Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ ~ ~ v ..~ 3 ~
HOECHST ARTIENGESELLSCHAFT HOE 89/F 225 Dr.DA/je
Description
Polyphenylene ether molding composition
The invention relates to a polyphenylene ether molding
composition which contains, to inhibit crystallization,
certain organic phosphorus compounds.
Polyphenylene ethers, in particular polyphenylene 6ul-
fide, number among the highly c~rstalline polymers, owing
to their linear struc~ure. Under normal processing,
crystallization occurs very rapidly and, among other
effects, prevents transparent articles being produced
from these polymers. ~oreover, the high crystallization
rate interferes with processes in which molecular orien-
tations, for example during stretching, play a part.
Inhibited crystalli~ation would be advantageous, in
particular in the production of fibers and films, since
then a wider processing range would be available in the
amorphous region.
Many proposals have already been made for controlling the
crystallization of highly crystalline polymers using
crystallization inhibitors.
~or instance, a process for the crystalliza ion of
polyarylene sulfide has been disclosed in which the
polymer is treated with polyvalent metal cations, prefer-
ably in aqueous solution (cf. European Patent 144,987).
Although this treatment depresses the crystallization
temperature in the melt and reduces the crystallization
rate, an additional process st~p using aqueous solutions
is at the same time necessary.
The object is therefore to provide an agent for inhibit-
ing the crystallization of polyphenylene ethers, which
can be incorporated into ~he polymer at low cost.
: , :
.
2 ~ i Y.li ~ ~
It has been found that this object can be achieved by
using certain organic phosphorus compounds.
The present i~vention accordingly provides a polyphenyl-
ene ether molding composition essentially composed of a
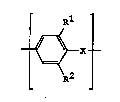
S polymer which contains units of the formula I
Rl
_ [ ~ X~
R2
in which Rl and R2 are identical or different and are a
hydrogen atom or a straight-chain or branched Cl-C4-alkyl
radical and
O X i8 an oxygen atom or a sulfur atom, and
a crystallization inhibitor, wherein the molding composi-
tion contains
0.05 to 5 parts by weight, rela~ive to the polymer, of an
organic phosphorus compound of the formula II
O - R4
\ ~ R5 (II)
in which R3, R4 and R5 are identical or different and are
a phenyl radical, which may be substituted by one or more
Cl-C4-alkyl groups, of the formula III
R7 o/ ~ P\ (III)
in which R6, R7, R3 and R9 have the meaning, of R3, R4 and
,
-:, . .
-- ~ . .... . -.. :. ~- .
-- 3 --
R5,
or of the formula IV
R10 0 P ~ --R12 ~' ~ P - O - Rll
\ o ~ ~ (IV)
in which Rl and R11 are identical or different and are a
S straight-chain C~-C22-alkyl radical or a phenyl radical
which may be substituted by one or more Cl-C4-alkyl
groups, and Rl2 iS a pentaerythrityl radical.
The polyphenylene ether molding composition according ~o
the invention is essentially composed of a polymer which
contains units of the formula I
~ X ~ (I)
In this formula, R1 and R2 are identical or different and
are a hydrogen atom or a straight-chain or branched Cl-C4-
alkyl radical. Rl and R2 are preferably a hydrogen atom or
a C1-C2-alkyl radical, in particular a hydrogen atom or a
methyl group. X is an oxygen atom or a ~ulfur atom,
preferably a sulfur stom. The polymer is preferably
composed entirely of units of the formula I. The mole-
cular weight is generally about 5,000 to about 200,000,
correspondin~ to 20 to 2,000 units of the formula I.
Suitable polyphenylene ethers are poly(thio-l,4-phenyl-
ene~ and poly(2,6-dimethyl-1,4-phenylene ether). Part-
icular preference i8 given to polyphenylene sulfide.
Polymers of this type are commercially available even
unstabili~ed and free from other additives. However, they
.. . .
., - . . :-: . -
.: - . : -. -,
, . . . , ~
?~ L,'`'`~
can also be prepared by the process according to European
Patent 144,987.
The molding composition according to the invention
contains 0.05 to 5, preferably 0.1 to 1, part~ by weight,
relative to the polymer, of a cr~stallization inhibitor.
This crystallization inhibitor is an organic phosphorus
compound of the formulae II, III or IV:
O
P~3 _ o _ p/
\0 - R5 (II)
R6 o\ ~{3 8 ( III )
R - O - P~ R12~ \P - O - Rll ( IV)
In these formulae R3, R4, R5, R6, R7, R~ and R9 are identi-
cal or different and are a phenyl radical which may be
substituted by one or more Cl-C4-, preferably branched
C4-, alkyl groups. R3, R4, R5, R6, R7, R8 and R9 are prefer-
ably a 2,4-di-t-butylphenyl group.
Rl and R11 are identical or different and are a C12-C22-,
preferably Cl8-Cl~-, alkyl radical or a phenyl radical
which may be substituted by one or more C1-C4-, preferab~y
branched C4-, alkyl radicals. Preferably, R10 and R11 are
~0 a stearyl rad~ical or a 2,4-di-t-butylphe~yl group.
Rl2 is a pentaerythrityl radical.
:
, . ~ ~ :`
. .
;~ v f~
-- 5 --
Particularly preferred phosphorus compvunds are tris-
(2,4-di-t-butylphenyl) phosphite and tetrakis-(2,4-di-t-
butylphenyl) 4,4'-bisphenylenediphosphonite.
Besides the phosphorus compounds which are to be used
according to the invention, the polyphenylene ether
molding composition may addil:ionally contain anti-
oxidants, ~V absorbers, light stabilizers, metal de-
activa~ors, stabilizers, fillers, reinforcing agents,
lubricants, pigments, optical bxighteners, flame retar-
dants or antistatic agents.
The incorporation of the phosphorus compounds into thepolyphenylene etheræ is carried out by the usual plastic
compounding procedures. ~or instance, the phosphorus
compounds can be incorporated in the form of pulverulent
solids wi~h the plastic powder or granules or can be
metered in in the form of a concentrate containing up to
50 % by weight of active ingredient or in the form of a
dispersion or emulsion in a suitable dispersing agent
which is subsequently removed.
The addition of the phosphorus compounds of the formulae
II, III or IV allows crystallization to be significantly
delayed. Moreover, these oompounds additionally act as
heat stabiliæers.
The invention is explained using the following example.
Example
Polyphenylens sulfide (density 1.34 g/cm3~ m.p. 283C)
was dried in a vacuum drying oven for 12 h at 14GC. A
slowly rotating stirrer was used to admix 0.5 parts by
weight of the pulverulent phosphorus ~ompound 1 or 2 per
100 parts by weight of the pulverulent polyphenylene
sulfide. These mixtures were used to prepare platelets of
dimensions 1 x 60 x 60 mm on an injection molding machine
(temperature profile 300-310-320-330C; mold temperature
.: : : ,. .
. .
.
.. . . :...... . .. . , - .
- 6 - 2 $ ~, t, " ~ ~
100C). ~he crystallinity was assessed by measuring the
transparency of the test pieces using a transparency
meter (type LT12/Dr. B. Lange, Berlin). The crystalliz-
ation process was investigated using differential scan-
ning calorimetry (DSC) and not only the post-
crystallization during the first heating step was moni-
tored but also the crystallization during the defined
cooling of the sample.
DSC conditions:
Temperature range: 10C - 310C
Heating/cooling rate: 10 K/min
Purging: nitrogen
Sample size: in the range of 6-7 mg
Measuring apparatus: ~ettler type TA 3000
15 The results of the measurements are summarized in the
table.
Phosphorus compound 1 = tris-(2,4-di-t-butylphenyl~
phosphite
C(CH3)3
p _ O ~C ( CH3 ) 3 3
Phosphorus compound 2 = tetrakis-(2,4-di-t-butylphenyl)
4,4'~biphenylenediphosphonite
R o/ ~ /O R R _ ~ C(CH3~3
,
.
~ .
7 ~ ? j ' ~
~xperimental result~
a) Post-crystallization during the initial heating step
Despite the high crystallinity of polyphenylene sulfide,
complete crystallization does not occur during in~ection
molding. Consequently, post-crystallization can be
observed at a higher temperature in the DSC.
Pure PPS has a pos~-crystallizaltion peak at 120C. On
adding phosphorus compound 1, this crystallization peak
is observed at 127C. Phosphorus compound 2 shift~ the
~0 post-crystallization peak to 12~C.
The greater liberated heat flows from each of the modi-
fied types is likewise evidence of lower crystallinity.
b) Crystallization during cooling
To investigate this crystallization, the polymer melts
are cooled in a defined manner.
The unmodified PPS has a crystalli~ation peak maximum at
246C. ~he sample modified with phosphorus compound 1 has
a peak maximum which is 30 g l~wer, i.e~ the crystalliz-
ation peak maxLmum is at 216~C.
c) Transparency
The addition of 0.5 part of the organic phosphorus com-
pound used allows 1 mm test platelets to be obtained
having a light transmission of up to 60 %. The unmodified
test pieces are opaque after processing.
'- :,. . ~ :, ,~
., ~ , .
- 8 - 2 $ 2 ~ l3 3 ~
Table
Results of the DSC analysis
PPS PPS PPS
without + 0.5 part ~ 0.5 part
additive of phosphorus of phosphorus
compound 1 compound 2
1. Heating
Post-crystalliz-
ation
Beginning C 100 108 107
Maximum C 120 127 129
End C 130 142 144
Heat transfer J/g 11.5 29.0 27.0
Melting temp.
Maximum C 283 283 284
Heat of fusion J/g 45 44 47
.
Cooling
20 Crystallization
Beginning C 260 237 249
Maximum C 246 216 233
End 200 192. 202
Heat transfer J/g 51 49 52
Transparencyopaque 60.6 4~.5
% transmission
.
- .