Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
P~OCESS FOR GE~ERATING CHLORIC ACID
AND CHLORINE DIOXIDE
TECHNICAL FIELD
This invention relates to a process for the
production of chloric acid and chlorine dioxideO
BACKGROUND ART
Chloric acid is a strong oxidant which is an
intermediate in the production of chlorine dioxide which
in turn is used as a bleaching agent in the pulp and
10 paper industry. Chloric acid is formed through the
action of concentrated sulphuric acid solutions on sodium
chlorate. The process leaves sodium sulphate as a
by-product which may or may not be usable in other parts
of the pulp and paper process.
Ano-ther method of generating chloric acid is by
the action of a soluble chloric acid salt, (e.g. barium
chlorate) which forms a precipitable salt (barium sul-
phate) with a suitable acid (sulphuric acid). The method
is of laboratory interest only but a varia-tion of the
20 method could be used indus-trially. An ion exchange
membrane could be used as the "precipitable" salt and
then reyenerated with a suitable acid, (e.g. sulphuric
acid) after the passage of the chloric acid salt. The
salt (e.g. sodium sulphate) formed through the
25 regeneration process and present in the waste stream
again may or may not be usable in other parts of the pulp
and paper process.
Membrane systems involving stacked pairs of
membranes have been recommended for various applications.
30 All these applications appear to stem from a disclosure
in C.A., Vol. 53, 11070b (1959) (Oda et al) of two
' .'' " '
' ' ' ' - ' '
,
,
20~278
compartment electrodialytic water splitting of aqueous
neutral salts. Examples of individual applications in
two and three compartment cell configurations are Mani
and Chlanda (U.S. Patent Nos. 4,504,373 and 4,391,6~0),
Gancy et al (U.S. Patent Nos. 4,238,305 and 4,219,396),
Chlanda et al (U.S. Patent Nos. 4,116,889, 4,107,015 and
4,028,835), and Dege et al (U.~. Patent No. 4,024,043).
Neither the separation of chloric acid from sodlum
chlorate nor the generation of chlorine dioxide from
10 chloric acid either inside or outside a cell are
envisaged in any o~ this prior art.
Other literature describes the generation of
chlorine dioxide from sodium chlorate. All this
literature, however, describes the same initial step of
15 acidifying sodium chlorate with a strong acid before
various reduciny agents are applied to the resulting
solution. The step of initially generating chloric acid
separately in an electrochemical cell is not specified in
the prior ar-t.
By using -the process in this invention chlorine
dioxide can be formed with sodium hydroxide as the
coproduct. Sodium hydroxide cannot be yenerated as a
coproduct in any of the other methods described and
sodium hydroxide is a chemical, -the need for which is
25 presently high and will grow in the future.
DISCLOSURE OF l'HE INVENTION
This invention seeks to provide a method for
the production of chloric acid.
This invention also seeks to provide a method
30 for the production of chlorine dioxide. In accordance
with the invention it has been found that chloric acid
and an alkali metal hydroxide can be generated from an
alkali metal chlorate by using an electrochemical cell
comprising a three compartment water splitter employing a
cation membrane, an anion mem~rane and a pair oE bipolar
membranes.
It has also been found that -the chloric acid
above a certain concentration forms chlorine dioxide in
this type of cell stack and depending on the current use
and the desired concentration of the coproduct, very high
current efficiencies can be aehieved with such cell.
Thus in accordance with one aspect of the
invention there is provided a process for producing
ehloric acid from which ehlorine dioxide ean be generated
whieh comprises establishing an electroehemieal eell
having an anode and a eathode and at least one unit
disposed between the anode and eathode. The unit eom~
prises an aeid eompartment, a salt compartment and a base
eompartrnent. An aqueous alkali metal chlorate solution
is fed to the salt compartment and wa-ter is fed to the
acid and base compartments and a direct elec-tric current
is applied across the unit between the anode and cathode.
The salt eompartment is defined by an anion
permseleetive membrane and a ea-tion permseleetive
membrane. The acid eompartment is defined by the anion
permseleetive membrane and a first bipolar membrane whieh
has a eation portion faeing the aeid eompartment and an
anion portion faeing the anode. The base eompartment is
defined by the cation permselective membrane and a second
bipolar membrane which has an anion portion facing the
hase eompartment and a eation portion facing the eathode.
The establishmen-t of the flow of direet current
effeets several phenomena in the eell: alkali metal
eat.ions move or migra-te from the salt eompartment, in the
direetion of the eathode, through the eation perm-
selective membrane to the base compartment; chlorate
anions move or migrate from the salt compartment, in the
direction o~ the anode, through the anion permselective
membrane to the acid compartment; water dissociates
within the first and second bipolar membranes and hydro-
gen cations move or migrate through the cation portion of
the bipolar membranes, in the direction of the cathode,
and accumulate in the acid compartment, and hydroxide
anions move or migrate through the anion portion of the
10 bipolar rnembranes, in the direction of the anode and
accumulate in the base compartment.
In this way chloric acid derived from the
hydrogen ions and chlorate ions accumulates in the acid
compartment; and alkali metal hydroxide derived ~rom the
alkali metal ions and hydroxide ions accumulates in the
base compartmen-t.
In operation of the cell spent chlora-te
solution is removed from the salt compartment and chloric
acid and alkali metal hydroxide solutions may be with-
drawn from the acid and base compartments, respectively.As an alternative to withdrawing chloric acid frcm -the
acid compartment, the concentration of the acid may be
allowed to increase in the acid compartment until it
dissociates -to liberate chlorine dioxide which can then
25 be recovered from the acid compartment.
DESCRIPTION OF PREFERRED EMBODIMENTS
The cell suitably includes a mul-tiplicity of
the units be-tween the anode and cathode, in such case the
invention involves a process which includes the following
steps a) feeding an aqueous alkali metal chlorate
solution into a three compartment water splitter composed
of repeating anion, cation and bipolar membranes; this
.
.
.
7 ~
solution is introduced between the cation and anion
membranes (compartment l); b) feeding a water solution
into each compartment between a cation membrane and the
anion si~e of a bipolar membrane (compartment 2); c)
feeding a water solution into each compartment between an
anion membrane and the cation side of a bipolar membrane
(compartment 3); d) passing a direct current through the
water splitter thereby causing the transfer of alkali
me-tal cations and chlorate ions from the salt solution in
all compartments numbered 1), e) bleeding from compart-
ments numbered 2) an alkali metal hydroxide solution, f)
bleeding from compar-tments numbered 3) a chloric acid
solution which can then be fed to a conventional chlorine
dioxide generator.
lS The alkali metal chlorate solution, and the
alkali metal chlorate itself in step a) is, in parti-
cular, free or substan-tially free of alkali metal
chloride and more especially the solution consists
essentially of alkali me-tal chlora-te in water.
The addition of a reducing agent to the chloric
acid externally of the cell, for example, NaCl, HCl,
CH30H or S02 suffices for the formation of chlorine
dioxide from the chloric acid. Alterna-tively the chloric
acid solution in each compartmen-t 3) can be raised to a
high enough concentration in -the stack for chlorine
dioxlde to be formed directly, for this purpose it is
appropriate to raise the chloric acid content in each
compartment 3) -to a concentration of above about 1.0
molar.
The three compartment wa-ter split-ter referred
to in steps a) to e) incorporates a large number of
cation, anion and bipolar membranes arranged in an
2 ~ 7 ~
--6--
alternating fashion between two electrodes to provide
alternating base, acid and salt compartments that form an
electrodialytic stack.
sipolar membranes are composite membranes
consisting of three parts, a cation selec-tive reyion, an
anion selective region and an interface region between
the ion selective regions.
The bipolar membranes are permeable or porous
to neutral species, for example, water and consequently
water migxates from the acid and base compartments
through the ion selective regions to the interface
region.
When a direct current is passed across a
bipolar membrane with the cation selective side towards
lS the ca-thode, electrical conduction is achieved by the
transport of H and OH ions which are obtained from the
dissociation of water within the interface region.
Hydrogen cations migrate from the interface
region through the cation selective region in the
direction of the cathode, and hydroxide anions migrate
from the interface region through the permselective
region in the direction of the anode.
Hydrogen ions migrating to the cathode produce
hydrogen which is drawn off from the cell at the cathode,
and hydroxide ions migrating to the anode produce oxygen
which is drawn off from the cell at the anode.
The water splitter employs suitable bipolar
membranes, that can be of the type described, for
example, in U.S. Patent No. 2,829,095 to Oda et al. In
general, stacks that are suitable for electrodialysis can
be used for -the water splitter. Such stacks are avail-
2 ~ 8
able commercially from Asahi Glass Co., Chiyoda Ku,Tokyo, Japan; Ionics, Inc., Watertown, Massachuse-tts and
other commercial sources.
In general, for efficient operation, it is
preferred to establish an acid content in the acid
compartment and an alkali content in the base compartment
prior to applying the direct current. This is achieved
by introducing an acid and an alkali, respectively, to
the acid and base compartments.
Suitably the start-up acid for the acid com-
partment is chloric acid and the start-up alkali for the
base compartment is the same as the alkali to be
generated, however, this is not essential and may depend
on the intended use and purity required, in the products
of the cell.
In general it is preferred that the start-up
acid be solely chloric acid and that the start-up base be
solely the base which is to be genera-ted in the base
compartment, for example, sodium hydroxide.
As the alkali metal chlorate there is prefer-
ably used sodium or po-tasslum chlorate.
BRIEF DESCRIPTION OF DRAWINGS
The invention is illustrated in par-ticular and
preferred embodiments by reference to the accompanying
drawings in which:
FIG. 1 illustrates schematically an elec-tro-
chemical cell for use in the process of the invention;
FIG. 2 illustra-tes schematically a process
system in accordance with a preferred embodiment of the
invention; and
7 ~
FIG. 3 illustrates schematically a process
system in accordance with another embodiment of the
invention.
MODES FOR CARRYING OUT THE INVENTION
5The operation of the water splitter and process
of the invention is further described below by reference
to Fig. 1. The concentration of the solution of aqueous
alkali metal chlorate fed in-to the salt compartment of
the three-compartment cell may be as low as 0.3 molar and
lO as high as the saturation concentratlon for the parti-
cular salt. However a 2 to 5 molar solution is pre-
ferred. Solutions of low concentration should be avoided
because of diminished conductivity in such solutions.
The solution fed to the acid compartment
15 preferably contains more than 0.3 molar chloric acid and
is free of other acids such as hydrogen chloride.
Solutions of concentrations above 1.3 molar should be
used with care because of the reactions which genera-te
chloride dioxide from the chloric acid. This solution
20 may be a stream exiting from a chlorine dioxide generator
which will be depleted in chloric acid.
The solution fed to the base compartment
preferably contains alkali metal hydroxide, for example,
sodium hydroxide, preferably at a concentration between 1
25 and 5 molar. This concentration may be achieved by
recycling the stream until the desired concentration is
reached. The movement or migra-tion of the various ions
is illustrated schematically in Fig. l.
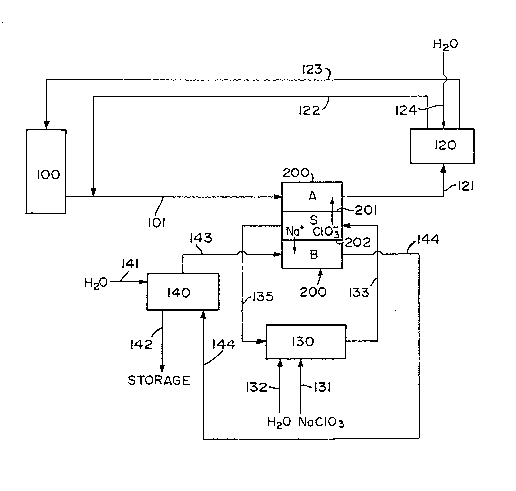
Fig. 2 schematically illustrates the preferred
30 embodimen-t of the process of the present invention which
uses a three compartment electrodialytlc water splitter.
A portion of the spent chlorine dioxide generator
~2~78
solution is taken from a generator 100 via line 101 to
the acid compartment A of a three compar-tment electro-
dialytic water s~litter. Alternatively if the process
described here is used to generate chlorine dioxide
directly the line from the generator 100 is excluded.
The three compartment electrodiaiytic water spli-tter has
unit cells defined by four membranes, including two
bipolar membranes 200, an anion permselective membrane
201 and a ca-tion permselective membrane 202 which form
acid A, salt S and base B compartments. Chlorate ions
rnigrate from the salt compartment S into the acid com-
partment A and therein combine with hydrogen ions
generated at the cation face of the bipolar membrane 200.
An aqueous chloric acid solution enriched in chloric acid
is removed from the acid compartment A via line 121 to a
reservoir 120 which has two lines coming from it. The
first line 122 leads back to line 101 while the second
line 123 leads to the generator 100. A third line 124
delivers water to the reservoir 120. The rates of flow
through the lines 101, 121, 122, 123 and 124 determines
the concentration of the solution in line 123 which is
either feeding the chlorine dioxide generator 110 or is
being taken to a stripper where chlorine dioxide
genera-ted in the stack is removed.
2~ Sodium chlorate is added via line 131 to the
salt compartment recycle tank 130 in the form of a solid,
slurry or aqueous solution. Make-up wa-ter, if necessary,
is added to recycle tank 130 via line 132. An aqueous
solution of sodium chlorate is removed from the recycle
tank 130 and forwarded via line 133 -to salt compartment
S. ~odium cations migrate through -the ca-tion perm-
selective membrane 202 from salt compartment S into base
compartment B and chlorate ions migrate from sal-t com-
2 ~ i 8
--10--
partment S through anion permselective membrane 201 to
acid compartment A. An aqueous sodium chlorate solution
containing a decreased amount of sodium chlorate is
removed from salt compartment S via line 135.
Water is added via line 141 to the base com-
partment B recycle tank 140. Two lines lead from the
tank. Line 142 leads to a storage tank ~not shown) ~rom
where the sodium hydroxide can be taken for use in the
mill processes. Line 143 leads to the base compartment
B. Sodium ions migrate from the salt compartment S
through the cation permselective membrane 202 where they
combine with hydroxide ions introduced at the anion face
of bipolar membrane 200 to form aqueous sodium hydroxide.
An aqueous sodium hydroxide solution containing an
increased amount of sodium hydroxide is removed from the
base compartment B via line 144.
General Experimental
Chloric Acid Genera-tion
The electrodialytic water splitter used in -the
experiments (Fig. 3) was a cell equipped at each end with
platinum electrodes 6 and 7 connected to a DC power
source. Several types of compartment were set up:
anolyte 1, base 2, acid 3, salt 4 and catholyte 5. The
compar-tments 2, 3 and 4 form a uni-t which was repeated 8
times. Each compartment was separated by ion exchange
membranes with an exposed area of 1000 cm2. Membranes 8
and 11 were Nafion (Trade Mark) 110 membranes manu-
factured by DuPont; 9 was a bipolar membrane manufactured
by Aquatech; and anion exchange membrane 10 waC~ com-
mercially available from Ionics Inc., under the code204-UZI,-386. Pumps 15, 16 and 17 were used to circulate
solutions through the cell. The anolyte/catholyte
.
reservoir 12 was charged with 0.5M Na~SO~. This solution
was circulated to the anolyte compartment as stream 26
and returned to the reservoir 12 via line 29 and to the
catholyte compartment as stream 27 and returned to the
reservoir via line 30. The base compartmen-t 2 was fed
from reservoir 13 by stream 35 and was returned to the
reservoir 13 via line 31. The salt compartment 4 was ~ed
from reservoir 14 by stream 36 and was returned to -the
reservoir 14 vla line 32. The acid compartment 3 was fed
from reservoir 19 by stream 37 and was returned to the
reser~oir 19 vla line 33.
Chlorine Dioxide Generation
The cell stack was allowed to run until the
chloric acid concentration increased to the point where
chlorine dioxide formed. Alterna-tively, chloric acid was
slowly hea-ted with a number of reducing agents in a glass
beaker.
Example 1
The salt tank was charged with 1 molar NaC103.
The acid tank contained 0.3 molar HC103 and the base tank
contained 0.3 molar NaOH. The circulation rates in the
base, acid and sal-t loops were 3 L per minute. The
voltage
has malntalned below 30 volts by varyin~ the cl rent. Table I shows
that over a pOEiod of 80 minutes, the conc~ntration o~ NaClO, in the
~alt loop was dlminished hhile the concentration of HClO, in the acid
loop increased. Llkewise the NaOH concentration in the base loop
lncreased.
TAELS I
Tlme, Current, Voltage, Concentration,
min A V moles~L
NaClO, HCl03 NaOH
7.3 2~.0 1.0 0.3 0.3
12.2 29.5 0.7 0.6 0.6
11.6 2g.5 0.5 0.8 0.8
9.3 29.0 0.3 1.0 1.0
The chloric acid solutlon was then treated with a number oE reducLng
agent The results are shown in Table II. To model the Holst proce~,
60 mL o~ LM Chlorlc acld WQg ~lowly heated while stirriny. During this
period 802 ~as bubbled through the ~olution. ~etween 40 and 50~C the
solutlon turned yellow indicatlng the productlon o~ chlorine dioxlde.
The colour lncreased with tlme and was confirmed to be due to chlorine
dioxlde by titratlon.
To mcdel the R3 process, the sa~e conditlons were used as ln the ~olst
proce s except that S~2 was replaced wlth the ~tolchlometrlc quantity of
NaCl. The solution turned yellow bet~een 50 and 60OC, a slightly hlgher
temperature than ln the Holst process model. The presence of chlorine
dioxide was again confirmed by titratlon.
The R8 proce~s was modelled as above e.Ycept methanol WQS the reductant.
Na~l was added to the ~olution to give a concPntration o~ 0.02 MVL a~
required by the R~ proce~- The solution turned yellow at 65~C.The
pre3ence of chlorlne dloxide was again confirned by tltration.
.
2 ~7~
-13-
TAELE II
ProcP s Reducing AgentTemperature at
which ClOz was
obse~ved,
oc
Holst SO~ 45
R3 NaCl 5~
R8 CH30H 65
Example 2.
10 The 5alt tank was charged with 1 molar NaCl03. m e acid tank contained
0.64 mol æ HClO, and the h~e tan~ contained 0.3 molar NaOH. The
circulation rates in the three loops were 3 L p~r rninute . The voltage
was maintained ~elow 30 ~olts by varying the current. Table ~II shows
that over a period of 120 minutes, the conc~ntration of ~ClO~ in the
15 acld loop lncreased until at a concentration of about 1.3 molesJL
chlorine dioxide was generated in the cell stack.
TAaLE III
Tlrne, Current, Voltage, Concentration,
min A V mole JL
H~O,
0 13.0 29.0 0.65
13.0 29.0 0.94
110 13.0 29.0 1.29
120 13.0 29.0 ClO~ formed