Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
FIELD OF THE INV~;N~l~lON
2 This invention relates to a hollow fiber bundle element
3 for use in an adsorber to separate at least one adsorbable
4 component from a ~ixture of fluid components.
BACKGROUND OF THE INVENTI~N
6 Adsorption processes are widely used in industry for
7 separation of fluid mixtures ~gas or liquid). The separation is
8 based on preferential adsorption of selective components on the
9 surface of solid adsorbents. For efficient separation, the
adsorbent material must have large surface areas to provide
11 reasonable adsorptive capacities. The commonly used adsorbents,
12 such as molecular sieve zeolites, activated carhon, alumina and
13 silica gel, have surface areas of at least 200 m2/g.
14 Most industrial adsorption processes are carried out
in fixed-bed type columns. ~he adsorbent granules are packed and
16 immobilized in a cylindrical vessel. As the fluid mixture to be
17 separated is passed through the packing via the void spaces among
18 the granules, the adsorbable components in the mixture are taken
19 up and retained by the adsorbent.
Since the adsorbent has a limited adsorption capacity,
21 it will become gradually saturated with adsorbate, and periodic
22 adsorbent regeneration is required. For continuoua processing
23 of a feed mixture, a multi-bed system is used in which each bed
24 goes through the adsorption/regeneration cycle in sequence.
Several different regeneration methods have been used
1 commercially. Chief a~ong them are the thermal swing adsorption
2 (TSA) and pressure swing adsorption (PSA) processes. In the TSA
3 process, the saturated adsorbent is regenerated by purging with
4 a hot gas. Each heating/cooling cycle usually requires a few
hours to over a day. In the PSA process, the adsorbent
6 regeneration is effected by purging with a portion of the
7 purified product gas at reduced pressure. The throughput is
8 higher than that of the TSA since faster cycles, usually in
9 minutes, are possible.
Apart from the adsorptive capacity of the adsorbent,
11 the adsorption rate and pressure drop are two important factors
12 that must be considered in adsorber design.
13 Pressure drop through the adsorber column should be
14 ;nl~ized, because high fluid pressure drop can cause movement
or fluidization of the adsorbent particles, resulting in serious
16 attrition and loss of the adsorbent.
17 The adsorption rate has a significant bearing on the
18 efficiency of the adsorption process. This rate is usually
19 determined by the mass transfer resistance to adsorbate transport
from the bulk fluid phase to the internal surfaces of the
21 adsorbent particles. Slow adsorption rate due to large mass
22 transfer resistance will result in a long mass transfer zone
23 (MTZ~ within which the adsorbent is only partially saturated with
24 adsorbate. The adsorbent in the region upstream of the MTZ is
substantially saturated with adsorbate, while that downstream of
26 the MTZ is essentially free of adsorbate. As the fluid continues
27 to flow, the MTZ advances through the adsorber column in the
28 direction of the fluid stream. The adsorption step must be
29 terminated before the MTZ reaches the adsorber outlet in order
1 to avoid the breakthrough of adsorbate in the effluent stream.
2 A long mass transfer zone, which contains a large quantity of
3 partially utilized adsorbent, will, therefore, result in a short
4 adsorption step and inefficient use of the adsorb~nt capacity.
These effects are especially serious for the pressure swing
6 adsorption process.
7 Both the pressure drop and the mass transfer resistance
8 are strongly influenced by the size of the adsorbent particles.
9 Changing the particle size, unfortunately, has opposite effects
on these two important factors. This is elaborated below:
11 (1) The pore sizes of the void spaces among the
12 adsorbent particles in the fixed-bed are
13 proportional to the size of the particles. Since
14 the resistance to the fluid flow through the
lS adsorber is inversely proportional to the pore
16 size of the packed bed, the use of small adsorbent
17 partic'e will cause high pressure drop. For this
18 reason, the SiZ8S of commercial adsorbents for
19 fixed-bed operation are generally larger than 2
mm in equivalent diameter. Adsorbent of smaller
21 particle sizes, such as zeolite crystals (less
22 than 10 microns), are pelletized using binding
23 material to suitable sizes.
24 (2) Almost all the surface areas of commercial
adsorbents are located at the interior of the
26 adsorbent particle. For adsorption to occur, the
27 adsorbate needs to be transported from the
28 external fluid phase to the interior surface of
29 the particle. The transport rate is dominated by
2~
1 two mass transfer mechanisms in series: (a)
2 interfacial mass transfer - diffusion through the
3 fluid boundary layer surrounding the external
4 surface of the adsorbent particle; and (b)
intraparticle mass transfer -- diffusion through
6 the internal pore space (micropores and
7 macropores) of the particle to its interior
8 surface where adsorption takes place. The size
9 of the particle has significant effects on the
rates of these two diffusion processes. Small
11 particles offer large fluid/solid contact areas
12 in the fixed bed for interfacial mass transfer and
13 reduce the path length for the intraparticle
1~ diffusion. Hence, small adsorbent particles will
increase adsorption rate and result in a narrow
16 mass transfer zone for fast and efficient
17 operation of adsorption/desorption cycles.
18 The above discussions and analysis show that small
19 adsorbent particles are desirable for efficient adsorption
processes, but the minimum particle size is limited by acceptable
2I hydrodynamic operating conditions of the fixed bed adsorber.
22 That is, one wants to avoid fluidization and excessive pressure
23 ~drop.
24 It would there~ore be desirable to provide an adsorber
containing adsorbent characterized by a relatively small particle
26 size and yet still able to operate with an acceptable pressure
27 drop.
28 At this point, it is appropriate to shortly describe
29 the structure and operation of a known separation device used for
2 ~ J ~
1 permeation and absorption and referred to as a hollow fiber
2 module. As will become clear below, this module is similar in
3 many respects to a shell and tube heat exchanger. The device is
4 used to separate at least one component (e.g. CO2) from a second
S 'carrier' component (e.g. natural gas) with which it forms a feed
6 mixture. A typical module comprises a cylindrical vessel
7 encapsulating a bundle of small-diameter, elongate~, hollow
8 fibers. The fibers are formed of a material having a
9 permeability which, in the case of a permeation module, is
selected to allow the component to be extracted to diffuse
11 therethrough but to substantially reject the carrier component.
12 In the case of an absorption module, the entire feed mixture may
13 readily diffuse through the fiber wall. The fibers are "po-tted"
14 at their ends in closure means, such as epoxy tube sheets, so
that the ends of the fibers project therethrough, leaving their
16 bores or "lumina" open. The tube sheets function to seal the
17 void space between the fibers at the two ends. The tube sheets
18 further seal or are sealed by means, such as an O-ring~ against
l9 the inside surface of the vessel. The vessel is provided with
a first inlet and first outlet communicating with the ends of the
21 fiber lumina. It further has a second inlet and second outlet
22 communicating with the ends of the void space. In operation, the
23 feed mixture of gases is fed through the second inlet into the
24 void space. In the case of an absorption module, absorbent fluid
is fed into the lumina. The absorbate (CO2) diffuses through the
26 fiber walls from the void space, is collected by the absorbent
27 fluid, and exi~s through the first outlet. The carrier gas,
28 reduced in CO2, leaves through the second outlet.
~o~
1 With this background in mind, it i6 now appropriate to
2 describe the present invention.
3 SUMMARY OF THE INVENTION
4 The present invention involves use of a known article,
namely a module comprising a bundle of hollow fibers contained
6 in an impermeable casing. The fibers each have a microporous
7 permeable wall having pore openings in the range of about 0.05 -
8 5 micrometers ~known as the "microfiltration range"). Minute
9 adsorbent solid particles are emplaced in a first of two
passageways, either the lumina of the fibers or the void space
11 between the fibers. The particles are sufficiently densely
12 packed substantially throughout the length and breadth of the
13 passageway, so as to have a density equal to or greater than the
14 free-standing bulk density of the particles. The particles are
sufficiently small or minute so as to provide fast mass transfer
16 of adsorbate to the interior surface of the adsorbent particles
17 where adsorption takes place. They are "free" particles, not
18 being bonded together by binder or the like. The first
19 passageway containing the particles is sealed at its ends, for
example by an epoxy tube sheet. The pore openings of the fiber
21 wall are smaller than the adsorbent particles involved. These
22 openings, however, are large enough to permit the fluid to
23 diffuse therethrough.
24 The particles are emplaced in the module in a unique
fashion. More particularly, a suspension of the particles in a
26 liquid or gas carrier is pumped under pressure into one of the
27 passageways. The carrier filters through the fiber walls into
28 the other passageway and exits the module, leaving the particles
~ 3
1 trapped in the original passageway. By this process, a dense
2 uniform dispersion of particles is emplaced in the original
3 passageway throughout its length. The particles are individually
4 free but are collectively immobilized in the original passageway
due to the completeness of the packing.
6 The final product, comprising the casing, the hollow
7 fibers, the end closures, and the charge of adsorbent particles,
8 is hereafter referred to as the "element".
9 As a result of assembling the foregoing, minute
iO adsorbent solid particles having fast mass transfer rate are
11 immobilized in the sealed first passageway of the element. Yet
12 an adsorbate component (e.g. C~2 ) of a fluid mixture (e.g.
13 natural gas containing CO2), that is introduced into the other or
14 second passageway, can still reach the adsorbent by diffusing
through a fiber wall to enter the first passageway, wherein it
16 is collected and retained by the adsorbent.
17 The pore openings of the fiber wall are sufficiently
18 large to enable the carrier liquid or gas to filter readily
19 therethrough during the fabrication step of emplacing the packing
of particles in one of the passageways.
21 In this fashion, it is feasible to fabricate the
22 element without high expense and it is possible to use very small
23 adsorbent particles having a very high mass transfer rate, in
24 connection with a pressure-driven fluid mixture to be processed,
without having fluidization occur. And the availability of the
26 second passageway, for the passage therethrough of the fluid
27 mixture, has ensured that only a relatively low pressure drop
28 will occur across the element.
~ 3
1 DESCRIPTION OF THE DRAWINGS
2 Figure 1 is a schematic showing the arrangement used
3 to emplace adsorbent particles in the lumina of an element;
4 Figure 2 ls a sahematic showing the arrangement used
to emplace adsorbent particles in the void space between the
6 fibers;
7 Figure 3 is a schematic showing an element, having the
8 adsorbent particles in the lumina, being used as an adsorber; and
9 Figure ~ is a schematic showin~ an element, having the
adsorbent particles in the void space between the fibers, being
11 used in conjunction with a vessel as an adsorber.
12 DESCRIPTION OF THE PREFERRED EMBODIM~NT
13 The adsorber A can take one of two forms, shown in
14 Figures 3 and 4 (which are not~to scale).
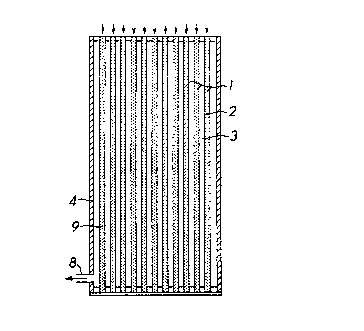
In Figure 3 the adsorber A is the element itself and
1~ comprises a bundle o~ fibers 1, each fiber having a bore or lumen
17 2. The plurality of fibers form a void space 3 between them.
18 An impermeable cylindrical casing 4 contains the bundle. The
19 bundle has top and bottom closures 5, 6 which seal the lumina 2
and void space 3. An inlet 7 is provided at one end of the
21 casing 4, for introducing the feed mixture, comprising a carrier
22 ~luid and an adsorbate fluid, and an outlet 8 is provided at the
23 opposite end of the casing for exhausting a stream comprising the
24 carrier fluid reduced in adsorbate fluid. Particles 9 of
adsorbent are packed in the lumina 2. The fiber walls have sub-
26 micron sized pores which enable the adsorbate to diffuse readily
27 therethrough but the pores are smaller than the adsorbent
2~ particles 9. As a result of providing fiber walls that prevent
~ ~3 3 ~
1 the particles 9 from movin~ therethrough and sealing the ends of
2 the lumina 2 with the closures 5,6, the particles 9 are
3 immobilized in the lumina 2.
4 In Figure 4, the adsorber B has the particles 9
S disposed in the void space 3 between the fibers 1. ~losures 5a,
6 6a are provided and leave the ends of the lumina 2 open but seal
7 the ends of the void space 3. The element 10 of Figure ~,
8 comprising the bundle of fibers 1, closures Sa, 6a and casing 4,
9 is positioned in a vessel 11 having a top inlet 12 and bottom
outlet 13. The inlet 12 and outlet 13 communicate with the ends
11 of the lumina 2. The element 10, as described, combines with the
12 vessel 11 to form the adsorber B.
13 From the foregoing, it will be noted that each of the
14 adsorbers provides a continuous longitudinal flow passageway.
In the case of the adsorber A, the passageway is the void space
16 3. In the case of the adsorber B, the passageway i5 provided by
17 the lumina 2. For separation of fluid mixtures, the feed is
18 directed to flow through the flow passageway. Since the thin and
19 porous fiber wall has negligible mass transfer resistance, the
fluid is always in intimate and substantially uniform contact
21 with the aasorbent particles 9. The adsorbers A, B are adapted
22 for use with PSA and TSA systems in accordance with known
23 technology.
24 Typically the hollow fibers will have a lumen diameter
less than 2 mm. The fiber wall will typically have pore openings
26 of about 0.5 micrometer in equivalent diameter.
27 The adsorbent particles preferably will be selected
28 from the group consisting of molecular sieve zeolites, silica
29 gel, activated alumina, carbon black, and activated carbon. The
1 particle size preferably will be less than 30 microns, most
2 preferably 1 - 30 microns. The surface area preferably should
3 be at least about 200 m2/g.
4 The solid adsorbent particles or crystals (referred to
collectively as "particles") can be packed into the lumina 2 or
6 void space 3 using one of several techniques. More particularly,
7 in the case of non-soluble particles, they are first suspended
8 by agitation in a liquid or gas carrier, such as alcohol, water
9 or air. The suspension is then pumped into the lumina 2 or void
space 3, as shown in Figures 1 or 2. The li~uid or gas carrier
11 is able to permeate readily through the microporous ~iber wall.
12 In the case of pumping the slurry into the lumina 2 (Figure 1),
13 the top ends of the lumina are open, to receive the feed and the
14 bottom ends are sealed. The adsorbent particles 9 become trapped
in the lumina while the carrier diffuses through the fiber walls
16 and exits through an outlet 8 in the casing ~. In this fashion,
17 a charge of densely packed particles may be accumulated to fill
18 the lumina substantially throughout its length. The top ends of
19 the lumina can then be sealed to immobilize the particles.
Similarly, in the case of pumping the slurry into the void space
21 3 (Figure 2), the top ends of the lumina 2 and the void space 3
22 are closed and the bottom ends of the lumina are left open. The
23 slurry enters the void space, the carrier passes through the
24 fiber walls and exits out the bottom of the lumina, and the
particles 9 remain trapped in the void space 3. In bo-th cases,
26 adsorbent loading may be facilitated by vibration by immersing
27 the module in an ultrasonic bath.
2~ In the case of soluble adsorbent materials, the
29 adsorber, having fibers that will not be wetted by the solvent,
2~
1 can be packed by filling a first passageway of the module with
2 the solution and then drying or leaching out the solvent by
3 circulating air or non-solvent through the second passageway of
4 the module.
Still another class of materials that can be used as
6 the adsorbent are those that can be cast in-situ to form a
7 microporous structure by the sol-gel phase inversion techniques.
8 (See Example 2 and Robert E. Kesting, "Synthetic Polymeric
9 Membrane", 2nd Edition, John Wiley, N.Y., 1985). A typical sol-
gel process ~or forming porous structure comprises: preparing
11 a solution of polymeric material, solvent, non-solvent and
12 swelling agent; evaporating or leaching ~he solvent with non-
13 solvent; and drying the non-solvent.
14 The present hollow fiber adsorber has certain
advantages over conventional packed bed adsorbers, namely:
16 (1) In the hollow fiber adsorber, the fluid pressure
17 drop through the adsorber is independent of the
18 particle size of the adsorbent, because the fluid
19 flow path is separated from the particles by the
microporous fiber walls;
21 (2) The hollow fiber adsorber can use very fine
22 adsorbent particles, such as micron sized crystals
23 of molecular sieve zeolites. This will reduce
24 mass transfer resistance, because the use of small
particles increases the fluid/solid interfacial
26 mass trans~er areas and reduces the intraparticle
27 diffusion path length. In addition, the binder
2~ materials contained in the larger pelletized
29 adsorbents used in conventional adsorbers is
12
C~ ~3 ~
1 eliminated, resulting in higher adsorptivs
2 capacities;
3 (3) The hollow fiber adsorber broadens the choice of
4 adsorbent materials for the adsorption proaess.
It can use a wide range of powder materials that
6 have adsorptive properties. If the particle size
7 is small enough, the adsorbent need not be of
8 porous material, because small particles have
9 large external surface areas;
(4) ~he hollow fiber adsorber can use microporous and
11 adsorptive structure that can be cast into either
12 the lumina or void space of the module. Many
13 plastic materials can be converted to microporous
14 matrices by the so-called phase inversion
techni~ue (see Example 2). The fiber wall
16 provides a partition between the matrix and the
17 flow passageway in the fiber module;
18 (5) The microporous hollow fibers provide efficient
19 and uniform contact between the adsorbent
particles and the fluid mixture for a wide range
21 ~ of flow rates, thereby avoiding the channelling
22 problems that can affect the conventional
23 adsorber,
24 (6) The fast mass transfer and low pressure drop of
the hollow fiber adsorber enables the PSA process
26 to be operated efficiently at fast cycle and high
27 feed rates.
28 The invention is illustrated by the following examples:
1 Example I
2 This example sets forth in detail an embodiment of the
3 best mode presently known to applicants for packing one of the
4 passageways with a charge of particles. It further describes the
character of the charge so emplaced.
6 Two hollow fiber modules were made using microporous
7 polypropylene Celgardl hollow fibers manufactured by the Hoechst
8 Celanese Corporation (Charlotte, N.C.). The physical parameters
9 of these modules are given in the following Table. Element 1 was
packed with molecular sieve zeolite crystals in -the fiber lumina
11 (see Figure 1) using cyclohexane as the carrier fluid. Element
12 2 was packed with activated carbon powder in the void space
13 between fibers (see Figure 2) using methanol as the carrier
14 fluid. Both elements were packed using 20 psi slurry solution
of adsorbent particles suspended in the carrier fluid, driven by
16 a diaphragm pump. The slurry pumping operation was then followed
17 by dry nitrogen circulation to dry out the carrier fluid from
18 adsorbent partiales. As shown in the ~able, the resulting hollow
19 fiber elements have adsorbent particle packing density
considerably greater than the free standing particle bulk
21 density. The packing was uniform throughout the length and
22 breadth of the packing space.
23 ITrade-Mark
14
~ ~ 2 6~ ~ ~r;~ ~
1TABLE 1
2Physical Parameters of Hollow Fiber Adsorher Elements
3 ~ollow Fiber ModuleElement 1 Element 2
4 casing, ID, cm .48 .45
fiber typeCelgard2 X20-400 Celgard3 X20-200
6 fibsr number 60 132
7 active fiber length, cm 65 64
8 fiber ID/ m.icrometer400 200
9 fiber OD, micrometer460 260
fiber wall porosity, %40 40
11 fiber wall pore opening
12 microme-ter
13 (width x length).065 x .19 .065 x .19
14 Adsorbent Packings
packing locationfiber lumina outside fibers
16 adsorbent type Union Carbide 5A Darco4 KB carbon
17 particle size, micrometer <10 <30
18 par-ticle bulk density, g/cc
19 (free standing) .49 .25
packing density, g/cc.53 .40
21 total packing weight, g 2.6 2.3
22 2Trade-Mark
23 3Trade-Mark
24 4Trade-Mark
1 Example II
2 This example illustrates the use of very fine, non-
3 soluble adsorbent particles in a hollow fiber adsorber for gas
4 separation.
Two hollow fiber modules were made containing
6 microporous polypropylene Celgards X10-400 hollow fibers. The
7 fiber had a 400 micron internal diameter lumen and 30 micron
8 thick wall. The fiber wall had 30% porosity provided by .065 x
9 .19 microns pore openings. Each of the test modules had 30 open-
ended fibers of 50 cm length encased in a 3/16 inch OD stainless
11 steel tube (.375 cm ID) with both ends of the fiber bundle potted
12 in 3 cm long polyurethane tube sheets.
13 The previously described filtration technique was used
14 to pack a type Y zeolite powder (less than 10 micron size) in-to
the modules. One module was packed with 1.3 g of powder in the
16 fiber lumen, and the other was loaded with 1.7 g of the same
17 powder in the void space between the fibers. The different modes
18 of adsorbent loading were chosen only to demonstrate the
19 workability of each version of the process.
The two modules were plumbed and instrumented to
21 operate as a cyclic pressure swing adsorption (PSA) system in
22 accordance with C. W. Skarstrom, U.S. patent 2,944,627. The
23 cyclic operation was automated with an 8 port valve directing
24 the gas to and from the inlets and outlets of the two adsorbers.
The valve was, in turn, driven by a solenoid controlled by a
26 programmable timer.
27 The PSA system was used to purify a feed stream
28 consisting of helium gas containing 1% CO2. In the first step of
29 5 Trade-Mark
16
~ ~ ~.J C~
1 the PSA cycle, the feed gas, at 200 psig and 23~C, was fed to the
2 first adsorber for CO2-removal at a rate of 200cc (STP)/min.
3 Simultaneously a portion (25cc/min.) of the purified helium was
4 throttled down to about 6 psig and supplied to the second
adsorber to pur~e previously adsorbed CO2. The remainder, still
6 at high pressure, was taken off as purified helium product.
7 After 3.5 minutes, the timer switched the system into
8 the second step of operation. At the beginning of this step, the
9 Eirst adsorber was de-pressurized to atmospheric pressure and the
10 second adsorber was pressurized with feed gas. It then started
11 the adsorption and purging operations for the second and first
12 adsorbers, respectively. The duration of the second step was the
13 same as the first step, and the system was alternated between
14 these two steps in cyclic fashion. The gas flow direction in
15 each adsorber Eor adsorption and pressuri~ation cycles was
16 countercurrent to that for purging and de-pressurization cycles.
17 ~ thermal conductivity gas analyzer was used to measure
18 the CO2 concentration in helium. ~he test results showed that
19 the microporous hollow fiber module, packed with minute adsorbent
20 particles, in both versions, was effective for gas purification
~1 by pressure swing adsorption, because no C~2 could be detected in
22 the purified effluent helium.
23 Example III
24 This example illustrates the use oE the sol-gel phase
25 inversion techni~ue for casting a microporous matrix into the
26 hollow fiber module for use as an adsorbent.
27 A hollow fiber module was made using microporous
28 polypropylene Celgard hollow fibers of 2~0 micron ID and 30
17
~ 3
1micron wall thickness. The fiber wall had 30% porosity with .065
2x .19 micron pore openings. The module had 60 50-cm long fibers
3encased in a 3/16 inch OD nylon tube, with both ends of the fiber
4bundle potted in 3 cm long polyurethane tube sheets.
5A microporous cellulose acetate matrix structure was
6cast into the void space between the fibers by first filling it
7with a cellulose acetate solution (made of 22 g cellulose
8acetate, 132 g acetone, 30 g water and 10 g ZnCl2), and then
9circulating water through the fiber lumina to leach out the
10acetone, followed by dry air circulation to remove water.
11The element was tested for gas dehydration. The water
12content in the gas was measured using a hygrometer. An air
13containing .04% water vapour at 80 psig and 23~C was fed to the
14module through the lumina at a rate of about 400 cc (STP)/min and
15dry air, containing only 20 ppm of water, was obtained from the
16element outlet.
17The moist air started to break through the element
18outlet only after about 20 minutes of operation. The water
19saturated cellulose acetate was able to be regenerated by purging
20the element with 6 psig dry air at 100 cc/min for about 20
2Iminutes.
22Example IV
23This example illustrates the use of non-porous soluble
24particles as an adsorbent in the hollow fiber adsorber. A hollow
25fiber module similar to the one described in Example 2 was packed
26with CuCl2 powder by filling the void space between the fibers
27with a 60~C concentrated aqueous CuCl2 solution (67% CuCl2 by
28weight) followed by dry air circulation through the fiber lumina
18
2 ~
1 to remove water. The module was tested for air dehydration, as
2 described in Example 2. An alr containing .052% water vapour was
3 fed to the module through the fiber lumina at 80 psig, 23~C, and
4 500cc(STP)/min. Dry air containing 110 ppm of water was obtained
from the outlet of the element. The moist air started to break
6 through the element outlet after about 24 hours of operation.
7 The water-saturated CuCl2 was regenerated hy purging the element
8 with 100 cc/min. dry air at 100~C for 12 hours.
9 Example V
This example illustrates the efficiency of the hollow
11 fiber adsorber in the fast-cycle pressure swing adsorption
12 process for high feed gas flow rates.
13 A hollow fiber module was made containing polypropylene
14 Celgard hollow fibers. The fiber had a 200 micron ID and 30
micron thick wall. The fiber wall had 40% porosity provided by
16 about .065 x .19 micron pore openings. ~he module had 132 open-
17 ended fibers of about 70 cm length encased in a 1/4 inch OD nylon
18 tube (0.44 cm ID) with both ends of the fiber bundle potted in
19 3 cm long epoxy tube sheets.
The previously described filtration technique, with
21 the aid of ultrasonic vibration, was used to pack 2.3 g Darco KB6
22 activated carbon powder (particle size less than 30 microns~ into
23 the void space between the fibers.
24 6 Trade-Mark
19
1 The element was plumbed and instrumented as a pressure
2 swing adsorber operating according to the following sequential
3 steps in cycle:
4 (1) Adsorbing adsorbate from a high pressure feed gas
for a predetermined time period to obtain purified
6 gas from the adsorber outlet;
7 (2) Depressurizing the gas remaining in the adsorber
8 (after the adsorption step) through its outlet and
9 into a first gas storage vessel having an internal
volume approximately equal to the internal void
11 volume of the adsorber;
12 (3) Further depressurizing the gas in the adsorber
13 into a second gas storage vessel having the same
14 internal volume;
(4) Venting the remaining gas in the adsorber through
16 its inlet;
17 (5~ Purging the adsorber using the gas stored in the
18 second storage vessel; the purge gas flow
19 direction being countercurrent to the feed gas
direction in the adsorption step;
21 (6) Pressurizing the adsorber using the gas stored in
22 the first storage vessel; the remaining gas in the
23 storaye vessel is then removed as low pressure
24 product;
(7) Further pressurizing the adsorber to feed gas
26 pressure using a portion of the purified high
27 pressure product gas, and thus readying the
28 adsorber for the next adsorption cycle.
1 The aforementioned hollow fiber adsorber containing
2 2.3g of minute activated carbon particles was used to purify a
3 314 psia hydrogen gas containing about 10% C~2 using the above
4 pressure swing adsorption steps. In the tests, we varied the
feed gas flow rate and determined the corresponding maximum
6 permissible adsorption step time without any CO2 breakth~ough
7 from the adsorber outlet. The following results were obtained:
8 Maximum Permissible
9 Feed Rate (Without
10Adsorption Step TimeC~2 Breakthrough)
11Seconds cc (STP)/min.
12 180 200
13 120 300
14 72 ~00
36 1,000
16 17 2,000
17 10 3,600
18 It is seen that the maximum permissible feed gas rate
19 is inversely proportional to the adsorption step time. The
corresponding hydrogen recovery for each of these flow rates is
21 virtually identical and e~ual to about 76%.
22 ~hese test results clearly indicate that the feed gas
23 throughput of a hollow fiber adsorber can be effectively
24 increa~ed without loss of separation efficiency by simply
shortening the PSA cycle time. The high adsorption efficiency
26 at short adsorption c~cle time and high feed rate is made
27 possible by the fast mass transfer rate and low gas pressure drop
2~ in the hollow fiber adsorber using minute adsorbent particles.
29 The scope of the invention is defined by the claims
now following.
21