Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02022728 1999-10-27
PROCESS OF PRODUCING PHOSPHATE COATINGS ON
METALS
DESCRIPTION
This invention relates to a process of
phosphating iron and steel surfaces according to the low-
zinc technology with phosphating solutions which are free
of nitrite and contain phosphate and nitrate and to the
use of that process in preparing iron and steel surfaces
for painting.
The zinc phosphating process is used on
a large scale in the metal-working industry. The phosphate
layers produced by that process on the treated metal sur-
faces serve particularly to facilitate sliding and non-
cutting cold-working as well as to afford protection
against corrosion and as a adhesion base for paint.
As a pretreatment before painting, phos-
phating processes using low-zinc technology afford
special advantages. The bath solutions used for that
purpose contain zinc in concentrations of only about
CA 02022728 1999-10-27
- 2 -
0.4 to 1.7 g/1 and on steel produce phosphate layers
having a high proportion of phosphyllite, which results
in a better adhesion of paint and in a higher resistance
to migration under paint than hopeite derived from phos-
phating baths having a higher zinc content.
Nitrite, chlorate and organic vitro
compounds have proved particularly satisfactory as ac-
celerators in low-zinc phosphating baths and result in
a formation of uniformly covering phosphate layers of
high quality in a short time. It is also known to use
peroxides as accelerators in low-zinc phosphating baths.
Whereas peroxides would be preferably to the above-
mentioned accelerators for the sake of working place
hygiene and protection of the environment, their accele-
rating action is not sufficient under the previously
employed treating conditions. A further disadvantage of
the per compounds resides in that that even a treatment
for a long time will result only in relatively thin
phosphate layers, which afford only a moderate protection
against corrosion.
It is an object of the invention to
provide for the zinc phosphating of iron and steel,
optionally together with galvanized, zinc alloy-coated
and aluminized steel and aluminium by means of nitrite-
free low-zinc phosphating solutions a process which
does not have the known disadvantages, particularly
CA 02022728 1999-10-27
- 3 -
those mentioned hereinbefore.
In the process of the kind described
first hereinbefore that object is accomplished in
accordance with the invention in that the surfaces are
contacted at 30 to 65 °C with an aqueous acidic phos-
phating solution which contains
0.4 to 1.7 g/1 Zn
7 to 25 g/1 P205
2 to 30 g/1 N03
and in which the weight ratio of free P205 to total
P205 is adjusted to a value in the range from 0.04 to
0.20, and H202 or alkali perborate is added to the
phosphating solution in such an amount that - being in
working condition - the peroxide concentration is
not in excess of 17 mg/1 (calculated as H202) and
the Fe(II) concentration respectively is not in excess of
60 mg/1 (calculated as Fe).
The process in accordance with the invent-
ion is intended for the surface treatment of iron and
steel. But low-alloy steel, galvanized steel, zinc
alloy-coated steel, i.e., e.g., steel coated with
ZnAl, ZnFe and ZnNi, aluminized steel, aluminum and its
alloys may be treated together with iron and steel.
Phosphating is affected at temperatures
in the range from 30 to 65 °C. Below 30 °C the phosphat-
ing rate will not be sufficient for modern series pro-
CA 02022728 1999-10-27
- 4 -
duction. Temperatures above 65 °C will result in disad-
vantages, e.g., in a stronger incrustation of the plant.
As is usual in processes of the so-called
low-zinc technology, the weight ratio of Zn to P205 in
the phosphating solution is preferably (0.075 to 0.015)
:1.
The content of perioxide or Fe(II) in
the phosphating solution is determined in a conventional
manner, e.g., by a titration with potassium permanga-
nate. In accordance with a preferred feature of the .
invention the surfaces are contacted with a phosphat-
ing solution in which the addition of H202 and/or
alkali perborate is controlled in dependence on the
electrochemical potential determined by a redox elec-
trode. For instance, a platinum electrode and a suit-
able reference electrode, such as a calomel or a
silver-silver chloride electrode, can be used for that
purpose. Such an electrode system may be used for a
continuous monitoring of the phosphating solution and
peroxide may be added in such a manner that the steady-
state concentration of Fe(II) ions and the steady-state
concentration of hydrogen peroxide are maintained within
the above-mentioned limits.
The kinds and quantities of the cations
and anions contained in the phosphating solution used
in the process in accordance with the invention are so
selected that the ratio of free P205 to total P205 is
:=:'
~i~ e~
,. ,
between 0~0~- and 0.20~ .As a rul8~ a higher bath tempe-
rature and/or a higher zinc concentration will require.
that ratio to be selected in the upper part of th~
above-mentioned range and a lower bath temperature
and/or a lower zinc concentration will require said
ratio to be selected in the lower part of said ranges
In accordance with a preferred 'feature
of the process in accordance with the invention9 the
surfaces are contacted with a phosphating solution to
which H202 and/or alkali perborate have been added in
such an amount that the maximum peroxide concentration
is 8 mg/1 and the maacimum Fe(7CI, concentration
respectively is 30 mg/1.
In accordance with a further preferred
feature of the invention the surfaces nre contacted
with a phosphatxng solution which in addition contains
up to 3 g/l manganese, up to 3 g/1 nickel and%lor cobalt
up t~ 3 g/1 magnesium and/or up to 3 g/1 calcium. The
co-use of manganese and/or maginesium and/or calcium
will result in phosphate coatiaags which in addition to
zinc and optionally :.~ron(I~) contain also skid catione.
Such miaced phosphates dist3.nguish by a higher resistance
to alkali and are particularly suitable as a adhesion
base for paint. Nickel and/or cobalt are preferably
added in order to increase the aggressiveness of the
phosphating solution en steel and - where zinc surfaces
CA 02022728 1999-10-27
- 6 -
are treated too - to improve the phosphating of zinc
surfaces. An optional addition of small amounts of
copper will increase the accelerating activity of
the phosphating solution. Alkali and/or ammonium are
mainly used for the adjustment of the desired acid
ratio.
In another desirable embodiment
of the invention the surfaces are contacted with a
phosphating solution which contains up to 3 g/1 fluoro-
borate (calculated as BF4) and/or up to 3 g/1 silico-
fluoride (calculated as SiF6) and/or up to 1.5 g/1
fluoride (calculated as F). In general, the anions
fluoroborate, silico-fluoride and/or fluoride act to
increase the phosphating rate and, in addition, will
be of advantage if a treatment of aluminum-containing
zinc surfaces is intended. The presence of free fluoride
(F ) is essential for the formation of crystalline
phosphate coatings on aluminum and its alloys.
Chloride and sulfate may be used
to adjust the phosphating solution to an electrically
neutral state and, in special cases, to increase the
agressiveness. An optional co-use of, e.g., polyhydroxy-
carboxylic acids, such as tartaric acid and/or citric
acid, will permit an influence on the thickness of the
resulting phosphate coatings and/or their weight per
unit of surface area.
~~~~~~,>~f~~ r~~-
If the phosphating solution contains
also manganese and/or nickel and/or cobalt and/or magrie- ,
sium, the weight ratio of Mn:Zn, of (Ni and/or, Co):Zn,
of ~g:2n and/ofi Ca:Zn should not be in excess of 2:1 in
each case.
In accordance w3.th a further desirable
embodiment of the invention the surfaces are contacted
with a phosphating solution in which the content of
free P205 or the ratio of free P2 5 to total P~05 is
ad~justed__during the processing by an addition of man
ganese carbonate, zinc carbonate and/or zinc oxide.
In that case it will be desirable to add said components
in the form of an aqueous dispersion.
The process in accordance with the
invention may be carried out by spraying, dipping,
spray, dipping or flooding.
In accordance with a further desirable
embodiment of the invention the metal surfaces are con-
tatted with a phosphating solution from which water is
removed and the removed water is replaced by an addition
o~'rinsing water from the succeeding rinsing stage or
rinsing stages. hater can be removed from the phosphating
bath, e.g., by evaporation, reverse osmosis and/or elec-
trodialysis. Particularly if H202 is used as a peroxide
component, these steps will permit the process in accord--
ante with the invention to be carried out in such a manner
J ~ ~~ fs!
that a sewage which is contaminated with phosphate
will not. be obtained as an effluent from the rinsing
step which succeeds the phosphating. The rinsing
stages suitably constitute a cascade of rinsing water,
which flows oppositely to the workpieces from each
rinsing stage to the next and is then supplied to
the phosphating bath. In the phosphating bath the
water thus supplied replaces the water which has been
removed from the phosp2~ating solwtion as mentioned
above" The water which has been removed from the phos-
phating bath, e.g., by reverse osmosis or electrodialy-
sis, may be recycled to the rinsing stages.
In another desirable embodiment of
the process in accordance with the invention the sur-
faces are contacted with a phosphating solution which is
replenished by an addition of phosphate in which the
ratio of free P205 to total P205 is (-0,54 to +0~20):1.
In that definition of the ratio of free P205 to total
P205, the minus sign means that there is no free P205
but a paxt of th~ phosphate consists of secondary phos-
pha'te. for instance, a value of minus 0~19 means that
1~6 of the total P205 are present as a secondary phos-
ph ate .
In accordance with another definition
the phosphate comprnents during replenishing ~
limited by a content of SCr~ secondary phosphate and
,. t ..~ a~ x." <l r J . .
,.
~$J $~d ;~ fd~'!~. ~...
5096 priaaa.r;~ phosphate (calculated, as P20~), on the
one hand, and by a content of 80~ primary phosphate and 20%
free phosphoric acid. (calculated as P20~) on the other
hand,
Because liquid replenishing concen-
traces are.wot stable in the stated range of free P2C3~
to total F~~5, the replenishing is usually effected in
the process in accordance With the invention by means
of at least two separate concentrates.
The process in accordance with the
invention9 particularly in its preferred embodiment in
e~hich the coating phosphating solution is replenished'
can be carried out for a long time~to form satisfactoz°y
coatings not onl;~ on iron and steel but also on accom-
panying surfaces, namely, gavanized, zinc alloy-coated
and aluminized steel and aluminumo
the process in accordance pith the
invention is of special advantage in pretreating sur-.
faces bef ore-th.ey are painted9 particularly by dip electro-
coating, and is of special significance for the cataphore-
tic dip electrocoating.
The invention will be explained more
in detail and by way of example in the follloeving
~,.,~' samples .
~~~~r~ , rJ
-lo-
Example 1
A phosphating solution to be sprayed
contained
0.8 g/1 Zn free X205 - 1.0~ g/1
1.0 g/1 Ni total P205 - 13 g/1
1.0 g/1 Mn free acid - 0.9 points
2.6 g/1 Na
13.0 g/1 p~05 total acid _ 23 points
2 .'1 g/ 1' LV03
The concentration of H202 in said solution was varied
between 10 and 70 mg/1 H~02 by an addition of H2Q2 and
in the absence of H202 the concentration of iron(II) was
varied between 10 and 90 mg/1 Fe(II) by a processing of
sheet steel.
Steel sheets which had been degreased
with organic solvent were sprayed with said baths at
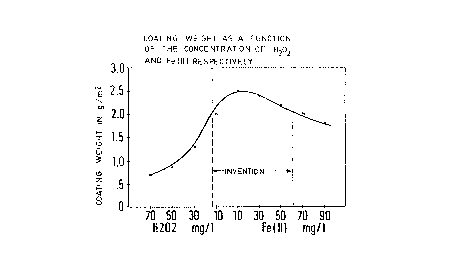
58°C. In Figure l9 the weight of the phosphate coating
is plotted which had been formed after a fraying time
of 3 minutes. Figure 2 indicates the minimum phosphating
times which have been determined in said experix~entse i.e.y
the phosphating times which were required to deposit
uniformly covering phosphate coatings on the sheets. ,,
~3oth figures represent the desirable result which is
achieved with the process in accordance with the invention.
sample 2
In a phosphating apparatus having a
cubic capacity of 5 liters, previously degreased sheets
CA 02022728 1999-OS-03
11
of steel (80%) and electrolytically galvanized steel (20%)
were phosphated in alternation with a phosphating solution
having the following composition:
0.8 g/1 Zn free acid: 0.9 points
1.0 g/1 Ni total acid: 23 points
1.0 g/1 Mn
2.6 g/1 Na
13.0 g/1 P205
2.1 g/1 N03
The solution was at a temperature of 55 to 60°C.
The treatment was effected by spraying for 3 minutes. The
throughput amounted to 3 m2/liter of bath volume at a
throughput rate of 0.1 m2/h. The composition of the bath
was maintained by an addition of zinc carbonate and a
suitably composed replenishing solution throughtout the
processing.
The replenishing concentrate contained, by
weight,
23.4 % P205
1.89 % Na
1.74 % Mn
1.34 % Ni
3.39 % Zn
0 . O1 % Fe (III)
3.09 % N03
and for replenishing to constant points was required in
a rS ~) c; ~f'1 ~t
IvI id ~~ f~.Y
-12-
an amount of 1~ g per square meter of surface area.
To adjust the ratio of free P~ 5 to total P205, basic
zinc carbonate (53~5 Zn) was added to the bath in an
amount of 1.8 g%m2. That replenishment corre~onds to a
ratio of free P205 to total P205 of (-0.18) :1 .
In dependence on 'the measured electro--
chemical potential. hydrogen peroxide was supplied to
such a rate that the steady-state concentration of Fe(II)
ions and the H202 concentration in the bath were not in
excess of 10 mg/1 each. The resulting phosphate coatings
were uniform and closed throughout and had a weight of
2.0-r0:2 g/m2 for steel and of 2.5-~~0.2 g/m2 on electroly-
tically galvanized steel.
sample 3
In a phosphating apparatus having a
cubic capacity of 5 liters, previously degreased sheets
of steel {60~,), electrolytically galvanized steel (30gb)
and aluminum{1096) of the A,lMg3 grades were treated in
alternation with a phosphat3.ng solution which contained
0.8 g/1 Zn free acids 1.1
1.0 g/1 Ni total acid: 23
1.0 g/1 Mn
3.2 g/1 Na
13.0 g/1 P205
2.l g/1 N03
0.5 g/1 P
rd '! ) f t j ~)
27 ~ t~e :~ r.a
_13~
V~hen the conditions mentioned aboee and
steady-state concentrations of Fe(II) and F3202 not in
excess o~ 6 m~/1 were maintained, uni.~orm and. closed
coatings were formed on all three materials and had the
following vrei~hts:
Steel: ~ 2.10.2 g/m2
electrodeposited 2.60.2 g/m2
zinc:
AlSi: 2.9_+0.3g/m~
Al~g3 : 3 a Z+V ~/~~ .
.3