Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~033~
~ ~l
_, _
METHOD OF MANUFACTURE OF METAL MATRIX COMPOSITE
MATERIAL INCLUDING INTERMETALLIC COMPOUNDS
WITH NO MICROPORES
Background of the Invention
Field of the invention
'the present invention relates to a composite material, and more
particularly, to a method of manufacture of a metal matrix composite
material having high integrity of microstructure available by high
affinity between materials to compose the composite material and
generation of intermetallic compounds therein.
Description of the prior art
In Canadian Patent Application No. 597,974 filed by the same
applicant as the present application it has been proposed to manufacture
a metal matrix composite material in which aluminum, aluminum alloy,
magnesium or magnesium alloy forming a base matrix is reinforced by
micro reinforcing elements such as short fibers, whisker, particles or
mixture of these made of alumina, carbon silicate, nitrogen silicate or
the like, by first forming a porous preform from such micro
reinforcing elements, and then infiltrating the porous preform with a
melt of the matrix material, wherein the novel concept resides in that
a third powder material is incorporated as mixed in the reinforcing
micro elements in the process of forming the porous preform, said
third material being metal such as Ni, Fe, Co, Cr, Mn, Cu, Ag, Si, Mg,
AI, Zn, Sn, Ti or an alloy or alloys of these metals when the matrix
metal is A1 or A1 alloy, said third material being metal such as Ni, Cr,
Ag, Al, Zn, Sn, Pb or alloy or alloys of these metals when the matrix
metal is Mg Mg alloy, or said third material being oxide of metal such
as W, Mo, Pb, Bi, V, Cu, Ni, Co, Sn, Mn, B, Cr, Mg Al or mixture of
these when the matrix metal is Al, A1 alloy, Mg or Mg alloy.
According to this method of manufacture, the third powder
material expedites the infiltration of the molten matrix metal into the
interstices of the porous preform not only by the good affinity or
wettability of the third material itself with the molten matrix metal but
also by increased fluidization of the molten matrix metal due to the
heat generated by the reaction between the third powder material and
the molten matrix metal.
_2_
In various experimental researches on this method, however, it
was found that under certain manufacturing conditions there were
formed micropores in the composite material. For example, when a
composite material was manufactured by forming a preform consisting
of 596 by volume SiC particles (10 microns average particle diameter),
3096 by volume aluminum alloy powder (Al - 129b Si, 40 microns
average particle diameter) and 309:0 by volume pure copper powder (30
microns average particle diameter) and immersing the preform in a
melt of aluminum alloy (JIS standard AC8A) at 575 C° for 15 seconds,
inspection of its section under the optical microscope revealed
micropores in the composite structure which are guessed to have been
caused by imperfect wetting of the aluminum alloy.
Summary of the Invention
In the process of various experimental researches to seek
conditions to avoid the generation of such micropores it was found that
when a porous preform is formed of 6096 to 8096 by volume aluminum
or aluminum alloy, 196 to 1096 by volume nickel, copper, nickel alloy or
copper alloy and 196 to 109b by volume titanium or titanium alloy so that
the total percent by volume of such fragments is 6296 to 959:0, and such
preform is infiltrated with molten matrix metal such as aluminum, '
aluminum alloy, magnesium or magnesium alloy by at least a part of
said preform being contacted with a melt of such matrix metal, a
highly integrated metal matrix composite material having reinforcing
nuclei made of intermetallic compounds and including no micropores is
obtained with no application of pressure to the melt of the matrix
metal.
Accordingly, it is a first object of the present invention to provide
a method of manufacture of a metal matrix composite material having
a highly integrated composite structure reinforced with nuclei of
intermetallic compounds generated therein and including no micropores
therein.
It is a second object of the present invention to provide a method
of manufacture of a composite material in which a conventional
reinforcing material such as fibers, whisker or particles is in tight
contact with a matrix material which itself is further reinforced with
nuclei of intermetallic compound generated therein so that no voids are
left between the reinforcing material and the matrix as well as in the
body of the matrix.
~~z0~~~
-3-
The above-mentioned first object is accomplished according to the
present invention by a method of manufacture of a metal matrix
composite material comprising the steps of forming a porous preform
including 609'o to 8096 by volume fine fragments essentially made of
aluminum, 19o to 1096 by volume fine fragments essentially made of
nickel, copper or both, and 19o to 100 by volume fine fragments
essentially made of titanium so that these fine fragments occupy in
total 629:o to 95~ by volume of said preform, and contacting at least a
part of said preform with a melt of a matrix metal selected from
aluminum, aluminum alloy, magnesium and magnesium alloy, thereby
infiltrating said porous preform with said melt under no substantial
application of pressure to said melt.
Further, the above-mentioned second object is accomplished
according to the present invention by that said preform is formed
further to include dispersed reinforcing material.
Since the fine fragments essentially made of aluminum such as
pure aluminum or aluminum alloy have excellent affinity to the melt of
aluminum, aluminum alloy, magnesium or magnesium alloy, while since
the fine fragments essentially made of nickel, copper or both such as
Zp pure nickel, pure copper, nickel alloy or copper alloy have low
tendency to form oxides, these two kinds of fine fragments cooperate
to provide excellent wetting for the melt of aluminum, aluminum alloy,
magnesium or magnesium alloy in contacting with the fragments of
pure aluminum or aluminum alloy while protecting surfaces of the fine
fragments of pure aluminum or aluminum alloy from forming oxide
layer. Further, when a part of the preform , is heated by contact with
the melt of matrix metal, the aluminum in the fine fragments of pure
aluminum or aluminum alloy and the aluminum or magnesium in the
melt of matrix metal reacts with the nickel or copper in the fine
3p fragments of pure nickel, pure copper, nickel alloy or copper alloy so
that intermetallic compounds are produced with generation of heat
which fuses those fine fragments of pure aluminum o,r aluminum alloy
and pure nickel, nickel alloy, pure copper or copper alloy.
On the other hand, according to such generation of heat, the
titanium in the fine fragments of pure titanium or titanium alloy which
is highly reactive with nitrogen and oxygen at elevated temperature
absorbs air existing in the interstices of the preform so as to change
it into volumeless liquid nitrides and oxides, thereby expediting intimate
-4- ~~~0~~
contact of the fine fragments of aluminum, etc with the melt of
aluminum, etc..
Under such circumstances, when the volume proportion of the fine
fragments of pure aluminum or aluminum alloy is selected to be 60°yo to
8090 so as to leave a relatively low ratio of cavity in the preform, the
fine fragments of pure nickel, pure copper, nickel alloy or copper
alloy and the fine fragments of pure titanium or titanium alloy at such
ratio as 1'yo to 109'o by volume operate most effectively in protecting the
fine fragments of pure aluminum or aluminum alloy from oxidization
while decreasing the volume of air remaining in the spaces between the
fine fragments of aluminum, etc. so that the melt of aluminum, etc can
easily enter the spaces between such fine fragments.
According to the present invention, a satisfactory composite
material is available if the temperature of the melt of matrix metal is,
expressing the melting point of the matrix metal by T C°, in a range
of the temperature for coexistence of liquid and solid such as T -
T+50 C°. In this case, however, it is desirable that the solid
phase
proportion of the melt is not more than 70%, particularly not more than
509b.
The fine fragments of metals used in the present invention may
be in the form of powder, short fibers or whisker, and it is desirable
that their sizes are, in the case of powder, an average particle
diameter of 1 to 500 microns, particularly 3 to 200 microns, and in the
case of short fibers or whisker, an average fiber diameter of 0.1
micron to 1 mm, particularly 1 to 200 microns and an average fiber
length of 1 micron to 10 mm, particularly 1 to 200 microns.
Further, the reinforcing material used in the present invention
may be in the form of short fibers, whisker or particles, and it is
desirable that their sizes are, in the case of short fibers or whisker,
an average fiber diameter of 0.1 to 20 microns, particularly 0.3 to 10
microns and an average fiber length of 5 microns to 10 mm, ,
particularly 10 microns to 3 mm, and in the case of particles, an
average particle diameter of 0.1 to 100 microns, particularly 1 to 30
microns.
It is desirable that the content of nickel in the nickel alloy when
it is used in the present invention is at least 50% by weight,
particularly more than 80% by weight, and, although any elements other
than nickel, excepting inevitable impurities, may be included, they are
- 5 - z~~~~~~
particularly silver, aluminum, boron, cobalt, chromium, copper, iron,
magnesium, manganese, molybdenum, lead, silicon, tin, tantalum,
titanium, vanadium, zinc and zirconium.
Similarly, it is desirable that the content of copper in the copper
alloy when it is used in the present invention is at least 500 by weight,
particularly more than 80Xo by weight, and, although any elements other
than copper, excepting inevitable impurities, may be included, they are
particularly silver, aluminum, boron, cobalt, iron, magnesium,
manganese, nickel, lead, silicon, tin, tantalum, titanium; vanadium,
zirconium and zinc.
Similarly, it is desirable that the content of titanium in the
titanium alloy when it is used in the present invention is at least 500
by weight, particularly more than 80~ by weight, and, although any .
elements other than titanium, excepting inevitable impurities, may be
included, they are particularly aluminum, vanadium, tin, iron, copper,
manganese, molybdenum, zirconium, chromium, silicon, and boron.
Brief description of the Drawings
In the accompanying drawings,'
Fig. 1 is a perspective view of a preform comprising alumina
silica short fibers, aluminum alloy powder, pure titanium powder and
pure nickel powder; and
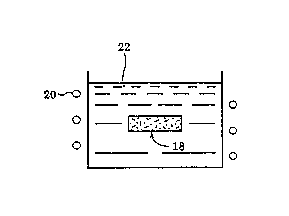
Fig. 2 is a sectional view schematically showing the preform
shown in Fig. 1 immersed in the molten aluminum alloy.
Description of the Preferred Embodiments
The present invention will now be described in detail with respect
to several preferred embodiments with reference to the accompanying
drawings.
Embodiment 1
Alumina-silica short fibers having 3 microns average fiber
3p diameter and 1.5 mm average fiber length (manufactured by Isolite
Kogyo KK), aluminum alloy powder (JIS standard AC8A) having 150
microns average particle diameter or aluminum alloy powder ( JIS
standard AC7A) having 100 microns average particle diameter, pure
titanium powder having 20 microns average particle diameter, and pure
nickel powder having 20 microns average particle diameter were mixed
in various proportions and subjected to compression forming to produce
preforms such as shown in Fig. 1 having 45 x 25 x 10 mm dimensions
and including the alumina-silica short fibers 10 at 0%, 59'0, 10%, 15~ or
-6- ~~~0~~5
200 by volume, the aluminum alloy powder 12 at 4096, 500, 60~, 700 or
8090 by volume, the pure titanium powder 14 at 096, 196, 59'0, 10~ or 159'0
by volume, and the pure nickel powder 16 at O~O, l~, 3~, 596, 796, 109'0
or 15°.o by volume, respectively, except such cases that the total
volume
proportion would exceed 9590.
Next, as shown in Fig. 2, each preform 18 was immersed in a
melt 22 of aluminum alloy (JIS standard ACBA) maintained at 570 C°
by a heater 20, was held there for 10 seconds, and then was removed
from .the melt, and then the molten metal infiltrated in the preform
was solidified without further treatment.
Next, each composite material thus formed was sectioned, and by
observation of the section, the penetration of the melt was investigated.
The results are shown in Table 1 and Table 2 in which <DOUBLE
CIRCLE> indicates that there were no micropores at all, <CIRCLE>
indicates that there were an extremely small quantity of micropores,
and <TRIANGLE> indicates that there were a small quantity of
micropores. Table 1 shows the results when the volume proportion of
the alumina-silica short fibers was OYo, 5~0, 100, 15~ or 2090, and the
volume proportion of the pure nickel powder was 0% or 150, and
Table 2 shows the results when the volume proportion of the alumina-
silica short fibers was O~O, 5~, 10~, 1590 or 20~, and the volume
proportion of the pure nickel powder was 196, 39'O, 5~, 7~ or 10~.
From Table 1 and Table 2 it will be seen that irrespective of the
composition of the aluminum alloy powder, it is desirable that the
volume proportion of the aluminum alloy powder is between 6096 and
800, and the volume proportions of the pure nickel powder and the
pure titanium powder are between 1~ and 10~, respectively.
Further, as a result of X-ray analysis of sections of those
composite materials indicated by <DOUBLE CIRCLE> in Table 2, it
was confirmed that the pure nickel powder had reacted almost
completely with aluminum so as to produce fine intermetallic
compounds such as NiAl3 and NiAI, that in the case where the volume
proportion of the alumina-silica short fibers was 09'o the aluminum
alloy matrix was compositely reinforced by these fine intermetallic
compounds, and that in the case where the volume proportion of the
alumina-silica short fibers was between 59'o and 209'o the aluminum alloy
matrix was compositely reinforced not only by the alumina-silica short
fibers but also by these fine intermetallic compounds.
._ _ 7 _ ~~2~J~J~
Fmfwiimnnt 7
596 by volume silicon carbide whisker (manufactured by Tokai
Carbon KK, having 0.3 micron average fiber diameter and 100 microns
average fiber length) as a reinforcing material, 709b by volume pure
aluminum powder (50 microns average particle diameter), 596 by
volume pure nickel powder (30 microns average particle diameter) and
5~ by volume pure titanium powder (30 microns average particle
diameter) were mixed and subjected to compression forming to produce
four preforms, and composite materials were manufactured in the
same manner and under the same conditions as in Embodiment 1, except
that the melts of matrix metal were aluminum alloy (JIS standard
A2024) at 550C°, 600C°, 650C°, 700C° and
750C°, and by observation of
sections of these materials, the penetration of the melt was
investigated.
As a result, it was confirmed that whatever the temperature of
the melt of matrix metal was, satisfactory composite materials were
formed with no the generation of rnicropores.
Embodiment 3
1030 by volume silicon carbide particles (manufactured by Showa
Denko HIC, 30 microns average particle diameter) as a reinforcing
material, 60~ by volume aluminum alloy powder ( JIS standard A2024,
150 microns average particle diameter), 89o by volume pure nickel
powder (30 microns average particle diameter), and 3°Yo by volume pure
titanium powder (30 microns average particle diameter) were mixed
and subjected to compression forming to produce preforms, and
composite materials were manufactured in the same manner and under
the same conditions as in Embodiment 1, except that the melt of matrix
metal melt was a semi-molten aluminum alloy (Al - 300 Cu) at a
temperature of approximately 550C°, and the immersion time of the
3p preform in the melt was 30 seconds, and then by observation of
sections of this material, the penetration of the melt was investigated.
As a result, it was confirmed that also in this embodiment,
satisfactory composite materials including no micropores were formed.
Further, as a result of X-ray analysis of sections of the
composite materials formed in Embodiments 2 and 3, it was confirmed
that the pure nickel powder had reacted almost completely with
aluminum so as to produce fine intermetallic compounds such as NiAl3
and NiAI, and that the aluminum alloy matrix was compositely
-s- ~~~~3~~
reinforced not only by the reinforcing material but also by these
intermetallic compounds.
Embodiment 4
1596 by volume alumina short fibers ("Safil RF" manufactured by
ICI, 3 microns average fiber diameter, 1 mm average fiber length) as
a reinforcing material, 65°Xo by volume aluminum alloy fibers
(manufactured by Aisin Seiki KK, A1 - 596 Mg, 60 microns average
fiber diameter, 3 mm average fiber length), 596 by volume pure nickel
fibers (manufactured by Tokyo Seiko KK, 20 microns average 'fiber
diameter, 1 mm average fiber length), and 1096 by volume pure titanium
fibers (manufactured by Tokyo Seiko KK, 20 microns average fiber
diameter, 1 mm average fiber length) were mixed and subjected to
compression forming to produce a preform.
Then, this preform was disposed within a die ( JIS standard No.
10) at 400C°, molten magnesium alloy (SAE standard AZ91) at
650C°
was poured into this die, and the preform infiltrated with the molten
magnesium alloy was cooled to room temperature under supply of
sulfur hexafluoride gas over the surface of the melt to prevent
oxidation of the magnesium alloy.
Then, the composite material thus formed was sectioned, and by
observation of sections of this material, the penetration of the melt
was investigated. As a result, it was confirmed that also in this
embodiment a satisfactory composite material including no micropores
was formed.
Further, as a result of X-ray analysis of sections of the
composite material formed in this embodiment, it was confirmed that
the matrix at a central portion was an aluminum alloy while the
matrix at peripheral portions was a magnesium alloy, that the nickel
fibers had reacted with aluminum so as to produce intermetallic
3p compounds such as NiAl3 and NiAI, that particularly at peripheral
portions the pure nickel fibers had reacted also with magnesium so as
to produce intermetallic compounds such as Mg2Ni and MgNi2, such
intermetallic compounds being higher in density toward outer peripheral
portions, and the matrix was compositely reinforced not only by the
reinforcing material but also by these intermetallic compounds.
Further, when a composite material was produced in the same
way except that the nickel fibers were replaced by the nickel powder
used in Embodiment 3 or the molten magnesium alloy was replaced by
_ 9 _ ~fl~fl~3~
molten pure magnesium at 680C°, in both cases satisfactory composite
materials including no micropores were formed.
Embodiment 5
7296 by volume pure aluminum powder (50 microns average
particle diameter), 69b by volume pure nickel powder (30 microns
average particle diameter), and 5°~o by volume pure titanium powder (30
microns average particle diameter) were mixed and subjected to
compression forming to produce preforms, and composite materials
were manufactured in the same manner and under the same conditions
as in Embodiment 1, except that the melt of matrix metal was an
aluminum alloy (JIS standard A2024) at 650C°.
Then, by observation of sections of the materials thus formed,
the penetration of the melt was investigated, and as a result, it was
confirmed that satisfactory composite materials including no micropores
1S were formed. Further, as a result of X-ray analysis of sections of
the composite materials, it was confirmed that the matrix at a central
portion and peripheral portions were substantially pure aluminum and
aluminum alloy, respectively, that the pure nickel powder had reacted
almost completely with aluminum so as to produce intermetallic
compounds such as NiAl3 and NiAI, and that the matrix was compositely
reinforced by these intermetallic compounds.
When in this embodiment the melt of matrix metal was replaced
by a pure magnesium melt at 680C°, the composite material formed in
the same way had again a satisfactory composite structure including no
micropores.
Fmfwiimonf /,
Composite materials were formed in the same manner and under
the same conditions as in Embodiment 1, except in that the pure nickel
powder was replaced by pure copper powder having 30 microns average
particle diameter, and by investigation of sections of the composite
materials thus formed, the penetration of the melt was investigated.
The results obtained were similar to those obtained in
Embodiment 1. In other words, regardless of the composition of the
aluminum alloy powder, it is desirable that the volume proportion of
the aluminum alloy powder is between 60 and 80~, and the volume
proportion of each of the pure copper powder and the pure titanium
powder is between 1 and lOYO, respectively.
Further, as a result of X-ray analysis of sections of the
- to - ~~2~33~
composite materials thus, it was confirmed that the pure copper
powder had reacted almost completely with aluminum so as to form
intermetallic compounds such as CuAl2, that when the volume proportion
of the alumina-silica short fibers was 0~, the aluminum alloy matrix
was compositely reinforced by these intermetallic compounds, and that
when the volume proportion of the alumina-silica short fibers was
from 59o to 200, the aluminum alloy matrix was compositely reinforced
not only by the alumina-silica short fibers but also by the intermetallic
compounds.
Embodiment 7
Composite materials were formed in the same manner and under
the same conditions as in Embodiment 2, except that the pure nickel
powder was replaced by pure copper powder having 30 microns average
particle diameter.
As a result, it was confirmed that at all temperatures of the melt
of matrix metal satisfactory composite materials were obtained with no
generation of micropores.
Embodiment 8
Composite materials were manufactured in the same manner and
under the same conditions as in Embodiment 3, except that the pure
nickel powder was replaced by pure copper powder having 30 microns
average particle diameter.
As a result, it was confirmed that in this embodiment also
satisfactory composite materials including no micropores were formed.
As a result of X-ray analysis of sections of the composite
materials formed in Embodiment 7 and Embodiment 8, it was
confirmed that the pure copper powder had reacted almost completely
with aluminum so as to form intermetallic compounds such as CuAl2,
and that the aluminum alloy of the matrix was compositely reinforced
3Q not only by the reinforcing material but also by these intermetallic
compounds.
Embodiment 9
A composite material was manufactured in the same manner and
under the same conditions as in Embodiment 4, except that the pure
nickel fibers were replaced by pure copper fibers (manufactured by
Tokyo Seiko KK, 20 microns average fiber diameter, and 1 mm
average fiber length), and by observation of sections of the composite
material thus formed, the penetration of the melt was investigated.
-11-
As a result, it was confirmed that also in this embodiment a
satisfactory composite material including no micropores was formed.
Further, as a result of X-ray analysis of sections of the
composite material thus formed, it was confirmed that a central
portion of the matrix was aluminum alloy while peripheral portions of
the matrix was magnesium, that the pure copper fibers had reacted
with aluminum so as to form intermetallic compounds such as CuAl2,
that particularly in the peripheral portions the pure copper fibers had
also reacted with the magnesium so as to form fine intermetallic
compounds such as MgCu2, and that the proportion of these
intermetallic compounds was higher toward the peripheral portion.
Thus it was confirmed that the matrix was compositely reinforced not
only by the reinforcing material but also by these intermetallic
compounds.
When in this embodiment the composite material was formed in
the same manner except that the pure copper fibers were replaced by
the pure copper powder used in Embodiment 8 or the melt of
magnesium alloy was replaced by a melt of pure magnesium at 680C°,
in both cases satisfactory composite materials including no micropores
were obtained.
Embodiment 10
Composite materials were formed in the same manner and under
the same conditions as in Embodiment 5, except that the pure nickel
powder was replaced by pure copper powder having 30 microns average
particle diameter.
Then, by examining sections of the composite materials thus
formed, the penetration of the melt was investigated, and as a result it
was confirmed that satisfactory composite materials including no
micropores were formed. Further, as a result of X-ray analysis of
sections of the composite materials, it was confirmed that the pure
copper powder had reacted almost completely with aluminum so as to
form intermetallic compounds such as CuAl2, and that the matrix was
compositely reinforced by these intermetallic compounds.
When in this embodiment composite materials were formed in the
same manner except that the melt of matrix metal was replaced by a
melt of pure magnesium at 680C°, satisfactory composite materials
including no micropores were also obtained.
-12-
Embodiment 11
Alumina-silica short fibers having 3 microns average fiber
diameter and 1.5 mm average fiber length (manufactured by Isolite
KK), aluminum alloy powder (JIS Standard AC8A) having 150 microns
$ average particle diameter or aluminum alloy powder (JIS Standard
AC7A) having 100 microns average particle diameter, pure titanium
powder having 30 microns average particle diameter, pure nickel
powder having 30 microns average particle diameter, and pure copper
powder having 30 microns average particle diameter were mixed in
various proportions and subjected to compression forming to produce
preforms having 45 x 25 x 10 mm dimensions and including the
alumina-silica short fibers at 0%, 5%, 10%, 15% or 20% by volume, the
aluminum alloy powder at 40%, 50%, 60%, 70% or 80% by volume, the
pure titanium powder at 0%, 1%, 5%, 10% and 15% by volume, the pure
copper powder at 0.5% by volume, and the pure nickel powder at 0.5%
to 15% (in steps of 0.5%) by volume, respectively, except such cases
that the total volume proportion would exceed 95'0.
Moreover, preforms were prepared in the same manner as above ,
to have 45 x 25 x 10 mm dimensions except that the volume proportion
2p of nickel powder was 0.5% and the volume proportion of pure copper
powder was 0.5% to 15% (in steps of 0.5%).
Then, composite materials were formed in the same manner and
under the same conditions as in Embodiment 1, except that the above
preforms were used, and by examination of sections thereof the
penetration of the melt was investigated.
As a result, as in Embodiment 1, it was confirmed that
regardless of the composition of the aluminum alloy powder, it was
desirable for the volume proportion of the aluminum alloy powder to be
between 60 and 80%, for the volume proportion of the pure nickel
30 powder plus the pure copper powder to be between 1 and 10%, and for
the volume proportion of the pure titanium powder to be between 1 and
10%.
Further, as a result of X-ray analysis of sections of the
composite materials formed with the volume proportions of the
35 aluminum alloy powder, the pure nickel powder plus the pure copper
powder, and the pure titanium powder within the above described
preferable ranges, it was confirmed that the pure nickel powder and
the pure copper powder had reacted almost completely with aluminum
-13 - ~~~033~
so as to form intermetallic compounds such as NiAl3 and NiAI and
CuAl2, respectively, and that in the case where the volume proportion
of the alumina-silica short fibers was 090, the matrix of aluminum
alloy was compositely reinforced by these intermetallic compounds, and
in the case where the volume proportion of ~ alumina-silica short fibers
was between 5 and 209, the matrix of aluminum alloy was compositely
reinforced not only by these alumina-silica short fibers but also by the
intermetallic compounds.
Embodiment 12
Composite materials were formed in the same manner and under
the same conditions as in Embodiment 2, except that the pure nickel
powder was replaced by 2.5% by volume pure nickel powder (5
microns average particle diameter) and 2.590 by volume pure copper
powder (30 microns average particle diameter).
As a result, it was confirmed that regardless of the temperature
of the melt of matrix metal satisfactory composite materials including
no micropores were formed.
Embodiment 13
Composite materials were manufactured in the same manner and
under the same conditions as in Embodiment 3, except that the pure
nickel powder was replaced by 39'O by volume pure nickel powder ( 10
microns average particle diameter) and 39o by volume pure copper
powder (20 microns average particle diameter).
As a result, it was confirmed that in this embodiment satisfactory
composite materials including no micropores were also obtained.
As a result of X-ray analysis of sections of the composite
materials formed in Embodiment 12 and Embodiment 13, it was
confirmed that the pure nickel powder and the pure copper powder had
reacted almost completely with the aluminum so as to form
intermetallic compounds such as NiAI~ and CuAl2, respectively, and that
the matrix of aluminum alloy was compositely reinforced not only by
the reinforcing material but also by these intermetallic compounds.
Embodiment 14
A composite material was manufactured in the same manner and
under the same conditions as in Embodiment 4, except that the pure
nickel fibers were replaced by 59'o by volume pure nickel fibers (30
microns average fiber diameter and 3 mm average fiber length) and 59'0
by volume pure copper fibers (20 microns average fiber diameter and
-14- ~~~~~~J
1 mm average fiber length), and by examination of sections of the
composite material thus formed, the penetration of the melt was
investigated.
As a result, it was confirmed that in this embodiment a
satisfactory composite material including no micropores was also
formed.
As a result of X-ray analysis of sections of the composite
material, it was confirmed that a central portion of the matrix was
aluminum alloy while peripheral portions of the matrix was magnesium,
that the pure nickel fibers and the pure copper fibers had reacted with
aluminum so as to form intermetallic compounds such as NiAl3 and
CuAl2, respectively, that particularly in the peripheral portions the pure
nickel fibers and the pure copper fibers had reacted also with the
magnesium so as to form intermetallic compounds such as NiMg2 and
MgCu2, respectively, and that the matrix was compositely reinforced
not only by the reinforcing material but also by these intermetallic
compounds.
When in this embodiment a composite material formed in the
same manner with the nickel fibers and the copper fibers being
2p replaced respectively by the pure nickel powder and the pure copper
powder used in Embodiment 13, or when the melt of magnesium alloy
was also replaced by a melt of pure magnesium at 680C°, in both
cases satisfactory composite materials including no micropores were
formed.
Embodiment 15
Composite materials were formed in the same manner and under
the same conditions as in Embodiment 3, except that the pure nickel
powder was replaced by 4~ by volume pure nickel powder (15 microns
average particle diameter) and 49'o by volume pure copper powder (25
microns average particle diameter).
Then, by observation of sections of the composite materials thus
formed, the penetration of the melt was investigated, and as a result it
was confirmed that satisfactory composite materials including no ,
micropores were formed. Further; as a result of X-ray analysis of
sections of the composite materials, it was confirmed that the pure
nickel powder and the pure copper powder had reacted almost
completely with aluminum so as to produce intermetallic compounds
such as NiAl3 and CuAl2, respectively, and that the matrix was
-15-
compositely reinforced not only by the reinforcing materials but also by
these intermetallic compounds.
Embodiment 16
Composite materials were formed in the same manner and under
the same conditions as in Embodiment 5, except that the pure nickel
powder was replaced by 59o by volume pure nickel powder (15 microns
average particle diameter) and 5% pure copper powder (25 microns
average particle diameter).
Then, by observation of sections of the composite materials thus
formed, the penetration of the melt was investigated, and as a result it
was confirmed that satisfactory composite materials including no
micropores were formed. Further, as a result of X-ray analysis of
sections of the composite materials, it was confirmed that a central
portion and peripheral portions of the matrix were substantially pure
aluminum and aluminum alloy, respectively, that the pure nickel powder
and the pure copper powder had reacted almost completely with
aluminum so as to form intermetallic compounds such as NiAl3 and
CuAl2, respectively, and that the matrix was compositely reinforced by
these intermetallic compounds.
When in this embodiment the melt of matrix metal was replaced
by a melt of pure magnesium at 680C° and composite materials were
formed in the same manner, satisfactory composite materials including
no micropores were also obtained.
Although the fine fragments of some particular compositions were
used in the various embodiments described above, in the present
invention the fine fragments may have other compositions. The
composition of the aluminum alloy may be, for example, JIS Standard
AC7A, JIS Standard ADC12, JIS Standard ADT17, or 89'o A1 - 3.50 Mg,
and so forth, the composition of the nickel alloy may be, for example,
Ni - 50~'o Al, Ni - 300 Cu, Ni - 39.5% Cu - 22.1 Fe, 8.8% B, and so
forth, the compasition of the copper alloy may be, for example, Cu -
50% Al, Cu - 29.6% Ni - 22.1% Fe - 8.80 B, and so forth, and
particularly when the nickel alloy or the copper alloy is a nickel-
copper alloy, the nickel and copper contents may have any proportions,
and further, the titanium alloy may be, for example, Ti - 1~'o B.
As will be clear from the above descriptions, according to the
present invention the molten matrix metal satisfactorily infiltrates into
the preform, and by the reaction of titanium with oxygen and nitrogen
_16_
in the preform, air is substantially removed from the preform, and as
a result an even more satisfactory composite material including no
micropores is manufactured.
Further, according to the present invention, since the temperature
of the molten matrix metal may be relatively low, and since the time
duration for the preform to be in contact with the molten metal is
shortened as compared with the case where no fragments of nickel,
copper, nickel alloy, copper alloy, titanium or titanium alloy is included
in the preform, a composite material can be manufactured at lower
cost and at higher efficiency as compared with the above-mentioned
prior proposal.
Although the present invention has been described in detail in
terms of several embodiments, it will be clear to those skilled in the
art that the present invention is not limited to these embodiments, and
various other embodiments are possible within the scope of the present
invention. For example, all or some of the fine fragments of nickel,
nickel alloy, copper or copper alloy may be replaced by fine fragments
of silver or silver alloy or fine fragments of gold or gold alloy.
25
35
_1~_ ~~~~33~
TABLE 1
VOLUME Ti ER
PROPORTION POWD (
OF %
)
0 1 5 10 15
40 ~ D D D ~
VOLUME 5p O O O
ORTION
O
PR 60 Q O O O O
P
OF AI
POWDER ~p O O O O O
g0 O O O O O
TABLE 2
VOLUME ER
PROPORTION (
OF %
Ti )
POWD
0 1 5 10 15
40 ~ D D O D
VOLUME 5p O O O O O
N
PROPORTIO 60 O Oo OO OO O
OF A1
POWDER O Oo po OO O
(off) ?0
80 Q OO OO OO O