select crude petroleum oil, and an emulsifler having the general
'ormula (I) above, and water, and transporting, as by a pump or truck, such emulsion to
a desired location, and, at such a location, subjecting such emulsion to conditions
sufficient to chemically change the emulsifler to "deactivate~ such emulsifier and thereby
break the emulsion and allow ready separation of the oil from the admixture. In certain
10 embodiments of the foregoing feature of the invention, conditions suffflcient to chemically
change the emulsifier so as to deactivate the same involve heating the emulsion to
temperatures of at least about 1 00CC. In yet certain other embodiments of this feature of
the invention, conditions sufficient to chemically change the emulsifler so as to deactivate
the same, involve adJusting the pH of the emulsion to a value below about 5. A further
5 preferred embodiment of this feature of the invention includes recovering the chemically
changed (deactivated) emulsifler and adjusting the pH of the so-recovered deactivated
emulsifler to a value above about 7.5 so as to "reactivate" said emulsifier for further use
in transporting crude petroleum oil.
It is a further object of the invention to provide an emulsifler/dispersant for crude
20 petroleum oil, with th~ emulsifler/dispersant having the general formula (I) above.
It is yet a further object of the invention to provide an improvement in the method
of scouring textiles which comprises admixing an amount up to about 50 percent of a
- 2(~5~4
wetting agent/emulsifier into a textile scouring bath with the wetting agents/ emulsifier
having the general formula (I) above. Somewhat similarly, it is the object of the invention
to provide improvements in treating textiles/fabrics wherein emulsifiers/lubricants/
softeners/dispersants and the like are utilized, such as in spin-finishing, fiber-finishing,
coning, winding, dyeing and the like processes or baths or the like, by adding to the
treatment process/bath a surfactant having the general formula (I) above.
It is yet a further object of the invention to provide an improvement in the method
of preparing pulp from a lignocellulosic material, such as wood chips, wherein chips or
the like are subjected to an alkaline liquor at select temperature, pressure and time
conditions to obtain a pulp having a select Kappa number range and comprises adding
s an amount up to about 10 percent of a surfactant having the general formula (I) above,
to the alkaline pulping liquor, discharging the pulping liquor and the at least partially
delignified lignocellulosic material and displacing the pulping liquor from the partially
delignified material with water or an aqueous liquor to obtain a pulp having the select
.. 15 Kappa number range. Of course, the above surfactants, per se or in an aqueous solution
may- be sprayed onto wood chips or the like prior to subjecting such chips to the alkaline
: liquor.
It is also an object of the invention to provide an improvement in the method ofproducing polyurethane/isocyanurate foam by admixing an amount up to about 10
percent of a surfactant having the general formula (I) above with a
polyurethane/isocyanurate foam-forming composition and forming a foam from the
admixture.
18
2~958'~
Other and further features, objects and embodiments of the inventions will be
apparent to those skilled in the art from the following description and claims.
Description of Preferred Embodiments
The invention provides surfactants, methods of producing such surfactants,
s compositions containing such surfactants and methods of utilizing such surfactants in a
variety of technical applications.
In accordance with the principals of the invention, surfactants of the present
invention comprise compounds having the following general formula:
¦~ R I
wherein R, and R2 are independently selected from the group consisting essentially of H
or C~ - C~0 linear or branched, substituted or unsubstituted alkyl, cycloalkyl, alkylene,
alkaryl, aryl or R3~R4 groups and mixtures thereof, with R3 and R4 being independently
selected from the ~roup consisting of C~ - C22 linear or branched, substituted or
unsubstituted alkyl, cycloalkyl, alkylene, alkaryl, aryl groups and mixtures thereof; y is an
integer of a value satisfying the valency of M; and M is a cation.
In certain embodiments of the invention, the R, and R2 moieties in formula (1)
above, are typically hydrocarbon moieties and may inciude moieties wherein one or more
of the carbon atoms in the respective carbon chains are replaced by a hetero atom, such
19
as, for example, oxygen and/or nitrogen. Further, in certain embodiments of the invention
the aromatic moiety in the above formulation may be substituted with various functional
groups, which include but are not limited to, halides, carboxylic acids and derivatives
thereof, nitro, nitroso, amines, amidos, hydroxyls, sulfonic acids and derivatives thereof,
alkyls, ethers, esters, nitriles and mixtures thereof.
In certain embodiments of the inventions, the cation, M, in formula (I) above, may
have a valency ranging from 1 to about 4 and may be selected from the group consisting
essentially of Na, K, NH4 [including (CH3CH2)3NH, (CH3CH2)2NH2, (HOCH2CH2)3NH,
(HOCH2CH2)2NH2 and similar ammonium derivatives], Ba, Ca, Mg, Al, r,, Zr and mixtures
o thereof. In certain preferred embodiments of the invention, M may be selected from the
group consisting essentially of Na, K, NH4 [including (CH3CH2)3NH, (CH3CH2)2NH2,(HOCH2CH2)3NH, (HOCH2CH2)2NH2 and similar ammonium derivatives] and mixtures
thereof.
In certain preferred embodiments of the surfactant having formula (I) above, R~ and
R2 may be independently selected from the group consisting essentially of H or C~ - C30
alkyl groups and more preferrably C, - C22 alkyl groups and M may be selected from a
group consisting essentially of Na, K, NH~ [including (CH3CH2)3NH, (CH3CH2)2NH2,(HOCH2CH2)3NH, (HOCH2CH2)2NH2 and similar ammonium derivatives] and mixtures
thereof.
2 o Specifically preferred embodiments of the surfactants having the formula (I) above,
include compounds wherein R~ and R2 may be independently selected from the groupconsisting essentially of H or C1 - C,8 alkyl groups and M may be selected from the group
2~95~
consisting essentially of Na, ~<, NH4 [including (CH3CH2)3NH, (CH3CH2)2NH2,
(HOCH2CH2)3NH, (HOCH2CH2)2NH2 and similar ammonium derivatives]. These
preferred compounds have many technical utilities, including as cleansers, emulsifers,
wetting agents, lubricants, surfactants, etc., in such diverse applications as caustic-stabile
surfactants useful, for example, in textile scouring baths or textile spin-finishing operations;
chlorine-stabile surfactants useful, for example, in hard-surface cleaners containing bleach,
or in liquid automatic dishwashing detergents, or in domestic laundry cleansing
formulations containing chlorine, etc.; as foodstuff emulsifiers useful in emulsifying various
edible oils and the like into foodstuff compositions, such as baked goods, mayonnaise,
dog/cat food, etc.; as emulsifiers for skin/hair-care products and useful in emulsifying
various emollients or conditioning/protective agents used in treating skin and/or hair; as
fabric softeners for treating domestic laundry; as industrial emulsifiers useful in emulsifying
various oily materials, such as crude petroleum oils, linseed oils, vitamin acetates, etc.,
including agricultural chemicals such as oil or water soluble or dispersable pesticides
and/or herbicides; as latex polymerization surfactants useful in producing water-insensitive
pigment and/or adhesive coatings, etc.; as pulp digestive additives; as polyurethane/
polyisocyanurate foam surfactant additives, etc. Exemplary specifically preferred
embodiments of the invention include:
sodium N-dodecylphthalamate,
20 sodium N-octadecylphthalamate,
sodium N-hexadecylphthalamate,
sodium N-tallowphthalamate,
sodium N-cocophthalamate,
sodium N-isododecyloxypropylphthalamate,
21
2 ~ 8 4
sodium N-methyl-N-octadecylphthalamate,
sodium N-methyl-N-hexadecylphthalamate,
sodium N-methyl-N-tallowphthalamate,
sodium N-methyl-N-cocophthalamate, mixtures thereof and their corresponding
5 potassium, ammonium, triethylammonium and triethanolammonium salts.
Embodiments of the i~vention include synthesis routes useful in manufacturing
surfactants of formula (I) above at relatively high yields, with little or no purification
r reqUirements.
In certain of these embodiments, molten phthalic anhydride may be contacted withe at least a primary (R5NH2) amine, wherein R5 is selected from the group consisting
essentially of C~ - C40 linear or branched, substituted or unsubstituted, alkyl, cycloalkyl,
alkylene, alkaryl, aryl, or R3-O-R4 groups, with R3 and R~ being independently selected
from the group consisting essentially of C, - C22 linear or branched, substituted or
unsubstituted, alkyl, cycloalkyl, alkylene, alkaryl and aryl groups, under reaction conditions
15 conducive for formation of an imide between the anhydride and the amine, and thereafter
adding to the resulting imidej a- nucleophilic source yielding an alkali (i.e. Na, K, NH4)
cation under reaction conditions conducive to the formation of the corresponding alkali
salt. In instances where an alkaline-earth metal salt is desired, a hydroxide of a desired
alkaline-earth metal may be added to the above alkali salt solution to form the
20 corresponding alkaline-earth metal (i.e., Ca, Ba or Mg) salt. Somewhat similarly, in
instances where an Al, Ti, and/or Zr salt is desired, the alkali salt solution may be
acidified, as by addition of a mineral acid, the phthalamic acid isolated and reacted with
8~
a C, - C4 alkoxide of Al, Ti, Zr, or mixtures thereof or at least one C1 - C4 amine may be
added to form the corresponding salt. Reaction conditions conducive to formation of the
imide and the desired salt generally include applying heat in the range of about 90 to
about 150C while stirring, preferably under an inert atmosphere, such as nitrogen, over
s a period of time, typically at least about 30 minutes and less than about 300 minutes.
During imide format;on, water may be removed and during salt formation water may be
added.
In certain other of these embodiments, surfactants of formula (I) above may be
:~ produced by contacting molten phthalic anhydride with a least a secondary (RsR6NH)
lO amine wherein R5 and R6 are independently selected from the group consisting essentially
of C, - C40 linear or branched, substituted or unsubstituted, alkyl, cycloalkyl, alkylene,
alkaryl, aryl, or R3-O-R4 groups, wherein R3 and R~ are defined as above, under reaction
conditions conducive for formation of phthalamic acid between the anhydride and the
amine, and thereafter adding a source yielding a cation selected from the group consisting
essentially of Na, K, NH4 [including ~CH3CHJ3NH, (CH3CH2)2NH2, (HOCH2CH2)3NH,
(HOCH2CH2)2HN2 and similar ammonium derivatives], Ba, Ca, Mg, Al, Ti, Zr and mixtures
thereof under reaction conditions conducive for formation of the corresponding salt.
In yat certain other of these embodiments, surfactants of formula (I) above, maybe produced by contacting phthalic anhydride with at least a tertiary C1 - C40 amine so as
20 to obtain an admixture, typically a substantially uniform admixture, and adding to such
admixture a primary or secondary (RSR6NH) amine, with Rs and R6 being independently
selected from the group consisting essentially of H or C1 - C~0 linear or branched,
23
2~5~
substituted or unsubstituted, alkyl, cycloalkyl, alkylene, alkaryl, aryl, or R3-0-R~ groups,
wherein R3 and R~ are defined as above, under reaction conditions conducive for
formation of an alkylphthalamate salt.
In certain reaction schemes, the foregoing steps may occur sequentially or
substantially simultaneously.
W7th ~e foregoing general discussion in mind, there will now be presented a
- number of detailed exemplary embodiments of the invention and workers skilled in the art
will appreciats that the following examples are non-limitive embodiments of the invention
and are included merely as specific exemplification of ths invention.
EXAMPLE I
Synthesis of Alkylphthalamate Wth Primary Amines
A number of alkylphthalamate surfactants are produced from phthalic anhydride
and various fatty primary amines in accordance with the following reaction scheme:
~O ~ R NH2 ~ ~N--Rs ~ Ma~ ] M
wherein Rs is selected from C, - C,0 linear or branched, substituted or unsubstituted alkyl,
cycloalkyl, alkylene, alkaryl, aryl, or R3~R~ groups, with R3 and R~ being independently
selected from C~ ar or branched, substituted or unsubstituted alkyl, cydoalkyl,
alkylene, alkaryl, and aryl groups and M is an alkali cation (i.e. Na, K, NH~, etc.).
24
8 ~
In all synthesis runs of this Example, select reactants may be placed in a suitable
reaction flask, which typically may be fitted with a stirrer, and Allihn condenser, a Dean-
Stark trap, a controlled inlet interconnected to a nitrogen gas source, a thermometer and
an addition funnel, or their equivalent. Phthalic anhydride may be placed in the reactor
5 flask, heated to a melt (about 131C), generally under a nitrogen blanket, and an amount
of a select fatty amine may be brought into contact with the molten phthalic anhydride.
Heating may be continued over a period of time ranging from at least about 30 minutes
to about 300 minutes, with removal of water aided by a nitrogen or other inert gas sparge.
Thereafter, deionized water may be added and the temperature of the reaction mixture
o may be adjusted to about 100C and a select nucleophilic source, i.e., a hydroxide,
yielding a desired cation may be added relatively quickly. This reaction mixture may be
heated with stirring for a period of time varying from at least about 30 minutes to about
300 minutes to obtain a high yield, typically about 90% or more, of an alkylphthalamate
without requiring purification. Of course, ff desired, purification may be effected.
Phthalic anhydride is a well-known chemical and is commercially available, for
example from Stepan Company, Northfield, Illinois, (assignee of the present invention).
Fatty primary amines are likewise well known and are commercially available from various
sources, including, for example, Aldrich Chemical Company.
Exemplary fatty primary amines include: alkyl, cycloalkyl, andtor alkylene amines,
20 such as hexylamine, cyclohexolamine octylamine, decylamine, dodecylamine,
tetradecylamine, hexadecylamine octadecylamine, eicosylamine, docosylamine,
cocoamine, tallowamme, and similar linear or branched, substituted or unsubstituted alkyl,
cycloalkyl or alkylene fatty amines; alkaryl amines, such as 4-methylaniline,
2,4-dimethylaniline, 3,4-diisopropylaniline, 3,5-dimethylaniline, and similar linear or
branched, substituted or unsubstituted alkaryl fatty amines; aryl amines, such as
aniline, 2-bromoaniline, 2-chloroaniline, 3-bromoaniline, 4-chloroaniline, 3,4-diethylaniline,
- 5 3,4,5-tribromoaniline and similar substituted or unsubstituted aryl fatty amines; fatty ether
amines, such as isododecyloxypropylamine, isodecyloxypropylamine,
hexyloxypropylamine, and similar fatty ether amine compounds.
Exemplary nucleophilic sources yielding alkali cations include: NaOH, KOH,
NH40H. Exemplary sources yielding alkaline-earth metal cation include: Ca(OH)2,
o Ba(OH)2, Mg(OH)2. Exemplary sources yielding Al, n, or Zr cations include:
Al[OCH(CH3)2]3, n(OCH2CH2CH3)4, Zr(OCH2CH2CH3)~ and similar alkoxide compounds.
Specific synthesis of sodium N-octadecylphthalamate, ammonium
N-dodecylphthalamate, potassium N-cocophthalamate, sodium N-dodecylphthalamate,
sodium N-isododecyloxypropylphthalamate, calcium N-octadecylphthalamate and
aluminum N-octadecylphthalamate will now be set forth and workers in the art will
recognize that similar syntheses may be performed to attain a desired alkyl, cycloalkyl,
alkylene, dialkyl, dialkylene, alkaryl, aryl, etc. phthalamate salt.
EXAMPLE IA
Sodium N-Octadecylphthalamate
Phthalic anhydride (1 mole, 148g) was placed in a two liter, three neck, round
bottom reaction flask fmed with a magnetic stirrer, an Allihn condenser, a Dean-Stark
trap, an inlet for nitrogen, a thermometer and an addition funnel. The phthalic anhydride
26
2~ ~5~
was heated under a nitrogen blanket to a melt (about 131C). Octadecylamine (1 mole,
278g) was added slowly over about 30 minutes to the molten phthalic anhydride. Heating
was continued at about 131C for about 3 hours, with the nitrogen blanket changed to
a nitrogen sparge to aid in the removal of water. A clear pale yellow liquid was obtained,
from which a small sample was withdrawn, allowed to solidify (off-white) and characterized
by IR spectroscopy (Beckman 983 spectrometer) as comprising N-octadecylphthalimide.
One mole (408g) of this reaction product, N-octadecylphthalimide, was transferred
into a twelve liter, 4-neck, round bottom flask fitted with a magnetic stirrer, an Allihn
condenser, an addition funnel and an inlet for connection to a nitrogen source (gas
cylinder). Deionized water (about 6.1 L) was added to the N-octadecylphthalimide and the
mixture was heated to about 100C to obtain a fairly homogenous mixture. Stirring was
then commenced under a nitrogen blanket and 1 N sodium hydroxide (about 1.1L, 10%
excess) was added relatively quickly over a period of a few minutes. The reaction mixture
was stirred with heating for about three hours. A clear light yellow reaction product was
obtained and a relatively small sample was removed, dried in a vacuum oven for about
1 hour (1 atm, 40C) and characterized by IR spectroscopy as comprising essentially
sodium N-octadecylphthalamate.
EXAMPLE lB
Ammonium N-Dodecylphthalamate
Ths above synthssis is, ssssntially, duplicated sxcept that dodecylamine is
substituted for octadecylamine and ammonium hydroxide is substituted for sodium
hydroxide. The final product is characterized by IR spectroscopy as cornprised essentially
of ammonium N-dodecylphthalamate.
~0~8~
EXAMPLE 1C
Potassium N-Cocophthalamate
The synthesis route of Example 1A above is repeated, except that cocoamine is
utilized in place of octadecylamine and potassium hydroxide is used in place of sodium
hydroxide. The final product is similarly characterized as comprising essentially potassium
N-cocophthalamate.
EXAMPLE 1D
Sodium N-Dodecylphthalamate
The synthesis route of Example 1A was essentially repeated, except that
dodecylamine was utilized in place of octadecylamine. The final product, straw yellow,
was characterized by IR spectroscopy as comprising sodium N-dodecylphthalamate. A
sample of the reaction product was removed and weighed 52.2980 g as about 11.3
percent solids in water. This sample was quantitively converted to the corresponding
phthalamic acid and using conventional calculations, it was determined that the percent
yield of the acid (or percent purity of the sample) was about 94.16 percent.
EXAMPLE 1 E
Sodium N-lsododecyloxypropylphthalamate
The synthesis route of Example lA was, essentially, repeated except that
isododecyloxypropylamine was used in place of octadecylamine and hydrolysis withNaOH occurred at about 70C, instead of 100C. The final product was characterized by
IR spectroscopy as comprising essentially of sodium N-isododecyloxypropylphthalamate.
~Ql~
EXAMPLE 1F
Calcium N-Octadecylphthalamate
A portion of the sodium N-octadecylphthalamate from Example 1A above is
acidified with a mineral acid, i.e., hydrochloric acid, to a pH of about 3 and filtered to
5 separate the semi-solid acid. This acid is then admixed with deionized water and a slurry
of calcium hydroxide is added, with stirring until a relatively stabile pH of about 8 or 9 is
attained. The final product is confirmed by IR spectroscopy as comprising essentially of
calcium N-octadecylphthalamate.
Similarly, the calcium salt may also be obtained by adding calcium hydroxide
10 directly to the alkali alkylphthalamate. Also a magnesium or barium salt may be produced
by substituting Mg(OH)2 or Ba(OH)2 for the Ca(OH)2 in the above neutralization
scheme.
Neutralization of alkyl and/or alkylene phthalamic acids may be accomplished in
water or in a non-aqueous solvent, such as methanol, isopropanol, or the like, by addition
15 of a suitable base (including various amines) to obtain a pH of about 8 or 9. When a neat
final product is desired, a low-boiling, non-aqueous solvent such as methanol, isopropanol
or the like may be used, with subsequent easy removal of the solvent by vacuum
stripping.
EX~MPLE 1G
20 Aluminum N-Octadecylphthalamate
Aluminum isopropoxide (0.0117 moles, 2.38g) and about 200 mL of isopropanol
were placed in a 1L four-neck, round bottom reaction flask provided with a nitrogen
29
2 ~ 8 ~
sparge, an Allihn condenser, a magnetic stirrer and a thermometer. This mixture was
heated to a temperature of about 78 C, with stirring for about 1 hour. Then
N-octadecylphthalamic acid (0.0353 moles, 15.0g)-obtained via the procedure of Example
lA and acidified with HCI to a pH of about 3 and filtered for separation - was slowly added
as a powder. The resultant mixture was heated at reflux (about 78~) for about 3 hours.
Upon conflrmation of the reaction product by IR spectroscopy, the isopropanol was
stripped-off under vacuum. A white solid final product was obtained.
EXAMPLE ll
Synthesis of Alkylphthalamates ~Ith Secondary Amines
A number of alklyphthalamates are produced from phthalic anhydride and various
fatty secondary amines in accordance with the following reaction scheme:
~ ' R~NH ~ R6: MOH ~
wherein Rs and R6 are independently selected from C~ - C~0 linear or branched,
substituted or unsubstituted alkyl, cycloalkyl, alkylene, alkaryl, aryl, or R3~-R~ groups with
R3 and R~ bein~ independently selected from C~ - C22 linear or branched, substituted or
unsubstituted alkyl, cycloalkyl, alkylene, alkaryl, and aryl groups and M is a cation
selected brom Na, K, NH~ [including (CH3CH2)3NH, (CH3CH2)2NH2, (HOCH2CH2)3NH,
(HOCH2CH2)2NH2 and similar ammonium derivatives], Ba, Ca, Mg, Al, T, 2r, and mixtures
thereof.
,, 30
The equipment and conditions for the synthesis runs of this Example are,
essentially, identical to those described in Example I above. (Except that the Dean-Stark
trap may be eliminated).
Phthalic anhydride is a well known chemical compound and is commercially
5 available, for example, from Stepan Company, Northfield, Illinois (the assignee of the
instant invention). Fatty secondary amines are likewise known and are commercially
available from various sources, for example, Aldrich Chemical Company.
Exemplary fatty secondary amines include: alkyl, cycloalkyl, and/or alkylene
amines, such as; N-dodecyl-N-methylamine, N-tetradecyl-N-methylamine, N-hexadecyl-
10 N-methylamine, N-octadecyl-N-methylamine, N-methyl-N-cyclohexalamine, N-coco-N-
methylamine, N-tallow-N-methylamine, N,N-dicocoamine and similar linear or branched,
substituted or unsubstituted alkyl, cycloalkyl, or alkylene fatty secondary amines; alkaryl
amines, such as; N-methyl4-dodecylaniline, N-methyl-4-octadecylaniline, N-methyl-4-
hexadecylaniline, N-methyl-4-tallowaniline, N-methyl4-cocoaniline and similar linear or
15 branched, substituted or unsubstituted alkaryl secondary amines; aryl secondary amines,
such as: N-methylaniline, N-propylaniline, and similar substituted or unsubstituted and aryl
secondary amines.
Exemplary sources yielding select M cations include: NaOH, KOH, NH40H,
Ca(OH)2~ Ba(OH)2~ Mg(OH)2, Al[ocH(cH3)2]3~Ti(ocH2cH2cH3)4~ Zr(OCH2CH2CH3)4
20 and similar sources.
2 ~ 8 ~
Specific syntheses of sodium N-dodecyl-N-methylphthalamate and
triethanolammonium N,N-dicocophthalamate will now be set forth and workers skilled in
the art will recognize that similar synthesis routes may be performed to obtain other
desired phthalamate salts.
EXAMPLE IIA
Sodium N-Dodecyl-N-methylphthalamate
Phthalic anhydride (0.10 mole, 14.8g) was placed in a 500 mL reaction flask
similarly configured and equipped to that described in Example 1A. The phthalic
anhydride was heated to a melt (131C) and N-dodecyl-N-methylamine (0.10 mole, 19.9g)
o was added to the molten anhydride over a period of about 15 minutes. Heating was
continued at about 131C for about 3 hours. A clear amber, viscous liquid was obtained,
which was characterized by IR spectroscopy as N-dodecyl-N-methylphthalamic acid. This
reaction product (about 30g) was diluted with methanol (ca. 50g) and neutralized with a
methanolic sodium hydroxide (1 N) to a pH of about 8.65. The methanol was then
stripped off using a rotary evaporator (water aspirator, 30C bath). A white powder was
obtained and was characterized by IR spectroscopy as essentially comprising of sodium
N-methyl-N-dodecylphthalamate. Methanol was utilized for sake of convenience in
producing a 100 percent active material. Water may be utilized if desired to obtain lower
active material Ot ff higher temperature, vacuum, etc., are utilized.
2 ~
EXAMPLE IIB
Triethanolammoniurn N~N-Dicocophthalamate
Phthalic anhydride (0.2 moles, 29.69) was placed in a reaction flask configured and
equipped similarly to that described above in Example IIA, heated to a melt and N, N-
dicocoamine (0.2 moles, 76.6 9) was added over a relatively brief time period. HeaUng
was continued at about 131C for about 3 hours. A liquid reaction product was obtained
and characterized by IR spectroscopy as comprising essentially N,N-dicocophthalamic
acid. About 309 of this acid was diluted with water (ca. 509) and neutralized with
triethanolamine to a pH of about 9. The water was then stripped-off as before and the
white paste obtained was characterized by IR spectroscopy as comprising essentially
triethanolammonium N,N-dicocophthalamate.
When a non-aqueous product is desired, neutralization can occur in a non-aqueoussolvent, such as methanol or isopropanol, which can then be removed after neutralizaUon
by vacuum.
EXAMPLE lll
Synthesis of Alkylphthalamate with Tertiary Amines
A number of alkylphthalamate surfactants may be produced from phthalic
anhydride and various fatty primary or secondary amines in the presence of a tertiary
amine in accordance with the following reaction scheme:
20~9~84
~0 I R5 NH R R R N I[~N R ¦
wherein Rs and R6 are independently selected from H or C, - C~0 linear or branched,
o substituted or unsubstituted alkyl, cycloalkyl, alkylene, alkaryl, cyclic including aryl, or R3-
O-R~ groups, with R3 and R~ being independently selected from C, - C22 linear orbranched, substituted or unsubstituted alkyl, cycloalkyl, alkylene, alkaryl and aryl groups
and R; R; R are independently selected from C, - C~0 linear or branched, substituted
or unsubstituted alkyl, cycloalkyl, alkylene, alkaryl, aryl or R3-O-R~ groups, with R3 and R~
being as defined above.
When a cation other than an ammonium derivative is desired, the above-obtained
reaction product may be acidifiad as set forth earlier, isolated, and then neutralized with
a suitable source yielding the desired cation, i.e. Na, K, Ca, Ba, Mg, ~1, n, Zr or mixtures
thereof.
The equipment forthe synthesis runs of this Example is, essentially, identical to that
described earlier, except that an ice bath may be required to control the exothermic nature
of this reaction.
34
2 ~ 8 ~
As indicated earlier, phthalic anhydride is commercially available from Stepan
Company, Northfield, Illinois. Primary and/or secondary amines are likewise known and
commercially available from various sources as set forth earlier. Tertiary amines are
5 likewise commercially available.
Exemplary primary and secondary amines are set forth above and exemplary
tertiary amines include: triethylamine, triethanolamine, trimethylamine, trimethanolamine,
N,N-dimethyl cyclohexylamine, N,N-dimethylaniline and similar alkyl, cycloalkyl, alkylene
alkaryl and aryl tertiary amines.
Specific synthesis of triethylammonium N-cocophthalamate,
N,N-dimethylcyclohexylammonium N-isododecyloxypropylphthalamate and
triethalonammonium N-isododecyloxypropylphthalamatewill now be setforth and workers
skilled in the art will recognize that similar synthesis routes may be followed to obtain
other desired phthalamate salts.
EXAMPLE IIIA
Triethylammonium N-Cocophthalamate
Phthalic anhydride (0.2 moles, 29.6g) and triethylamine (0.2 moles, 20.2g) were
introduced into a 500 mL, three-neck, round bottom flask, equipped with a magnetic
stirrer, Allihn condenser, an inlet for nitrogen, a thermometer and an addition funnel.
20 This mixture was stirred until a fairly uniform mixture was attained. Cocoamine (0.2 moles,
40.8 g) was then slowly added at a rate which maintained the temperature of the reaction
mixture below about 35C. After completion of the cocoamine addition, the mixture was
2 Q ~
stirred for about 1 hour while the temperature of the reaction mixture was maintained at
about 30C. A clear yellow liquid product was obtained, cooled to a pale yellow paste and
was characterized by IP as essentially comprising triethylammonium N-cocophthalamate.
EXAMPLE IIIA- 1
5 Triethylammonium N-Cocophthalamate
The procedure of Example IIIA above is repeated except that triethylamine and
cocoamine are added substantially simultaneously to the phthalic anhydride. The reaction
may be quite exothermic and an ice bath may be utilized to maintain control.
EXAMPLE IIIB
10 N.N-Dimethylcyclohexylammonium N-lsododecyloxypropylphthalamate
The procedure of Example IIIA was, essentially, repeated, except that
N, N-dimethylcyclohexylamine was substituted for triethylamine and
isododecyloxypropylamine was substituted for the cocoamine. The final product was
characterized by IR spectroscopy as comprising essentially of N,N-dimethyl-
lS cyclohexylammonium N-isododecyloxypropylphthalamate.
EXAMPLE IIIC
Triethanolammonium N-lsododecyloxypropylphthalamate
The synthesis route of Example IIIA was, essentially, repeated, except that
triethanolammonium was substituted fortriethylammonium and isododecyloxypropylamine
20 was substituted for the cocoamine. The final product was characterized by IR
spectroscopy as comprised essentially of triethanolammonium
N-isododecyloxypropylphthalamate .
36
2~9~84
E)(AMPLE A
Foaming and Wetting Characteristics
Standard Ross-Miles Foaming and Draves Wetting tests were preformed on
various fatty phthalamates with the results set forth in Table 1 below. In all tests, an active
5 level of 0.1% was utilized.
As may be surmised from the data presented in Table 1, the various sodium
alkylphthalamates tested exhibited better foam and wetting characteristics in deionized
water than in tap water. It is also apparent that the C,2 alkyl moiety exhibited the best
wetting and foaming characteristics within the alkyl moiety range tested. Further, the
10 alkoxypropylphthalamates appeared to exhibit better wetting characteristics in tap water
than in deionized water, with the inverse being true for foaming characteristics. For
example, it will be noted that sodium isododecyloxypropylphthalamate exhibited a wetting
time of 4.5 seconds with tap water and a foam height of only 5.5 cm. Thus, at least some
alkoxypropylphthalamates may have good utility in tap water applications (i.e. domestic
15 laundry detergents, etc.).
`-` 20.t9~8~
o o o o r~
d ~
~ ~ ~ ~ ~ O
o ~ -- ~ ~ _ . . . .
~_ :~ d :> ~ ~C _ ~ ~-- r~ ~ .
O "
O O ~ ~ N O
-- .~ o ~n o o -- o o o
~ _ , , , _ _ . . _
a ~ ~ O~ d ~ ~ ~
C ~ ~ ~ ~ ~ O ~ ~ ~ O
c O ~ . . . O N . . O
IY ~¦ 2 _ 0~ d
o
-
>
O
U o o o o o~~r
_ 3 N N~~ N O 1
_ ~ ~ ~~~ ~ I~_ N
U
O O
_
a o
c ~ O OO O ~ d ~ 1
NN O d N
~ ~ ~¦~ dN~ ~ N0.~ _ d N
G
C
o
O
O ~ O
-- C
-- -- ~ _ ~
a~ -- c
,o~ Co~ _
~ _-- ~.o. ~ ~ ~ C
o ~ ~ V~ o ~ O O
C C ~
. _ _ ~~o ~ ~ ~
Cc ~ o-- ~ o ~ ~ o
-- K
C ~ U O ~ O
~ ~~ C ~ -- u a
C _~ U U ~ ~ ~ ~
U ~ ,~ ~ U
U O O U O O U ~ ~ N
O ~--o CO~J ~ , -- -- ~ o o
.~ C ~ 2 ~ ~ O ~ "
~I! ~C e E E E E -- E e E ~
-- _O _ _ _ _ _ C ~
E ~ ~ O _ ~ .
o oooOOOOCoooC~
` O Ln
`
,' . ~ ~ .
8 ~
EXAMPLE B
Emulsion Stability Characteristics
In order to obtain reproducible results, an emulsion stability test, modified from the
standard used in the art for testing surfactants was developed. In the modified procedure,
5 0.59 of a select surfactant (instead of lg) was dissolved in 90 mL of deionized water in
a graduated cylinder containing 5.0 mL of xylene, which was then sealed and rocked
between a vertical/horizontal position for 30 cycles, sllowed to settle for about 5 to 10
minutes and subjected to the same rocking cycle with the results of the second sequence
recorded (instead of the first sequence).The results obtained for various
0 alkylphthalamates are set forth in Table ll below:
TABLE ll
~ompound Emulsion Break Rate (mL/sec)
Sodium N-octylphthalamate 0.111
Sodium N-decylphthalamate 0.034
Sodium N-dodecylphthalamate 0.068
Sodium N-tetradecylphthalamate 0.085
Sodium N-hexadecylphthalamate 0.102
Triethanolammonium N-octylphthalamate 0.043
~ Triethanolammonium N~decylphthalamate 0.046
20 Triethanolammonium N-dodeylphthalamate 0.039
, .
39
2019584
As is apparent from the above data, the triethanolammonium salts exhibited better
(i.e., slower) emulsion break rates relative to the sodium salts. However, in personal-
care formulations, (which are materially different systems from a xylene/water system)
the sodium salts appeared to perform as well or better than the triethanolammonium salts.
EXAMPLE C
Caustic Stability Characteristics
In order to determine caustic stability of the phthalamates, 5g of octylphthalamic
acid was dissolved in 450 mL of water containing an equivalent of NaOH. This mixture
was heated at reflux for about 24 hours and five separate 90mL samples were withdrawn
at 0 hours, 1 hour, 2 hours, 16.5 hours and 23.7 hours. The resulting individual samples
were acidified and the sodium N-octylphthalamic acid was isolated and analyzed (filtered,
dried, weighted and m.p. and neutralization equivalents were obtained). The results are
set forth in Table lll below:
v¦ N N N N NN N
U
o
E N U~ o 0~ 0
_ O O O O
~n OOOoo
_ O U~ O
11 ~ O~ 0
~ O
0 0~_ 0~_ O. O
~ 0 0 0 0 0
S 0 0 0 0 0
~ 0~ N N `O
.' C ~ ~ 0 ~0 ~
OOOOO
u~ t~ a
_ O _ N _ N
E
n
U>
I _ N 10
i
~19~
From the foregoing data it is apparent that alkylphthalamates are relatively stabile in a
caustic environment and that sodium N-octylphthalamate was stabile at a pH of at least
10.
EXAMPLE p
Thermal Stability Characteristics
Thermal stability studies were conducted with neat (100% actives) sample of
ammonium N-dodecylphthalamate mulled on sodium chloride plates. These plates were
then placed in an oven heated at about 100C for various time periods (0 to 30 minutes)
and thereafter characterized by IR spectroscopy. Conversion of a portion of the
0 ammonium N-dodecylphthalamate to the N-dodecylphthalimide was very apparent after
only 10 minutes, with almost total conversion to the N-dodecylphthalimide after only 20
minutes. These results are very similar to results obtained for various other
alkylammonium alkylphthalamates.
On the other hand, similar studies with sodium alkylphthalamates exhibited a
relatively higher degree of thermal stability. For example, sodium N-dodecylphthalamate
was stabile at 120C over about 28 hours, while sodium N-octadecylphthalamate exhibited
only a very small degree of imidation after 3.5 hours at 150C. Nevertheless, after 64
hours at 1 50C significant formation of N-octadecylphthalimide was noted. These results
show the destructible nature of these surfactants thermally.
; 20 EXAMPLE E
Solubility and Temperature Stability Characteristics
Sodium N-octadecylphthalamate, 1% actives, was evaluated for solubility in various
solvents and under cold storage (4C) and freeze-thaw conditions. The results are
summarized in Table îV below.
42
I
~u <I
o~7
~1 "
N
.' l oo _ ~
: ` ~ ~1
'`
0~ _ _ _ _ _ _ _ _ _ _ ~ _ _
O
~: -- ~ O
t ~ ~ ~ o U
~ ~ m m o o t s ~ o
.~
In o
9~
' l ' ' ~ ~
~ o~ C
~ , O
.. , ,~
. l ....
. ~ U
.. oo ~ ~ ~ " .
V
' i
,,
C ,
. , o , 1,
o ,.~ U -- ~ ~ ~ ' '
~1 ~ O _ N O 11-
~ ~ U -~
U7 O
- ~0~ ~84
1 NEOBEER M-5 = Stepan Company's registered trademark for caprylic/capric
- triglyceride.
2 NEOBEER M-20 = Stepan Company's registered trademark for propylene glycol
dicaprylate/dicaprate.
3 NEOBEER 18 = Stepan Company's registered trademark for safflower oil.
4 WECOBE~ = Stepan Company's registered trademark for hydrogenated
vegetable oil.
5 ALPHA-STEPR ML-40 Stepan Company's registered trademark for sodium alpha-
sulfo methyl ethyl laurate.
10 6 STEPANO~ WA-EX = Stepan Company's registered trademark for sodium lauryl
sulfate.
7 STEOLR CS-330 = Stepan Company's registered trademark for sodium laureth
sulfate.
8 NINOLR 40-C = Stepan Company's registered trademark for
N,N-diethanolcocomide.
9 AM MONYX R LO = Stepan Company's registered trademark for laurylamine oxide.
10 AMPHOSOLR CG = Stepan Company's registered trademark for cocamide propyl
betaine.
11 MAKOI~ = Stepan Company's registered trademark for nonylphenol
polyethoxylate.
12 STEPANATER X = Stepan Company's registered trademark for sodium xylene
sulfonate.
-- 2 ~ 8 ~
EXAMPLE F
Bleach (Chlorine lon~ Stability Characteristics
Select N-alkyl and N,N-dialkyl phthalamates were incorporated into a standard test
formulation containing a common bleach. Substantially identical formulations were
prepared with a commercially available surfactant promoted for bleach stability
'(DOWFAXR-2A1) and with a commercially available surfactant known to exhibit relatively
poor bleach stability 2(MAKONR-10), along with a control formulation (no surfactant). The
standard formulation comprised 4.0% actives of NaOCI; 0.25% actives NaCI; 1.0% actives
NaOH (50/O); 2.0% actives surfactant and O.S. 100% deionized water.
o The various formulations were individually placed in tightly capped jars and
subjected to a 90C water bath for about 5 hours and then observed for formulation clarity
and foaming characteristics. Each sample was run in duplicate. The control formulation
remained clear yellow throughout the test. The MAKONR-10 formulation was initially clear
yellow but exhibited clouding and phase separation at about 1.5 hours and showed no
foaming aRer about 5 hours. The DOWFAXR-2A1 formulation was clear yellow throughout
the test and exhibited stabile foam afler about 5 hours. A formulation containing sodium
N-methyl-N-dodecylphthalamate was likewise clear yellow throughout the test but the
foam was characterized as unstabile afler about 5 hours. On the other hand, a
formulation containing sodium N, N-dicocophthalamate exhibited poor solubility, with phase
separation and no pre-bath or post-bath foam. Further, a sodium N-dodecylphthalamate
formulation was initially clear yellow but became cloudy and exhibited phase separation
as the test progressed.
46
2~9~8~
1. DOWFAXRR-2A1 is Dow Chemical Company's registered trademark for
sodium branched C,2 diphenyloxide disulfonate.
2. MAKONR- 10 is Stepan Company's registered trademark for a nonylphenol
polyethoxylate.
It was concluded from the above that N,N-dialkylphthalamates, particularly
sodium N-methyl-N-dodecylphthalamate exhibited good bleach stability and better
than that exhibited by the MAKONR- 10 formulation.
.~ EXAMPLE G
Particulate Material Suspension Characteristics
In this test, potassium N-octadecylphthalamate was utilized as a suspending
agent in a typical shampoo base with known particulate anti-dandruff materials,
zinc pyrithione and coal tar. Particulate (about 500 mesh) zinc pyrithione and
particulate (about 350 to 400 mesh) coal tar were suspended in shampoo base
formulations and stored at room temperature, at about 1.6C and at about 43.3C.After 5 days the zinc pyrithione formulation exhibited phase separation at 43.3C
with pyrithione settling noted. On the other hand, the coal tar formulation did not
show phase separation and coal tar was successfully suspended and exhibited
excellent stability after 5 days at room temperature, at 1.6C and at 43.3C.
About 2 percent potassium octadecylphthalamate was incorporated with
about 50 to 70 percent particulate coal (average size about 200 mesh) and about
30 to 50 percent water at ambient temperature. The resulting admixture was a free
flowing, substantially homogeneous suspension which was easily pumped between
select locations.
This illustrates that small particle, powdered coal can be fluidized for
transport to storage areas or use areas, such as at coal-burning power plants.
EXAMPLE H
Emulsification Characteristics in Foodstuffs
Sodium N-octadecylphthalamate was added to a standard cup-cake recipe
;! at actives levels of 0% (control), 1%, 2%, 3%, 4%, and 5%. The N-octadecyl-
phthalamate was admixed into a typical batter as a partial replacement for the
shortening, margarine or butter called for in the recipe, baked at a uniform
temperature (about 176C) and time (about 25 minutes). After cooling, the various
cupcakes were examined, with the following results; 0% - tended toward dryness
and was crumbly; 1% - appeared and felt moister than the control, retained shapebetter and exhibited a denser interior; 2% - increased density, more rnoist; 3% -
similar to 2% sample except slightly sunken in middle; 4% - not fully cooked, more
` sunken in middle; 5,6 - further cooking required, sunken in middle.
A mayonnaise formulation containing about 1% by weight, based on a 100%
total weight of formulation, of sodium N-octadecylphthalamate was prepared, withthe N-octadecylphthalamate being substituted for egg yolk in a given recipe. A
fluffy creamy white emulsion formed as the salad oU was incorporated into the
formulation, which exhibited excellent stability.
48
:;
A dog food was prepared by admixing about 200g of ~Generic~ beef-flavored
dog food with 39 of 100% actives sodium N-octadecylphthalamate. Samples ofthis
dog food were exposed to various temperatures (li.e., 0C, 43C and 50C).
Heated samples, when cooled to room temperature, exhiblted no visible oily
5 pockets but after refrigeration for 24 hours and storage for 8 hours at room
temperature, showed visible oil pockets. However, aRer a further 24 hour storageat room temperature these samples showed no oil pockets.
- EXAMPLE I
Anionic Emuisification Characteristics for Personal-Care Formulations
: lo Four typical personal-care formulations were parepared wRh different
N-alkylphthalamates. In each instance, the ingredients listed under Part A were
- combined to produce a substantially uniform mixture with application of heat in the
range of about 74C to 77C (165F to 170F). Similarly, the ingredients listed
, under Part B were combined with heat so as to obtain a uniform mixture.
15 Thereafter, the Part A mixture was added to the Part B mixture, with good agXation
and continued heating to acheive a substantially uniform mixture. The pH of the
final mixture was then adJusted with citric acid (although other acids may be
utilked) to a pH in the range of aoubt 7.0 to 8.5. ~he resultant mixture was then
allowed to cool to about 35C (45F) and evaluated. As can be seen from the data2 o preserlted in Table V below, these surfactants exhibit excellent emulsion quality and
provide excellent performance quality.
49
2Q195~
< o o o . o o o U U
s I C~ _ O _ ~ a w w
., ~ :~
ot I : ,, _ O o o J J
o '~ ~ ~ ^ -- X X
~ ~ _ . o O
_
o J
~C I . ~0 N O O O L~ U
I O~1 _ o ~ N a O ~X~ X O
g 3 -
. . . . . . o U ..
. o ~, o
o '
.
J J
,, J ., " ~ o ~ ~
W ~ ~ o o o .
o ~ S ~ J ~~ X D ~. ~ ~ ~ ,
-- ~ J ~' O ~ ~ ~ W U~ J O
U ~ ~ ~ ~ -- -- ~ ~ L' -- ~
~ J K ~-- ~ J O O U J -- ~-- 'J
u c~ ~ o o O ~ O ~
'I -1 , ~
O Lt~
2 0 ~
EXAMPLE J
Rapid Breakina Emulsification Characteristics for Personal-Care Formulations
A number of N-alkylphthalamates were formulated into typical personal-care
compositions and evaluated.
The effective usage level of the N-alkylphthalamates in these compositions
is about 0.01% to about 10% by weight, with the preferred level of about 1.0% toabout 4.0% An effective pH range for these compositions is from about 6 to about10, with a preferred pH range being between about 7.6 to about 9.5. While
C, - C,0 chain lengths are useful, a preferred chain length is about C8 to C,8 and
o a more preferred chain length was found to be C,6, tallow (C,6 - C,8) and C,8. The
respective results are set forth in Tables Vl-A through Vl-C below.
51
1 -- -- ~
. . O ~ _ N ~ _ _
¦ V O o ~ _ N ~ C O
O ~ _ N _ O O N ~ ~ _
O N O _ _ O O ~ C 7
r O ¦ ~ , O _ O O ~ ~, c N O N
'1 - o o v o v u c~ o
O I _ O O 1~ ~ _ N ~0 ~1 _ _ O
N 1~ N O O > ~ O _ 7
"~ O 000 _ O O ~ 41 ~ _ _o
~ ~ o C
~ O 7 ~ O _~0
~U O ~ ~ o ~ O ~ C _ ~ ~ C
~ lc _
O U~ O
2019~
V~ o
, ..
~ ~ N
7 ~
~ ~ O
.. o~ ~
IU N
I~ O
o~ .
~ . O
O ~ O ~
"~ g O O~_ _N
O
t ~
O
O ~ o
~ ~ ~ O " ~U r ~
o _ , < ,.. .,
~ U ~
Q ~ o~ W ~ ~U
L~O ~- O
~ O ~ ~_ ~I ~
< ~uo Y ou. Ot ~ o ~ n
. ~. .
-- N 1'1~ V~ ~ ~ _ N
O U~
.
.~Q~95~
., , o ~ L
~ o o o ~n O ~ < ~ ~
o . O O ~ -- N 1-- ~ ~--
O N _ _ -- O O
< O
g _ ~ 0 0 o ~ o .~ O
o ~ ~~ -- N ~
_
L'l ~ 0 ,, , . O .
_ ~t ~n o o o ~ o
~ ~ ~' ~ ~ -- N
1l~ ~ o N _ _ _ o o v~
< C _ . O
_ g ~,~ o o o ~n o ~ m J L
O _ O O O r~-- N ~ ~ N
O N o -- _O o
O ~ n o
~
O N O ~ _ O O n W N
~ &
s ~ < O
~ :c s ~: &
.~ ~ O
C~ ~ U~_ & ~u o ~ ~
U ~ < ~ ~ ~ ~-
O ~ L~ X oO L~ L~O ~ ~,
-- ~ O -- o O O -- ~ t
O ' ~ ~ ~ ~ ~ ~ O ~ ~ ~ _
J ~ ~ ~ ~ ~ o L~O o _ ~ _
L~ L~~ o ~ _ ~ ~~
~u o o o o o ~ Y ~ ~ t L~ ~. ~ _ _
V~ Y Y ~ N J O _ _
O _ N S ~Y
O U7 0
2 ~ g ~
Various commercial personal-care products (skin-care lotions) suggest that
their formulations provide quick penetration into skin areas brought into contact
- therewith. It is known in the emulsion technology art that the faster breaking
emulsions are oil-in-water, nonionic or anionic soap-based emulsions which contain
CarbopolR (a registered trademark of B.F. Goodrich Company for certain
polymerked vinyl resins, see EPA 026816A2) as the primary emulsifier. CarbopolR
materials appear unstabile in the presence of salts (electrolytes) so that when
emulsion products containing such materials are applied to skin, which includes
salt from perspiration or the like, the emulsion breaks and emollients and the like,
in the product then quickly penetrate the skin. Emulsions without CarbopolR,
regardless of type (anionic or nonionic), do not break down as readily when
applied to skin.
The N-alkylphthalamates of the invention comprise excellent cosmetic
emulsifiers and are water dispersable (i.e., high HLB) emulsifiers. Hydrophilic-lipophilic balance or HLB Ts a widely accepted rneasure of the polarTty of a
surfactant and of its relative affinity for aqueous or hydrocarbon media. it is
generally held lsee, for example, NonTonic Surfactants, edited by Schick (MarcelDekker Inc., N.Y. 1967) pages 606 608] that HLB ranges for different surface active
functions are as follows:
Water-in^oil emulsifier 3 - 6
Wetting Agent 7 - 9
~9
'i
Oil-in-water emulsifier 8 -15
Detergent 13- 15
Solubilizer 15 - 18
The N-alkylphthalamates of the invention are characterked as being relatively high
5 HLB emulsHiers or primary emulsifiers having an HLB value in the ran~e of about
7 to 15, and when utilked to formulate cosmeUc emulsions are combined with
secondary or relatively low HLB emulsifiers, such as glyceryl stearate to produce
rapidly breaking, loosely emuls'~ied emulsions. The resulting optimal emulsions
were found to exhiblt excellent shelf-life stabil'ty while breaking down extremely
10 quickly when applied to skin. As shown in Table Vl-D below, emulsion
compositions containing a N-alkylphthalamate broke substantially quicker than
various commerdal products tested.
TA8LE Vl-D
EVAWATION OF BREAKDOWN PROPERTY
lS FOR VARIOUS PRODUCTS
BREAKDOWN TIME*
,PRODUCT TESTED (SEC)
FORMULATION J-11* 27
1VASEUNE INTENSIVE CARE LOTIONR 37
2SOFT SENSE LOTION~ 52
' 3KERI LOTION~ 47
JVISIBLE DlfFERENCE LOTIONR 52
5OIL OF OLA`tA 43
56
2 ~ 8 4
,,.
These tests were performed in triplicate and comprised washing select skin area
with soap and water, drying the washed skin arlea, applying; 0.1g of formulation;
rubbing until dry; and recording the elapsed time from initial rubbing until non-
tacky with no drag observed.
5 *See Table Vl-C
1. Registered trademark of Chesebrough Pond's Inc.
2. Registered trademark of S.C. Johnson & Son, Inc.
3. Registered trademark of Westwood Pharmaceuticals, Inc.
4. Registered trademark of Elizabeth Arden, Inc.
lO 5. Registered trademark of Olay Company, Inc.
Personal care product compositions (such as skin-care lotions or creams)
containing the surfactants of the invention exhibit excellent salt tolerance (i.e., up
to about 3% of sodium chloride can be incorporated into select compositions
without breaking the emulsions).
Further, typical emulsion compositions exhibit excellent solvent tolerance.
Stabile emulsion compositions having up to 20% SD~0 alcohol and/or SD-3A
alcohol have been successfully prepared.
57
The N-alkylphthalamate surfactants of the invention can be incorporated into
personal-care product compositions via an oil phase or a water phase. Final
emulsions are preferably prepared using a normal two phase hot process.
A wide variety of personal-care emollients, such as petrolatum light, mineral
s oil, isopropyi palmltate, caprylic/capric triglyceride, avacado oil, sesame seed oil,
safflower oil, corn oil, peanut oil, lanolin A~ deodorized, acetylated lanolin,
melanin, a variety of sunscreens and vitamins, etc., are readily emulsified by acombination of the relatively high HLB emulsifiers of the invention with a relatively
low HLB emulsifier, such as glycerol mono-stearate and a cosmetic emollient of
o choice so as to attain a ratio of emollient to emulsifier in the range of about 3:1 to
about 30:1.
EXAMPLE K
Hair Conditionina Characteristics
Sodium N-octadecylphthalamate was incorporated at 5%, 3% and 1%,
15 respectively, into a standard hair shampoo formulation. These formulations
comprised 12% active ammonium lauryl suHate, 2.5% N,N-diethanol cocoamide
and Q.S 100 deionked water, along with the above N-alkylphthalamate. These
three hair shampoo compositions were then evaluated on hair swatches by a panel
for wet compatibility and dry compatibility, along with three commercially available
20 hair shampoo formulations. Each swatch was rated from 1 (Worct) to S (best) and
the average resuits are set forth below:
58
28~ ~8~1
EORMULA~ON WETCOMBING D~YCOMBI~
5% PHTHALAMATE 4.28 3.72
3% PHTHALAMATE 2.28 3.44
1% PHTHAlAMATE 3.67 3.17
1PERT PLUS~ 3.70 3.70
2PRELLR 1.80 1.98
3SUAVE GOLDENR 1.39 2.64
1. Pert Plus~ is a registered trademark of Procter and Gamble Company for a
hair conditioning shampoo.
10 2. PrellR is a registered trademark of Procter and Gamble Company for hair
cleaning shampoo.
3. Suave GoldenR is a registered trademark of Helene Curtis, Inc. for a hair
cleaning shampoo.
As can be se~n from the above data the 5% phthalamate formulation readily
15 outperformed all three commercial shampoos. Additionally, the 3% and 1%
phthalamate forrnulations outperformed at least two of the commercial shampoos.
EXAMPLE L
Latex Polymerization Surfactant Characteristics
Latex or emulsion polymers, typlcally derived from ethylenically unsaturated
20 monomers, rnethacrylate esters, vinyl esters and similar known latex-forming
monomers are widely used ln a variety of appr~ons, such as binders for
59
2~ 9~8~
pigmented or unpigmented protective paints or coatings, as adhesive masses,
binders for paper coatings and for non-woven textiles, binders for carpet backing
materials and plastisols, binders for synthetic rubbers, as concrete or portiandcement additives, printing ink additives, floor polish and sim-~ar wax additives and
5 in other similarly related areas. Most conventional emulsion (iatex) polymers are
produced by an emulsion poiymerkation process wherein select monomeric
materials are poiymerked while they are dispersed in an aqueous medium via
surfactants. Such surfactants may be anionic in nature, such as soaps or varioussuHates or suHonates of various organic substances. Alternatively, such surfactants
10 may be nonionic, such as ethylene oxide derivatives of organic substances or
cationic, such as alkyl ammonium halides. Further, the polymerkation reactions
frequently are effected in the presence of water-soluble protective colloids or
stabilizing agents. Any one of the above emuls-~ying or stabileing agents may lead
to the presence of a water-sensitive materiai in the vinyl polymeric latex, which in
15 many instances is highiy undesirable. The art has long theorized that a means of
avoiding the presence of water-sensitive materials in a poiymeric latex mass is to
employ a fugi~ve or destructible surfactant. Such as surfactant would function as
an emulsifier and solubilker during the poiymerkabon process and as a stabilker
for the latex produced therefrom. Drying of such a latex, as in produdng a latex20 coatiny, espedaliy forced drying, would drive off or somehow chemicaliy destroy
or chan~e the surfactant and render the latex water~nsensitive. To date, necessary
2 ~
surface active properties have been found only in molecules too large to be volatile
so that destruction of the surfactant appears to be preferred. Some workers in this
art (i.e. see U.S. Patents 4,544,726 and/or 3,928,423) suggested surfactants which
are incorporated into the polymeric mass during the polymerization process so as
5 to render them incapable of migrating to the surface of the polymeric mass and
thereby reducing the water-sensitivlty of such polymeric mass. However, none of
these or similar materials have been completely satisfactory, largely due to the fact
that all remain water-sensitive, even as bound components in the polymeric mass.
As indicated earlier, the invention provides a new and useful class of latex
o polymerization surfactants having the general formula (I) above and which are
readily destroyed or chemically changed during application (coating/heating) so
as to render latex compositions containing such substantially water-insensitive. In
preferred embodiments of the foregoing surfactant, Rt and R2 may be
independently selected from the group consisting essentially of H or C, - C,8 alkyl
15 or R3-O-R4 groups and mixtures thereof, with R3 and R4 being independently
selected from the group consisting essentially of C, - C22 linear or branched,
substituted or unsubstituted alkyl, cycloalkyl, alkylene, alkaryl, aryl groups and
mixtures thereof, with the cation, M, being selected from alkali metals (including
ammonium and various amine derivatives) and alkaline earth metals. In even more
20 preferred embodiments, R~ and R~ may be independently selected from the group
consisting essentially of H or C, - C,8 alkyl moieties and M may be selected from
ammonium or an amine derivative thereof which boils below about 1 25C. An
,:
61
2 ~
exemplary preferred surfactant comprises triethylammonium N-cocophthalamate.
It is theorized and generally confirmed by infrared (IR) spectroscopy that the
Iatex polymerization surfactants of the invention, once subjected to drying
conditions, including a temperature in the range of about 10 to about 300C for5 a period of time undergo a chemical change and form an imide, which is
substantially water-insensitive and does not exhibit appreciable surface activity.
A number of exemplary latex compositions were prepared and subjected to
a water resistance test which involved dipping two microscope slides into each
Iatex composition and allowing the so-coated slides to dry at ambient conditions10 for 24 hours, while suspended in a vertical position. One of each pair received no
further treatment while the other slide of each pair was baked for about 15 minutes
in a circulating air oven maintained at about 150C and allowed to cool to room
temperature. All the slides were then immersed in deionked water for a period oftime of not less than three weeks and observed for adhesive failure (blistering,15 pulling away from the glass surface, etc.). The results are tabulated below in Table
Vll.
L~atex composition #1 was prepared by charging into a suitably set-up
reactor 205.5 9 of deionized water, 53.2 9 of dimethylethanolammonium
N-dodecylphthalamate (7.52% solids) and 2.0 9 of NH~OH and heated to about
20 70C with stirring under a nitrogen blanket. Thereafter, about 20% of a totalmonomer charge and all of an initiator charge tmonomer charge comprised of
100.0 g of butyl acrylate and 100 g of methylmethacrylate; initiator charge
2 ~ 8 ~
comprised of 0.2g of K2S2O8 and 20.0 g of deionized water) were added to the
reactor and allowed to react. Next, the remainder of the monomer charge was
added continuously over about 1 hour along with a separate, continuous and
concurrent addition of a feed comprised of 30.0 g of deionized water, 0.8 g of
K2S2O8 and 2.0 g of NH~OH, with continued heating at aoout 70 to about 75C.
This polymerization reaction mixture was heated for an additional 1 hour at about
70 to 75C with stirring. Thereafter, the finished and relatively stabile latexcomposition was cooled to ambient temperature and discharged for storage and
testing.
Latex compositions #2 was essentially similar to composition #1 above and
:~ was prepared in a substantially identical matter, except that triethylammmonium
N-dodecylphthalamate (about 5.44% solids) was used in place of the
dimethylethanolammonium N-dodecylphthalamate, along with minor adjustments
of water content.
Latex composition #3 was prepared in an essentially similar manner, of
substantially identical materials as that described in composition #1 except that
styrene was used in place of the methylmethacrylate monomer.
Latex composition i~4 was prepared in a substantially identical manner and
with substantially ider~tical materials to that of composition #2, except that styrene
was used in place of the methylmethacrylate.
Latex compo~itions #5, #6 and #7 were prepared in a substantially identical
manner and with substantially identical ingredients as described for composition
63
#1, except that in each respective formulation, sodium lauryl ether sulfate
containing 30 moles of ethylene oxide; sodium lauryl ether sulfate containing 4
moles of ethylene oxide and sodium lauryl ether sulfate containing 12 moles of
ethylene oxide were utilized in place of the dimethylethanolammmonium
N-dodecylphthalamate.
Latex composition #8 was prepared by charging into a suitably set-up
reactor 49.0 g of deionized water, 1.5 9 of K2S2O8 and 2.0 9 of triethylamine and
heated to about 80C with stirring under a nitrogen blanket. A pre-emulsion
composition comprised of 280.4 g of triethylammonium N-dodecylphthalamate,
o 2.0 9 of triethylamine, ~80.0 g of 2-ethylhexylacrylate, 90.0 g of methylmethacrylate
and 30.0 g of hydroxyethylacrylate, and an initiator solution comprised of 0.5 g of
K2S2O8 and 25.0 g of deionized water were made-up and each was separately,
continuously and concurrently added to the reactor over about 3 hours, with
continued heating at about 80 to 85C. The resultant mixture was held for an
additional 1 hour at about 80 to 85C with stirring. Thereafter the so-formed
stabile latex composition was cooled for storage and testing.
Latex composition #9 was somewhat similarly prepared by charging into a
reactor 81.9 g of deionked water, 2.5 g or triethylamine, 1.7 9 of triethylammonium
N-cocophthalamate (60.0% solids), 0.4 9 of sodium formaldehyde sulfoxylate and
0.4 9 of t-butyl hydroperoxide. A pre-emulsion composition comprised of 133.3
g of deionized waterl 8.0 g of tr~ethylamine, 13.2 9 of triethylammonium
N-cocophthalamate, 110.0 g of butyl acrylate, 82.0 9 of methylmethacrylate, 28.5g
64
2~9~8~
of hydroxyethylmethacrylate and 2.1 g of t-butyl hydroperoxide was prepared and
about 26 mL of this pre-emulsion composition was added to the reactor and
heated, with stirring, to about 63 to 65C under a nitrogen blanket. Once the
emulsion polymerization reaction began, the remainder of the pre-emulsion
composition and, as a separate feed, an initiator solution comprised of 2.1 g ofsodium formaldehyde sulfoxylate and 30.0 9 of deionized water were separately,
continuously and concurrently added to the reactor over a period of about 2 hours,
with continued stirring and heating at 63 to 65C. The resultant polymerizationmixture was heated for an additional 1 hours, with continued stirring. Thereafter,
o the formed stabile latex was cooled to ambient temperature for storage and testing.
Latex composition #10 was prepared from substantially identical ingredients
and in a similar manner as that described for composition #9, except that the
surfactant utilized was a mix of 50% triethylammonium N-dodecylphthalamate and
50% triethylammonium N-octylphthalamate (60.0% solids).
Latex composition #11 was prepared from substantially identical ingredients
and in a similar manner as that described for compositions #5 and #6, except that
sodium lauryl sulfate was utilized as a surfactant in place of the ethoxylated sodium
Iauryl sulfates.
Latex composition #12 was prepared by charging into a suitable set-up
reactor 73.5 9 of deionked water, 2.0 9 of triethylamine, 1.8 9 of triethylammonium
N-cocophthalamate ~60.0% solids), 0.4 9 of sodium formaldehyde sulfoxylate and
2Q~8~
0.4 9 of t-butyl hydroperoxide. About 20 mL of a pre-emulsion composition
comprised of 119.8 9 of deionized water, 7.0 9 of triethylamine, 14.7 9 of
triethylammonium N-cocophthalamate, 123.2 9 of butyt acrylate, 81.2 9 of styrene,
10.1 9 of methylmethacrylate, 32.2 9 of hydroxyethylmethacrylate and 2.4 9 of
5 t-butyl hydroperoxide, were added to the reactor and heated to about 63 to 65C,
with sbrrin~ under a nitrogen blanket. Once the emulsion polymerkabon reaction
began, the remaining pre-emulsion composition and an initiator solution comprised
of 2.4 9 of sodium formaldehyde su~oxylate and 35.0 9 of deionized water were
added, as separate feeds, continuously and concurrently ovsr about 2 hours, with10 continued stirring and heating at about 63 to 65C. The reactor charge was
heated at this temperature for an additional 1 hour, cooled and discharged for
storage and tesbng.
Latex composition #13 was prepared by charging into a suitably set-up
reactor 73.5 9 of deionked water 2.0 9 of triethylamine and 1.8 9 of
15 triethylammonium N~ocophthalamate (60.0% solids) and heating thTs mixture to
about 75C under an inert gas blanket. 0.2 9 of K2S208 was then added to the
reactor and, as separate feeds, a pre-emulsion composition comprised of 118.9
of deionized water, 7.0 9 of triethylamlne, 14.7 9 of triethylammonium
N-cocopthalamate (60.0% solids), ~23.29 of butyl acrylate, 91.3 9 of
2 0 methylmethacrylate and 32.2 ~ of hydroxyethylmethacrylate and an initiator solution
comprised of 1.0 9 of K2S20~ and 30.0 g of deionked water, were continuously and
concurrently added to the reactor over about a 2 hour period, with heating at about
75 to 80C and stirring. This reaction m~dture was heated at the above
temperature for an addiUonal 1 hour, cooled to arnbient and discharged for storage
and testing.
67
TABLE ~/11
LATEX CONDITIONS*
COMPOSITION BAKED NOT BAKED SURFACTANT
9** 2 dimethylethanolammonium
N-dodecylphthalamate
2 7 4 triethylammonium
N-dodecylphthalamate
3 8 2 dimethylethanolammonium
N-dodecylphthalamate
4 - - triethylammonium
N-dodecylphthalamate
1 1 sodium lauryl suHate
with 20 moles of E.O.
6 1 - sodium lauryl suHate
with 4 moles of E.O.
7 2 - sodium lauryl suHate
with 12 moles of E.O.
8 - - triethylammonium
' 20 N-dodecylphthalamate
` g 9 - triethylammonium
N-cocophthalamate
9 - 50/50 mix of triethylammonium
N-dodecylphthalamate and
triethylammonium
N-cocophthalamate
11 3 - sodium laurylsuHate
12 - - triethylammonium
N-cocophthalamate
30 13 - - triethylammonium
N-cocophthalamate
68
TABLE Vll CONTINUED
*CONDITIONS Baked = 150C for 15 minutes and water immersed for at least 3 weeksNot Baked = dry at ambient for 24 hours and water immersion for at least 3 weeks
** Numerical values assigned on a scale of 1 (worst) to 10 (best~, with 10 representing total
adhesion and 1 representing all or most of latex film lifted off of glass slide. Values in between
indicate various degrees of blistering, lifting, etc.
From the foregoing data, it was concluded that latex compositions containing the emulsion
polymerization surfactants of the invention consistently outperformed the more traditional latex
surfactants (in compositions 5, 6, and 7). It was also concluded that a dramatic difference in water
0 resistance in coating integrity existed between heat-treated and non-heat treated coatings containing
the inventive surfactants. This difference was attributed to the "destructible" nature of the inventive
surfactants whereby the N~alkylphthalamate, which exhibits surface active properties in water, when
subjected to drying conditqons, is chemically converted to N-alkylphthalimide, which is substantially
devoid of surface active properties and is substantially water insoluble. It was also noted that
15 dimethylethanolammonium N-dodecylphthalamate, triethylammonium N-cocophthalamate,
dimethylethanolammonium N-dodecylphthalamate and a 50/50 mixture of triethylammonium N-
dodecylphthalamate and triethylammonium N-cocophthalamate exhibited exceptionally good
emulsification properties during latex formation and, coatings containing these particular surfactants
exhibited excellent water-insensitivity after drying.
69
201.nl58
EXAMPLE M
Fabric Softening Characteristics
Sodium N-tallowphthalamate and triethanolammonium octadecylphthalamate were
evaluated as fabric softeners and compared against two commercially available fabric
5 softeners (ARQUADR 2HT and STEPANTEX~ 6~30B) in a convenUonal domestic laundrymachine having typical wash and rinse cydes. White cotton ~oop) terry cloth hand towels
were prepared for evaluation by CSMA methods DCC-13A and in DC~13B. The new
towels were stripped of mill textile conditioners by washing three times with a standard
detergent and 20 g of sodium phosphate, followed by five w ashings without detergent (all
10 washes were full cycle, hot wash, cold rinse). For each softener tested, as well as for a
' control (no softener), three towel bundles were added to either the wash cycle or to the
rinse cycde. The softeners, at 5 percent solids were dispersed in water and sdded to the
wash or rinse cycle at a use level at about 0.2 percent active soRener/weight of towel.
After completion of the wash and/or rinse cyde, the towels were dried in a typical
15 domestic dryer for about 50 minutes and evaluated for harshness/softness, with ratings
from 1 (harshest) to 3 (softest) and the average results are set for~h below:
SOFrENER AVERAGE.WASH CYCLE AVERAGE. RINSE CYCLE
1ARQUADq 2HT 2.5 3
2STEPANT~CR 6530B 2.3 2.8
Trlethanolammonium
N-octadecylphthalamate 2 2.1
sodiun N-tallowph~lamate 2 2.2
CONTROL
.
1. ARQUADR 2HT - a registered trademark of AKZO (ARMAK GROUP), Inc. for a
dimethylditallow ammonium chloride.
2. STEPANTEXR 6530B - a registered trademark of Stepan Company for a dialkyl
quaternary ammonium methyl sulfate.
s As can be seen from the above, the fabric so~teners of the invention (alkyl/alkylene
phthalamates) exhibited softening characteristics in both wash and rinse cycles, with
better results exhibited in the rinse cycle. Further, the fabric softeners of the invention
compared favorably with the commercial softeners tested.
EXAMPLE N
Reversible Crude Oil Emulsion/Dispersion/Suspension Characteristics
Sodium N-dodecylphthalamate, sodium N-cocophthalamate, sodium N-
isodecyloxypropylphthalamate and sodium N-isododecyloxypropylphthalamate, along with
a control (tap water only), were evaluated as emulsifying and/or dispersing and/or
suspending agents for a given, heavy (viscous) crude petroleum oil (Dome crude oil,
David Field, California) in different water samples, one having a hardness (expressed as
CaCO3) of 150 ppm, another having a hardness of 500 ppm, along with a synthetic sea
` water (NaCI-containing tap water, 150 ppm). In addition, various admixtures of sodium
N-dodecylphthalamate and lauryl amine oxide were similarly evaluated with the above
petroleum crude oil and synthetic sea water for synergistic emuls~lcation characteristics.
llle respective surfactants were prepared as aqueous solutions with the various
water samples at a concentration of about 0.1 percent actives. Measured samples
(50 mL) of the surfactant solutions were placed in 100 mL graduated cylinders, 2 drops
71
3 ~ ~ ~
of the crude oil added, the cylinders stoppered and relatively vigorously shaken manually
for about 10 to 20 seconds. The degree of emulsification/dispersion, suspension was
noted and recorded. The data is set for in Table Vlll below.
From the data observed, it was noted that some of the emulsions/dispersions or
5 suspensions did not appear very stabile and sometimes separated within minutes.
However, the lauryl amine oxide/sodium N-dodecylphthalamate solutions provided more
stabile emulsions which did not separate even after 2 hours. From the data obtained, it
was concluded that N-alkylphthalamates emulsified/dispersed crude oil in relatively hard
water and that the N-alkyletherphthalamates at least dispersed oil in relatively hard water
10 and in sea water. Additionally, it was concluded that there was a synergistic effect in
producing emulsions with a combination of an alkyl amineoxide and
N-alkylphthalamtes .
72
~0~9584
o ~ o
.. , , ~
;
2 ~
In order to demonstrate the "reversible~' nature of the emulsification properties of
the surfactants of the invention, the pH of a sodium N-dodecylphthalamate solution (0.1%
actives in 50 mL of 150 ppm hardness water) containing about 2 drops of the aforesaid
crude oil was repeatedly adjusted. At relatively high pH values (i.e., above about 7)
5 emulsification of the crude oil readily occurred and at relatively how pH values (i.e., below
about 5) de-emuls~lcation of the crude oil readily occurred. A control was prepared from
a sample of the 150 ppm hardness water without surfactant but with 2 drops of the above
crude oil.
Adjusting the pH of the control from about 13 to about 3 did not produce any
0 emulsions or dispersions. However, when the above N-alkylphthalamate solution was
adjusted to a pH of 8.5 the oil readily emuls-~ed. The pH of this emulsion was then
dropped by the addition of 1N HCI to a value of about 2.6 and upon agitation, the
emulsion broke, almost instantly, with floccing. The pH of this acidified mixture was then
raised by addition of 1N NaOH, to a value of about 13.4 and with agitation (about 10-15
15 seconds of manual shaking) an emulsified solution was noted. The foregoing cycle
between relatively high pH and relatively low pH was repeated an additional three times,
with essentially identical emulsification/de-emulsification results. During a low (4.9) pH de-
emulsification cycle, separated crude oil was removed, by decantation and a pH of the
`i remaining (used) phthalamic solution was adJusted to about 13.5 and fresh crude oil
20 added. Upon agitation, very stabile emulsion was quickly formed. The pH of this
emulsion was then dropped to about 7.0 without noticeable de-emulsification. The pH
was then further dropped to about 5.2, with a slight flocculation noted. The pH value was
74
2 ~ 8 4
then dropped to about 3.5 and the emulsion broke. Upon addition of additional fresh
sodium N-dodecylphthalamate, the emulsion re-formed.
From the foregoing dala it is concluded that N-alkylphthalamates and
N-alkyletherphthalamates are useful in emuls-~ying viscous petroleum crude oils so as to
5 enable such oils to be transported between select locations. Further, such emulsifications
can be readily de-emulsified by adjusting the pH of the emulsion to a value below about
pH 5 and the ~deactivated~ emuls~ier reactivated by increasing the pH thereof to a value
above about pH 7.
EXAMPLE O
10 Compatibilization/Surfactant Characteristics in Polyurethane/lsocyanurate S~stems
Various aromatic polyester polyols are utilized in a variety of polyurethane/
isocyanurate foam systems employed in, for example, production of laminated boards,
insulating foams, etc. During production of such foam systems, blowing agents such as
fluorocarbons, water, carbon dioxide, etc., are utilked and must be blended into the so-
15 called polymer blends. However, blowing agents are typically insoluble or non-dispersable
in these blends so that compatibilizing agents or surfactants must be included. For further
details see Wood U.S. Patent 4,595,711, (assigned to the Instant assignee and which is
incorporated herein by reference).
A typical polyol blend was formulated with no surfactant (control) and with three
20 different surfactants of the invention. ~he tNo sodium salts (defined below) were solids
while the triethylammonium salt was a liquld. The sodium salts were difficult to solubilize
in the polyol blend and did not appear to compatibilke the blowing agent into the resin
201~5~ 1
mixture. However, these sodium salts did not appear to interfere with the foam reaction
- and exhibited fair cell structures, friability, density (reactivity) and overall cell integrity (see
Table IX-A, below). On the other hand, the triethylammonium salt solubilized quite well
in the polyol mixture and demonstrated good compatibilization or surfactant characteristics
with the blowing agent tested (see Table IX-B, below).
76
2 ~
TABLE IX-A
COMPONENT FORMULATIONS: Ingredients as % by weight
Control #9 - 1 #9 - 2 #9-3
~POLYOL 100 9.5 9.5 92.5
PHTHALAMATE 0.00 *5.0 **5.0 ***7.5
2DABCO K-15 4.2 4.2 3.5 2.4
3PoLYCAT - 8 1.4 1.4 1.16 0.8
4B-8433 2.25 2.25 2.25 2.25
sR-11 47.0 47.0 40.0 23.0
ISOCYANATE 132.20 126.33 123.9 117.35
CREAMTIME, sec. 7 5 6 7
GEL TIME, sec. 11 20 23 26
TACK-FREETIME, sec. 20 21 n/a 28
END OF RISETIME, sec. 25 50 77 90
15 CuP DENSITY
(Ibs./cubic ft.) n/a 1.43 1.7 n/a
sCELL STRUCTURE E F F P
6FRIABILITY E F F F
61NTEGRITY E P F P
___________ _ _ _ _ _ _
20 *Sodium N-tallowphthalamate
**Sodium N-dodecylphthalamate
***Triethylammonium N-isododecyloxypropylphthalamate
~19~
TABLE IX-A CONTINUED
1. Polyol - prepared substantially in accordance with Example A of Wood, U.S. Patent
- 4,595,711 except that the hydroxyl number was adjusted to 185 and the final
product had a viscosity of about 22,000 centiposes at 25C.
5 2. DABCO K-15 - trade name designation of Air Products & Chemicals, Inc.,
Allentown, PA for potassium octoate.
3. POLYCAT - 8 - trade name designation of Air Products & Chemicals, Inc.,
Allentown, PA for dimethyl cyclohexylamine.
4. B - 8432 - trade name designation of Goldschmidt Chemical Corp., Hopewell, VA for a polysiloxane surfactant.
5. R-11 - Freon 11, tricholorofluoromethane obtained from E.l. du Pont de Nemours
& Company, Wilmington, DE.
6. Visual inspections, E = excellent, G = good, F = fair, P = poor
TABLE IX - B
Formulation
Control 9 4
1POLYOL 100 92.5
PHTHALAMATE 0 7.5
57R-11, max. soluble, g.9.10 24.7
:
1. ibid.
5. ibid.
7. R - 11 added to polyol in small increments until incompatibility was reached.
Endpoint is marked by a slight white/blue haziness, additions beyond this point
2 5 cause the mix~ure to become a mi~ky, opaque white.
78
9 5 8 ~
- From the foregoing data it is concluded that the N-alkylphthalamates of the
invention function as surfactants in polyurethane/isocyanurate foam systems. Further, it
is apparent that the longer chain N-alkylphthalarnate exhibited excellent compatibilization
characteristics with the blowing agent tested. Yet further, potassium salts of the inventive
5 surfactants are of potential interest since such appear to have a beneficial effect on the
catalysis of these foam systems.
EXAMPLE P
Industrial Oily Materials Emulsification Characteristics
Certain industrial oily materials, such as linseed oil, tall oil, soybean oil alkyd,
10 linseed oil alkyd, various cuts of distilled petroleum oil, heavy paraffinic oil, asphaltene oil,
etc., are useful in a wide variety of applications, such as in road construction, as resin
binders, etc., but are extremely difficult to manipulate due to their inherent viscosity,
adhesiveness, lack of spreadibility, etc. Attempts to emulsHy these oily materials have
met with only limited success and typically involved the use of a plurality of high HLB and
low HLB emulsmers and non-aqueous solvents which, at best, form non-stabile emulsions.
- For example, linseed oil is used in road constructions as a binding material prior
to repairing at least certain roadways. Linseed oil is an extremely sticky and heavy
material and is very dHficult to apply in a somewhat unHorm manner. Heretofore, linseed
oil, per se, or in combination with non-aqueous solvents, was loaded onto a specialized
20 truck having a heated tank maintained at a fairly high temperature (i.e, about 90 to
100C). The tank truck included feed pipes leading to spray nozzles extended along a
manifold to cover a given surface area (i.e., a road lane). The heated linseed oil was
2~ o~
pumped through the nozles, which in some instances were also connected to separate
emulsion/solvent solution storage tanks so as to briefly form an emulsion at the nozle
head. This emulsion was then sprayed onto the work surface for manual spreading.Once the linseed oil cooled down and/or the emulsion broke, the linseed oil became very
5 d~ficult to spread unHormly with manual spreading tools. As will be appreciated, with this
type of system, the spray nozles tend to dog-up and are difficult to clean and workers
are exposed to organic vapors and excessive heat.
In accordance with the principles of the invention, linseed oil was readily emulsified
'!, into an aqueous system utilizing the phthalamate surfactants (a relatively high HLB
emulsifier) in combination with a relatively low HLB emulsifier, such as glycerol stearates,
preferably glycerol monostearate, and water. The resulting emulsions were very easy to
spray and spread to a desired area. Emulsified linseed oil was stored over extended
periods of time without noticeable separation. Further, emulsified linseed oil does not
require high temperature for application, thereby providing an economical as well as an
5 environmental advantage.
Linseed Oil Emulsion Formulation
Component Wt.%
A. LINSEED OIL 60.0
SODIUM N-OCTADECYLPHTHALAMATE 2.0
1KESSCOR GLYCERYL 1.0
MONOSTEARATE PURE
B. WATER Q.S. 100
1. KESSCOR - Registered trademark of Stepan Company for various organic esters.
2 ~ 8 ~
Components A, above, were charged into a heatable vessel and heated with
agitation to a temperature of about 77 to 80C. Component B, above, was charged into
a separate heatable vessel and also heated to about 77 to 80C. Thereafter, component
A was added to component B with agitation and continued heating for about 30 minutes
5 or until the emulsion formed. Thereafter, agitation was continued and the emulsion was
allowed to cool to about 32C. Agitation was stopped and emulsion transferred for
storage and evaluation. Emulsion stability was evaluated at about 43C for a 1 month
; period, at room temperature for a 3 month period and at about 2C for a 1 month period.
In each instance, the emulsion was stabile and was easily applied onto glass slides.
lOSimilarly, other industrial oily materials can readily be emulsified by combining the
relatively high HLB phthalamate emuls7fiers of the invention with a relatively low HLB
emulsifier, up to about 80 percent by weight of an oily material and Q.S. 100 water.
As is apparent from the foregoing specification, the invention is susceptible of being
: embodied with various alterations and modifications which may differ particularly from
those that have been described in the preceding specification and description. For this
reason it is to be fully understood that all the foregoing is intended to be merely illustrative.
It is not to be construed or interpreted as being restrictive or otherwise limiting of the
present invention, excepting as it is set forth and defined in the hereto-appended claims.
81