Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ '3
TITLE OF INVENTION
SUPPR~SSION OF DIOXIN PRODUCTION IN THE
INCINERATION OF ~AST~ ~TERIAL
FIELD OF INVENTION
The present invention relates to the incineration
of waste materials and, in particular, to the
suppression of chlorinated organics including dioxins
and furans on the flyash and in the stack emissions from
such incineration, as well as to the suppression of acid
gases, in particular, HCl and SO2, in the stack
emissions from such incineration.
BACKGROUND TO THE INVENTION
Incineration, an attractive alternative to burying
in a landfill for the disposal of urban garbage, is
practiced throughout the world and results in a
considerable decrease in waste volume and the recovery
of energy in the form of steam or electricity. One of
the significant drawbacks to the incineration procedure
is that several hundred stable and toxic compounds,
including polychlorinated dibenzo-p-dioxins
(collectively commonly termed "dioxins") and
-polychlorinated dibenzofurans (collectively commonly
termed "furans"), are formed and are presented in parts-
;~25 per-million concentrations both in the flyash formed
during combustion and in the stack emissions.
A large city may incinerate 3 to 5 million tons of
garbage annually. For every million tons of urban waste
incinerated, about 34,000 tons of flyash are produced by
the typical incinerator. Between 95 and 99% of th-e
flyash is precipitated alectrostatically and buried in
landfills. The remainder is emitted from the
`~incinerator stacks along with the gaseous by-products,
namely water vapour, ~Cl, SO2, CO2, air and volatilized
organic compounds. The gaseous stack emission
introduces dioxins, furans and other toxic chlorinated
compounds to the atmosphere. Landfill disposal of
h~Pj~
flyash introduces dioxins, furans and other hazardous
organic chlorinated compounds into the earth, from where
they may be leached into ground water systems.
The primary hazard of the most toxic of these
organic compounds, dioxins and furans, to humans may be
cancer in the long term, but dioxins exert a much higher
impact on the general environment and are considered
undesirable. There exists, therefore, a need for a
means to decrease the dioxins, furans and other
chlorinated compounds content of both solid and gaseous
by-products from incinerator systems. It is also
essential to decrease emissions of acid gases produced
in the incineration process.
4~ 7f There has been previously described in U.S. Patent
/~ 15 No. 4,793,270, naming two of us as inventors, the
disclosure of which is incorporated herein by reference,
the surprising discovery that the flyash which is formed
during the incineration of solid municipal waste
catalyses the formation of dioxins from chlorinated
phenols formed from combustion products of plastics,
paper and chemicals, and several other di~xin precursors
in the gaseous combustion products. Accordingly, as
described in that patent, a material acting as a
catalyst inhibitor is provided in association with the
flyash so as to inhibit the catalytic activity of the
flyash towards the formation of chlorinated compounds
including dioxins and furans.
SUMMARY OF INVENTION
It has now been found that alkanolamines and
inorganic bases (herein termed inhibitor mixtures) are
very effective in inhibiting dioxin formation as well as
the formation of other chlorinated chemicals, and, at
the same time, are effective in decreasin~ the acid
(mainly HCl and SO2) content of the incineration gas
emissions. Ammonia (NH3) also may be employed as an
inhibitor in place of the inhibitor mixture and may be
readily introduced as a gas.
Accordingly, in one aspect, the present invention
is directed towards the suppression of the formation and
hence occurrence of chlorinated organic compounds,
including dioxins and furans on flyash produced during
municipal solid waste incineration.
In another aspect, the present invention is
directed towards the suppression of dioxins and acid
gases, such as HCl and S02, in the stack emission o~
municipal solid waste incineration.
In accordance with one aspect of the present
invention, therefore, there is provided a method of
disposal of organic material, such as municipal waste or
industrial waste, which is combustible to form gaseous
chlorinated organic compounds, including toxic dioxins
and dibenzofurans, which comprises a plurality of steps.
The organic material is incinerated in an
incineration operation, which often i5 self-sustaining,
to form gaseous products of incineration containing
flyash, precursors for the formation of chlorinated
organic compounds, and acid gases, and the gaseous
products o incineration are passed to a precipitation
step wherein the flyash is precipitated ~rom the gaseous
products of incineration.
The surface of the flyash during the passage of
the gaseous products of incineration to the
precipitation step is contacted with a small quantity of
at least one inhibition substance which reacts with
-~ catalytically-active sites on the surface of the flyash,
so as to inhibit catalytic effects of the flyash
towards the formation of chlorinated organic compounds,
including toxic dioxins, from the precursors during the
passage.
Chlorinated organic compounds and the acid gases
are reacted with the at least one inhibitor substance in
the gaseous products of incineration to suppress the
3,~
chlorinated organic compounds and acid gases in a vented
product gas stream. The product gas stream is vented
after the precipitation of flyash from the yaseous
products of incineration.
The prior patent exemplified the process employing
carbon disulphide, sulphur dioxide and thiophenes as
inhibitors. The materials Pmployed herein possess
certain advantages in comparison with the prior art
materials. In particular:
10 (i) the quantity of chemical required herein
to achieve a greater than 9S~ inhibitor
effect is much less than was observed in
the prior art and the chemicals employed
herein tend to be much less costly than
the prior art ones, leading to overall
economies in the process
(ii) The chemicals employed herein are
generally water soluble and the
resulting solutions are readily employed
and handled in the operating plant,
whereas the prior art chemicals are less
readily employed and handled, and
(iii) The chemicals employed herein are basic
in nature and exhibit an acid gas
decreasing effect, whereas the prior
chemicals tend to generate acid gases.
The present invention is described herein mainly
with respect to the inhibition o~ the formation o~
dioxin and other chlorinated compounds and to decreasing
the acid gas content of the vent gas stream in municipal
solid waste incinerators. However, the prssent
invention is broadly applicable to achieving such
effects in the incineration of any material that is
organic in origin, including not only municipal solid
waste, but also sewage sludge and industrial waste, and
which is combustible to form gaseous chlorinated organic
compounds, including precursors for dioxin formation.
BRIEF DESCRIPTION OF DRAWINGS
Figure 1 is a schematic representation of a typical
municipal solid waste incinerator, showing locations for
introduction of inhibitor mixtures (points 1, 2 and 4)
according to the process of the invention;
Figure 2 i~ a bar graph showing 13C-labelled
polychlorinated dibenzo-p-dioxins (PCDD) produced from
13C-labelled pentechlorophenol (13C-PCP) by the
catalyti~- activity of the flyash samples from various
incinerators located in different countries;
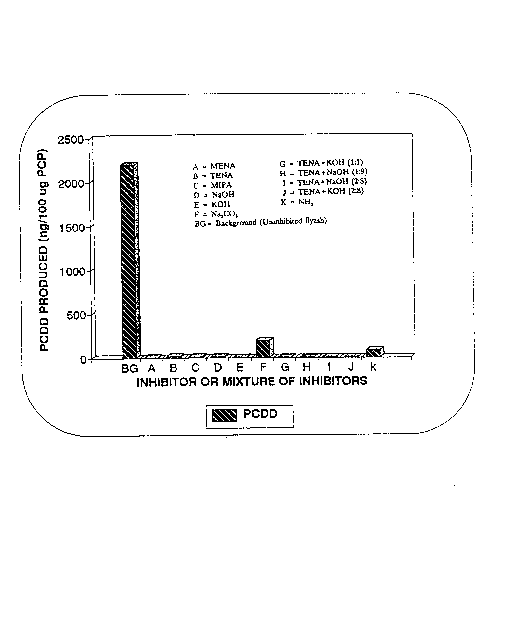
Figure 3 is a bar graph showing the amounts of 13C-
PCDD formed in laboratory tests from 13C-PCP by the
catalytic activity of uninhibited flyash (background=BG)
and flyash treated by various uninhibitors, where BG =
PCDD produced on uninhibited flyash, A to K are the
different inhibitors added to the flyash prior to
inhibition test. A = MENA, B = TENA, C = MIPA, D =
NaOH, E = KOH, F = Na2CO3, G = TENA + KOH (1:1 Mixture
in Water), H = TENA ~ NaOH (1:9 mixture in water), I =
TENA + NaOH (2:8) mixture in water), J = TENA + KOH
(2:8 mixture in water), K = anhydrous ammonia (2 wt.%
of flyash injected during test). The amount of
inhibitor used was 2 to 4~ by wt. of flyash;
Figure 4 is a bar graph showing the comparison of
the amounts of PCDD and PCDF formed on the flyash during
the normal operation (background=BG~ and during the
introduction of inhibitor mixture 1 in the municipal
solid wa~te incinerator. During the plant test,
inhibitor (0.2 wt.% of the solid waste) introduction was
started at 10.00 am and terminated at 1.30 pm;
Figure 5 is a chromatogram showing the response of
electron captor detector for the chlorinated compound~
in samples extracted from uninhibited and inhibited
flyash in the plant test represented by data in Figure
4;
J JL ~ ~J ~
Figure 6 is a bar graph showing the stack emissions
of HC1 and S02 prior (normal operation) and during the
introduction of inhibitor in the plant test;
Figure 7 is a bar graph showing stack emissions of
PCDD and PCDF prior (normal operation) and during the
introduction of inhibitor in the plant test;
Figure 8 is a bar graph showing the comparison of
the amounts of dioxins and furans, tetra- to octa-
congeners and total PCDD and PCDF that were present in
the stack emissions during the normal operation ~without
ammonia~ and during the introduction of ammonia (O.2
wt.% of solid waste) in the operating incinerator; and
Figure 9 is a bar graph showing the comparison of
the amounts oX PCDD and PCDF formed on the flyash during
the normal operation (background=BG) and during the
introduction of inhibitor mixture 2 in the municipal
solid waste incinerator. During the plant test,
inhibitor (0.2 wt.% of the solid waste) introduction was
started at 8.00 am and terminated at 4.30 pm.
GENERAL DESÇRIPTION OF INVENTION
In the present invention, there are employed
certain inhibitor mixtures to effect dioxin inhibition.
Su~h inhibitor mixtures may comprise at least one
alkanolamine.
Alkanolamines that have been found to be
particularly effective are monoethanolamine (MENA),
triethanolamine (TENA) and monoisopropanolamine (MIPA).
The inhibitor mixtures may also comprise at least
one inorganic ~ase. Inorganic bases found to be
effective are sodium hydroxide (NaOH), potassium
hydroxide (KOH), sodium carbonate (Na2C03), calcium
oxide (CaO), magnesium oxide (MgO), sodium orthosilicate
(Na4SiO4) and sodium metasilicate (Na2SiO3).
Such inorganic bases may be employed separately or
in different combina~ions with alkanolamines, preferably
as an aqueoue solution thereof or in solid particulate
form.
Laboratory tests using such inhibitors (1 to 4~ by
wt. of flyash) separately or in mixtures thereof show
that more than 98% inhibition of dioxin formation occurs
S on the flyash from reaction of a known dioxin precursor,
pentachlorophenol (PCP), at 300C (see Examples below
for detail~). The inhibitor mixtures, such as
alkanolamines combined with inorganic bases, may b~
applied to the municipal waste matsrial prior to
combustion (i.e. feed point 4 in Figure 1) or during
combustion to the flyash between boiler and precipitator
(i.e~ feed points 1 and 2 in Figure 1).
Several mixtures of above-desrribed specific
inhibitor materials were tested in laboratory inhibition
experiments and also in a large operating incinerator
(see Examples below for details). The inhibitor
mixtures employed in the operational incinerator were 1)
MENA + TENA + water, 2) TENA + KOH + water, 3) MENA
TENA ~ CaO. A reduction of dioxins in the flyash by 80%
or more was observed. In addition, a reduction of
dioxins in the stack emissions by 78% or more and a
reduction of HCl and SO2 by 78 to 80~ or more in the
stack emissions have been observed.
In this way, inhibitors effectively poison the
catalyst sites on the flyash, so that the ~uantity of
YariOUS dioxins, furans and other chlorinated compounds
present in the tail gas stream and on the precipitated
flya~h is significantly decreased.
Dioxins are a family of chlorinated products having
the general formula:
Cl ~ ~ Cly
w~
while tbe rela~ed ~urans have ~he general .ormula:
~,~ .
Clx Cl~
The dioxins and the furans exhibits varying degree of
toxicity, depending on the number of chlorine atoms
present, with those compounds having gr~ater n~mbers of
chlorine at~-~s being more benign than tho~e with lesser
n~mbers of chlorine atoms.
It is known that dioxin formation can occur though
thermal reaction of precursors, such as
pentachlorophenol, which ar fo~med as combustion
pro~ucts from various organic materials in the solid
waste material to form the oc~achlorodioxin, as follows:
Cl Cl
Cl ~ ~ Cl ~ C
Cl Cl
Cl C1
C~ Cl
Cl Cl
We have found that, through catalytic reactions on the
flyash octachlorodioxin forms at lower temperatures
(approximately 200 to 400C), and much faster, and the
octachlorodioxin then can be catalytically converted to
dioxins having ~es~ex numbers of chlorine atoms on the
,J il~ iJ ~ i~' tJ ~3
benzene rings by the action of the flyash. Other
precursors, such as polychlorophenols also can form
dioxins through such catalytic reaction sequences.
Many other precursors of dioxins and furans have
been identified by our research, including products of
combustion of chlorinated polymeric materials, such as
polyvinyl chloride and inked newsprint. We have recent
evidence to support the concept that dioxin precursors
are simply intermediate compounds formed from more
primary combustion products, such as XCl, CO, H2O, H2,
C2H2 and C2H4, by catalytic reactions on the flyash.
Accordingly, inhibition of catalytic properties of the
flyash decreases the formation of all chlorinated
compounds (see Figure 5).
By using the inhibitors of the present invention,
such catalytic effects are decreased and minimized,
hence decreasing the formation of all chlorinated
compounds and thereby the more toxic lesser chlorinated
dioxins and furans, and resulting in a decreased
concentration of such materials on the flyash and in the
vent gas stream from the incinerator.
In addition, by preventing formation of the lesser
chlorinated species, the overall amount of dioxins
entering the flue gases is decreased by virtue of the
lower vapour pressure of the octachlorodioxin. Only
small quantities of inhibitor mixtures are required to
achieve the desired result, generally from about 0.01 to
about 0.2 wt.% of the solid waste material incinerated.
One significant benefit that results from the use
of the mixtures of inhibitors herein, in addition to the
decrease in dioxin content of the combustion gas stream,
is a decrease of the acid gas content, in particular HCl
and SO2, of the combustion gas stream.
EXAMPLES
Exam~le 1
This Example shows that the flyash samples from
r,~ J ~
different incinerators promote the production of
chlorinated dioxins under simulated incinerator
conditions.
An experimental test apparatus was set up
consisting of a vertically oriented oven with a glass
tube (25 x 1 cm I.D.) surrounded by the oven. Part of
the flow tube was a reservoir containing flyash from the
municipal solid waste incinerator (MSWI). The flyash
sample had been exhaustively extracted with solvent
heated at 300C to remove all organic compounds.
In each experiment, 1.5 g of pre-cleaned flyash
was placed in the glass tube. A volume of 50
microliters (ul) of 5 ug/ul 13C-PCP solution in methanol
was deposited on- the glass beads on the top of the
flyash and the solvent allowed to evaporate. The
section of the tube containing flyash and PCP was heated
at 300C for 60 minutes using 3 ml/minute flow of dry
air. After completing the experiment, the flyash was
spiked with an internal standard for recovery estimates.
Organic compounds formed on the flyash and the internal
standards were extracted by eluting with ~20 ml toluene.
Extracts were concentrated by rotary evaporation to a
few ml and ~inal concentration in a sample vial to 500
ul under a gentle stream of N2.
Figure 2 shows the bar graph for the amounts of
PCDDs praduced by the catalytic activity of flyashes
from Canada (Ontario), U.S.A. (Sunlakes), German~
(Krefeld) incinerators.
Exam~le 2
This Example illustrates that the catalytic
production of dioxins from PCP can be suppressed. Based
on the data presented in Example 1 and the data in the
previous patent (U.S.A. 4,793,~70) and similar results
from our experiments, it is clear that the formation of
dioxins occurs on the flyash due to catalytic activity
of the flyash.
~, ~,f
11
Inhibition of the ~lyash catalytic activity,
therefore, should prevent the catalytic effect and thus
pr0vent the formation in the incineration process, of
some or all of the chlorinated compounds, including
dioxins. Separate tests were conducted on the flyash
from U.S.A. incinerator, in which separately or in-
combination A) monoethanolamine (MENA), B)
triethanolamine (TENA3, C) monoisopropanolamine (MIPA),
D) sodium hydroxide (NaOH), E) potassium hydroxide
(KOH), F) sodium carbonate (NazC03), G) TENA + KOH, (1:1
mixture), H) T~NA + NaOH (1:9 mixture), I) TENA + NaOH
(2:8 mixture), J) TENA + KOH (2:8 mixture) and K) NH3
were added to the flyash prior to the catalytic activity
test performed as described in Example 1. Anhydrous
a~monia (K) was injected upstream to the flyash in the
air stream during the test of its inhibition ability.
The results of thess laboratory inhibition tests
are presented in Figure 3. Organic inhibitors alone
were highly effective when used in an amount of 2% by
wt. of flyash. To obtain similar effect, a mixture of
organic and inorganic inhibitor of 4% by wt. of flyash
was required.
Example 3
This Example illustrates that the production of
dioxins in operating municipal solid waste incinerator
(MSWI) can be suppressed using organic inhibitors.
A schematic of the MSWI is shown in Figure 1.
Based on the laboratory tests and results thereof, plant
tests were conducted in an operating MSWI. A mixture of
30 25% monoethanolamine, 25% triethanolamine (about 0.1 to
0.2 wt.% of solid waste) and 50% water was introduced in
the post combustion zone of the incinerator (Figure 1,
Point 2), at a temperature of about 350C. Flyash
samples were collected prior to and during the injection
of the inhibitor mixture.
Amounts o~ dioxins and furans detected in various
~3~
flyash samples collected under different conditions are
shown in Figure 4. The amount of inhibitor injected
(~.1 to 0.2 wt.~ of refuse feed) was calculated from the
amount of flyash produced per unit time. The bar graph
of Figure 4 clearly indicates that the suppression in
PCDD/PCDF occurred during injection of inhibitors.
Figure 5 shows the plots of gas chromatography/electron
capture detector response for various chlorinated
compounds produced prior to and during introduction of
the inhibitor mixture in the operating incinerator. It
is clear from Figure 5 that the suppression of all
chlorinated compounds occurred during the inhibitor
mixture introduction.
ExamPle 4
15This Example illustrates the decreased acid gas
content on the gas stack during the plant test of
Example 3, flue gases at stacks (point 3 in Figure 1)
were analyzed for HCl and S02. The level of HCl and S02
prior to and during injection of the inhibitor mixture
as described in Example 3 are shown in Figure 6. It has
been observed that HCl levels up to 78% and SO2 levels
up to 80% were suppressed during introduction of the
inhibitor mixture.
At the same time, the plant flue gas (point 3, in
Figure 1) was analyzed for dioxins and furans. The
levels of PCDD and PCDF prior and during introduction of
the inhibitor mixture (Example 3) are shown in Figure 7.
It has been obser~ed that dioxin/dibenzofuran levels up
to 78% were suppressed during inje~tion of the
inhibitor mixture.
Exam~le_5
This Example illustrat2s that the production of
dioxins in operating municipal solid waste incinerator
(MSWI) can be minimizèd using ammonia as an inhibitor.
35Based on the laboratory tests and results thereof
plant tests were conducted in the operating MSWI shown
in Figure 1. Gaseous ammonia (0.1 to 0.2 wt.% of solid
waste) was introduced in the post combustion zone of the
incinerator (Figure 1, Point 2). PCDDs/PCDFs were
analyzed in the stack emissions prior to and during the
injection of the inhibitor ammonia. The amount of PCDDs
and PCDFs detected under different conditions are shown
in Figure 8. The amount of inhibitor to be injected
(0.1 to 0.2 wt.% of refuse feed) was calculated from the
amount of flyash produced per uni~ time. The bar graph
in Figure 8 clearly indicates that the suppression in
PCDDs/PCDFs occurred during injection of ammonia.
Example 6
Example 3 was repeated except that a mixture of
inhibitors (TENA ~ KOH + water : 4% ~ 30% + 66%, total
lS inhibitors 0.1 to 0.2 wt.% of the solid waste), was
injected at point 1, Figure 1 where the temperature was
about 400C in the plant test. The amounts of dioxins
and dibenzofurans produced are shown in Figure 9. An
important aspect of this ~xample is that it illustrates
the possibility of injecting the inhibitor mixture at
temperature higher than 350C, such as at point 1 in
Figure 1.
SUMMARY OF DISCLOSURE
In summary of this disclosure, the present
invention provides a method for inhibiting the formation
of chlorinated compounds, including dioxins and furans,
and for decreasing acid gas formation, in the combustion
gas stream from a municipal solid waste incineration
procedure by employing certain alkaline compounds,
including organic and inorganic inhibitors, such as
alkanolamines and various alkaline inorganic compounds
of sodium, potassium, magnesium and their mixtures.
Modifications are possible within the scope of this
invention.