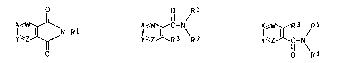Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2 ~ i3 ~
O.Z. ~050J41171
Pyridine derivative~ and their use for controllinq
unde3irable plant qrowth
The present invention relates to nov~l pyridine
derivatives, processes for their prepara-tion and their
use for controlling undesirable plant growth.
N-substituted pyridinedicarboximides and their
derivatives ara known. EP-A-128 006 descr.ibes, inter
alia, N-cycloalkylenepyridinecarboximides and their use
as soil funyicide~.
DE-A 31 22 635 discloses 2,6-dic:hloropyridine-
3,~-dicarboxylic acid N~alkylimides which are suitablQ as
coupling components for azo dyes.
US-A-3 539 568 descri~es a process for the
preparation of 2,3- and 3,4-pyridinedicarboximides and
their conversion into isomeric dicarboxamides, which can
be used as intermediateq for herbicîdal pyrimidinediones.
US-A-4 261 730 discloses 3-carboxypyridine-2-N-
(aryl)-carboxamides and phthalamic acids having a growth-
regulating action.
US-A-4 658 030 discloses a proces~ for the
preparati~n of herbicidal 2-(imidazolin-2-yl~-nicotinic
acids based on 3-carbo~ypyridin-2-(N-carbamido-3-methyl-
2-bu~yl)-carbo~amides.
~elv. ChLm. Ac~a 71 (19~8), 486 and 493 diseloses
a cycloaddition proces~ for the preparation of pyridine
2,3-dicarboxLmide~. These substances are not known to
have herbicidal properties.
WQ have found that pyridine derivatives of the
formulae I'a, I'b and I'c
I ~N~ J ~ 1 1 ~ R3 R1 ~
Y~t ~ Y~ R3 R2 y~ C-N
I'a I'b I'c
where W, X, Y and Z are each C-R4, N or N ~ O, with the
proviso that tha r.ing contain~ only one hetero atom, and
2 0 ~
- 2 - O.Z. 0050/41171
Rl is hydrogen, hydroxyl, Cl-C4-alkoxy, Cl-C~-alkyl which
may carry from one to three of tho following group3:
C1-C4-alkoxy, Cl~C~-haloalkoxy, Cl C4-alkylthio, Cl-C4-halo-
alkylthio, C~-C4-dialkylamino, C3-C8-cycloalkyl) halogen,
cyano, C3-C8-cycloalkyl or phenyl which may be substi~uted
by halogen, cyano, nitro, Cl-C4-alkyl, Cl-C4-haloalkyl,
Cl-C4-alkoxy, Cl~C4-haloalkoxy, Cl-C4-alkylthio or Cl-C~-
haloalkylthio;
C3-C8-cycloal~yl which may carry from one to three of the
following groups: Cl-C~-alkyl, Cl-C~-haloalkyl, C~-C4-
alkoxy, C1-C4-haloalkoxy, halogen, nitro or cyano;
C3-C6-alkenyl or C3-C6-alXynyl, each of which may be
monosubstituted ~o trisubstituted by halogen and/or mono-
substituted by phenyl which may carry from one to three
of the following groups: C1-C4-alkyl, C1-C4-haloalkyl,
Cl-C4-alkoxy, Cl-C4-haloalkoxy, Cl-C4-alkylthio, Cl-C4-halo-
alkylthio~ halogen, cyano or nitro;
C1-C4-dialkylamino;
a 5-membered or 6-membere~ heterocyc}ic radical having
one or two heteroatom~ selected from the group can~isting
of oxygen, sulfur and nitrogen, which may be up to trisub-
stituted by C1-C4-alkyl or halogen;
phenyl which may carry from one to four of the following
groups: C1-C4-alkyl, Cl-C4-haloalkyl, C1~C4-alkoxy, C,-C4-
haloalkoxy, Cl-C4-alkylthio, C1-C4-haloalkylthio, halogen,
nitro, cyano, ormyl, C~-C5-alkanoyl, C~-Cs haloalkanoyl or
C2-C5-alkoxycarbonyl, or
naphthyl which may be monosubstituted to trisubstituted
by C1-C4-alkyl or ha~ogan;
R2 is hydrogen or Cl-C8-alkyl which may carry from one to
three of the following sub~tituen~: hydroxyl, halogen,
C1-C4-alkoxy, Cl-C4-alkylthio or C1-C4-dialkylamino;
C3-Ca-cycloalkyl which may be mono~ub~tituted to tri-
sub~tituted by C1-C4-alkyl, halogen or Cl-C4-haloalkyl,
or
R1 and R2 together form a radical having the structure
-(CH2)n-~Yp-(CH2)q-~ where n and q are each 1, 2 and 3, p i8
2 ~
_ 3 - O.Z. OOS0/~1171
0 or 1 and Y is oxygen or sulfur or N-methyl or the
radical of the formula -(CH2)3-CO-;
R3 is formyl/ 4,5-dihydrooxazol-2-yl or one of the radi-
cal~ -COAR9 or -CONR6R7;
R4 is hydrogen, halogen, nitro, cyano or C1-C6-alkyl which
may be substituted by one to five halogen atoms and/or
one or two of the following radicals: C~-C4-alkoxy, Cl-C4-
haloalkoxy, C1-C4-alkylthio, C1-C4-haloalkylthio, C3-C6-
cycloalkyl or cyano;
benzyl which may be monosubstituted to trisubstituted by
Cl-C4-alkyl, Cl-C4-haloalkyl, Cl-C4-alkoxy, Cl-C4 halo-
alkoxy, Cl-C4-alkylthio, C1-C4-haloalkylthio, halogen,
cyano or nitro;
C3-Ca-cycloal~yl which may be monosubstituted to trisub-
stituted by Cl-C4-alkyl or halogen;
C2-C~-alkenyl which may be mono~ubstituted to tri~ubstitu-
ted by halogen and/or monosubstituted by Cl-C3-alkoxy or
by phenyl which ma~ carry ~rom one to three of the
following groups: C1-C4-alkyl, Cl-C4-haloa}kyl, Cl C4-
alkoxy, Cl-C4-haloalkoxy, Cl-c4-alkylthio~ Cl-C4-haloalkyl-
thio, halogen, cyano or nitro;
C2-C6-alkynyl which may be monosubstituted to trisubstitu-
ted by halogen or Cl-C3-alkoxy and/or monosubstituted by
phenyl which may carry from one to thre~ of the following
groups: Cl-C4-alkyl, Cl-C4-haloalkyl, Cl-C4-alkoxy, Cl-C,,-
haloalkoxy, Cl-C4-alkylthio, Cl-C4-haloalkylthio, halogen,
cyano or n~tro;
Cl-C4-alkoxy, Cl-C4-alkylthio, Cl-C4-haloal}coxy, Cl-C4-
haloalkylthio, C2-Cg-alkenyloxy, Cz-Cs alkynyloxy, Cl-C4-
alkylsulfinyl, Cl-C4-alkylsulfonyl or C1-C4-haloalkyl-
sulfonyl;
phenoxy or phenylthio, each of which may be monosub~titu-
ted ~o trisubstituted by Cl-C4-alkyl, C1-C4-haloalkyl,
Cl-C4-alkoxy, Cl-C4-haloalkoxy, Cl-C4-alkylthio, Cl-C4-halo-
alkylthio, halogen, cyano or nitro;a S-membered or 6-membered heterocyclic radical having
one or two heteroatoms selec~ed ~rom the group con~isting
202 ~
_ 4 _ o.Z. 005~/41171
of oxygen, ~ulfur and nltrogen, which may carry one or
two substituent~ o~ the following groups: Cl-C3-alkyl,
halogen, C1-C3-alkoxy or C2-C4-alkoxycarbonyl, or
phenyl which may carry from one to three of the following
groups. C1-C8-alkyl, Cl-C6-haloalkyl, Cl-C6-alkoxy, Cl-C6-
haloalkoxy, Cl-C6-alkylthio, C1-C6-haloalkylthio, halogen,
nitro or cyano;
A is oxygen or sulfur;
R5 is hydrogen or Cl-C~-alkyl which may b~ sub~tituted by
one ~o fLve halogen atoms or one to five hydroxyl groups
and/or one of the following radical~: Cl-C4-alkoxy, Cl-C4-
alkoxy-C2-C4-alkoxy, cyano, trimethyl~ilyl, Cl-C3-alkyl-
thio, C,-C3-alkylamino, Cl-C3-dialkylamino, Cl-C3-alkyl-
sulfin~l, Cl-C3 alkylsulfonyl, carboxyl, C1-C3-alkoxy-
carbonyl, C2-C4 alkoxycarbonyl-Cl-C3-alkoxy9 C2-C4-alkoxy-
carbonyl-C;~-C4-alkoxycarbonyl,Cl-C3-dialkylaminocarbonyl,
Cl-C3-dialkoxyphosphonyl ,: alkanLminoxy, thienyl r furyl ~
tetrahydrofuryl, phthalimido, pyridyl, benzyloxy or
ben~oyl ~hich may carry ~rom one to three of the follow-
ing groups: Cl-C3-alkyl, Cl-C3-alkoxy or halogen;
benzyl which may carry from one to three of the following
groups: C1-C3-alkyl, Cl-C3-alkoxyt Cl-C3-haloalkyl, halo-
gen, nitro or cyano;
C3-Ca-cyc loalkyl;
phenyl which may carry from one to three of the following
groups: Cl-C4-alkyl, Cl-C4-alXoxy, C1-C4-haloalkyl, Cl-C4-
haloalkoxy, Cl C4-alkoxycarbonyl, halogen, nitro or cyano;
C3-C8-alkenyl, C5- or C6-cycloalkenyl, C3-C~-alkynyl, each
of which may be monosub~tituted by hydroxyl, C1-C4-alkoxy,
halogen or phenyl, which may carry from one to three of
the following group~: Cl-C4-alkyl, C1-C4-alkoxy, Cl-C4-
haloalkyl, halogen, nitro or cyano;
a 5-membered or 6-member~d heterocyclic radical having
one or two he~eroatoms selected from the group consisting
of oxygen, sulfur and nitrogen, or bensotriazolyl;
phthalimido, tetrahydrophthalimido, ~uccinimido or
maleimido;
2()2 ~ L95
- 5 - O.Z. 0050/~1171
one equivalent of a cation from the group consi~ting of
tha alkali or alkaline earth metal~, manganes~, copper,
iron, ammonium and substituted ammonium, or a radical
-N=CR8R9
where
RB and R9 independently of one another are each hydrogen,
Cl-C4-alkyl, C3-C6-cycloalkyl, phenyl or furyl or together
may form a methylene chain having 4 to 7 mamber~;
R6 is hydrogen, C1-C6-alXyl or C3-Ca-cycloalkyl and
R7 is hydrogen or C~-C6-alkyl, or R6 and R7 may fsrm a
methylene chain ha~ing 4 or 5 members,
and salts thereof which can ~e used in agriculture
possess herbicidal activity and are ~elective with
respect to crops.
Pyridine derivative~ of the formulae Ia, Ib and
Ic
7~ ~ N-Rl I ~ C-N~ I ~ R3 /RI
Y~ R3 R2 R2
Ia Ib Ic
where W, X, ~ and Z are each C-R4, N or N ~ O, with the
proviso that the ring contains only one heteroatom, and
R1 is C1 C4-alkoxy or is C3-C6-alkyl which may ~arry from
one to three of the following group~: Cl-C4-alko~y, Cl-C4-
haloalkoxy, C1-C4~alkylthio, C1-C4-haloalkylthio, C1-C4-
dialkylamino, C3-C8-cycloalkyl or halogen,
C3-C8-cycloalkyl which may carry from one to ~hree of the
following groupss Cl-C6-alkyl, Cl-C6-haloalkyl, Cl-C4-
alkoxy, C1-C4-haloalkoxy, halogen, nitro or cyano,
C3-C6-alkenyl or C3-C6-alkynyl, each of which may be
monosubstitutad to trisubstituted by halogen,
R2 i8 hydrogen,
R3 is formyl, 4l5-dihydrooxazol-2-yl or one of the radi
cals -Co-A-R5 or -Co~NR6R7,
A is oxygen or sulfur,
2~
- ~ - O.~. 0050/41171
R4 i~ halogen, nitro, cyano or C1-C6-alkyl which may be
subs~ituted by one to five halogen atom~ and~or one or
two of the following radicals: C1-C4-alkoxy, C~-C4-halo-
alkoxy, Cl-C4-alkylthio, C1-C4-haloalkylthio, C3-C~-cyclo-
alkyl or cyano,benzyl which may be monosub~tituted to trisubstituted by
C~ C4-alkyl, Cl-C4-haloalkyl, Cl-C4-alko.xy, Cl-C4-halo-
alkoxy, Cl-C4-alkylthio, C1-C4-haloalkylthio, halogen,
cyano or nitro,
C3-C8-cycloalkyl which may be mono ubstitutad to tri~ub-
stituted by C1-C4-alkyl or halogen,
C2-C6-alkenyl which may be monosubstitut~d to trisub~titu-
ted by halogen and/or monosubstituted by C1-C3-alkoxy or
by phenyl which may carry from one to three of the
following groups: Cl-C4~alkyl ~ Cl-C4-haloalkyl, Cl-C4-
alkoxy, C1-C4-haloalkoxy, C1-C4-alkylthio, Cl-C4-haloalkyl-
thio, halogen, cyano or nitro,
C2-C6-alkynyl which may be monosubætituted to tri~ubstitu-
ted by halogen or Cl-C3 alkoxy andJor monosub~tituted by
phenyl which may carry from one to three of the following
groups: Cl-C4-alkyl, C1-C~haloalkyl, Cl-C4-alkoxy, C1-C4-
haloalkoxy, Cl-C4-alkylthio, C1-C4-haloalkylthio, halogen,
cyano or nitro,
Cl-C4-alkoxy, Cl-C4-alkylthio, Cl-C4-haloalkoxy, Cl-C4-
haloalkylthio, C2-Cs-alkenyloxy, C2-C5-alkynyloxy, Cl-C4-
alkylsulfinyl, C1-C4-alkylsulfonyl or Cl-C4-haloalkyl-
sulfonyl,
phenoxy or phenylthio, each of which may be mono~ubstitu-
ted to trisubstituted by Cl-C4-alkyl, C1 C4-haloalkyl,
Cl-C4-alkoxy, Cl-C4-haloalkoxy, Cl-C4-alkylthio, Cl-C4-halo-
alkylthio, halogen, cyano or nitro,
a 5-membered or 6-membered heterocyclic radical having
one or two heteroatoms selected from the group consi~ting
of oxygen, sulfur and ni~rogen, which may carry one or
two ~ubstituen~ o~ the following groupss ClC3-alkyl,
halogen, C~-C3-alkoxy or C2-C4-alkoxycarbonyl,
pheny} which may car~y from one to three of the following
20~i~1 95
_ 7 _ o.Z. 0050/41171
group~: Cl-C~-alkyl, C~-C5-haloalkyl, Cl-C6-alkoxy, C1-C6-
haloalkoxy, Cl-C6-alkylthio, C1-C6 haloalkylthio, halogen,
nitro or cyano,
R5 is hydrogen or C1-C6~alkyl which may be substi~uted by
one to five halogen atoms or one to fi~e hydroxyl groups
and~or one of the following radicals: Cl-C4-alkoxy, Cl-C4
alkoxy-C~-C4-alkoxy, cyano or Cl-C3-alkylthîo,
benzyl which may carry from one to three of the following
groups: C1-C3-alkyl, Cl-C3-Alkoxy~ C1-C3-haloalkyl, halo-
gen, nitro or cyano,
C3 -C8-cyc loalkyl,
phenyl which may carry from one to three of the following
groups: C1-C4-alkyl, C~-C4-alkoxy, C1-C4-haloalkyl~ Cl-C4-
haloalkoxy, C2-C5-alkoxycarbonyl, halogen, nitro or cyano;
one equivalent of a cation from the group consisting of
the alkali or alkaline earth metals, manganese, copper,
iron, ammonium and substituted ammonium, or
a radical -N=CRaR9,
R8 and R9 independently o~ one another are each hydrogen,
Cl-C4-alkyl or C3-C6-cycloalkyl or to~ether may ~orm a
methylene chain having 4 to 7 members,
R6 and R7 are each hydrogen or Cl-C6-alkyl or together may
form a methylene chain having 4 or 5 members,
with the proviso
that X and Z in formula Ia are not simultaneou~ly C-R4,
where R4 is halogen, or independen~ly of one another C-R4,
where R4 is halogen or hydroxyl, when Y i~ N,
and that X in ormula Ia is not C-R4, where R4 is phenyl,
when Y is N,
and salts thereof which can be used in agriculture are
novel.
The pyridine deriva~ives Ia, Ib and Ic or I'a,
I'b and I'c may form addition salts, for example with
inorganic and organic acid~ or wi~h alkyl halides, or, if
one of the ~ubstituents ha~ acidic properties, ~aid
derivatives can be reacted with inorganic and organic
base~ to form salt~. The pre~ent invention al~o relates
2~12;^~:~9~
- B o.~. 0050~41171
to the corresponding salts.
Pyridine derivatives which are preferred as
herbicidal active ingredients are those of the formulae
I'b and I'c where R2 i5 hydrogan.
Further preferred pyridine derivatives of the
formulae I'b and I'c are those in which one of the ring
member~ W, X, Y and Z i5 N and the others are Pach C-R4,
Rl is C3-C6-alkyl, C3-C~alkenyl, C3-C6-alkynyl or C3~C8-
cycloalkyl, R2 is hydrogenr ~3 iS a radical -Co-A-R5 and
R4 is hydrogen, halogen, nitro, cyano, Cl-C4-alkyl, Cl-C4-
alkoxy, Cl-C4-alkylthiol Cl-C4-alkylsulfonyl, C1-C4-halo-
alkyl, Cl-C4-haloalkoxy or C,-C4-haloalkylthio. In these
compounds, R5 is preferably hydxogen, C1-C4-alkyl, phenyl
which is unsubstituted or substitu~ed by C~-C4-alkyl,
C1-C4-haloalkyl, C1-C4-alkoxy, Cl-C4-alkylthio or halogen,
or a radical -N=CR8R9, where Ra and R9 are each prefarably
C1-C~ alkyl. A is preferably oxygen; the C~-C4-haloalkyl
radical R4 may be substituted by one to five, preferably
3, halogen atoms.
The pyridine derivatives of the formulae Ia, Ib
and Ic can ~e prepared by various methods:
1. . The compounds Id and Ie are converted into the
pyridine derivative~ of the foxmula Ia by dehydration
with water-eliminating agents, for example acetic an-
hydride or inorganic acid halides. ~he reaction is
advantageously carried out by a procedure in which the
carboxamide3 in an inert organic solvent are initially
taken and about molar amounts of a water-eliminating
agent, if necessary likewise dissolved in an inert
solvent, are added dropwise. The mixture can be worked
up in a conventional manner, for example by hyd~olysi~
with water and filtration o~ the product under 3uction or
by extraction of the product wi~h an organic solvent and
evaporation o~ the organic solvent:
202 ~5
- 9 - O. Z . 0050/~L1171
X~C--NHRl X~CO2H AcOAc
Y~Z~CO2H Y~Z~fi-NHRl or SOCI z Ia
O PHal 3, PHal 5
Id re COCl 2/DMF
Advantageously, solvent~ such a~ halohydro-
carbon~, eg. tetrachloroethane, methylene chloride,
chloroform,dichloroethane,chlorobenzene and 1, 2 -dichloro-
benzene, ethers, eg. diethyl ether, methyl tert-butyl
ether, dimethoxye~hane, diethylene glycol diethyl ether,
tetrahydrofuran and dioxane, dipoIar aprotic solven~s,
eg. acetonitrile, dimethylformamide, dime~hylacetamide,
dLmethyl sul~oxide, N-methylpyrrolidone, 1,3-dimethyl-
tetrahydro-2(lH)-pyrimidinone and 1,3-dimethylimidazol-
idin-2-one, aromatics, eg. benzene, toluene, xylene,
pyridine and quinoline, ketones, eg. acetone and methyl
ethyl ketone, and corresponding mixtures are u ed for
these reactions.
~he reaction can be carried out at from -10C to
the reflux temperature of the particular solvent, pre~er-
ably from 0 to 150C.
The molar ratios in which the required starting
compounds are reacted with one another are in general
from 0.9 : 1 to 5 t 1 for the ratio of water-eliminating
agent to acid amide.
The concentration of ths educt~ in the solvent
(mixture) is in general from 0.1 to 5/ preferably from
0.2 to 2, mol/l.
2. A process for the preparation o~ compounds oP the
formulae Ib and Xc in which R3 is a radical -Co-A-R5, ~ i8
oxygen and R5 i3 hy~rogen, is based on the reactlon o~ a
~ubstituted pyridinedicarboxylic anhydride with an amine.
The reaction is advantageously carried out by a
procedure in which the anhydride II in an inert solven~
2~2 ~9~
- 10 - o.z. 0050/41171
is initially taken and about molar amount~ of an amine
III, if necessary likewise di~olved in an inert solvent,
are added dropwise. After the end of the reaction, the
reaction product i~ filtered off under suction or i~
isolated by evaporating the solvent used, the amides Ib
or their i~omer~ Ic being obtained.
y~ ~ C~O HN~ D I b ~ I c
O R2
Il IIi
The i~omer di~tribution is essentially determined
by the position of the heteroatom. For example, æaN
leads to preferential formation of Ic wherea~ the ~or-
matio~ of Ib takes place preferentially in the case of
Y=N.
AdvantageouYly, solvents such as halohydro-
carbonsl eg. tetrachloroethane, methylene chloride,
chloroform, chlorobenzene and 1,2-dichlorobenzene,
ethers, eg. diethyl ether, methyl tert-butyl ether,
dimethoxyethane, diethylene glycol dLmethyl ether,
tetrahydro~uran and dioxane, dipolar aprotic solvent~,
eg. acetonitrile, dimethylformamide, dimekhylacetamide,
dLmethyl sulfoxide, N-methylpyrrolidone, 1,3-dimethyl-
tetrahydro-2(1H)-pyrimidinone and 1,3-dimethylimidazol-
idin-2-one, aromatics, eg. benzene, toluene, xylene,
pyridine and quinoline, ketones, eg. acetone and me~hyl
ethyl ketone, and corresponding mixtures are used for
these reaction~.
The reaction can be carried out at from -10C to
the re~lux temperature of the particular ~olvent or
~olvent mixture, pre~erably from -20 to 120C.
~he molar ratio~ in which the required ~tarting
compound~ are reacted with one another are from 0.9 : 1
202 7~9~
~ O.Z. OOS0/~1171
to 3 : 1 for the ratio of amine III to anhydride II. The
concentration of the educts in the solvent is from 0.1 to
5, preferably from 0.2 to ~, mol/l.
The pyridinedicarboxylic acids or anhydrides
required as starting materials for this process are
commercially available or known from the literature or
can be prepared by conventional methods. An overview
appear~ in BellstQin H 22, 150-160, E I 531-536, E II
104-111, H 27 261, E I 319, E II 299, R.C. Elderfield,
Heterocyclic Compounds, Vol. I, Chapter 8, J. Wiley and
Sons, N.Y., and E. Klingberg, Pyxidine and its Deriva-
tive~, Part 3, Chapter X, in rhe Chemistry of Hetero
cyclic Compounds, 1962, Interscience Publishers, and in
EP-A-299 362.
3. A proces~ for the synthesis of compounds of the
formulae Ib and Ic, in which R3 is a radical -Co-A-R5, A
is oxygen and R5 is hydrogen or C1-C8-alkyl which is
unsubstituted or substituted by one to five halogen atom~
or one to five hydroxyl groups and/or one of the radicals
Cl-C4-alkoxy, Cl-C4-alkoxy-C2-C4-alkoxy, cyano or Cl-C3-
alkylthio, comprises reacting a pyridinedicarboxylic
mono- or diester IV with an amine III.
Particularly suitable mono- or dialkyl esters IV
are lower alkyl e~ers, pref~rably dimethyl esters or
diethyl ester~, The reaction i3 carried out by a method
in whicA a dicarboxylic mono- or dialkyl e~ter IV i~
treatad with about one equivalent of a primary or secon-
dary amine III at from 0 to 130~C, preferably from 50 to
100C, in an organic solvent. After the reaction is
complete~ the mixture is cooled and tha product i~
filtered off under ~uction or the solution i~ evaporated
down. Tha resulting products of the formulae Ib and Ic
can be further purified by conventional standard methods,
~uch a~ recrystallization or chromstography.
2~i7~5
- 12 ~ O.Z. 0050~1171
Rl
X~h~y~C02R5
I ll + HN\ ~ Ib ~ Ic
Y~Z-~Co2R5 R2
IV III
The i~omer di tribution i5 essentially determined
by the po~ition of the heteroatom. For example, in the
case of a dialkyl ester, Z=N lead~ to the preferential
formation of Ic whereas tha formation of Ib take~ place
preferentially when Y=N. In the case of a monoalkyl
e~ter IV, the particular ester radical i5 di~placed
regardles~ of the position of the heteroatom.
Ethers, eg. diethyl ether, methyl tert-butyl
ether, dimethoxyethane, diethylene glycol dLmethyl ether,
tetrahydrofuran and dioxane, aromatics, eg. benzene,
toluene, ~ylene and mesitylene, alcohol~, eg. methanol,
ethanol, n-propanol~ isopropanol and tert butanol, and
corresponding mixtures are u~ed as solvents for the~e
reactions.
The molar ratio in which the mono- or die~tax IV
and the amine III are used i8 from 0.9 ~ 1 to 2 : l,
preferably from l : l to l : 1.2.
The concentration of the educt~ in the solvent
(mixture) i~ in general from 0.1 to 5, preferably from
0.2 to 2, ~ol/l.
4. Compounds o the formula Ia may al~o be prepared
by reac~ing a monoalkyl pyridinedicarboxylate V with a
halogenating agent and then reacting the product with an
amine III.
X~h~r~C02R5 SOCt2 X~W~r~C02R~ HNRlR2
Y~Z ~ C02H COCl2/DMFY~Z ~ COHal III
or PHal 3, PHal 5
V VI
2 ~
- 13 - o.Z. 0050/41171
The rQaction i~ carried out by a method in which
a half ester V i~ converted in a conventional manner with
an inorganic acid halide, ~uch as thionyl chlori~e,
phosgene, phosphorus trichloride, phosphoru~ tribromide
or phosphoru~ pentachloride, preferably thionyl chloride,
into the acid halide VI.
Advantagaously, the inorganic acid halide i~ u~ed
in an amount of from 1 to 5, preferably from 1 to 2,
molar equivalents, ba~ed on carboxylic acid V used.
It is also po~sible to carry out the raaction
without a solvQnt or in the pre~ence of an inert inor-
ganic solvent; halohydrocarbon~, eg. tetrachloroethane,
methylene chloride, chloroform, dichloroethane, chloro-
benzene and 1,2-dichlorobenzene, and aromatics, eg.
ben2ene, toluene and xylene, are advantageou~ly u~ed.
The reaction i~ carried out at from oac to the boiling
point of the inorganic acid halide or of the solvent
used, prPferably at from 20 to 120C.
In some ca~es, the addition of a catalyst, ~uch
a~ dLmethylfonmamide or 4-dimethylaminopyridine, may be
advantageous. The concentration of th~ cataly~t i~ from
0.3 to 20 mol %, based on carbo~ylic acid V used.
The reaction i~ par~icularly preferably carried
out without a solvent, in thionyl chloride a the inor-
ganic acid halide, a~ from 60 to 90C, in the presence offrom 1 to 10 mol % of dimethylformamide as the catalyst.
The conver~ion of the carbon~l halides VI into
the pyridine derivatives Ia is carried out by a procedure
in which the carbonyl halide, in an inert organic 501-
vent, such a3 dichloromethane or an ether, eg. diethylether or methyl tert-butyl ether, i~ reacted with the
amine III, likewise di~solved in an inorganic solvent.
The amine i~ ad~antageously u~ed in from 2 to 5 time~ the
molar amount, in order to bind the hydrogen halide
formed. I~ ic al~o pos~ible to carry out the reaction in
the presence o~ an auxiliary base, for example a tert~ary
amine (triethylamine). In thi~ ca~e, from 1 to 1.5 molar
2~ 13 2 ~ 1 9 r~
- 14 O.Z. 0050~41171
equivalents of amine are su~ficLent. The reackion
temperature may be from 0 to 50C, preferably from 0 to
20C~ The reaction is generally complete after from 1 to
12 hours. The mixture can be worked up in a conventional
manner, for example by hydrolysis with water and extrac-
tion of th~ product Ia with an organic solvent and
evaporation of thQ organic ~olvent. The product of the
formula Ia can be purified by recrystallization or
chromatography.
5. A process for the preparation of compounds Ib in
which R3 i5 formyl or a radical -CO-A-Rs, where A i~
oxygen and R5 is hydrogen, is based on the reaction of a
pyridinecarbonyl halide VII with an amine III. Preferred
carbonyl halides are the chloride~.
lS In an advantageous procedure, the carbonyl halide
VII, in an inert organic solven~, such as dichloromethalle
or an ether, eg. diethyl ether or methyl tert-butyl
ether, is reacted with an amine III, likewise dissolved
in an oxganic solvent. The amine i~ advantageou~ly used
in from 2 to 5 times, preferably from 2 to 3 times, tha
molar amount in order to bind the hydxogen halide formed.
It iæ also possible to carry out the reaction in the
presence of an auxiliary base, for ex~mple a tertiary
amine ~triethylamine). In this case, from 1 to 1.5 molar
equivalents of amine are sufficient. ~he reaction
temperature may be from 0 to 50C, preferably from 0 to
20C. The reaction is complete in general after from 1
to 12 hours. The mixture can be worked up in a conven-
tional manner, for example by hydrolysis wi~h water and
extraction of the produ~t VIII with an organic solvent
and evaporation of the organic solvQnt.
~RI
~COIldlHIIRIR2 I~COI'~ 1. Alkyl-Li Ib
~Z~ I~I Y~Z~ R2 2. C02 or
DMF
VII VII~
202i~,~95
- 15 - O.Z. 0050/41171
Th~ pyridinedicarboxylic acid semi-amides of the
formula Ib are obtained from the pyridineamide~ VIII by
reaction with a~kyllithium, preferably with the addition
of a solvent which is inert under the reaction condi-
tions, such as diethyl ether, methyl tert-butyl ether,
dimethoxyethane, diethylene glycol dimethyl ether,
dioxane or tetrahydrofuran. As a rule, the reaction is
carrièd out under a nitrogen atmosphere at from -80 to
30C. In this process, the alkyllith:ium compound is
generally used in from ~ to 3 times the molar amount,
based on amide of the formula VIII used. After the
reaction is complete, the mixture is treated with carbon
dioxide, preferably in an inert ~olvent, such as diethyl
ether or, for example, tetrahydrofuran, the desired
products of the formula Ib, wher~ R3 is carboxyl, being
obtained.
Suitable alkyllithium compounds are methyl-
lithium, n-butyllithium, sec-butylli~hium and tert-
butyllithium. In this proce~s, tha organometallic base
is used in from 2 to 4 ~Lmes, preferably from 2 to 2.5
time~, the molar amount, based on amide VIII employed.
Pyridinecarboxamides of the formula Ib, in which
R3 is formyl, can also be obtained by the same process if
dimethylformamide i~ u~ed instead o~ car~on dioxide.
Substituted pyridinecarboxamide~ or formylpyridinecarbox-
amides of the formula Ib are obtained after working up in
a conventional manner. The concentration of the educts
in the solvent i~ in general from 0.1 to 5, preferabLy
from 0~2 to 2, mol/l.
6. A further process for the preparation of the
compounds Ib comprises treating a semi-amide of the
formula If with an alcohol of the formula IX in the
presence of a strong mineral acid, eg. hydrochloric acid
or sulfuric acid.
2~2 ~5
- 16 - O.Z. 0050/41171
O R 1
X~W~y~C~N~~R2 H~
Y~Z ~ C02H HoR5 ~ b
If IX
The process leads to compounds Ib in which R3 i~
a radical -Co-A-R5, A is oxygen and R5 i~ C1-C6-alkyl which
may be ~ubstituted by one to five halogen atom~ or one to
five hydroxyl groups and/or one of the following radicalss
C1-C4-alkoxy, C1-C4-alkoxy-C2-C4-alkoxy, cyano or C1-C3-
alkylthio;
benzyl which may carry from one to thrPe of the following
groups: C~-C3-alkyl, C1-C3-alkoxy, Cl-C3-haloalkyl, halo-
gen, nitro or cyano,
C3-C~-cycloalkyl;
phenyl which may carry from one to three of the following
groups: ~1-C4-alkyl, C1-C4-alkoxy, C~-C4-haloalkyl, C1-C4-
haloalkoxy, C2~C5-alkoxycarbonyl, halogen, nitro or cyano;
a radical -N=CR8R9,
where R8 and R9 independently of one another are each
hydrogen, Cl-C4-alkyl or C3-C~cycloalkyl or together may
form a methylene chain having from 4 to 7 member~.
A~ a rule, the alcohol is used in excess, for
example from ~ to 50 moles per mole of amide If. How-
ever, it is al~o possible to use an inert solvent, such
as methylene ehloride, dichloroethane, chlorobenzene or
: 1,2-dichlorobenzene, an ether, eg. diethyl ethex, methyl
ter~-butyl ether, diethylena glycol dimethyl eth~r,
tetrahydrofuran or dioxane~ or corre~ponding mixtures.
The reaction can be carried out at from 0 to
100C, preferably from 20 to 60C.
7. A further proces~ ~or ~he preparation of the
compounds of formula Ib compri~e~ reac~ing an acid I f
with an alcohol or thiol ~ in ~he presence of a dehydrat-
ing agent (eg. dicyclohaxylcarbodiimide (DCC)) at ~rom
-20 to 50C, preferably from 0 to 30C. ~8 a rule, the
2 ~ 5
- 17 - O.Z. 0050/41171
~tarting material~ are reacted in roughly ~toichiometric
amounts. The reaction is preferably carried out in th~
presence of one of the aboveme~tioned inert solvent~, eg.
tetrahydrofuran, dichloromethane or toluenQ.
O Rl
X~W~r~C-N--R2 ~CC
Y~Z ~ C02H HAR5 ~ ~~~ Ib
I~ X
A i~ oxygen or sulfur and R5 has the meanlng~
stated for proce~ 6.
The molar ratio in which the requixed starting
compounds are reacted with one another are in general
from 0.9 : 1 to 2 : 1 for the ratio of carboxylic acid If
to alcohol or thiol and from 1 : 1 to l ~ 3 for the rat:io
of carboxylic acid If to dehydrating agent.
The concentration of the educts in the solvent :i~
in general from 0.1 to 5, preferably from 0.2 to 2,
molll.
8. A ~ur~her proce3s for the preparation of the
compounds of the formula Ib comprises reacting a pyridine
deri~ati~s Ia with an alcohol in the pre~ence of an
organic base and of an inert ~olvent, for example in one
of the abovementioned hydrocarbon~ or an alcohol at ~rom
0 to 80C, preferably from 20 to 50C. Examples of
suitable organic bases are trie~hylamine, tri-n-butyl-
amine, pyridine, N,N-dLmethylaniline and N,N-dimethyl-
cyclohexyl~mine.
~N-RI HoR5 -- ~ Ic
Ia IX
The novel sub~tituent i8 preferentially intro-
duced meta ~o the heteroatom. Compounds Ic in which R2
202~5
- 18 - O.Z. 0050/41171
is hydrogen, R3 is a radical -CO-A-Rs, A is oxygen and R5
has the meaning~ stated for formula Ic can be prepared in
this manner.
The molar ratios in which the required starting
compounds are reacted with one another are in general
from 0.9 : 1 to 200 : 1 for the ratio of alcohol IX to
pyridine derivative Ia, depending on whether the alcohol
is used directly as a solvent, and from 0.9 : 1 to 2 : 1
for the ratio of tha base to the pyridine derivative Ia.
The concentration of th~ educts in the solvent is
in general from 0.1 to 5, preferably from 0.2 to 2,
mol/l.
9. A proce~s for the preparation of compound~ of the
formulae Ib and Ic in which R2 i~ hydrogen and R3 i~ a
radical -Co-NR6R7 comprises reacting a pyridine derivative
Ia with an amine XI in the presence of one of the above-
mentioned inert solvents, for example an ether or an
alcohQl~ at from 0 to 80C, preferably from 10 to 40C.
The weaker basic amine is preferentially introduced meta
to the heteroatom. Isomeric compound~ can be separated
in a conventional manner, for example by chromatography.
The molar ratios in whlch the required starting
compounds are reacted with one another are in general
from 0.9 : 1 to 20 : 1 for the ratiQ of amine XI to
pyridine derivative Ia. The concentration of the educts
in the solvent is in genexal from 0.1 to 5, preferably
from 0.2 to 2, mol/l.
o
R~
~ll--RI ~ R7 Ib + IC
O
Ia XI
10. Compounds of the formula Ic in which R3 is a
radical CO-A-Rs and R5 i~ one equivalent of a cation are
obtained by reacting a pyridine derivative Ic, where R3
2~2'~ ~5
~ O.Z. 0050~41171
i~ a radical -Co-A-R5 and R5 i~ hydrogen, with one equiva-
lent of the corr~spondin~ salt-formi~g cation. If thi~
i~ an inorganic cation, eg. ~odium, potas3ium or calcium,
the acid Ic is advantageously di~solved or suspended in
water or in a lower alcohol or in a mixture of these, and
one equivalant of the salt-forming cation is added. The
salt-forming cation can be used, for ~xample, in the form
of its hydroxide, carbonate or bicarbonate, preferably in
the form of its hydroxide. The reaction is generally
complete after a few minute~ and t~e reactioll mixture can
be worked up in a conventional manner, for example by
precipitation and filtration undex suction or by evapora-
ting down the solution. For the preparation of compounds
Ic in which R5 is ammonium or organic ammonium, the acid
Ic i~ di~solved or suspended in an organic solvent~ eg.
diethyl ether, tetrahydrofuran or dioxane, and the
mi~ture is treated with one equivalent of amm~nia, an
amine or a tetraalkylammonium hydroxide.
Example~ of amine~ which may be u~ed are methyl-
amine, ethylamine, n-propylamine, isopropylamins, n-
butyl~mine, isobutylamina, sec-butylamine, n-amylamine,
isoamylamine, hexylamine, heptylamine, octylamine,
nonylamina, decylamine, undecylamine, dodecylamine,
tridecylamine, tetradecylamine, pentadecylamine, hexa-
decylamine, heptadecylamine, octadecylamina, methylethyl-
amine, methylisopropylamine, methylhexylamine, methyl-
nonylamine, methylpentadecylamine, methyloctadecylamine,
ethylbutylamine, ethylheptylamine, ethyloctylamine,
hexylheptylamine, hexyloctylamin0, dimethylamine, di-
ethylamine, di-n-propylamine, diisopropylamine, di-n-
amylamine, diisoamylamine, dihexylamine, diheptylamine,
dioctylamine,trimethylamine,triethylamine,tri-n-propyl-
amine, trii~opropylamine, tri-n-butylamine, trii~obutyl-
amine, tri sec-bu~ylamine, tri-n-amylamine, ethanolamine,
n-propanolamine,isopropanolamine,diethanolamine,N,N-di-
ethylethanolamine, N-ethylpropanolamine, N-butylethanol-
amine, allylamine, n-buten-2-ylamine, n-penten-2-ylamine,
2 ~ 5
- 20 - a.z. 0050/41171
2,3-dLmethylbutsn-2-ylamine, dibuten-2-ylamine, n-hexen-
2-ylamina, propenylen~diamine, tallow amine, cyclopsntyl-
amine, cyclohexylamine, dicyclohexylamine, piperidine,
morpholine and pyrrolidine.
In the case of the tetraalkylammonium hydroxides,
it is po~ible to use, for example, tetramethyl-, tetra-
ethyl- or trimethylb~nzylammonium hydroxide. As a rule,
the ammonium salt or organic ammonium salt i~ precipi-
tated from the solution and can be isolated by conven~
tional method~. Alternatively, the salt of the formula
Ic can also be obtained by evaporating down the solvent.
The reaction can be carried out at from -10 to
80C, preferably from 20 to 40C.
11. A proce~s for the preparation of the compound~ of
the formula Ia in which one of the ring members X, Y and
Z is N and another is C R4 where R4 is halogen comprises
first reacting a pyridine derivative of the formula Ia
where one of the ring member~ Y, X and Z i5 N with an
oxidizing agent, for example m-chloroperbenzoic acid or
hydrogen peroxide, in an inert organic olvent, for
example one of the abovementioned halohydxocarbons, such
a~ methylene chloride, or an alkanecarboxylic acid, such
as glacial acetic acid~ to give the N-oxide at from 0 to
100~, preferably from 20 ~o 60Ct and then reacting said
N-oxide with a pho~phorus oxyhalide, for example pho~-
phorus oxychloride, to give the halogen compound at from
80 to 120C.
O O O
I ~N--R 1 OXidatiOn I ~N--R 1 ~ 1~N R 1
Y~N~ Y n~
O O O O
Ia
The molar ratio~ in which the requ.ired ~tarting
compound~ are reacted with one another are in general
2~ 7:~95
- 21 - O.Z. 0050/41171
from 1 : O.9 to 1 : 1.5 for the ratio of imide Ia to the
oxidizing agent. In the subsequent halogenation step, an
inert solvent, such as chlQroben~sne, may ba used, but
the reaction is advantageously carried out directly in
excess phosphorus oxyhalide as a reaction medium.
The concentration of the educt~ in the solvent
(mix~ure) is in general from 0.1 to 51 preferably fxom
0.2 to 2, mol/l.
The individual prncess ~teps axe known from the
literatuxe or can be carried out by rn~thod~ ganerally
known from the literature (J. Org. Chem. 19 (1954),
1633).
12. A process for the preparation of the compounds of
the formula Ia compriseq reacting a haloLmide of the
formula Ia where one or more of the ring member~ W, X, Y
or 2 are C-R4 in which R4 is halogen with the salt of an
alcohol or of ? thiol XII in the presence of excess
slcohol or o an inert organic ~olvent.
o
X 11~
--+y ~ c~N~R1 ~ MeR4 - ~ ~a
la XII
In formulae Ia and XII, R4 i~ Cl-C4-alkoxy or C~-C4-alkyl-
thio, each of which may be monosubstituted ~o trisub
stituted by halogen,
C3- or ~4-alkenyloxy,
C3- or C4 alkynyloxy
or phenoxy or phenylthio, each of which may be monosub-
stituted to trisubstituted by Cl-Cb-alkyl, Cl-C4-haloalkyl,
Cl-C4-alkylthio, C,-C4-haloalkylthio, halogen, cyano or
nitro.
Me is- an alkali metal or alkalino earth metal
ion, for example lithium, sodium, potas~ium, magnesium or
2~)2'7~95
- ~2 - O.Z. 0050/~1171
calcium.
Solven~ such a~ halohydrocarbons, eg. tetra
chloroethane, methylene chloride, chloroform, dichloro-
ethane, chlorobenzene and 1,2-dichlorobenzene, ethers,
eg. diethyl ether, methyl tert-butyl ether, dimethoxy-
ethane, diethylene glycol dimethyl ether, tetrahydrofuran
and dioxane, dipolar ~protic solvents, eg. acetonitrile,
dimethylformamide, dimethylacetamide, dLmethyl ~ulfoxide,
N-methylpyrrolidone, 1,3-dimethyltetrahydro-2(lH)-
pyrimidinone and 1,3-dimethylimidazolidin-2-one, aroma-
tics, eg. ben~ene, ~oluene, xylene, pyridine and quino-
line, ketones, eg. acetone and methyl ethyl ketone,
alcohol~, eg. methanol, ethanol, isopropanol and tert-
butanol, and corresponding mixtures are advantageously
used for these reaction~.
The reaction can be carried out at from 20C to
the reflux temperature of the particular solvent or
solvent mixture, preferably at from 40 to 150C.
The ba~e~ used are hydride~ and alkoxide~ o~
alkali metal and alkaline earth metal cations, in par-
ticular Na~, KH, CaH2, LiH and potassium butoxide. It is
sometLmes al~o useful to use combinations of the above-
mentioned bases.
The molar ratio~ in which the required starting
compounds are reacted with one another are in general
from 0.9 : 1 to 1.5 : 1 for the ratio of alcohol or thiol
to halogenated imide Ia and from 1 ~ 1 to 1 : 3 for the
ratio of alcohol or thio} to the effective base.
The concentrations of the educts in the solvent
are in general from 0.1 to 5, preferably from 0.2 to 2,
mol/l.
The proce~s can be carried out by me~hods gener
ally known from the literature (US-A 4647301).
13. Pyridine derivati~es Ia in which R4 in C-R4 i~
C~-C4-haloalkyl,C1-C4-haloalkoxyorCl-C4-haloalkylmercapto
can be obtained in a firs~ step by haloganation of a
pyridi.nedicarboxylic anhydride of the formula II, where
2~2i~95
- 23 - O.Z. 0050/41171
R4 i~ C1-C4-alkyl, C~-C4-alkoxy or Cl-C4-alkylthio, at from
60 to 150C, preferably from 80 to 130C, in the presence
of one of the abovementioned inert aromatic halohydrocar-
bons, eg. chlorobenzene, and in the presence or absence
S of a free radical initiator, ~uch a3 ~ azoisobutyro-
nitrile. Thi~ is followed, in a second step~ by halogen
exGhange by antimony(III) fluoride in the presence or
absence of a catalytic amount of antimony(V~ chloride.
For this purpo~e, a mixture o~ anhydride II' and
antLmony(III) fluoride is heated to about 80C, if nsces-
sary the catalyst is then added a little at a time and
the mixture is heated further until the exothermic
reaction continue~ by itself, preferably at from 110 to
140C. After stirring has been carried out for from 20
~o 60 minutes, the reaction solution is taken up in a
chlorohydrocaxbon, decomposition is effected in a conven-
tional manner with hydrochloric acid and the organic
pha~e i8 worked up. The anhydride II~' thus obtained i~
then converted in~o tha correspondiny Lmide Ia by
proces~es 1 and 2.
q~_+~ ~ o ~ H~l-Ri-+ ~ O SbF3
Y~Z Hal - Cl, Br Y~ SbC~5
O ' O
Il II'
~Y+~`~c~O I HN\ p~esses 1 ~r Z , 1
8 R2
The molar ratios in which the required starting
compound~ are reacted with one another are in general
from 0.9 ~o 3 moles of halogen for the C-H bond to be
halogenated in II and from 0.25 to 0.33 mola of antimony-
~III) fluoride for the halogen equivalent to be exchanged
~27~95
- 24 - ~.Z. 0050/41171
in II.
The concentration of the educts in the solvent
(mixture) is in general from 0.1 to 5, preferably from
0.2 to 2, mol/l.
The process can be carried out by methods gener-
ally known from the litarature (DE-A-2 91~ 915), and the
degree of fluorlnation can be controlled by the amount o~
fluorinating agent.
1~. Compounds of the formula Ia in which R4 in C-R4 i~
Cl-C4-haloalkyl are obtained if ~nides I~a ~ubstituted ~
to the heteroatom by Cl-C~-alkyl are reacted, in a fixst
step, with an oxidizing agent, for example m-chloroper-
b~nzoic acid or hydrogen peroxide, in an inert organic
solvent~ for example one of the aboveman~ioned halohydro-
carbons, such as methylene chloride or chlorobenzene, or
an alkanecarboxylic acid, ~uch a glacial acetic acid, or
an aromatic, such as toluena, to give the corresponding
N-oxides at from 0 to 100C, preferably ~rom ~0 to 60C,
and ~he latter are then conver~ed into the halogen
compounds I''~a with a phosphorus oxyhalide, for exampls
phosphorus oxychloride, a~ ~rom 80 to 120~C. For ~he
pyridine-2,3-dicarboximides, the reaction takes place in
accordance with the following scheme:
O O O
x~~ Rl x~e~ 1 x~
R4~ 4J~fi~ R4J~
o o o o
l"a I"'a
(R4 = Cl-C6- alkyl) (R4 - Cl-C4-nalo~
The molar ratios and the procedure are based on
the reaction conditions of process 11. The individual
process steps- can be carried out by methods generally
Xnown from the literature.
- 25 - O.Z. 0050/41171
In view of the intended uYe of the compound~ I~a,
I'b and I'c, for example, the following radical~ are
suitable substituen~s:
W, X, Y and Z are each C-R4, nitrogen or N-oxide;
S Rl is hydrogen, hydroxyl, C1-C4-alkoxy, such as methoxy,
ethoxy, propoxy, l-methylethoxy, butoxy, l-methylpropoxy,
2-methylpropoxy or 1,1-dimethylethoxy, in particular
methoxy, ethoxy, l-methylethoxy or 1,1-dimethylethoxy;
Cl-C6-alkyl, such as methyl, ethyl, propylg l-methylethyl,
butyl, 1-methylpropyl, ~-methylpropyl, 1,l-dimethylethyl,
pentyl, l-methylbutyl~ 2-methylbutyl, 3-methylbutyl, 1,1-
dimethylpropyl, 1,2-dimethylpropyl, 2,2-dLmethylpropyl,
1-ethylpropyl, hexyl, l-methylpentyl, 2-methylpentyl, 3-
methylpentyl, 4-methylpentyl~ dLmethylbutyl, 1,2-
dimethylbutyl, 1,3-dimethylbutyl, 2,?-dimethylbutyl~ 2,3-
dLmethylbutyl, 3,3-dimethylbutyl, l-ethylbutyl, 2-ethyl-
butyl, 1,1,2-~rimethylpropyl t 1,2,2-trimethylpropyl, 1-
ethyl-l-methylpropyl or 1-ethyl-2-m0thylpropyl, in
particular methyl, ethyl, propyl, 1-methylethyl or l,l
dLmethylethyl, which may carry from one ~o three of the
following groups:
: C1-C4-alkoxy as stated above, in particular methoxy or
ethoxy;
haloalkoxy, ~uch a~ difluoromethoxy, trifluoromethoxy,
chlorodifluoromethoxy, dichlorofluoromethoxy, 1-fluoro~
ethoxy, 2-fluoroethoxy, 2,2-difluoroethoxy, 1,1,2,2-
tetrafluoroethoxy,2,2,2-trifluoroethoxy,2-chloro-1,1,2-
trifluoroethoxy or pentafluoroethoxy, in particular
trifluoromethoxy or pentafluoroe~hoxy;
alkylthio, such as mathylthio, e~hyIthio, propylthio~
1-methylethylthio, n-butylthio, 1-methylpropylthio,
2-methylpropylthio or l,l-dimethy].ethylthio, in particu-
lar methylthio or ethylthio;
haloalkylthio, such as difluoromethylthio, trifluoro-
methylthio, chlorodifluoromethylthio, l-fluoroethylthio,
2-fluoroethylthio, 2,2-difluoroethylthio, 2,2,2-tri-
fluoroethylthio, 2-chloro-2,2-difluoroethylthio,
2 ~
- 26 - o.z. 0050/~1171
2,2-dichloro-2-fluoroethylthio, 2,2,2-trichloroethylthio
or pentafluoroethylthio, in particular trifluoromethyl-
thio or pentafluoroethylthio;
dialXylamino, such a~ dimethylamino, diethylamino,
dipropylamino, diisopropylamino, dibutylamino or methyl-
ethylamino, in particular dimethylamino or methylethyl-
amino;
cyano;
halogen, such as fluorine, chlorine, bromine or iodine,
in particular fluorine or chlorine;
cycloalkyl, ~uch as cyclopropyl, cyclobutyl, cyclopentyl,
~yolohexyl, cycloheptyl or cyclooctyl~ in particular
cyclopropyl, cyclopentyl or cyclohaxyl, or phenyl which
may be substituted by halogen, cyano, nitxo, alky:L as
stated above, in particular methyl or ethyl, haloalkyl
as stated above, in particular trifluoromethoxy;
alkoxy, a~ stated above, in par~icular methoxy or e~hoxy,
haloalkoxy a~ stated above, in partizular trifluoro-
methoxy, or haloalkylthio;
C3-C8-cyclo lkyl a~ stated above, in particular cyclo-
propyl, cyclobutyl, cyclopentyl or cyclohexyl, which may
carry from one ~o three of ~he following groups: alkyl
as stated above, in particular methyl, ethyl or i30-
propyl, haloalkyl a~ stated above, in particular tri-
fluoromethyl, alkoxy as stated above, in particular
methoxy or ethoxy, haloalkoxy as stated above, in par-
ticular trifluoromethoxy, halogen as stated above, in
particular fluorine or chlorine, nitro or cyano;
C3-C6 alkenyl, such as 2-propenyl, 2-mPthylethenyl, 2-
~utenyl, 3-butenyl, 1-methyl-2-propenyl, 2-methyl-2-
propenyl, 2-pentenyl, 3-pentenyl, 4-pentenyl, 3-methyl-
2-butenyl, 1-methyl-2-butenyll 2-methyl-2-butenyl, 1-
methyl-3-butenyl,2-methyl-3-butenyl,3-methyl-3-butenyl,
1,1-dLmathyl-2-propenyl, 1,2-dimethyl-2-propenyl, 1-
ethyl-2-propenyl, l-hexenyl, 2-hexenyl, 3-hexenyl, 4-
hexenyl, 5-hexenyl, 1-methyl-2-pentenyl, 2-methyl-2-
pentenyl, 3-methyl 2-pentenyl, 4-methyl 2-pentenyl,
2 0 ~
- 27 - O,~. 0050/41171
l-methyl-3-pentenyl, ~-methyl-3-pentenyl, 3-methyl-3-
pentenyl, 4-methyl-3-pentenyl/ 1-methyl-4-pentenyl, 2-
methyl-4-pantenyl, 3-methyl-4 pentenyl, 4-methyl-4-
pentenyl,l,l-dimethyl-2-butenyl t 111-dimethyl-3-butQnyl,
1,2-dimethyl-2-butenyl, 1,2-dimethyl~3-butenyl, 1,3-
dimethyl-2-butenyl~1,3-dimethyl-3-butenyl r 2,2-dimethyl-
3-butenyl, 2 r 3-dimethyl-2-butenyl, 2,3-~imethyl-3-
butenyl, l-ethyl-2-butenyl, 1-ethyl-3-butenyl, 2-ethyl-
2-butenyl,2-ethyl-3-butenyl,1,1,2-trLmethyl-2-propenyl,
1-ethyl-1-methyl-2-pentenylorethyl-2-methyl-2-pentenyl,
in particular ethenyl, 2-propenyl, l-methyl~thenyl, 2-
butenyl, 3-butenyl, l-methylpropyl, 1-methyl-2-propenyl,
2-methyl-2-propenyl, which may be monosub~tituted to
tri ubstituted by halogen as stated abo~e, in particular
f luorine or chlorine, and/or monosubstituted by phenyl,
where the phenyl radical in turn may carry from one to
three of the following g.roups: alkyl as ~tated above~ in
particular trifluorome~hyl or chlorodifluoromethyl,
alkoxy as stated above, in particular mathoxy or ethoxy,
haloalkoxy a~ stated above, in particular tri1uoro-
methoxy, trichloromethoxy or pentafluoxoethoxy, alkylthio
as stated above, in particular methylthio or ethylthio,
haloalkylthio as stated above, in particular difluoro-
methylthio, trifluoromethylthio or pentafluoromethylthio,
halogen as stated above, in particular fluorine or
chlorine, cyano or nitro;
C3-C6-alkynyl, such as propargyl, 2-butynyl, 3-butynyl, 1-
methy}-2-propynyl, 2-pentynyl, 3-pentynyl, 4-pentynyl, 1-
methyl-3-butynyl~2-methyl-3-butynyl,1-methyl-2-butynyl,
3-methyl-1-butynyl, 1,1-dimethyl-2-propynyl, 1-ethyl-2-
propynyl, 2-hexynyl~ 3-hexynyl, 4-hexynyl, 5-hexynyl, 1-
methyl-2-pentynyl, 1-methyl-3-pentynyl, 1-methyl-4-
pentynyl, 3-methyl-4-pentynyl t 4-methyl-2-pentynyl, 1,1-
dimethyl-2-butynyl,l,l-dimethyl-3-butynyl,1,2-dimethyl-
3-butynyl, 2,2-dimethyl-3-butynyl, 1-ethyl-2-butynyl, 1-
ethyl-3-butynyl, 2-ethyl-3-bu~ynyl or l-ethyl-l-me~hyl-
2-propynyl, in particular 1-methyl-2-propynyl or
2 ~ 9 ~
- 28 - o.Z. 0050/41171
1,1-dimethyl-2-propynyl, which may be monosubstituted to
trisubstituted by halogen as stated above, in particular
fluorine or chlorine, and/or monosubstituted ~y phenyl,
where the phenyl radical in turn may carry from one to
three of the following groups: alkyl a~ stated above, in
particular methyl, ethyl or l-methylethyl, haloalkyl a~
stated above, in particular trifluoromethyl or chlorodi-
fluoromethyl, alkoxy as stated a~ove, in particular
methoxy or ethoxy, haloalkoxy as s~at~d above, in par-
ticular trifluoromethoxy, trichloromathoxy or penta-
fluoroethoxy, alkylthio as stated above, in pa.rticular
methyl~hio or ethylthio, haloalkylthio a~ stated above,
in particular difluoromethylthio, trifluoromethylthio or
pentafluoromethylthio, halogen a~ stated above, in par-
ticular fluorine or chlorine, cyano or nitro;
Cl-C4-dialkylamino as stated above, in particular di-
methylamino, diethylamino or diisopropylamino;
a 5-membered or 6-membered satuxated or aromatic hetero-
cyclic radical con~aining one or two hetero atoms selec-
ted from the group con~isting of oxygen, sulfur andnitrogen, such as tetrahydrofuryl, tetrahydropyranyl,
furyl, thienyl, thiazolyl, pyrazolyl, pyrrolyl, pyridyl,
morpholino, piperidino or pyrLmidyl, for example 2~
tetrahydrofuranyl, 3-tetrahydrofuranyl, 2-tetrahydro-
thienyl, 3-tstrahydrothienyl, 2-tetrahydropyranyl, 3-
tetrahydropyranyl, 4-te~rahydropyranyl, 2-furyl, 3-furyl,
2-thienyl, 3-thienyl, 3-isoxazolyl, 4-isoxazolyl, 5-
isoxazolyl, 3-isothiazolyl, ~ o~hiazolyl, S-isothiazol-
yl, 2-oxazolyl, 4 oxazolyl, 5-oxazolyl, 2-thiazolyl, 4-
thiazolyl~ 5-thiazolyl, 2-imidazolyl, 4-imidazolyl/ 5-
imidazolyl, 2-pyrrolyl, 3-pyrrolyl, 3-pyrazolyl, 4-
pyrazolyl~ 5-pyraæolyl, 2 pyridyl, 3-pyridyl or 4-
pyridyll which may carry from one to thrae o the follow-
ing sub3~ituent~: alkyl a~ ~tated above, in particular
methyl or ethyl, or halogen a~ ~tated above, in par-
ticular fluorine or chlorine;
phenyl which may carry from one to four of the following
~2 ~195
- 29 - O.Z. 005~/41171
groups: alkyl as stated above, in particular methyl,
ethyl or isopropyl, haloalkyl as stated above, in par-
ticular trifluoromethyl or chlorvdifluoromethyl, alko~y
as stated above, in particular methoxy or ethoxy, halo
alkoxy as stated above, in particular trifluoromethoxy,
trichloromethoxy or pentafluoroethoxy, alkylthio as
stated above, in particular mekhylthio or ethylthio,
haloalkylthio as stated above, in particular difluoro-
methylthio, trifluorome~hylthio or pentafluoromethylthio,
halogen a~ stated above, in particular fluorine or
chlorine, cyano, nitro, formyl, Cl-C4-alkanoyl, such as
acetyl, propionyl or butyryl, in particular acetyl,
haloalkanoyl, such as trifluoroacetyl, trichloroacetyl,
or pen~afluoropropionyl, in particular trifluoroacetyl/
lS or alkoxycarbonyl as stated under Rl, in particular
methoxycarbonyl;
naphthyl which may be monosubstituted to trisubstituted
by alkyl as stated under R1, in particular methyl or
ethyl, or halogen as stated under Rl, in particular
fluorine or chlorine;
R2 i~ hydrogen;
C1-C6-alkyl as stated under R1, in particular methyl,
ethyl, l-methylethyl, which may carry from one to three
of the following substituent~: hydroxyl, halogen as
stated under Rl, in particular fluorine or chlorine,
alkoxy as stated under Rl, in particular metho~y or
ethoxy, alkylthio a~ stated under Rl, in particular
methylthio or ethylthio, or dialkylamino a~ stated under
R5, in particular dimethylamino;
C3-Ca cycloalkyl as stated under Rl, in particular cyclo-
propyl, cyclobutyl, cyclopentyl or cyclohexyl, which may
be monosub~tituted to trisubs~i~uted by alkyl a~ ~ated
under R1, in particular methyl, ethyl or isopropyl,
halogen as stated under Rll in particular ~luorine or
chlorine, or haloalkyl as stated under Rl, in particular
trifluoromethyl,
or
2 :12 7i9~
- 30 - o.Z. 0050~41171
Rl and R2 together form a C~-C~-methylene chain which may
he interrupted by oxygen, sulfur or N-methyl, such a~
- ( CH2 ) 3~ CH2 ) 4- ~ - ( C~2 ) 5- ~ - ( CHz)~-, -CH2-O-CH~ CH2-CH2-
O-CH2-CH2-, -CH2-S-CH2-, -CH2-CH2-S-CH2-CH2-1 -CH2-CH2-
N~CH3)-CH2-CH2-, in particular -(CH2) 5- or -CH2-CH2-O-CH2-
CH2-, or the radical of the formula -(C~I2)3-CO-7
R3 is formyl, 4,5-dihydrooxa~ol-2-yl or a radical -COARs
or -CoNR6R7;
A is oxygen or sulfur;
R5 is hydrogen;
alkyl as stated for Rl, in particular methyl, ethyl,
propyl, 1-methylethyl or hexyl, which may carry from one
to five halogen atoms as stated under R1, in particular
fluorine or chlorine, from one to five hydroxyl groups
and/or one of the following radicals: alkoxy a~ stated
under R~, in particular methoxy or ethoxy;
alkoxyalkoxy, such as methoxyethoxy, ethoxyethoxy or
pxopoxyethoxy, in particular methoxyethoxy, cyano,
trLmethylsilyl, alkylthio as stated under Rl, in par-
ticular methylthio or ethylthio, alkylamino, such as
methylamino, ethylamino, propyl~mino or 1-methylethy}-
amino, in particular methylamino or ethylamino, dialkyl-
amino, such a~ dLmethylamino, diethylamino, dipropyl~
amino, diisopropylamino or methylethylamino, in par-
ticular dLmethylamino or methylethylamino, alkylsulfinyl,
such as methyl~ulfinyl, ethylsulfinyl, propylsulfinyl or
l-methylethylsulfinyl, in particular methylsulfinyl or
ethylsulfinyl, alkylsulfonyl, such as methylsulfonyl,
ethylsulfonyl, propyl~ulfonyl or l-methylethylsulfonyl,
in particular methylsulfonyl or ethylsulfonyl, carboxyl,
alkoxycarbonyl, such as me~hoxycarbonyl or ethoxycar-
bonyl, alkoxycarbonylalkoxy, such a~ methoxycarbonyl-
methoxy, me~hoxycarbonylethoxy or ethoxycarbo~ylethoxy,
alkoxycarbonylalkoxycarbonyl, such a~ methoxycarbonyl-
methoxycarbonyl, methoxycarbonyle~hoxycarbonyl or ethoxy-
carbonylethoxycarbonyl, dialkylaminocarbonyl, such as
dimethylaminocarbonyl, diethylaminocarbonyl,
2 ~ 2 i~
- 31 - O.Z. 0050/41171
dipropylaminocarbonyl, diisopropylaminocarbonyl or
methylethylaminocarbonyl, in particular dLmethylamino-
carbonyl or diethylaminocarbonyl, dialkoxyphosphonyl,
such as dimethoxyphosphonyl, diethoxypho phonyl, di-
propoxyphosphonyl or diisopropoxyphosphonyl, in par-
ticular dLmethoxyphosphonyl or diethoxyphosphonyl,
alkanLminoxy, such as 2-propaniminoxy, thienyl, uranyl,
tetrahydrofuranyl, N-phthalimido, pyridyl, benzyloxy or
benzoyl, where thesa cyclic radicals may carry from one
to three of the following groups: halogen, in particular
fluorine or chlorine, alkoxy, in particular methoxy or
ethoxy, or alkyl, in particular methyl or ethyl;
benzyl which may car~y from one to three of the following
groups: nitro, cyano, halogen, in particular fluorine or
chlorine, alkyl as stated under R1, in particular methyl
or ethyl, alkoxy as stated under Rl, in particular meth~
oxy or ethoxy, or haloalkyl as stated in general and in
particular under R1;
cycloalkyl as stated under R1, in particular cyclopentyl
or cyclohexyl;
phenyl which may carry from one to three of the ~ollowing
groups: alkyl a~ stat~d under R1, in particular methyl
or ethyl, alkoxy as stated under R1, in particular meth-
oxy or ethoxy, haloalkyl as ~tated under-R1, in particular
trifluoromethyl, haloalkoxy as stated under R1, in par-
ticular trifluoromethoxy, alkoxycarbonyl as stated above,
in particular methoxycarbonyl, halogen as stated under R1,
in particular fluorine, chlorine or bromine, nitro or
cyano;
C3-C8-alkenyl as ~tated under Rl, in par~icular 2-propenyl
or 2-butenyl, Cs~ or C~-cycloalkenyl, such a3 2-cyclo-
pentenyl or 2-cyclohexenyl, in particu}ar 2-cyclohexe~yl,
C3-C8-alkynyl as st2ted under R1, in particular 2-
propynyl, where the three last-mentioned.groups may carry
one of the following radicals5 hydroxyl, alko~y as
stated under Rl, in particular methoxy or ethoxy, halo~en
as s~ated under R1, in particular fluorine or chlorine,
l g ~
- 32 - O.Z. 0050/41171
phenyl which in turn may carry from one to three of the
following groups: alkyl a~ ~tated under R1, in particular
methyl or ethyl, alkoxy as stated under R1, in particular
methoxy or ethoxy, haloal~yl a~ stated under Rl, in
particular trifluoromethyl, halogen as stated under R1, in
particular fluorine or chlorine, nitro or cyano;
a five-membered or six membared heterocyclic radical
having one or two hetero atom~ selected from the group
con~isting of oxygen, sulfur and nitro~en, a~ stated
undar Rl, in particular tetrahydrofuranyl or tetrahydro-
pyranyl, or benzotriazolyl;
N-phthalLmido, tetrahydrophthalimido, succinimido or
maleimido;
one equivalent of a cation from the group consisting of
the alkali metal or alkaline earth metals, m~nganese,
copper, iron, ammonium and substituted ammoni~m, or
-N=CR~R9, where
R8 and R9 are each hydrogen, al~yl a~ stated under R2, in
particular methyl, ethyl or isopropyl;
cycloalkyl as stated under Rl, in particular cyclopropyl;
phenyl or furyl,
or together form a methylena chain having ~rom 4 to 7
member~;
R6 i~ hydrogen, Cl-C5-alkyl as stated under Rl, in par-
ticular meth~l or ethyl, or C3-C~-cycloalkyl as stated
under R1l in particular cyclopropyl;
R7 is ~ydrogen or Cl-C~-alkyl as stated under Rl, in
particular methyl, ethyl, propyl, l-methylethyl or 1,1-
dimethylethyl, or
R~ and R7 together form a methylene chain having 4 or 5
memb0rs;
R4 is hydrogen, halogen a~ stated under R1, in particular
fluoEine or chlorine, nitro, cyano, alkyl as stated under
R1, in particular methyl, ethyl, propyl, 1-methylethyl or
1,1-dimethylethyl, which may carry from one to ive
halogen atom~-a~ stated under R1, in particular fluorine
or chlorine, and/or one or two of the following radlcal~:
2 ~ 2 i~
_ 33 _ o.~. 0050/41171
cyano, alkoxy as stated under R1, in particular methoxy,
ethoxy, 1-methylethoxy ox l,l-dimethylethoxy, haloalkoxy
as stated under R1, in particular difluoromethoxy or
trifluoromethoxy, alkylthio as stated under R1, in par-
ticular methylthio or ethylthio, haloalkylthio as stated
under R1, in particular difluoromethylthio or trifluoro-
methylthio;
cycloalkyl a~ stated under Rl, in particular cyclopropyl;
benzyl which may be monosub~tituted ~o ~risub~tituted by
alkyl of 1 to 4 carbon atoms a~ stated under R1, in
particular methyl, ethyl or l-methylethyl, haloalkyl as
stated under R1, in particular trifluoromethyl or chloro-
difluoromethyl, alkoxy as stated under R1, in particular
methoxy or ethoxy, haloalkoxy as stated under Rl, in
particular trifluoromethoxy, trichlorometho~y or penta
fluoroethoxy, alkylthio as ~tated under Rl, in particular
methylthio or athyl~hio, haloalkylthio as stated under
R1, in particular difluoromethylthio, trifluoromethylthio
or pentafluoromethylthio, halogen a~ ~tated under R1, in
particular fluorine or chlorine, cyano or nitro;
C3-C8-cycloalkyl as ~tated under Rl, in particular cyclo-
propyl, cyclopentyl or cyclohexyl, which may be mo~osub-
stituted to trisubstituted by alkyl as stated under Rl,
in particular methyl or ethyl, or halogen a~ stated under
R1, in particular fluorine or chlorine;
C2-CB-alkenyl a~ ~tated under R1, and l-ethenyl, 1-
propenyl, 1-methyl-1-propenyl, 2-methyl-1-propenyl, 1-
pentenylO l-methyll-butenyl, 2-methyl-1-butenyl, 1-
ethyl-l-propenyl, 1-methyl-1-pentenyl, 2-methyl-1-
pentenyl, 3-methyl-1-pentenyl, 4-methyl~1-pentenyl~ 1,2-
dimethyl-1-butenyl,1,3-dimethyl-1-butenyl,2,3-dimethyl-
l-butenyl, 3,3-dimethyl-1-butenyl, ethyl-1-butenyl, 2-
ethyl-l-butenyl or 1-ethyl-2-methyl-1-pentenyl, in par-
ticular ethenyl, l-propenyl, 2-propenyl, l-methylethenyl,
l-butenyl, 2-butenyl, 3-butenyl, l-methylpropenyl, 1-
methyl-2-propenyl, 2-methyl-1-propenyl or 2-methyl-2-
propenyl, which may be mono~ubstituted to trisub~tituted
_ 34 _ o.z. 00~0/41171
by halogen a stated under R1, in particular fluorine or
chlorine, or alkoxy as stated under R1, in par~icular
methoxy or ethoxy, and/or monosubstituted by phenyl,
where the phenyl radical in turn may carry from one to
three of the following group~s alkyl as stated under R1,
in particular methyl, ethyl or l-methylethyl, haloalkyl
as ~tated under R1, in particular trifluoromethyl or
chlorodifluoromethyl~ alkoxy as stated under R1, in
particular methoxy or ethoxy, haloalkoxy as stated under
13 Rl, in particular trifluoromethoxy, trichloromethoxy or
pentafluoroethoxy, alkylthio as stated under R1, in par-
ticular methylthio or ethylthio, haloalkylthio as ~tated
under R1, in particular difluoromethylthio, trifluoro-
methylthio or pen~afluoromethylthio, halogen as stated
under R1, in particular fluorine or chlorine, cyano or
nitro;
alkynyl as stated under R1, and ethynyl, l-propynyl, 1-
butynyl, 1-pentynyl, 1-hexynyl, 3-methyl-1 pentynyl or 4-
methyl-l-pentynyl, in particular ethynyl, 1 propynyl or
propargyl, which may be monosubstituted to trisubstituted
by halogen a~ stated above, in particular fluorine or
chlorine, or aIkoxy as stated a~ove, in particular
methoxy or ethoxy, and/or monosubstituted by phenyl,
where the phenyl radical in turn may carry from one to
three of the following groups: alkyl a~ stated above, in
: particular methyl, ethyl or l-methylethyl, haloalkyl as
stated above, in particular trifluoromethyl or chlorodi-
fluoromethyl, alkoxy as stated above, in particular
methoxy or ethoxy, haloalkoxy as stated above, in par-
ticular trifluoromethoxy, trichloromethoxy or penta-
fluoroethoxy, alkylthio as stated above, in particular
methylthio or ethylthio, haloalkylthio as stated above,
in particular difluorome~hylthio, trifluoromethylthio or
pentafluoromethylthio, halogen as stated above, in
particular fluorine or chlorine, cyano or nitro;
C~-C4-alkoxy a~ stated under R1, in partlcular methoxy or
ethoxy;
2 ~ 9 ~
- 35 - O.z. 0050/41171
C1-C4-haloalkoxy as stated above, in particular tri-
fluoromethoxy, trichloromethoxy or pentafluoroethoxy;
C2wC4-alkenyloxy, such as vinyloxy, 2-p~openyloxy, 1-
methylethenyloxy or ~-methyl-3-butenyloxy, in particular
2-propenyloxy or 2-methyl-3-butenyloxy;
C2-C4-alkynyloxy, such as ethynyloxy, 2-propynyloxy, 1-
methylethynyloxy or 2-methyl-3-butynyloxy, in particular
2-propynyloxy or 2-methyl-3-bu~ynyloxy;
C1-C4-alXylthio as statad under Rl, in particular methyl-
thio or ethylthio;
Cl-C4-haloalkylthio as stated und~r Rl, in particular
difluoromethylthio, trifluoromethylthio or pentafluoro-
ethylthio;
C1-C4-alkylsulfinyl, such as methylsulfinyl, ethylsul-
finyl, n-propylsulfinyl, isopropylsulfinyl, n-butylsul-
finyl or tert-butylsulfinyl, in particular methyl~ulfin-
yl;
C1-C4-alkylsulfonyl, such as methylsulfonyl, ethylsul
fonyl, n-propylsulfonyl, isopropylsulfonyl, n-butylsul-
fonyl or tert-butyl ulfonyl, in particular methyl~ulfon-
yl;
Cl-C4-haloalkylsulfonyl, such as trifluoromethyl~ulfonyl,
pentafluoroethylsulfonyl or monofluorobutylsul~onyll in
particular trifluoromethylsulfonyL;
phenoxy or phenylthio which may be monosubstitutecl to
trisub~tituted by alkyl as stated under R1, in particular
methyl, ethyl or isopropyl, haloalkyl as statsd un~er R1,
in particular trifluoromethyl or chlorodifluoromethyl,
alko~y a3 stated above, in particular methoxy or ethoxy,
haloalko~y a~ stated above, in particular trifluorometh-
oxy, trichloromethoxy or pentafluoroethoxy, alkylthio a~
~tated above, in particular methylthio or ethylthio,
haloalkylthio a~ stated above, in particular difluoro-
methylthio, tri~luoromethylthio or penta~luoromethylthio,
halogen as stated above, in particular fluorine or
chlorine, cyano or nitro;
a 5-membered or 6-membered saturated or aromatic
~ ~ 2 ~
- 36 - O.Z. OOSO/41171
heterocyclic rad1cal containing one or two hetero atoms
selected from the group consis~ing of oxygen, sulfur and
nitrogen, a~ stated under Rl, which may carry one ox two
of the following sub~tituents: alkyl as stated under R~,
in particular methyl, halogen a~ stat2d under Rl, in
particular fluorine or chlorine, alkoxy as stated under
Rl, in particular methoxy or ethoxy, or alkoxycarbonyl,
such as methoxycarbonyl or ethoxycarbonyl, in particular
methoxycarbonyl;
phenyl which may carry from one to three of the following
groups. alkyl as stated under R1, in particular methyl,
ethyl or isopropyl, haloalkyl as stated under R1, in par-
ticular trifluoromethyl or chlorodifluoromethyl, allcoxy
as stated under Rl, in particular methoxy or ethoxy,
haloalko~y as stated under Rl, in particular trifluoro-
methoxy, trichloromethoxy or pentafluoroethoxy, alkylthio
a~ stated under R1, in particular methylthio or ethylthiol
haloalkylthio as stated under Rl, in particular difluoro-
methylthio, trifluoromethylthio or pentafluoromethylthio,
halogen as ~ta~ed under R1, in particular fluorine or
chlorine, cyano or nitro.
Examples of herbicidal compounds of the formulae
I'a, I'b and I'c are shown in the Table~ belows
2 ~ 9 ~
- 37 - O.Z. 0050/4117
R4--~ CH~ or R4~ CIH3
Ia
R4 monosubstituted in the 2-, 5- or 6-po~ition, radicals
R4independently of one another di~ubstituted in the 2,5-,
2,6- or 5,6-position or trisubstituted in the 2,5,6-
position or monosub~tituted in the 4 , 5- or 6-position
or indepsndently of one another di~ubstituted in the 4,5-,
5,6- or 4,6-position or trisubstituted in the 4,5,6-
position
R4
H
F
Cl
Br
Methyl
Ethyl
n-Propyl
Isopropyl
n-Butyl
Isobutyl
sec-Butyl
tert-Butyl
Cyclopropyl
Cyclobutyl
Cyclopentyl
Cyclohexyl
Cycloh~ptyl
C~clooctyl
l-Methylcyclopropyl
Cyclopropylmethyl
l-(Cyclopropyl)-ethyl
Dichlorome~hyl
Trichlorome~hyl
Chlorodi~luoromethyl
~2 ~95
- 38 - O.Z. 0050~41171
R4
Trifluoromethyl
Pentafluoroethyl
Difluoromethyl
Methoxymethyl
l-Methylmethoxymethyl
l-Methyl-~-methoxyethyl
1-Methylethoxymethyl
Ethoxymethyl
Vinyl
Allyl
Methallyl
Crotyl
Propargyl
Cinnamyl
1-~ethyl-2-propenyl
1-Methyl-2-propynyl
Methoxy
Ethoxy
n-Propoxy
Isopropoxy
n-Butoxy
Isobutoxy
sec-Butoxy
tert-Butoxy
Methylthio
Ethylthio
Chlorodifluoromethoxy
Trifluoromethoxy
Chlorodifluoromethylthio
Trifluoromethylthio
Difluoromethoxy
Difluoromethylthio
Trichloromethoxy
Trichloromethylthio
Allyloxy
Allylthio
~ L9~
_ 39 _ o.Z. 0050/41171
_
Propargyloxy
Propargylthio
Cyanomethyl
2-Cyanoethyl
l-Methyl-2~cyano~thyl
l-Methyl-1-cyanoethyl
1,1-DLm~thyl-2-cyanoethyl
Methylsulfinyl
Ethylsulfinyl
n-Propylsulfinyl
Isopropyl~ulfinyl
Methylsulfony}
Ethylsulfonyl
n-Propylsulfonyl
Isopropylsulfonyl
Trifluoromethylsulfonyl
Chloromethyl
2-Chloroethyl
1-Methyl-2-chlorosthyl
l-~ethyl-1-chloroethyl
Nitro
Cyane
Phenyl
2-F phenyl
3-F-phenyl
4-F-phenyl
2-Cl-phenyl
3-Cl-phenyl
4-Cl-phenyl
2-CH3-phenyl
3-CH3-phe~yl
4-CH3-phenyl
2-CF3-phenyl
3-CF3-phenyl
4-CF3-phenyl
2-OCH3-phenyl
I 9 5
- 40 - O.Z. 0050/41171
R4 _ _
3-OCH3-phenyl
4-OCH3-phenyl
2,4-Dichlorophenyl
Phenoxy
- Phenylthio
2-C1-phenoxy
3-Cl-phenoxy
4-Cl-phenoxy
2,4-dichlorophenoxy
Benzyl
~-Cl-benzyl
3-Cl-benzyl
4-Cl-benzyl
2-Thienyl
3-Thienyl
2-Pyridyl
3-Pyridyl O O
R4 ~ N ~ or R4 ~ N
~ Ia
R4 monosub~tituted in the 2-, 5- or 6-position, radicals
R4 independently of one anothsr di~ub~tituted in the 2,5-,
2,6- or 5,6-position or trisubstituted in the 2,5,6-
position or mono~ubstituted in the 4-, 5- or 6-position
or independently of one another disub~tituted in the 4,5-,
5,6- or 4,6-po~ition or trisub~tituted in the 4,5,6-
po~ition
R4 _ .
H
Cl
Br
I
Methyl
Ethyl
~S~5
- 41 - O. Z . 0050/41171
lR4 _ - -
n-Propyl
I sopropyl
n-Butyl
Isobutyl
sec -Butyl
tert -Butyl
Cyclopropyl
Cyc lobutyl
Cyc lopentyl
Cyclohexyl
Cycloheptyl
Cyclooc~yl
1-Methylcyclopropyl
Cyclopropylmethyl
l-(Cyclopropylj-ethyl
Dichloromethyl
Trichloromethyl
Chlorodifluoromethyl
Trifluoromethyl
Pentafluoroethyl
Difluoromethyl
Nethoxymethyl
l-Methylmethoxymethyl
1-Methyl-2-methoxyethyl
1-~ethylethoxymethyl
Ethoxymethyl
Vinyl
Allyl
MethalIyl
Crotyl
Proparyyl
Ci~lamyl
l-Methyl-2-prepenyl
1-~ethyl-2-propynyl
Methoxy
Ethaxy
2~ 7~ 9~
42 - O.Z. 0050/41171
_
n-Propoxy
Isopropoxy
n-Butoxy
Isobutoxy
sec-Butoxy
tert-Butoxy
Methylthio
Ethylthio
Chlorodifluoromethoxy
Trifluoromethoxy
Chlorodifluoromethylthio
Trif luoromethylthio
Difluoromethoxy
Difluoromethylthio
Trichloromethoxy
Trichloromethylthio
Allyloxy
Allylthio
Propargy~oxy
P.ropargylthio
Cyanomethyl
2-Cyanoethyl
l-Methyl-2-cyanoethyl
l-Methyl-l-cyanoethyl
1,1-Dimethyl-2-cyanoethyl
Methylsulfinyl
Ethylsulfinyl
n-Propyl~ulfinyl
Isopropylsulfinyl
Methylsulfonyl
Ethylsulfonyl
n-Propylsulfonyl
Isopropylsulfonyl
Trifluoromethylsulfonyl
Chloromethyl
2-Chloroethyl
2 ~ 9 5
_ 43 _ o.Z. 0050/41171
~,4 _ _ _ _
l-Methyl-2-chloroethyl
l-Methyl-l-chloroethyl
Nitro
Cyano
Phenyl
2-F-phenyl
3-F-phenyl
4-F-phenyl
2-Cl-phenyl
3-Cl-phenyl
4-Cl-phenyl
2-CH3~phenyl
3-CH3-phenyl
4-CH3-phenyl
2-C~3-phenyl
3-CF3-phenyl
4-CF3-phenyl
2-OCH3-phenyl
3-OCH3-phenyl
4-OCH3-phenyl
2,4-Dichlorophenyl
Phenoxy
Phenylthio
2-Cl-phenoxy
3-Cl-phenoxy
4-Cl-phenoxy
2,4-dichlorophenoxy
Benzyl
2-Cl-benzyl
3-C1-benzyl
4-Cl-benzyl
2-Thienyl
3-Thienyl
2-Pyridyl
3-Pyridyl
2~2 ~
- 44 - O.Z. 0050~41171
For example, fur~her compounds having the general
stru ::ture
O O Rl
x~N--R1 1~C--N\ I~R3 /RI
Y~Z R 3 R 2 y~z C--N
la Ib Ic
where W, ~, Y or Z are togath-or from orle to 3 radicals
C-R4, N or N ~ O, with the proviso that the ring contains
a he tero atom and, or example,
R4 is a radical from the group consisting of Ql to Q133,
R1 is a radical from the group con~isting of Ll to L200,
R2 i~ a radical from the group consisting of pl to p~1,
R3 is formyl, 4,5-dihydrooxazol-2-yl or a radical -Co-AR5
or -Co-NR6R7,
A is oxyyen or ~ulfur,
R5 is a radical from the group con~isting of ~1 to Mg,
R6 and R7 independently o~ one another are each a radical
from the group con~isting o~ Sl to S15 or together form a
radical ~rom the group consisting of s'6 to Sl9
and W, X, Y, ~, Q, ~, P, ~ and S may be combined as
desired,
can also be prepared in a ~imilar manner.
~sl~9~
_ 45 _ o.z. 0050/41171
R4~ Rl, R2, R5, R~ and R7 may be, for example, the fo11Ow-
ing radicals:
cQm~ound no. R4
, _ . . .
Ql H
Q2 F
Q3 ~l
Q4 ~r
Q5
Q6 CH3
Q7 C2Hs
Q8 n-C3H7
49 i~C3H7
QlO n-C4Hg
Qll i-C~Hg
Ql2 s-C4Hg
Ql3 tert.-C4Hg
Ql4 cyclo-C3Hs
Ql5 cyclo C4H7
Ql6 cyclo-CsHg
Ql7 cyclo-C6Hll
Ql~ cyclo-C7H13
419 cyclo-CgHls
Q20 l-Methyl-CyCl-C3HS
Q21 cyclo-C3~-m~thyl
Q22 l-~cyclo-C3Hs)-ethyl
Q23 CHCl2
Q2~ CCl3
Q25 CF2Cl
Q26 CF3
Q27 C2F5
Q28 Cf2~
Q29 CH20CH3
~2i,~
- 46 - O.Z. 0050/41171
R 4 _ _ _ _
Q30 CH [ CH 3 ~ OCH 3
Q31 CH(CH3)OC2~5
Q32 CH(CH3)CH2~CH3
Q33 CH 2OC 2H 5
Q34 CH=CH 2
Q35 C!l 2--CH=CH 2
Q36 CH ( CH 3 ) C~=CH 2
Q37 CH 2--CH=CH--CH 3
Q38 C--CH
Q39 CW=CH-C6H5
Q40 C=C-C6~5
Q41 Cil(CH3~C_CH
Q42 neo-c5Hl 1
Q43 CH 3O
Q44 C 2H5O
Q45 n-C 3H70
Q46 i -~: 3H70
Q47 n-5:4H9O
Q48 S-CbHgO
Q~9 i-C4~9Q
Q50 tert.-C4H90
Q51 CH3S
Q52 C 2H5S
Q53 n C 3H75
Q54 i -C 3H7S
Q55 s-C4HgS
Q56 tert.-C4HgS
Q57 CICF20
Q58 CF 30
Q59 CIC~ 2S
Q60 Cf 3S
2tt2 7 ~
_ 47 - O. Z . 0050/41171
ccn~ . no. R 4
Q5 I HCF 2
Q62 HCF 2S
Q63 CCI 30
Q64 ~CI 35
Q6 5 CH 2=C~CH 2
Q66 CH 2=CH--CH 2--S
Q67 HC~C~CH2~)
o,68 HC=C~CH 2--5
Q69 CH2--CH--CH(CH3)0
Q70 H-C_CH--CH ~ CH 3 ) O
Q7 l CH 2CN
Q72 CH 2Ctl 2CN
Q7 3 CH ( H 3 ) SH 2CN
Q74 C (CH3~ 2CN
Q75 C(C113) 2CH~CN
Q76 CH2CI
Q77 CH2CH2CI
Q78 CH ( CH 3 ) CH 2C I
Q79 I:(CH3~ 2CI
q80 N0 2
Q81 CN
Q82 C6H5
Q83 2-F-CçH4
Q84 3-f-C8H~,
Q85 4-f-CfiH~
Q86 2-CI-C6~4
Q87 3-CI-C~,H~
Q88 4-CI-C~H~
Q89 2-CH3-C6H~,
Q90 3-CH 3-C~H4
Q91 4-CH3-C~H~
~2 ~9~
- 48 - O. Z . 0o5~ 7
ca~p . no ' R 4 c~p . no ' R 4
Q92 2-CF3-C6H4 Q125 Methylsulfinyl
Q93 3-CF 3-C6H4 Ql 26 Ethy 1 su 1~1 nyl
Qg4 4-CF3-C6H4 Q127 n-Propylsulfinyl
Q95 2-OCH3-C6H4 Ql28 i-Propylsulfinyl
Q96 3-ocH3-c8H4 Q129 Methylsut.fonyl
Q97 4-OCH3-C6~4 Q130 Ethylsulfonyl
Q98 4-SCH3-C6H4 Q131 n-Propy1sulfonyl
Q99 4-SCF3-C6H4 Q132 i-Propylsulfonyl
Q 10 0 4 NO 2 - C 6 H 4 Q 13 3 Trif luc~ hylsulfonyl
Q101 4-CN-C6H4
Q102 2~4-~CI,CI)-C6H4
Q103 2~4- (cH3? CH33-C6H4
Q104 C6H5O
Q105 C6HgS
Q106 2-CI-~6H4O
Q107 3-CI-C6H4O
Q 108 4-C l -C6H4O
Q109 C6Hs-CH2
Q110 2-Cl-C6H4-CH2
Qlll 3-CI-C6H4-~H 2
Q112 4-CI-C6H4-CH2
Ql 13 4-F-C6H4-CH2
Q114 2-Thienyl
Q115 3-Thienyl
Q116 2-Furyl
Ql 17 3-Fury l
Q118 1-Methyl-5 pyrazolyl
Qll9 2 Oxazolyl
Q120 2-Th~azolyl
Q121 2-Pyridyl
Q122 3-Pyridyt
Ql 23 4-Pyridyl
Ql 24 2-retrahydrofury 1
2 ~
49 O. Z . 0050/41171
cc~rp.
L1
L~ CH3
L3 C2H5
L4 n-C3H~
L~ i-C3H7
L6 n-C4Hg
L7 1-C4Hg
L8 sec-C4Hg
L9 tert.-C4Hg
L10 n-CsHll
Lll -cH(cH3)c3H7
L12 -CH(C~Hs~C2~5
L13 n-C6H13
L14 -CH(SH3)C4Hg
L15 -CH(C2~s)~3H7
L16 n-C7H15
L17 -CH(CH3)~5H11
L18 -CH(C2H5)c4H9
Ll9 n-CgH17
L2~ -CH(CH3)C6H13
L21 -CH5c2~5)csHll
L22 -C(CH3)~CH2Ctc~3)3
L23 Cyclo-c3Hs
L24 cyclo~C4H7
L25 cyclo-CsHg
L26 cyctO-c6Hll
L27 cyclo C7H13
L28 cyclo-C8~15
L2g l-Methylcyctohexyl
L30 1-Ethylcycloh~xyl
L31 3,5-Dim~thytcyclohexyt
L32 3-Trifluoromethylcyclohexyl
L33 T~trahydropyran-4-yl
L34 4-M~thyl-tetrahydropyran-2-yl
L35 4-M6thyl-tetr~hydropyran-4-yl
L96 -CH2-CH~CH~
9 ~
O.Z. 0û50/41171
C~.
L3 7 -CH ( CH3 ) ~1 1=CH2
L38 -C ( ~H3 ) 2~ H=CH2
L3~ -C (CH3, C2H5) CH=CH2
Ll~0 -C (CH3) ~-C2H5
L41 -C (CH3, C2H5 ) C2H5
L42 -C (CH3) 2C3H7
L43 -c~sH3)2~ycloc6
L44 -CH2-C ( Ctl3 3 =CH2
L45 -CH2CH=CHCH3
L46 -CH ( CH3 ~ CH=CHCH3
L47 -C ( CH3 ) 2CHSCHCH3
L48 -CHzC_CH
L49 -CH ( CH3 ) C~C~
L50 -C (CH3) 2C=--CH
L51 -C (CH3, C2H5] C----CH
L52 -C (C2H5) 2C~CH
L53 -CH2C~CCH3
L54 -CH(CH3~C~ CH3
L55 -C (CH3) 2C~ CH3
L56 -CH2C6H5
L57 -CH ( CH3 ) C6H5
L58 -C ~CH3) 2C6H5
L59 -CH2CH2C6H5
L60 -CH2CH2SCH3
L6 1 -CH ( CH3 ) CH25CH3
L62 -C tCH3) 2CH2S~ H3
L63 -cH2cll2cH2scH3
L64 -CH2CH2C I
L55 -C~ H3)~:H2CI
L66 -C ¦ CH3 ) 21 H2C I
L67 -CH2CH20CH3
L68 -CH ( CH3 ~ CH20CH3
L69 -C ~ CH3 ) 2CH20CH3
L70 -CH2CH2N ~ CH3 ) 2
L71 -cH2cH2N ~C2H5) 2
L72 -cH2cH?cH2ocll3
~ 7~
- 51 ~ O.Z. 0050J41171
c~p.
n~
L73 -C112CH2CH2~ (CH3) 2
L74 -CII~CH2C3~2N (C2H5) 2
L75 2-CH3~C6~4
L76 3-CH3-C6H4
L77 4-CH3-C6H4
L78 2-c2H5-~6H4
L7g 3-~2H5-c6H4
L80 4-C2H5-C6H4
L81 3-tert.-C4H9~C6H4
L82 4-tert.-C4H9~C6H4
L83 2, 3- (CH3 ) 2-C6H3
L84 2, 4- (CH3) 2-C6H3
L85 2, 5- (CH3) 7-~6H3
L86 2, 6- ( CH3 ) 2-C6tl3
L87 3, 4- ~CH3) 2-~6~3
L88 3, 5- ( CH3 ) 2-C6~3
L89 2, 3, 4-~cH3)3-c6H2
L90 2, 3, 5-~CH3)3-C6H2
L91 2, 4, 5- (CH3) 3-C6H2
L92 2, 4, 6- (CH3) 3-C6H2
L93 3, 4, 5- (SH3 ) 3-C6tl2
L9b, 2-CF3-1 6H4
L95 3-CF3-c6H4
L96 4-Cf 3-C6H4
L97 2-F-C6H4
L98 3-F-C6H4
L99 4-F-C6H4
L100 2~CI -C6H4
Ll 01 3-ct-C6H4
L102 4-cl-c6H4
L103 2-Br-C6H4
L104 3-er-C6H4
L105 4-ar-C~,H4
L106 2, 3-F2-C6H3
L107 2, 4-F2-C6H3
Ll08 2, 5-F2-C6H3
9 ~
- 52 - O.Z. 0050/41171
cc~.
n
L109 2,6-F2-C6H3
L110 2,3-CI2-~6H3
Llll 2~4-CI~-C6H3
Ll12 2,5-C12-C6H3
L113 2,6-C12-~6H3
L114 3r4-cl2-c6H3
Lll5 3,5-Cl2-C6H3
L116 2,3,4-CI3-C6H2
L117 2,3,5-CI3-C6H2
L118 2,4,6-C13-~6~2
Ll19 3,4,5-Cl3-C6H2
L120 2-CN-C6~4
L121 3-cN-c6H4
L122 4 CN-C6H4
L123 2-OCH3-~H4
L124 3-ocH3-~6H4
L125 4-OCH3-C~H~
L126 2-OC2H5-C6H4
Ll27 3-OC2H5-C6H4
L128 4-OC2H5-C6H4
L129 2-O-n-C3H7~~6H4
L}30 3-~-n-C3H7-C6H4
L131 4-o-n-C3H7~C6H4
L132 2-o-i-c3H7-C6H4
L133 3-o-i-C3H7-C6H4
L134 4-o-i-c3H7-c6~4
L135 2,3-(OCH3~2-C6H3
L136 2,4-~OCH3)2-C6H3
L137 2,5-~OCH3)2 C6H3
L138 2,6-(0CH3)2-C6H3
L139 3~4-(ocH3)2-c6H3
L140 3,5 (ocH3)2-c6H3
Ll41 3,4,5-(0CH3)3-C6H2
L142 2-OCF3-C6H4
L143 3-ocF3-c6H4
L144 4-ocF3-~6~4
~ ~ 2 '~
- 53 - O.Z. 0050/41171
G~p .
no. R1
L145 2-0CF2CHF2-~6H4
L146 3-oCF2C~F2-~6H4
L147 4-ocF2cHF2-c6H4
L148 ~-SCH3-C6H4
L149 3-SCH3-C6H4
L150 4-SCH3-~5H4
L151 2-SC2H5-C6H4
L152 3-SC2H5-C`6H4
L153 4-SC2~5-C~4
L154 2-S-i-C3H7-C6H4
L155 3-s-i-C3H7 C6H4
L156 4-s-i-C3H7-c6H4
L157 2,4-(SCH3)2-C6H3
LI58 2-SCF3-C6H4
L159 3-scF3-c6H4
L160 4-scF3-c6H4
L161 2-N02-C6H4
L162 3-No~-c6H4
L163 4-N02-~6H4
L164 2,3-~N02)2-C6H3
L165 2,4-(N02)2-C6H3-
L166 2,5-(N02)2-~6H3
L167 2, 6- ( N02 ) 2-C6H3
L168 3l4-(No2)2-c6H3
L169 3~s-(No2)2-c6H3
L170 2-CH0-C6H4
L171 3-cHo-c6H4
L172 4-CH0-C~H4
L173 2-6CH3-C6
o
L174 3~llCH3_C6H~
O
L175 4_1C~CH3_C6
2 ~
- 54 - O.Z. 0050/~1171
carp .
no. Rl
L176 2-llC2Hs-C6H4
L177 3-1~C2Hs~C6H4
L178 4_llC2HS_c6~4
o
L179 ~ n-c3H7-c6H4
L180 3~ n_C3H7_C6H~
Ll~l 4~ n_C3H7_C6H4
L182 2-l~CF3-C6H4
o
L183 3-llcF3-c6~4
O
L184 4_llCF3_C6H~
o
L185 l-Niphthyl
L186 2-~aphthyt
L187 C6Hs
~188 Plp~rid1no
L189 T~trahydrofur-3-yl
Ll90 Thiazol-2-yl
Ll91 5-M~thyl-th1azol-2-yl
L192 5-Ethyl-thlazol-2-yl
L193 5~n-Propyl-thiazol-2~yl
- 55 - O. Z . 0050/41171
CC~p.
no.
L194 4-Methyl-5-carboxy thiazol-2-yl
L195 Cyclopropylm~thyl
L196 1-(Cyc1opropyl)-ethyl
L197 C~(CH3)CH2CN
L198 C(CH3)2~ N
L 199 C ( CH3 ) 2CH2CN
L200 C ( C~13 ) 2CH2F
2~'~'7~9~
` 56 - O.Z. 0050~41171
Pl H
P2 CH 3
p3 C 2H~
p~ n-C 3H7
p5 i-C3H7
P6 n-C~Hg
P7 s-C4Hg
P8 t-C4Hg
P9 CH 2-CH 20H
P10 CH2-t:H2Cl
Pll CH20C~3
P12 CH20C2H5
p 13 CH 2CH 20CH 3
P14 ~H25C~3
Pl 5 CH 2$C 2H5
P 16 CH 2C11 2SCH 3
P17 CH2-CH~-N(CH3) 2
pl~ CH2CH2-N(C2~5) 2
Pl9 cyclo-C3H5
p~O cyCl~-C6H11
P21 1-Methyl-cyclo-C6HIo
9 5
- 57 - O.Z. 0050~41171
M1 H
M2 CH3
M3 C2~5
M4 n~C3H7
M5 i-C3H7
M6 n-C4Hg
M7 s-C4Hg
M8 t-C4Hg
M9 CH (CH3 3 C~H13
M1 0 CH2CH20CH3
Ml I CH2CH20C2H5
Ml 2 Succ i n imi do
M13 Li~
MI4 Na~
M 1 5 K~3
M16 NH4~
M17 H3N~i-C3H7
Ml8 H2N~( i-C3H7) 2
Ml 9 H3N~H2CH2H
M20 CH2CH'CH2
M21 CH2-C (CH3) =CH2
M22 CH2-C ~ C I ) 'C~2
M23 CH2-C~CH
M24 CH2_~C_GH2oH
M25 -N=C (CH3) 2
M26 -N-C ~C2H5) 2
M27 CH2-CH2-N (CH3 ) 2
M28 CH2 CH2-N (C2H5) 2
M29 CH2-CH2N~9~ CJ~3 ) 3 Ie
M30 CH 2-t:F 3
M31 Phenyl
M32 Ph~ny I athy I
M33 cH2-CH2-S1 ~CH3) 3
M34 CH2-CN2-ON'C(clt3) 2
M35 CH2-PO~OC2H5) 2
M36 CH ~ CH 3 ) CH 20CH 3
M37 CH2-COI'l(C2H5) 2
~ 7.~9~
- 5~ O. Z . 0050/41171
M38 CH 2CH 2-N ( C 2~S ) 2
M39 CH 2-0CH 2-C6H$
M40 C H 2COOCH 3
M41 -N-c(cyc~a-c3Hs~ 2
jCH3
M42 --N=C\
C 2~S
M43 Cyc 1 ohe~;an i mi no
M44 CyclooctaniM{no
M4 5 CH 2-CH 2-C I
M46 CH ~-CH 2-CN
M47 CH2-Ccl 3
M48 Pyr~d-3-ylmethyl
M49 Th i ~n-2-y 1 -methy I
M 50 C H 2CW 20CH 2~ 20CH 3
M5 1 CH 2~ OH
CH2~
M52 CH2cH2ocH2cH2o-c~2cH3
M53 -CH(C6Hs)c02cH3
M54 cyclo-C6HIl
M55 -CH2-ocH2-(4-c~-c6~)
M56 Tetrahydropyran-2-yl
M57 Tetrahydrofur-2-y 1
M58 ( 4-~rano~oyl)methyl
M59 (4-Methoxybenzoyl)m~thyl
M60 CH~-CH2COOCH3
M61 Phthalimidom~thyl
M62 Fur-2-yIm~thyl
M63 Tetrahydrofur-2-yt-m~thyl
.
~2 ~19~
- 59 _ O.Z. 0050/41171
c~p. no. R 5 ",,,
M64 Pyrid-2~yl-methyl
M65 Pyr i d-4-y 1 -methy I
1'166 Pyrid-3-y1-methyI
M67 Thi~n-2-yl-m~thyl
M68 -cH(c6~slGooc~3
M69 Piperidino
M70 Phthalimido
M71 Benzotriazol-l-yl
M72 -NSCH-C6H5
M73 Fur-2-yl-methylenimino
M74 2-No2-4-F-c6H3
M75 3,5-(CF332-C6~3
M76 CH2-CH2-S-CH3
M77 4-NHCOC~3-C6H4
M78 2, 4-DichlQrobenzyl
M79 cyclo-C3Hs
M80 1-~cyclo-C3H7~-ethyl
M81 CH(CH3)CH=c~2
M82 CH~CH3)CHaCN
M83 C ( ~H 3 ) 2CN
M84 CH(C113)CH2
M85 C(C~3)2CH2Cl
M86 CH2CH2O-cH2co2~3
M87 CH 2CH2-ocH(cH33co2sH3
M88 CH2CH20cH(cH3)c02c2H5
M89 CH(CH3)C021:H2C02CH3
M90 CH(CH3)C02CH(CH3)C02CH~
2 ~ 9 5
- 60 - O.Z. OOsO/41171
Sl H
S2 CH3
S3 C 2H5
S4 n-C 3H7
SS i -C 31~7
S6 cyclo-C3H5
S7 n-C4Hg
S8 i-C4Hg
S9 s-C4Hg
510 tert.-C4Hg
Sl 1 cyclo-c5H9
S12 CYC1O-~6H1
S13 C6HS
514 2-FUrYI
515 3-FUrYI
516 ~CI~2) 4*
S17 ICH2)5* (*R6 and R7 t~gether~
S18 (CH2) ~*
S19 (CH2)7*
2 ~
- 61 - O.Z. 0050/4117
Prepara~ion Example~
1. 2-I~opropylaminocarbonyl-5-methylpyrldina-3-
carboxylic acid
3.25 g of isopropylamine ar~ added to 8.1 g of 5-
methylpyridine-2,3-dicarboxylic anhydride in 50 ml of
methylene chloride at from ~0 to 30C while s~irring, and
the stirred mixture is refluxed for 3 hours. The reac-
tion tnixture i~ evaporated down under reduced pressure
and the residue is stirred in a 1 : 1 : 1 mixture of
ether, methyl tert-butyl ether and petroleum ether.
Filtration under suction and drying g:ive 10.1 g of the
title compound as colorles~ crystals of melting point 95-
105C.
Active ingredient Example No. 1.026
2. N-isopropyl-5 methylpyridine-2,3-dicarboximide
3 g of the carboxylic acid from 1. in 30 ml of
acetic anhydride are refluxed for 3 hours while stirring.
The reaction mixture is evaporated down under reduced
pressure, the residue i~ -~tirred with water and taken up
in methylene chloride and ~he solution is dried over
magnesi~n sulfate. Chromatography over alumina, evapora-
tion under reduced pressure and washing with 1 : 1
petroleum ether~ether give 2.3 g of the title compound
of melting point 127 128C.
Active ingredient Example No. 3.05
3. a) 2-Carbomethoxy 6-methylpyridine-3-carboxylic acid
48.9 g of 6-methylpyridine-2,3-dicarboxylic
anhydride in 200 ml of methanol are refluxed for 3 hours.
while s~irring. Evaporating down the mixture under
reduced pre~sure give~ 57.9 g of the ~itle co~pou~d of
melting point 133-158C (decomposition).
b) 2-Carbomethoxy-6-methylpyridine-3-carbony}
chloride
44 g of thionyl chloride are added to 57.7 g of
the carboxylic acid from a) in 250 ml o 1,2-dichloro-
ethane at 60C while stirring, and the mixture i8 re1uxed
for 6 hour~. 64 g of the title cotnpound are obtained as
2 ~
- 62 - O.Z. 0050/41171
a semicrystalline mass. According to the NMR spectrum,
it conta.ins about 20% of the i~omeric 3-carbometh~xy
compound.
c) N-sec-butyl-6-methylpyridine-2,3-dicarboximlde
43 g of sec-butylamine are added dropwise to 64 g
of the acyl chloride from b) in 300 ml of methylene
chlorid~ at from 15 to 20C while stirring, and ~tirring
i~ continued for 12 hours at 25~C. ~ashing with watsr
and evaporating down under reduced pressure give 43 g of
a ~Pmicrystalline mas~. The latter i~ dissolved in
methylene chloride and ~he solution is stirred over
actiYe carbon and then chromatographed over alumina.
36 g of the title compound are ebtained as colorless
cry~tals of melting point 105-106C.
lS Active ingredient Exampl~ No. 3.017
4. ~-(2-Chlorophenyl)-aminocarbonyl-6-mQthylpyridine-
3 carboxylic acid
54.5 y of the title compound of melting point
155-160C are obtained from 32.6 g of 6-methylpyridine-
~,3-dicarboxylic anhydride and 28.1 g of 2-chloroaniline
by the method used in 1.
Active ingredient Example No. 1.010
5. Methyl 2-(2-chlorophenyl)-aminocarbonyl-6-methyl-
: p~ridine 3-carboxylate
12.8 g of N,N-dicyclohe~ylcarbodiLmide are added
to a mixture of lS g of carboxylic acid from 4., 100 ml
of diisopropyl e~her and 50 ml of methanol at from 15 to
20C, and stirring is carried out for 3 hours at 50C.
The precipitated urea i~ ~iltered off under ~uction, the
filtrate is evaporated down under reduced precsure and
the re~idue is chroma~ographed over alumina. Washing
with pentane gives 7.5 g of the titla compound a3 color-
less crystals of melting point 105-107C.
Active ingredient Example No. 1.007
6. N-~ec-butyl-S-meth~lpyridine-2,3-d~carboximide
i~ obtained as colorless cry3tals of mslting point 96-
99C in the preparation by the methods of Preparation
2 ~ 9 ~
- 63 - O.z. 0050/41171
Examples 1 and 2 in ring cleavage of pyxidine-2,3-dicarb-
oxylic anhydride with sec-~utylamine and subsequent
cyclization in aceti~ anhydride.
Active ingredient Example No. 3 . 018
7. Methyl 2-~ec-butylaminocarbonyl-5-methylpyridine-3-
carboxylate
3 g of tri~thylamine are added dropwise to 4.3 g
of the imide from 6~ in 130 ml of methanol a~ from 20 to
25C, and the mixture i 3tirred for 2 hours at 50C. The
reaction mixture i~ e~aporated down under reduced pre~-
: ~ure, the residue i~ taken up in methylene chloride and
the solution is chromatographed over ~ilica gel. 2.8 g
of the title compound of melting point 58-60C are ob-
tained as colorles~ crystals.
Active ingredient Example No. 1.023
8. 2-Sec-butylaminocarbonyl-5-methylpyridine-3~carbox-
amide
8 g of gaseous ammonia ara pa~sed into a solution
of 10 g of the imide from 6. .in 200 ml of i~opropanol in
the course of one hour at 0C, and stirring i~ carried
out for 12 hour~ at 25C. The mixture i~ evaporated down
under reduced pre~sure and the re~idue i~ stirred with
me~hyl tert-butyl ether. 8.3 g of the title compound of
melting point 119-123~C are obtained. According to the
NMR spectrum, 20% of the i~omeric 3-sec-butylamino~
carbonyl compound was formed at the ~ame time.
Active ingredient Example No. 7.003
9. ~-Tert-butylaminocarbonyl-6-methylpyridine 3
carboxylic acid
is obtained as colorle~ cry~tals of melting point 100-
102C on ring cleavage of 6-methylpyridine-2,3-dicarb-
oxylic anhydride with tert-butylamine by the method of
Preparation Example 1~
Active ingredient Example No. 1.003
lO. Tri-n butylammonium 2-tert-butylaminocarbonyl-6-
methylpyridine-3-carboxylate
8.5 ~ of tri-n-butylamine are added to 9.4 g of
- 64 - O.Z. OQ50/41171
the carboxylic acid from 9. in 100 ml of methylene
chloride at from 20 to 24C in the course o 10 minutes
while stirring, and stirring is continued fox a further
lO minutes. The reaction mixture is partition0d between
water and saturated sodium chloride solution. The
organic phase i5 ~ried and evaporated down under reduced
pressure and the residue i~ stirred with ether to give
17.7 g of the title compound of melting point 85-87C.
Active ingredient Example No. 1.020
11. N-isopropylpyridine 2,3-dicarboximide-1-oxide
79.7 g of 55~ strength 3-chloxoperbenzoic acid
are added to 22 g of N-i~opropylpyridine-2,3-dicarbox-
imide in lOO ml of methylene chloride in tha course of 2
hours while reflu~ing and stirring, and stirring is
continued for a further 2 hours. The reaction mixture i~
extracted three tLmes with 10~ strength sodium carbonate
solution and then with water and saturated sodium chlor-
ide solution, dried, and evaporated down under reduced
pressure. The residue is stirred with methyl tert-butyl
ether, 11.2 g of the title compound of melting point 138-
142C being obtained.
Active ingredient Example No. 5.003
12. N-i~opropyl-6-chloropyridine-2,3-dicarboximide
6.5 g of the imide from ll. are added a little at
a time to 100 ml of phosphorus oxychloride, and the
mixture is heated stepwise until it refluxe~ and is
stirred for 5 hours. The reaction mixture is evaporated
down under reduced pressure, the residue is stirred ~ith
water and is taken up in methylene chloride, and the
solution is washed in succe sion with 10% st~ength sodium
carbona~e solution, with water and with satu~ated sodium
chloride solution. Evaporation gives 4.1 g of the title
compound as colorle~s crystals of melting point 122-
123C.
Active ingredient Example No. 3.023
13. N-isopropylpyridine-3,4-dicarboximide-1-oxide
The title compound is obtained as colorless
2 ~ !i 5
- 65 - O.Z. 0050/41171
crystals of melting point 169-173C by tha method of
Pr~paration Exampl~ ll, in thQ oxidation of the N-
isopropyl-3,4~dicarboximide with 3-chloroperbenzoic acid.
Activ~ ingredient Example No. 6.001
14. N-isopropyl-6-chloropyridine-3,4-dicarboximide
The title compound is obtained as colorlass
crystals of melting point 94-96C by the method of
Preparation ~xample 12 on reacting the N-oxide from 13.
with phosphoru~ oxychloride. According to the NMR
~pectrum, about 15% of the isomeric l-chloro compound was
formed a~ the sam~ time.
Active ingredient Example No. 4.006
15. N-isopropyl-6-methoxypyridine-3,4-dicarboximide
12 g of 30% strength -~odium methylate solution
are added to 5 g of the chlorine compound from 1~. in
100 ml of methanol, and the mixture is refluxed for
16 hours. The reaction mixture is evaporated down under
reduced pres~ura and then partitioned between water/
methylene chloride. ~he organic phase is evaporated down
and the re~idue i~ ~tirred with ether/petroleum ether to
give 1.6 g of the title compound of melting point 150-
152C.
Active in~redient Example No. 4.011
The aqueous phase is neutralized with concent-
rated hydrochloric acid and evapora~ed down and the
residue i~ stirred with methanol. The organic extract i~
: evaporated down to give 4.4 g of sodium 3-isQpropylamino-
carbonyl-6-methoxypyridine-4-carboxylate of melting point
56C ~decomposition)~
Active ingredient Example No. 2.007
16. a) ~-Trichloromethylpyridine-2,3-dicarboxylic
anhydride
A mixture of 200 g o~ 6-methylpyridine-2,3-
dicarboxylic anhydride and 6~0 ml of 1,2-dichlorobenzene
i5 gassed at 120C for about 1 hour with hydrogen chlor-
ide, after which 2 g of ~,~-azoi~obutyronitrile are
added and chlorine ga5 i5 passed in over 10 houra. The
2 ~
- 66 - o.z. 0050t41171
reaction solution is evaporated down and the residue Ls
~tirred with n-pentane. 311.6 g of the title compound are
obtained as colorles~ crystals of melting point 126-127C.
b) 6-Chlorodifluoromethylpyridine-2,3-dicarboxylic
anhydride
A stirred mixture of 53.3 g of tha anhydride of
a) and 39.3 g of antimony~III) fluoride i9 heated to
120C and S ml of antimony(V) chloride are then ~lowly
added, the exothermic reaction being slowed down at 130C
by removing the heating bath~ Stirring is continued for
a further 30 minutes at 125C, the mixlture is cooled and
100 ml of l,~-dichloroethane are added. 100 ml o~ 6 N
hydrochloric acid are ~hen ~lowly run in at from 0 to
10C and the aqueou~ phase is extracted with twice lOû ml
of methylene chloride. The organic extract is wa~hed
once with 6 N hydrochloric acid. Drying and evaporation
give 29.5 g of the title compound a~ a yellowish oil [IR~
C=0 1796 cm~1~.
c) 2-Tert-butylaminocarbonyl-6-chlorodifluoromethyl-
pyridine-3-carboxylic acid
28.5 g of the ti~le compound are obtained as a
colorless mas~ from 23.~ g of the anhydride from b) and
8 g of tert-butyla~nine in 200 ml of dioxane by the method
o~ Preparation Example l; lH-NMR (CCl3) [ppmJ 1.52 (s,
9H), 7.9 (d, lH), 3.8 ~d, lH).
Acti~e ingredient Example No. 1.027
17. N-tert-butyl-6-chlorodifluoromethylpyridine- 2,3-
dicarboximide
15.5 g of the title compound are obtaine~d as
colorle~s crystals o~ melting point 138-140C from 28.3 g
of the amide from 16 c) in 300 ml of acetic anhydride by
the method of Preparation Example 2.
Active ingredient Example No. 3.031
18. N-tert-butyl-6-chloxomethylpyridine-3, 4-
dicarboximid~
31.5 g of N-tert-butyl-6-methylpyridin2-3,4-
dicarboximide-1-oxide from 6.004 are added a lit~le at a
!
9 5
- 67 - o.Z. 0050/41171
time to 300 ml of phosphorus oxychloride, and the mixture
is heated ~tepwise until it refluxes and is stirred for
3 hours. The reaction mixture is evaporated down under
reduced pressure, the re~idue i8 taken up in methylene
5 chloride and the solution is stirred into ice water. The
organic phase is washed several times with water, dried,
filtered over alumina and evaporated down under reduced
pressure. 28.9 g of the title compound of melting psint
62-64C are obtained~
Active ingredient Example No. 4.010
19. Methyl 4-tert-butylaminocarbonyl--6-methoxymethyl-
pyridine-3-carboxylate
44.8 g of 30% strength sodium methylate solut~on
are added to 21.0 g of the Lmide from la . in 250 ml of
methanol at from 25 to 30C in the course of 15 mimltes
while stirring, and the mixture is refluxed for 6 hours.
After cooling, ~he mixture i3 brought to a pH of about
6.5 with 2N methanolic hydrochloric acid and the preclpi-
tated sodium chloride is filtered o~f under ~uction. The
filtrate is evaporated down under reduced pressure and
the residue is tri~urated with 3 : 1 methyl tert-butyl
ether/petroleum ether to give 15.6 g of the title com-
pound of melting point 138-142C.
Active ingredient Example No. 9.001
The pyridine derivatives of tha formulae Ia, Ib
and Ic listed in the ~ables below were obtained by these
processes described in Examples 1 to 19.
2~7~5
- 68 - Q.z. 0~50J41171
.~ U ~ ~ o
uta:~ ~: oooococ~oooooo
o=~ o
~ T 3~ S C~ T
S ~ ~- -r T
,~ , o ~ o g O C ~ O O O o o o
.. ~0~171~
- 69 - O.Z. 0050/41171
o
E
x r.
E ~
a ~ r ~
, -_ 0 ;~ ~ o oo ~o u~ O 1~ C C~
~, ~, 0 ~ ~ o c~ o ~ ~ ~ r
.~
~cc O O ~ O O O O O O O O O O O O O
~ ~ ~ S :3 T
:: ~
S ~1 U T 2 C,~ 2 IL ~ ~
C S S S X S~ S ~ S
~ t~ V ~ ql~ ~ ~ '* ~ T ' t ~
-- L ~ ~ ~ ~ L L L.
OOOOOOOOO OO OOO OO
2~2'7195
- 70 - O. z . 0050/411 î 1
-
_,
z
-
E
C ~
.s o o o c~ o o o
Q: CJ t ~
s ~ ~ o~ ~ S ~ ~ r
Lt~ V I C~ I T
o ~ a~ o c~
T T 3
. ~ ~ T
g ,~
_ L 3~ 3 L L
n ~ ~ ~ ~ ~ ~ ~ ~ ~
~a . 0 0 0 ~ O O o o
E-~' ~7 .. . .. . . .
2 ~ 2 ~ 5
7 1 ~ 1 Z . û O S O / 4 1 1 7 1
o
$
~ ~ ~ 0 _
'~ O ~
U ~ ~
o o o ~ o o o
o~ o
~Z C ~ ~ Z
.
~ ~ I
J S~
~ T I
S 0~ ~: T
L ~1 ~d L tJ L ~.)
N ~ o J o O
20~ i~95
- 72 - O.Z. OOS0/41171
TABLE 3
R4 ~ ~ 0 N-R1 la
phys. data
No. Rl R4 mp ~C), IR(cm 1)
3.001 tert.-C4Hg H 58- 60
3.002 i-C3H7 H 102-104
3.003 tert.-C4Hg 6-CH3 160-161
3.004 i-C3H7 6-CH3 153-154
3.005 j-~3H7 5-CH3 127-128
3.006 tert.-C4Hg 5-CH3 120-121
3.007 3-CI-Phenyl H 188-190
3.008 Phenyl 6-CH3 200-205
3.009 C2Hs 6-CH3 169-170
3. 010 tert . -C4Hg 5-C2Hs 82- 83
3.011 3-CI-Phenyl 6-CH3 211-212
3.012 C2H5 lO9 111
3.013 4-Cl-Phenyl 6-CH3 244-245
3.014 i-C3H7 6-C2Hs 104-105
3.015 cyclo-Propyl 6-CH3 174-175
3.016 2-Cl-Phenyl 6-CH3 250-253
3.017 sec.-C4Hg 6-CH3 105-106
3.018 SeC~-C4Hg S-CH3 96- 99
3.019 4-Ct-Phenyl 6-CC13 181-182
3.020 sec. -C4Hg H 52- 53
3.021 tert.-C4Hg 6-CCt3 148-149
3.022 t~rt.-C4Hg 6-C1 127-129
3.023 i-C3H7 6-Cl 122-123
3.024 sec. -C4Hg 6-Cl 78- 80
3.025 s0c.-C4Hg 5-CH3, 6-C1104 105
3.026 tert.-C4Hg 5-C2Hs, 6-C113S-136
3.027 tert.-C4Hg 5-CH3, 6-C1118-121
3 . 028 tert.-C4Hg5-CC13 76- 81
3.029 tert.-C4Hg S-CH3, 6-CH3146-149
2 0 ~ 9 ~
- 73 -O.Z. 0050/41171
TABLE 3 (continued~
phys. data
No. Rl R4 19P (C~, IRtcm~l)
3-030 sec~-c4Hg 5-CC13 resin
IR:C=O 1720
3.031 tert.-C4Hg 6-CF2Cl 138-140
3.032 i C4H9 S-CH3 148-151
3.033 i-C4Hg H 93- 97
3.034 tert.-C4H9 4-~H3 131-132
3.035 i-C3H7 4-CH3 89- 90
3.036 1-(cyclo- 6-CH3 110-112
Propyl)ethyl
3.037 tert.-C4Hg 4-C2H5 130-132
3.038 i-C3H7 4-C2H5 84- 87
3.039 i-C3H7 H-CH~, ~-CI101-103
3.04~ gec.-C4Hg 6-tert.-C4~gn~3-1.5211
3.041 tsrt.-C4Hg 6-tert.-C4Hg63- 67
3.042 tert.-C4Hg 6-OCH3 131-133
3.043 tert.-C4Hg 6-CF3 82- 87
3.044 tert.-C4Hg 5-tert.-C4Hg128-132
3.045 sec.-C4H9 5-i-C3H7 ~3-1.5300
3.046 tert.-C4Hg 5-i-C3~7 60- 62
3.047 i-C3H7 5-cH2-c6H5 64- 67
3.048 tert.-C4Hg 5-cH2-c6Hsn~3=1.577
3.049 tert.-C4Hg 5-i-C3H7~ 93- 95
6-CI
3.050 seC~-C4Hg 6-OCH3 91- 95
3.051 C~cH3)2-c2H5 5-CH3, 6-CH384- 87
3.052 sec.-C~Hg 5-i-C3H7n~3~1.5361
6-OCH3
3.053 tert.-C4Hg 5-~2H5~ 105-107
6-S02CH3
3.054 tert.-C4Hg 5-C2Hs, 93~ 97
6-~CH3
3.055 tert.-C4Hg 5-C2H5~ 94~ 97
6-OCH3
3.0S6 C(CH3)~-~-C3H7 5-CH3, 6-OCH3 125-130
3.057 sec~-C4Hg 5-CH3, 6-OCH3 104-106
~i7~95
_ 74 _ o.z. 0050/41171
TABLE 3 ( continued)
phy~i. data
No. Rl R4 ~ (C), IR(cm~l)
3.058 tert.-C4Hg 5-CH3, 6-OC~13 124-1~7
3 . 05g tert . -C4Hg 6-OCH2CH-CH252- 57
3.060 C(CH3)(i~G3H7)CN H 92- 96
3.061 C(CH3) (CH20Cil3)CN H 152-156
~i7~ ~
~ 75 ~ O. ~ . 0050t~1171
T~BLE 4
R 4~ N-R I l a
C~
phys. data
No. Rl R4 mp.(C), IR(cm~
4.001 i-C3H7 H 103-106
4.002 sec.-C4Hg H oel
IR:C=O 1716
4.003 tert.-C4Hg H 46- 49
4.004 tert.-C4Hg 6-C1 73- 76
4.005 se~-C4Hg 6-C1 30- 35
4.006 i-C3H7 6-C1 94- 96
4.007 tert.-C4Hg 6-CH3 89- 91
4.008 i-C3H7 6-CH3 91- 94
4.0q9 i-C3H7 6~CH2C1 63- 65
4.010 tert.-C4Hg 6-CH2Cl 62-- 64
4.011 i-C3~7 6-OCH3 150-152
4.012 tert.-C4Hg 6-O-CH3 101-103
4.013 tert.-C4Mg 6-CH2-O-CH338- 41
4.014 n-C3H7 2,6-C12 74- 78
4.015 C(CH3)2CN H 84- 86
9 ~
- 76 - O.Z. 0050/41171
TABLE S
R4-~ ,N-Rl Ia
~ 11
O O
~o. Rl R4 mp.~C)
5.001 sec~ -C4H9 H 103-104
5.002 tert.-C4Hg H 118-122
5.003 i-C3H7 H 138-142
5.004 tert.-C4Hg 5-~H3 118-122
5.005 t2rt.-C4Hg 5~C2H5 128-131
5.0Q6 sec.-C4Hg 5-Ch3 195-198
TABLE 6
: 8
R4 ~ ~N-RI Ia
OP~ 11
No. Rl R4 mp.~C~
6.001 i-C3H7 H 172-174
6.002 tert.-C4Hg H l9~-1g9
6.003 sec~-C4Hg H 120-125
6.004 t~rt,-C4Hg ~-CH3 227-229
- 77 _ O. ~; . 0050/41171
TABI,E 7
O R6
,~ C N--R 7
R4~ ll Ia
~N~C-N--R 1
No. Rl R2R4 R6 R7~' (C)
7.001 sec.-C4Hg H 6-CH3 H H131-133
7 . 002 tert . -C41tg H 6-Cl H H 85 (deca~. )
7.003 sec.-C4Hg H 5-CH3 H H119-123 as a mixture wi'ch
20~ ~f isn~ic
:2--amide
7.004 i-C4Hg H 5-CH3 H H118-122 as ami.~cturew~th
20% of isaneric
2-amide
~ABl:E 8
I R 1
R4 - ~ ~ Ib
~N~ ~Il_A--R5
N~. Rl R2 R4 R5 A mp (C)
8.001 i-C~Hg H 5-CH3 H 0 125-129 (dec~p.)
8 . Q02 tert . -Cl~Hg H 6-tert . -C4Hg C~13 0 57- 61
2~2ijl~9~
7~ - O.Z. 0050/41171
TAE~LE 9
O Rl
C--N---R 2
R4~ Ib
N~8_A--R 5
R2 R4 R5 A ~ (C)
9.001 tert.-C~/~Hg H 6-CH2oCH3 i1 C 172-178
9.002 l:ert.-C4Hg H 6-CH2oCH3 CH3 0 138-142
7~
79 O.Z. 0050/41171
The compounds l'a, I'b and I'c, or herbicidal agents containing them, may
be applied for instance in the form of directly sprayable solutions, pow-
ders, suspensions (including high-percentage aqueous, oily or other sus-
pensions), dispersions, emulsions, oil dispersions, pastes, dusts, broad-
5 casting agents, or granules by spraying, atomizing, dusting, broadcastingor watering. The forms of application depend entirely on the purpose for
which the agents are being used, but they must ensure as fine a distrib-
ution of the active ingredients according to the invention as possible.
lO The compounds I'a, I'b and I'c are suitable for the preparation of sol
utions, emulsions, pastes and Q.l dispersions to be sprayed direct. Ex-
amples of inert additives are mineral oil fractions of medium to high
boiling point, such as kerosene or diesel oil, further coal-tar oils, and
oils of vegetable or animal origin, aliphatic, cyclic and aromatic hydro-
15 carbons such as toluene, xylene, paraffin, tetrahydronaphthalene, alkyl-
ated naphthalenes and their derivatives, methanol, ethanol, propanol,
butanol, cyclohexanol, cyclohexanone, chlorobenzene, isophorone, etc., and
strongly polar solvents such as N,N-dimethylformamide, dimethyl sulfoxide,
N-methylpyrrolidone and water.
Aqueous formulations may be prepared from emulsion concentrates, pastes,
oil dispersions, wettable powders or water-dispersible granules by adding
water. To prepare emulsions, pastes and oil dispersions the ingredients as
such or dissolved in an oil or solvent may be homogenized in water by
25 means of wetting or dispersing agents, adherents or emulsifiers. Concen-
trates which are suitable for dilution with water may be prepared from
active ingredient, wetting agent, adherent, emulsifying or dispersing
agent and possibly solvent or oil.
30 Examples of surfactants are: alkali metal, alkaline earth metal and
ammonium salts of aromatic sulfonic acids, e.g., ligninsulfonic acid,
phenolsulfonic acidj naphthalenesulfonic acid and dibutylnaphthalene-
sulfonic acid, and of fatty acids, alkyl and alkylaryl sulfonates, and
alkyl, lauryl ether and fatty alcohol sulfates, and salts of sulfated
35 hexadecanols, heptadecanols, and octadecanols, salts of fatty alcohol
glycol ethers, condensation products of sulfonated naphthalene and
naphthalene derivatives with formaldehyde, condensation products of
naphthalene or naphthalenesulfonic acids with phenol and formaldehyde,
polyoxyet~lylene octylphenol ethers, ethoxylated isooctylphenol, ethoxyl-
40 ated octylphenol and ethoxylated nonylphenol, alkylphenol polyglycolethers, tributylphenyl polyglycol ethers, alkylaryl polyether alcohols,
isotridecyl alcohol, fatty alcohol ethylene oxide condensates, ethoxylated
castor oil, polyoxyethylene alkyl ethers, ethoxylated polyoxypropylene,
lauryl alcohol polyglycol ether acetal, sorbitol esters, lignin-sulfite
waste liquors and methyl cellulose.
~12 7~
O.Z. 0050/41171
Powders, dusts and broadcasting agents may be prepared by mixing or
grinding the active ingredients with a solid carrier.
Granules, e.g., coated, imp~egnated or homogeneous granules, may be
5 prepared by bonding the active ingredients to solid carriers. Examples of
solid carriers are mineral earths such as silicic acids, silica gels,
silicates, talc, kaolin, attapulgus clay, limestone, lime, chalk, bole,
loess, clay, dolomite, diatomaceous earth, calcium sulfate, magnesium
sulfate, magnesium o~ide, ground plastics, fertilizers such as ammonium
10 sulfate, ammonium phosphate, ammonium nitr~te, and ureas, and vegetable
products such as grain meals, bar~ ~eal, wood mea~, and n~tshell meal,
cellulosic powders, etc.
The formulations contain from 0.01 to 95, and pre~erably 0.1 to 90, % by
15 weight of active ingredient. The active ingredients are used in a purity
of from 90 to 100, and preferably from 95 to 100, %.
The pyridine deriva-tives I'a, I'b and I'c may be formulated for instance
as follows:
2~
1. 90 parts by weight of compound no. 3.003 is mixed with 10 parts by
weight of N-methyl-alpha-pyrrolidone. A mixture is obtained which is
suitable for application in the form of very fine drops.
25 II. 20 parts by weight of compound no. 3.003 is dissolved in a mixture
consisting of 80 parts by weight of xylene~ 10 parts by weight of the
adduct of 8 to 10 moles of ethylene oxide and 1 mole of oleic acid-N-
monoethanolamide, ~ parts by weight of the calcium salt of dodecylbenzene-
sulfonic acid, and 5 parts by weight of the adduct of 40 moles of ethylene
30 oxide and 1 mole of castor oil. By pouring the solution into 100,000 parts
by weight of water and uniformly distributing it therein, an aqueous dis-
persion is obtained containing 0.02% by weight of the active ingredient.
III. 20 parts by weight of compound no. 3.004 is dissolved in a mixture35 consisting oF 40 parts by weight of cyclohexanone, 30 parts by weight of
isobutanol, 20 parts by weight of the adduct of 7 moles of ethylene oxide
and I mole of isooctylphenol, and 10 parts by weight of the adduct of
40 moles of ethylelle oxide and 1 mole of castor oil. ~y pouring the
solution into 100,000 parts by weight of water and finely distributing it
40 therein, an aqueous dispersion is obtained containing 0.02% by weight of
the active ingredient.
~ ~ 2 ~
81 O.Z. 0050~41171
IV. 20 parts by weight of compound no. 3~003 is dissolved in a mixture
consisting of 25 parts by weight of cyclohexanone, 65 parts by weight of a
mineral oil fraction having a boi1ing point between 210 and 280C, and
10 parts by weight of the adduct of 40 moles of ethylene oxide and I mole
5 of castor oil. By pouring the solution into 100,000 parts by ~eight of
water and uniformly distributing it therein, an aqueous dispersion is
obtained containing 0.02% by weight of the active ingredient.
V. 20 parts by weight of compound no. 3.004 is well mixed with 3 parts by
lO weight of the sodium salt of diisobutylnaphthalene~alpha-sulfonic acid,
17 parts by weight of the sodium salt of a lignin-sulfonic acid obtained
from a sulfite waste liquor, and 60 parts by weight of powdered silica
gel, and triturated in a hammer mill. By uniformly distributing the
mixture in 20,000 parts by weight of water, a spray liquor i5 ~btained
15 containing 0.1% by weight of the active ingredient.
Vl. 3 parts by weight of compcund no. 1.001 is intimately mixed with
97 parts by weight of particulate kaolin. A dust is obtained containing 3%
by weight of the active ingredient.
VII. 30 parts by weight of compound no. 2.002 is intimately mixed with a
mixture consisting of 92 parts by weight of powdered silica gel and
8 parts by weight of paraffin oil which has been sprayed onto the surface
of this silica gel. A formulation of the active ingredient is obtained
25 having good adherence.
VIII. 20 parts by weight of compound no. 3.010 is intimately mixed with
2 parts of the calcium salt of dodecylbenzenesulfonic acid, 8 parts of a
fatty alcohol polyglycol ether, 2 parts of the sodium salt of a phenol-
30 sulfonic acid-urea-formaldehyde condensate and 68 parts of a paraffinic
mineral oil. A stable oily dispersion is obtained.
The active ingredients or the herbicidal agents containing them may be
applied pre- or postemergence. If certain crop plants tolerate the active
35 ingredients less well, application techniques may be used in which the
herbicidal agents are sprayed from suitable equipment in such a manner
t~lat the leaves of sensitive crop plants are if possibla not touched, and
the agents reach the soil or the unwanted plants growing beneath the crop
plants (post-directed, lay-by treatment).
The application rates depend on the objective to be achieved, the time of
the year, the plants to be combated and their growth stage, and are from
0.001 to 5, preferably 0.01 to 2, kg of active ingredient per hectare.
~B~ 7~9~
82 o.Z. 0050/41171
In view of the numer~us application me~hods possible, the compounds ac-
cording to the invention may be used in a large number of crops for
removing unwanted plant growth.
5 To increase the spectrum of action and to achieve synergistic effects, the
compounds I'a, I'b and I'c may be mixed with each other, or mixed and ap-
plied together with numerous representatives of other herbicidal or
growth-regulating active ingredient groups. Examples of suitable com-
ponents are diazines, 4~-3,1-benzo~a~ine derivatives, benzothiadia~inones,
lO 2,6-dinitroanilines, N-phenylcarbamates, thiolcarbamates, halocarboxylic
acids, triazines, amides, ureas, diphenyl ethers, triazinones, uracils,
benzofuran derivatives, cyclohexane-1,3-dione derivatives, quinolinecar-
boxylic acids, (hetero)-aryloxyphenoxypropionic acids and salts, esters,
amides thereof, etc.
It may also be useful to apply the pyridine derivatives I'a, I b and I c,
either alone or in combination with other herbicides, in admixture with
other crop protection agents, e.g., agents for combating pests or phyto-
pathogenic fungi or bacteria. The compounds may also be mixed with
20 solutions of mineral salts used to remedy nutritional or trace element
deficiencies. Non-phytotoxic oils and oil concentrates may also be added.
Use examples
25 The herbicidal action of the pyridine derivatives I'a, I'b and I'c is
demonstrated in greenhouse experiments:
The vessels employed were plastic flowerpots having a volume of 300 cm3
and filled with a sandy loam containing about 3.0% humus. The seeds of the
3~ test plants were sown separately, according to species.
For the preemergence treatment, the active ingredients, suspended or
emulsi~ied in water, were applied through finely distributing nozzles to
the surface of the soil immediately after the seeds had been sown. After
35 the agents had been applied, the vessels were lightly sprinkler-irrigated
to induce germination and growth. Transparent plastic covers were then
placed on the vessels until the plants had taken root. The cover ensured
uniform germination of the plants, insofar as this was not impaired by the
active ingredients.
For the postemergence treatment, the plants were grown, depending on
growth form, to a height of 3 to 15 cm before being treated with the com-
pounds, suspended or emulsified in water.
~7~9~
83 O.Z. 0050/41171
The pots were set up in the greenhouse, heat-loving species at 20 to 35C,
and species from moderate climates at 10 to Z5C. The experiments were run
for from 2 to 4 weeks. During this period the plants were tended and their
reactions to the various treatm~nts assessed. The assessment scale was 0
5 to 100, 100 denoting nonemergence or complete destruction of at least the
visible plant parts, and 0 denoting no damage or normal growth.
The plants used in the greenhouse experiments were Abutilon theophrasti,
Bromus inermis, Chenopodium album, Chrysanthemum coronarium and Stellaria
lO media.
For instance pyridine derivatives 3.003 and 3.004, applied postemergence
at a rate of 1.0 kg/ha, provided excellent control of unwanted plants.