Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
o.z. 0050/~11~5
The preparation of isoxazole-4 5-dicarbox~~lic diesters
The present invention relates to a process for
preparing isoxazole-4,5-dicarbaxylic diesters of the
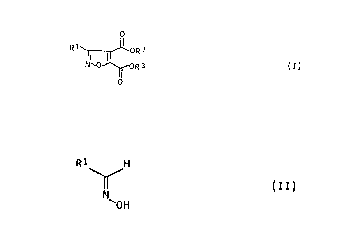
formula I
0
R1 NI 0 I ORS (I)
0
where
R1 is an aliphatic radical of 1 to 20, cycloaliphatic
radical of 3 to 10, aromatic radical of 6 to 10,
heteroaromatic ar heterocyclic radical of 3 to 10 or
aratiphatic radical of 4 to 12 carbon atoms, each
radical being unsubstituted or substituted by
substituents which are inert under the ruction
conditions, and where RZ and R3 are each alkyl, and
to novel isoxazate-4,5-dicarbaxytic diest~rs~.
Isoxazole derivatives, including isoxazale-4,5
dicarboxytic diesters, can be Qrepared by 1,3-dipotar
cycloaddiaion of nitrite oxides IV auto dauble ar, in
particular, triple bonds as shown in the general
equation (1).
R-CAN--s0 + X-CdC-Y -> R N°~0 I Y ( 1 )
IV
Because the nitrite axides IV required for the
reaction are very reactive and only few of those known
are stable after isolation, it is usually necessary to
generate those required for the reaction according to
equ~~tion (1). 3.n situ in the reaction mixture.
Tho generation of nitrite oxides generally starts
fro~a correspondingly substituted nltrome~thyl compounds
~~~~r~s~ ~.
- 2 - o.~. ao5~o4145
which react with activating agents such as phenyl iso-
cyanate, acetic anhydride or acetyl chloride in the
presence of catalytic or stoichiometric amounts of bases,
such as sodium acetate, sodium alcoholates or tart.
amines to give adducts which are unstable under the
reaction conditions and spontaneously decompose to the
corresponding nitrite oxides (cf. Chem. Pharm. Bull. z6
(1978) 3254-3256 and 28 (1980) 3296-3303; Tetrahedron ~0
(1974) 1365-1371 and 42 ((1986) 3825-3840; Bull. Chew.
Soc. Jpn. 59 (1986) 2827-2831). Furthermore, the nitrite
oxide IV can be obtained from the relevant nitramethyl
compounds by acid-catalyzed elimination of water (Bull.
Chem. Soc. Jpn. 57 (1984) 2531-2534).
These processes for preparing isoxazole deriv
atives have the disadvantage that many nitromethyl
derivatives are toxicologically unacceptable and the
preparation thereof is elaborate in some cases and often
uneconomic. Furthermore, the reaction of the nitromethyl
derivatives to give the corresponding nitrite oxides and
subsequently to give the relevant isoxazole derivatives
results in a considerable quantity of various by-products
which are very costly to remove. Accordingly, these
processes are generally not suitable for transfer to the
industrial scale.
This is why processes have been developed for
generating the nitrite oxides by oxidizing the corres-
ponding aldoximes using inorganic hypochlorites. These
processes (cf. DF-A 27 54 832; Synthesis (1982) 508-509)
have the advantage that the niarile oxides can be pre
pared from the aldoximes which are easily obtainable from
the corresponding aldehydes. The disadvantage of these
processes is that their applicability is limited. Thus,
to date it has been possible in this way to prepare only
isoxazole derivatives with alkyl or aryl substituents,
but not isoxazole-4,5-dicarboxylic diesters, One possible
reason for this is that the acetylenedicarboxylic di-
esters required as dipolarophile for preparing these
~L~~~~~~~~
3 - O.Z. 0050/41145
compounds are themselves so reactive that they react very
vigorously with the relevant aldoximes even in the
absence of the hypochlorite oxidizing agent (see Compara°
tive Experiment .~). Especially in the pxesence of cata-
lytic amounts of bases (hypohalites are bases) this
reaction takes place in an uncontrolled and almost
explosive fashion (see Comparative Experiment ~).
Since isoxazole-4,5-dicarboxylic diesters are
intermediates for preparing crop protection agents, it
was an object of the present invention to develop a
process which allows them to be prepared at low cost from
easily obtainable starting compounds, eg. aldoximes.
Another object was to find novel isoxazole-4,5-dicar-
boxylic diesters which are suitable as intermediates for
preparing crop protection agents.
We have found that this object is achieved by a
process for preparing isoxazole-4,5-dicarboxylic diesters
of the formula _T
0
R1 ORZ
N) ~OR3 (r)
(Y~0
where
Rl is an aliphatic radical of 1 to 20, cycloaliphatic
radical of 3 to 10, aromatic radical of 6 to 10,
heteroaromatic or heterocyclic radical of 3 to 10 or
araliphatic radical of 4 to 12 carbon atoms, each
radical being unsubstituted or substituted by
substituents which are inert under the reaction
conditions, and where '
RZ and R3 are each alkyl, which comprises reacting an
aldox3.me of the formula II
CA 02026761 2000-02-24
4
Ri H
(II)
N
OOH
with an acetylenedicarbox~:rlic diester of the formula III
R200C-C-C-COOR3 (III)
in solution of an organic solvant, in the presence of an
aqueous solution of a hypohalite in the pH range from 5 to
10.
Furthermore, novel isoxazole-4,5-dicarboxylic
diesters of the formula I'
0
li RZi
R ~ ~ ~U (I~)
N~R3~
where
R1~ is
C3-Cg-alkyl, ethenyl, isopropenyl, or monocyclic C3-Cg-
cycloalkyl or C5-Cg-cycloalkenyl which is unsubstituted or
substituted by 1, 2 or 3 Cl-C4-alkyl groups, or C1-C6-alkyl
or which is substitued by 1,2 or 3 C3-C~-cycloalkyl,
Cl-C4-alkoxy, halogen and/or cyano groups, or C2-C6-alkyl
which is substituted by 1, 2 or 3 unsubstituted phenyl
groups or 1, 2 or 3 phenyl groups substituted by halogen
and/or C1-C4-alkyl, or tetrahydrofuryl, tetrahydro-
pyranyl, dioxolanyl, dioxanyl or dioxepanyl, each of
which is unsubstituted or substituted by 1, 2 or 3
C1-C3-alkyl and/or halogen groups, and where R2~ and R3'
are identical or different and are each C1-C4-alkyl.
The process according to the invention makes it
possible for the first time to prepare isoxazole-4,5-
dicarboxylic diesters I from the aldoximes II and acetyl-
enedicarboxylic diesters III in accordance with
~~~~"~~~.
- 5 - O.Z. 0050/41145
equation (2):
R1 H
+ R ZOOC-CSC-COOR 2 h~°halite R 1 ~ ( COOK 2
N~OH N~0 C00R3
II III I
In this reaction, the aldoxime II is oxidized in
the reaction medium by the hypohalite to the correspond-
s ing nitrile oxide which is a very reactive 1,3-dipole and
is continuously removed, as it is produced, by the
dipolarophile acetylenedicarboxylic Blaster, which is
likewise present in the reaction medium, in a 1,3-Bipolar
cycloaddition to form the isoxazole compound I.
The hypohalites generally used in the process
according to the invention are hypobromites and hypo-
chlorites, preferably the latter. It is possible to use
for this purpose aqueous solutions of hypochlorous or
hypobromous acid, but preferably alkali metal or alkaline
earth metal hygochlorites or hypobromites are employed,
for example sodium hypochlorite, potassium hypochlorite,
calcium hypochlorite, magnesium hypochlorite, strontium
hypochlorite, barium hypochlorite or the corresponding
hypobromites. Sod~.um, potassium and calcium hypochlorite
are particularly preferred, specifically ~.n the form of
their commercial aqueous solutions. It is, of course,
also possible to use mixtures of various hypohalite
solutions in the process according to the invention.
Since the'hypohalites are generally added as
aqueous solutions to the reaction mixture, whereas the
acetylenedicarboxylic diesters xzL are usually insoluble
or only slightly soluble in the a~aeous phase, this
addition usually results in two phases. In order to avoid
reaction between the acetylenedicarboxylic Blaster III
and the aldoxime II, the compounds are exp~diently
dissolved in an organic solvent. It is possible to use
for this purpose both; solvents which arc immiscible with
- 6 - O.Z. 0050/41145
the aqueous phase and those which dissolve in both
phases, the organic and the aqueous, and thus produce a
homogeneous reaction medium.
Examples of solvents suitable for the process
according to the invention axe alcohols such as methanol,
ethanol, propanol or isopropanol, ketones such as acetone
or methyl ethyl ketone, ethers such as diethyl ether,
methyl tent-butyl ether, tetrahydrofuran or dioxane,
hydrocarbons such as pentane, hexane, cyclohexane,
petroleum ether, white oils or naphtha, aliphatic halo-
hydrocarbons such as methylene chloride, chloroform,
tetrachloromethane, dichloroethane, trichloroethane,
tetrachloroethane or perchloroethane, aromatic compounds
such as benzene, toluene, xylenes or chlorobenzenes,
esters such as ethyl acetate, and dimethylformamide, N-
methylpyrrolidone, dimethyl sulfoxide, sulfolane etc. It
is, of course, also possible to use mixtures of solvents.
The concentration of the solutions of the.aldox
ime and of the acetylenedicarboxylic diester in the
particular solvents is not in general critical for the
success of the process according to the invention, ie. it
is possible to employ both dilute and relatively concen-
trated solutions of these compounds. It is self-evident
that the concentration of the solutions of the aldoxime
and the acetylenedicaxboxylic diester also depends on the
solubility of these compounds in the particular solvent
used> However, it is expedient to use from 0.1 to 2 molar
solutions of the aldoximes and of the acetylenedi-
carboxylic diester~.
When solvents which are insoluble in water are
used it may be advantageous for the progress and result
of the reaction to add phase-transfer catalysts such as
quaternary ammonium or phosphonium salts, eg. triethyl-
benzylammonium chloride, tri,methylbenxylammonium bromide,
triphenylbenzylammonium chlorid~, rnethyltributylammonium
iodide, tetrabutylammonium bisulfate or benzyltributyl-
phosphonium bromide, to tha reaction medium in amounts
- 7 - O.Z. 0050/41145
of, in general, from 0.1 to 10 g/1 of reaction mixture.
It is expedient to stir the reaction mixture particularly
vigorously when the system contains two or more phases.
The temperature at which the reaction is carried
out can vary within wide limits. As a rule, the reaction
takes place at -15°C or even lower, and the upper temper
ature limit is determined in principle only by the
boiling point of the solvent used, because the reaction
is expediently carried out under atmospheric pressure. zt
is expediently carried out at from 0 to 40°C. The reac-
tion can also be carried out under elevated pressure,
especially under autogenous pressure, but atmospheric
pressure is preferred.
In order for the process according to the inven
tion to succeed, and in particular to avoid the side
reactions which have bean described above plus some
others, it is particularly important to carry out the
reaction in the pH range.fram 5 to 10 and particularly
advantageously from 6 to 8, ie. when using a two-phase
system that the pH of the aqueous phase is within this
range, while when using a homogeneous reaction mixture
that this is the pH of the aqueous/organic mixture.
It is expedient to adjust the desired pH of the
aqueous phase or of the aqueous/arganic solution using
buffer substances or solutions before addition of the
hypohalite. Then, dubing the addition of hypohalite, the
pH is advantageously checked continuously and kept in the
desired pH range, preferably constant, by adding further
buffers or acids o~ alkalis if necessary.
The buffer systems which can be used are in
principle all those able to exert their buffering action
in the stated pH range. However, conventional buffers
such as sodium bicarbonate, sodium acetate or the sodium
dihydrogen phosphate/disodium hydrogen phosphate buffer
system are preferably used. The buffers can be added to
the reaction mixture as solids,..but buff~r solutions are
expediently used. The strength of the buffer solutions
~~'~~'~~~.
._ 8 - O.Z. 0050/41145
can in principle be chosen arbitrarily, but in general
from 0.1 to 1 molar buffer solutions are used in order
not to have to handle excessive quantities of liquid.
The procedure for the reactions is usually such
that all the components of the reaction system, except
for the hypohalite, are introduced into the aqueous/
organic reaction mixture, and then the hypohal3.te solu
tion is added to this mixture while stirring vigorously
and continuously monitoring the pH. The optimal rate of
hypohalite addition depends in general on the reactivity
of the reactants and is expediently determined in a
preliminary test.
It may prove advantageous when reacting sensi
tive, ie. particularly reactive, compounds II and/or III
to introduce only one of these into the buffered reaction
mixture and then to add the other reactant at the same
time as the hypohalite. Another possibility in this case
is to introduce one of the reactants II or III completely
and the other reactant only in .a small amount, for
example one tenth of the amount required, and to add the
remainder of this reactant at the same time as the
hypohalite to the reaction mixture. The addition of the
hypohalite solution is advantageously controlled so that
the concentration of hypohalite and nitrila oxide in the
reaction mixture is never high.
To prepare the isoxazole-4,5-dicarboxylic di-
esters I it is expedient to react equimolar amounts of
the aldoxime II and of the acetyhnedicarboxylic di-
ester III with the hypohalite. The hypohalite can be
added in the stoichiometric amount to the reaction
mixture, but as a xule a slight excess, up to a 'two-fold
excess, will be metered into the reaction mixture. It may
be advantageous, for technical reasons, to limit the
conversion by using less than the stoichiometri,c amount
of hypohalite, for example from 50 to 90 mol-% of hypo-
halite per mole of II. It is likewise possible to use
amounts of the reactants II or III which are above or
- O.Z. 0050/4115
below stoichiometric.
The process according to the invention otherwise
displays no technical peculiarities so that further
details are not necessary. The process can be carried out
by conventional techniques, such as use of tube reactors
or stirred vessel cascades, and continuously. Since the
isoxazole derivatives I generally are preferentially
soluble in organic solvents, the working up of the
reaction mixture and the isolation of the isoxazole-4,5-
dicarboxylic diesters can usually be carried out in a
conventional manner, by extraction, distillation or
crystallization. Excess hypohalite, which may impede
working up, can be destroyed by adding reducing agents
such as iron(II) sulfate, thiosulfates or sulfites.
The aldoximes II required in the process accord-
ing to the invention are either known or can be easily
prepared by generally known processes (see, for example,
Houben-Weyl, Methoden der organischen Chemie, Vol. 10/4,
pages 55 to 66, Thieme, Stuttgart 1968) by reacting the
corresponding aldehydes with hydroxylamine. The aldox
imes II can, of course, be used both in the form of their
E or Z isomer and as mixtures of these stereoisomers. The
acetylenedicarboxylic diesters are available commercially
or by known methods ( see, for example, Organic; Syntheses,
Coll: Vol. 4, page 329).
The process according to the invention fox
preparing isoxazole-4,5-dicarboxylic diesters I can be
applied virtually universally. Thus, it is possible to
obtain compounds I from the correspanding aldoximes II
where R1 is an aliphatic radical of 1 to 20, a cycloali-
phatic radical of 3 to 10, an aromatic radical of 6
to 10, a heteraaromatic or heteracyclic radical of 3
to 10 or an araliphatic radical of 4 to 12 carbon atoms.
The upper limit on the number of carbons in Rg is deter-
mined so~.ely by the utilizability of the relevant com-
pounds and daes not derive from a lack of applicability
of the process according to th~ invention when Ri is
- 10 - O.Z. 0050/411A5
larger.
The R1 radicals can also be substituted. The
nature and the number of the substituents can in princi-.
ple be chosen as desired, naturally on the condition that
they are chemically possible, and on the condition that
the substituents are inert to the oxidizing agent, ie.
the basic hypohalite solution, and to the nitrite oxide
which is formed in situ under the reaction conditions.
Thus, the process according to the invention can be used
to prepare isoxazole derivatives I where the aliphatic,
araliphatic or cycloaliphatic radicals R1 contain double
bonds or in which the carbon chains are interrupted by
hetero atoms, especially oxygen atoms.
The nature of RZ and R3, which are introduced into
the compound I from the acetylenedicarboxylic diester, is
not in general critical for the progress of the reaction
and can accordingly be chosen as desired. However, RZ and
R3 are expediently each alkyl, in particular C1-C4-,alkyl.
R2 and R3 can be identical,or different. If they
are different, the cycloaddition of the nitrite oxide IV
with the acetylenedicarboxylic diester III generally
results in a mixture of the regioisomers Ia and Ib
it1 COORZ R1 COORS
N) I COOK 3 NI ( COOR 2
Ia I~
in a ratio which i~ essentially determined by the steric
requirements of R1, RZ and R3. This effect is not critical
and may even be desired depending on how the intermediate
compounds are to be further processed. however, in
general, acetylenedicarboxylic diesters III where R2
and R3 are identical axe preferably reacted to give
compounds I.
It is possible. and advantageous to prepare by the
process according t~ the invention isoxaxoha-4,S-di-
~~~~~'"~~.
- 11 - O.Z. 0050/41145
carboxylic diesters I where R1 is C1-Clo-, especially
C1-Cs-, alkyl, Cz-Clo°. especially Cz-Cs-, alkenyl, C3-CB-,
especially C3-C,-, cycloalkyl or cycloalkenyl, a 5- to 7-
membered aromatic or cycloaliphatic heterocyclic radical
which contains one or two oxygen, nitrogen and/or sulfur
atoms, especially oxygen and/or nitrogen, or phenyl or
benzyl.
R1 can be unsubstituted or carry substituents
which are inert under the reaction conditions.
Thus, alkyl or alkenyl can carry, depending on
the size of Rl, 1, 2, 3, 4 or 5, preferably up to 3,
identical or different substituents such as C3-C,-cyclo-
alkyl, C1-C3-alkoxy, halogen, cyano or phenyl, it being
possible for the phenyl in turn to be substituted by up
to 3, preferably one or two, of the substituents halogen,
C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy, C1-C4-halo-
alkoxy, cyano or vitro, and the substitution pattern of
these phenyl substituents not being critical in general.
R1 as alkyl can be straight-chain or branched. It
is possible and particularly advantageous to prepare
according tc~ the invention compounds I where R' is alkyl
which is substituted by C1-C4-alkoxy, halogen and/or
phenyl, the latter being substituted by, preferably,
halogen atoms and/or Ci-C4-alkyl groups.
It is self-evident to those skilled in the art
that the number of substituents depends on the number of
carbon atoms in the aliphatic radical R1. The substitution
pattern of the aliphatic radical R1 is not in general
critical for the ruction according to the invention.
It is also possible and advantageous to prepare
by the process according to the invention isoxazole.
derivatives I where one carbon atom is substituted by up
to 3 of the said substituents, in particular C1-C$-alkoxy
or halogen substituents. Thus, it is possible and advant-
ageous to prepare compounds I where the aliphatic radi-
cal, especially the alkyl radical, Rl contains the group
~~,~Hl :~.
- 12 - o.z. 0050141145
~c(oR4) a,-cM(oRw) a ox °c(oR~) 3
where RG is C1-C4-alkyl, ie. acetal, ketal or orthoester
moieties.
The cycloalkyl groups R1 can be substituted,
depending on their size, by 1, 2, 3, 4 or 5, preferably
by up to 3, identical or different C1-C4-alkyl, C1-C3
alkoxy or halogen substituents.
It is also possible and advawtageous to produce
by the process according to the invention isoxazole-4,5
dicarboxylic diesters where Rz is a 5- to 7-membered
heterocyclic radical which can b~ substituted by Z to 3
identical or different C1-C4-alkyl, C1-C4-alkoxy, and/or
C1-C4-alkoxycarbonyl, particularly preferably, Cl-C4-alkyl
groups: Heterocyclic R1 can be aromatic or cycloaliphatic
in nature. Heteroaromatic radicals R1 can contain 1 or 2
oxygen, nitrogen and/or sulfur atoms. It is possible and
advantageous to prepare by the process according to the
invention the isoxazole derivatives I where R1 is substi-
tuted or unsubstituted thienyl, pyridyl, pyrazinyl,
pyrianidinyl, pyridazinyl, pyrazolyl, imidazolyl, oxazo-
lyl, thiazolyl, isoxazolyl and isothiazolyl.
It is likewise possible and advantageous to
prepare isoxazole derivatives I according to the inven-
tion where R1 is a 5- to 6-membered cycloaliphatic radi-
cal which contains 1 or 2 nitrogen and/or, preferably,
oxygen atoms. Examples of such heterocycloaliphatic
radicals Rl are substituted or unsubstituted tetrahydro-
furyl, tetrahydropyranyl, 1,3-dioxolanyl, 1,3-dioxanyl,
1,4-dioxanyl, oxepanyl, 1,3-dioxepanyl, 1,4-dioxepanyl,
1,5-d~.oxepanyl, pyrrolidinyl, imidazolidinyl, piperidinyl
or piperazinyl.
It is also possible and advantageous to prepare
by th~ process according to the invention .isoxazole
CA 02026761 2000-02-24
- 13 -
derivatives I where R1 is substituted or unsubstituted
C6-Clo-aryl or C,-C12-aralkyl, in particular phenyl or
benzyl. The aryl radicals, especially phenyl, can carry
1, 2 or 3 identical or different C1-C6-alkyl, C1-C6-halo-
alkyl, C1-C6-alkoxy, C1-C6-haloalkoxy, halogen, nitro or
cyano groups.
It is also possible and advantageous to prepare
by the process according to the invention in particular
the novel isoxazole-4,5-dicarboxylic diesters of the
formula I'
0
Rl OR2~
NI O~OR 3'
I I0
where Rl~ is
C3-CB-alkyl, CZ-Clo-alkenyl, or monocyclic C3-Ce-cycl.oalkyl
or CS-Ce-cycloalkenyl which is unsubstituted or substitut-
ed by 1, 2 or 3 C1-C4-alkyl groups, or C1-C6-alkyl or
CZ-C6-alkenyl which is substituted by 1, 2 or 3 C3-C,-
cycloalkyl, C1-C4-alkoxy, halogen and/or cyano groups, or
Cz-C6-alkyl or -alkenyl which is substituted by 1, 2 or 3
unsubstituted phenyl groups or 1, 2 or 3 phenyl groups
substituted by halogen and/or C1-C4-alkyl, or tetrahydro-
furyl, tetrahydropyranyl, dioxolanyl, dioxanyl or dioxe-
panyl, each of which is unsubstituted or substituted by
1, 2 or 3 Cl-C3-alkyl and/or halogen groups, and where R2~
and R3' are identical or different and are each C1-C4-
alkyl.
These novel isoxazole-4,5-dicarboxylic diesters
are used as intermediates for preparing the novel
isoxazole-5-carboxamides with herbicidal activity which are
described in European laid-open Patent Application
EP-A-0337263.
The alkyl groups R1~ in the novel isoxazole-4,5-
dicarboxylic diesters can be straight-chain or branched.
Particularly preferable with a view to the activity of
-- 1~ - O.Z. 0050/1145
the herbicides prepared therefrom are C3- and C4-alkyl, in
particular isopropyl, sec-butyl arid isobutyl.
The CZ-C8-alkenyl groups R~' in the novel com
pounds I' can likewise be straight-chain or branched and
contain one or twa double bonds. Particularly preferable
with a view to the activity of the crop protection agents
prepared therefrom are ethenyl and isopropenyl.
Preferred cycloalkyl groups Rz' are cyclopropyl
and methyl-, ethyl- and dimethylcyclopropyl.
Particularly preferred substituted alkyl groups
R'' are C1-C3-alkoxymethyl, di-C1-C3-alkoxymethyl, a-C1-C3-
alkoxyethyl, a-C1-C3-alkoxypropyl and a-C1-C3-alkoxybutyl,
and ethyl, propyl, isopropyl, isobutyl, sec-butyl and n-
butyl each of which is substituted by 1 to 3 fluorine,
chlorine or bromine atoms.
Preferred isoxazole-4,5-dicarboxylic diesters I'
with heterocyclic radicals R~' are those where R1' is
tetrahydrofuryl, tetrahydropyranyl, 1,3-dioxanyl and
dioxolanyl.
EXAMPLES
COMPARATIVE EXPERIMENT At
1.8 g (0.01 mol) of diethyl acetylenedicarboxyl-
ate and 0.59 g {0.01 mol) of acetaldoxime were mixed at
room temperature, the solution immediately becoming
yellow. The temperature of the mixture rose to 60°C
within 8 min, after which it was cooled by immersing the
vessel in a dry ice/acetone bath (-60°C). A sample was
taken from the resulting tarry product after 1~ hours and
was analyzed. The c~as chromatogram showed a large number
of compounds, acetaldoxime being no longer detectable.
COIdPARATIVE EXPERIMENT Bs
5 g of dimethyl acetylenedicarboxylate and 5 g of
acetaldoxime were mixed, and one drop of 10 ~ by weight
sodium hydroxide solution was added. The mixture turned
yellow and heated up within 20 sec to 150 to 200°C (d)
so that it started to boil out of the reaction vessel.
After the reaction subsided, the flask contained a black
- 15 - O.Z. 0050/41145
tarry mass which was not investigated further.
EXAMPLE 1
A mixture of 59 g (1.0 mol) of acetaldoxim~,
17.8 g (0.1 mol) of disodium hydrogen phosphate dihy
drate, 15.6 g (0.1 mol) of sodium dihydrogen phosphate
dehydrate, 400 m1 of methylene chloride and 400 ml of
water was cooled to 0°C. The pH of the aqueous phase was
adjusted to 7, and then 14.2 g (0.1 mol) of dimethyl
acetylenedicarboxylate were added to the mixture. To this
vigorously stirred mixture were added dropwise, at from
0 to 10°C, simultaneously but separately 128 g (0.9 mol)
of dimethyl acetylenedicarboxylate dissolved in methylene
chloride (420 ml of solution) and 420 ml of a 13.4 ~ ?~v
weight aqueous solution of sodium hypochlorite (0.9 mol)
over the course of 2 hours, during which the pH was
continuously checked and maintained constant by adding
hydrochloric acid or sodium hydroxide solution. Subse-
quently a further 93 ml of sodium hypochlorite solution
(0.2 mol) were added dropwise. The mixture was then
stirred for 2 hours, after which the aqueous phase was
separated off and extracted twice with methylene chlor-
ide, and the organic phases were combined, washed with
water, dried over sodium sulfate and distilled to remove
solvent.
Dimethyl 3-methylisoxaole-4,5-dicarboxylate was
obtained in a yield of 98 ~ of theory.
The product was further purified by vacuum
distillation, after which the yield was 89.5 ~ of theory.
Melting point 34-35°C
ELE 2
100 ml of methylene chloride, 400 ml of water,
17.8 g (0.1 mot) of disodium hydrogen phosphate dehydrate
and 15.6 g (O.l mo1) of sodium dihydrogen phosphate
dehydrate were mixed and the pH of the aqueous phase was
adjusted to 6.5. At 15°C, 142 g (1.0 m1) of dimethyl
acetylenedicarboxylate were added, fallowed by 5.9 g
(0.1 mol) of aoetaldoxim~. Then, simultaneously but
- 16 - O.z. 0050/41145
separately, a solution of 53.2 g of acetaldoxime
(0.95 mol) in 550 ml of methylene chloride and 550 ml of
a 13.4 ~ by weight aqueous solution of sodium hypochlo-
rite (1.15 mol) were added dropwise over the course of
3 hours while the reaction mixture was vigorously stirred
and its pH was continuously checked. After the addition
was complete, the mixture was stirred for 2 hours and
then worked up as described in Example 1.
Yield: 91.5 ~ of theory.
EXAMPLE 3
To 4 . 2 g ( 0 . 03 mol ) of lpethylpyrazole-4-aldoxa.me
in 40 ml of methylene chloride were successively added,
at 0°C, 6.4 g (0.045 mol) of dimethyl acetylenedicar-
boxylate and 0.1 g of triethylbenzylammonium chloride.
Then 50 ml of an approximately 12 ~ by weight sodium
hypochlorite solution were added dropwise over the course
of one hour, after which the reaction mixture was stirred
overnight and then worked up as described in Example 1.
The crude product was purified .by chromatography on
silica gel (eluent: 3/1 (v/v) cyclohexane/ethyl acetate).
Yield of dimethyl 3-(1-ethyl-4-pyrazolyl)isoxa
zole-4, 5-dicarbaxylate: 30 ~ of theory ( see table for P1MR
data)
EXAMPhES 4 TO 29
Examples 4 to 29 were carried out in a similar
manner to Example 2. The results of these reactions are
listed in the table which contains, apart frori the
isoxazole derivatives I prepared, data on the reaction
temperature, yield, melting point (m. p.) for crystalline
compounds or boiling point (b. p.) if the products were
distilled, and the principal data of the 250 MHz 1H-1~1MR
spectra of these compounds in deuterochloroform (CDC13).
The following abbreviations are used in the
table:
Me: methyl;
Et: ethyl;
iPr: isopropyl
~0~~'~~~.
- 17 -- O.Z. 0050/41145
c-Pr: cyclopropyl;
t-Hu: tert-butyl;
s: singlet;
d: doublet;
to triglet;
q: quartet;
ms multiplet;
~i~~~r~~:~.
.- m -. o.z. oQ~o~~m5
..
M..~
g ~,
.
cV _ ~
~
N
i
.. NM u1
.
v
-
v' o ~ x
M M
_ ~,"~~ M N .-n r,~ N
s C9
U d . ~ s a
p .. v N~ V1
Md
V . a ~ ~ J . .,Qy
E Z ~ a .
S ~-~
~ 1 .,r
MM.-~ E
~ E MM
tIl ~ ~ f0 ~ w'-'ue....
N
NtplL1 X ~ ~, ~p C
i ~ N ICf .U M ~ W
O O~ 01
W
G! , o .
N~ N ~Mf~. i .
~'1 e
~
~
~ ~ ,- .-
a ..
...
..
N
fIl N
L L
a v c~ '~ '
U
. O O ~
\ \
.a ~ W f7
w M M M U U
I ~ i ~ ~ I
d M M M ,-a
E
8g ~ 3$
.
-C ,
41
.r Q1 .r Q M ~ .-a
CO O1 tb CO r.Q1 d1
U
O
~
v
c
~
~ ~ ~ Q o w m n
ro
r ~ " N a-a 161 N
6.
i-~ ~ tC1 O G7
C7
V N
d
t0
E
M ,
Oi
M
CC
~ ~ ~ ~ ~ ~ ~ ~
~
- a
47 N ~ ~ asa.,,
~ ~ t , s ~ w ~
~ .
d
m.
x ~. N c1 ~ a<1 GO
tD
n
f.. w
~fi~~~"~~ ~.
- 19 - O.Z, 0050/41145
s
.. . M
..
r o
n ~ ~
~ n
.r ~ ~ c c r~-
c l -eo
v
.. _ .. a
..
N x~ ~ VI r..l e~'
v E E
~ ~ _ ier
~
. c~ cs O o~M Ic
.
NZ U1 VI ~ Cr'1~9'M.? Zd
~tT1 _ td'1~....y..~. .. ..t,~ ..,
1 1
M ~ tT M~ Ch ~GO ~t .~~
.-, = m v e~ 2 ~ z Vi =
V9 r a
U -9"~ M M~ M -~M NM ~D~
O ,p~ ~" .... ..~ ."." ..
Y A
' ~ E ~ ~i ~
' g
- M zm as xui g x
-
N ~ MM lD 661M Id~M.~ .~.o.-..
~ " "~ .
.
QC a ~ .vr .~. s s a~ o~
~~ 'C9
.. vi 'v cn N N N x ~.-.
~ ~n x vi'o vy
x x
I wM d..r.H~.ta1i ! I .-r I
.-~M ~w.e ...,
t C~ ~Ohape u1 O~ON1Cpo tL"f .4'~9
v ; ' ~~
s
~
s ~-a'~1.~ie'r1M- . ~ s-
.~tC1.- a
r
L L
E
M N
d
, O O
"~ U
V
O O ! I 1 !
.~ O
a o N
E
. -D
N~ ~ d&, 8S ~ 'be 3e
8
~ ~ ~ ~
n ~ ~ ~
p ~
C p 161
i.i
p. <"" tf1 -4
c0
P ~ O O C id1 I I
L 0
~ "~ ,.,,.., P, I o
v 1 O
U 1 ~
a ~
c0
E
C7
4D
~,
~
-- 20 ~. O.Z. 0050/42.45
s
1
c~ N ~.
1 ~f9 o ~ O
p~pp
...M~6'1~~- ~ .
M ~ . ~'f~1~
NN"J ~ ....gN gn ~
N
m ~ Z
c..
,
of
~
~
,
~~ ~ M ,~ ~~
~
~
~ "~V
' Op
. O
r'Z M1 ~ VI 6 Cn a
Vi -..~'e
.
. e ~ ~
.
.p~ r r
. .~tp N
~ r
Z Z ~C~'t~~I~
~
o ~.r~ ~ , ~ ,
~ ..~
~v ve y,..
, +JZ~ g
tllZ'$,'
1 ~ ~~ ~ ~VM ~'y
p~ .~ c'~7 cs~
Hmne Nv
a
E
'P
v ...,
~ o
c " .e.
.r L
V
N ~
~ ~ ~ ~
N Qa 1 1 p
D; fir. ~ O p i
p
.~.
rn
~
N ~ ~ ~. ~
- al - o.z. 0o5o~~m~s
I ~ I C7.... .~. , ..
.....
cn ~ ~ ~ ~ ~'
_ r.
N 1'~ ° '..-a h..-~O
. a .,ps Z
Mh M ~
M~~
U9~ V1 w~..~~~.. .~ NB000
a , a.n ~.~-eP'o O ~ '°
.-~ C5 ~°°~~
OeC ~ ~~r1~flP~ 02 4 mt
V d't'~ ,t a.m .. ~.~.a..~ ~?Q0.-~
p . ~ ~G
.~~.~. :C r~ ~ ov ...,°°~ ~
g = ~ = t~'1l0'~n M h~
f'r1N MCn ~~'h C~le.e0p
VI~ y .w .~
tP1 E Z°~. ~C9e-~a~ t/1 ~~~
w IlIM
f79N OOtD h dfi 01 .v~ C9~ILlh v
Z s a a v vU1 i. d ~ ~h9~ ~
MI°. tT'Ih f,'1°~~R'~ F1~.
s
a
s
a
~ ~ ,
~ g:; 82 bk 3~ ~
.r M 9~ M IC1 ~
r A~ CCD ~ tt'r
...
~ V
0
C ~~..i .~..
n ~o
~r L
+, ~
t7 d u1 ~t7 ~P1 tC1
W 0 B O O dO
~ ~
fr 1.~.
g~
N
\ / \
1~
.P ~
C7
~ ~ ~ ~ ~ ~
~ ~ N N N N N
~~~t~r~~~.~.
- 22 Q.Z.0050/1145
-
z
N
.f,i ..
_ ~D 2
.-.
M
~.~ .. ..
~ ~~
t0 ~wp ~ N N
~ILI o M
M Z ~: ~..r
E o h' ~
Mtl1 M ~ N
I
NM Vih ~ .161rrMB'1
'r' V1~~aVI M
o a
M Of~ a ~ M N
V1 Vf
I a aw O . ..
v ' ..
M 00 ?M 01 .r-~.~..0~awa~...~
.
~" a ~ a .~ ~ Z N =
v a t/i x . ~
V M~ ..~ M~ w N~ 09.~w T
p .. x . ,.." "
. .. ~ "
. ~
$ =1P'9M ~T
QOM tlf.-iM aN MM 6C1iC1tf7111
~
0~ . ~ .r-v N
.rr . n
~ '~9 Vi "~f~N ,-a .a.~
VI T t/1~
=
x .......... ,~,--.-.M u, 1 m I I
I --
I MO~ ~N N Orow.-eOfn1M NM
. .
a v .VI s a a v
wVl a .
.r .-.Ma Mi'4Nr Mf~ NM .-.~'.-.~'
o L L
rp \ ~
O
.
.D 1 I 1 1 ~ .
,..,'\.t~7
it1
s 1 M.-b
w
a ao 00
o
o
a
a
~ as ae ae. a~ a~ ae ae
,~ ~ ~ t~ n 0~ /'m ~
r ~ ,n N M ao co ~
L.V
C
+~
w
L
~ g ~ O o n O
a
In .. ,. a-. .. .~ ...
v ~ ~ ~ o o a o
v
~
~.,
~ ~ a~ a~ as ar ~ .w
.
n ~ ~ ~ g a~ s~ ur
O L
.. r ~ ~ ~
~ . ~ p
~ \
,.
.,.
~ N N p
~ x N a P M trf
V '1