Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~' ~~'s~~°~:~
1 FIELD OF THE INVENTION
2 This invention relates to a hollow fiber bundle element
3 which may be packed in a first passageway with minute solid
4 particles, such that the particles may interact with fluid
components in a second passageway.
6 BACKGROUND OF THE INVENTION
7 Adsorption processes are widely used in industry for
8 separation of fluid mixtures (gas or liquid). The separation is
9 based on preferential adsorption of selective components on the
surface of solid adsorbents. For efficient separation, the
11 adsorbent material must have large surface areas to provide
12 reasonable adsorptive capacities. The commonly used adsorbents,
13 such as molecular sieve zeolites, activated carbon, alumina and
14 silica gel, have surface areas of at least 200 m2/g.
Most industrial adsorption processes are carried out
16 in f fixed-bed type columns . The adsorbent granules are packed and
17 immobilized in a cylindrical vessel. As the fluid mixture to be
18 separated is passed through the packing via the void spaces among
19 the granules, the adsorbable components in the mixture are taken
up and retained by the adsorbent.
21 Since the adsorbent has a limited adsorption capacity,
22 it will become gradually saturated with adsorbate, and periodic
2
,.~. ~~z.3
1 ~ adsorbent regeneration is required. For continuous processing
2 of a feed mixture, a multi-bed system is used in which each bed
3 goes through the adsorption/regeneration cycle in sequence.
4 Several different regeneration methods have been used
commercially. Chief among them are the thermal swing adsorption
6 (TSA) and pressure swing adsorption (PSA) processes. In the TSA
7 process, the saturated adsorbent is regenerated by purging with
8 a hot gas. Each heating/cooling cycle usually requires a few
9 hours to over a day. In the PSA process, the adsorbent
regeneration is effected by purging with a portion of the
11 purified product gas at reduced pressure. The throughput is
12 higher than that of the TSA since faster cycles, usually in
13 minutes, are possible.
14 Apart from the adsorptive capacity of the adsorbent,
the adsorption rate and pressure drop are two important factors
16 that must be considered in adsorber design.
17 Pressure drop through the adsorber column should be
18 minimized, because high fluid pressure drop can cause movement
19 or fluidization of the adsorbent particles, resulting in serious
attrition and loss of the adsorbent.
21 The adsorption rate has a significant bearing on the
22 efficiency of the adsorption process. This rate is usually
23 determined by the mass transfer resistance to adsorbate transport
24 from the bulk fluid phase to the internal surfaces of the
adsorbent particles. Slow adsorption rate due to large mass
26 transfer resistance will result in a long mass transfer zone
27 (MTZ) within which the adsorbent is only partially saturated with
28 adsorbate. The adsorbent in the region upstream of the MTZ is
29 substantially saturated with adsorbate, while that downstream of
3
1 the MTZ is essentially free of adsorbate. As the fluid continues
2 to flow, the MTZ advances through the adsorber column in the
3 direction of the fluid stream. The adsorption step must be
4 terminated before the MTZ reaches the adsorber outlet in order
to avoid the breakthrough of adsorbate in the effluent stream.
6 A long mass transfer zone, which contains a large quantity of
7 partially utilized adsorbent, will, therefore, result in a short
8 adsorption step and inefficient use of the adsorbent capacity.
9 These effects are especially serious for the pressure swing
adsorption process.
11 Both the pressure drop and the mass transfer resistance
12 are strongly influenced by the size of the adsorbent particles.
13 Changing the particle size, unfortunately, has opposite effects
14 on these two important factors. This is elaborated below:
(1) The pore sizes of the void spaces among the
16 adsorbent particles in the fixed-bed are
17 proportional to the size of the particles. Since
18 the resistance to the fluid flow through the
19 adsorber is inversely proportional to the pore
size of the packed bed, the use of small adsorbent
21 particles will cause high pressure drop. For this
22 reason, the sizes of particles of commercial
23 adsorbents for fixed-bed operation are generally
24 larger than 2 mm in equivalent diameter.
Adsorbent of smaller particle sizes, such as
26 zeolite crystals (less than 10 microns), are
27 pelletized using binding material to suitable
28 sizes.
29 (2) Almost all the surface areas of commercial
adsorbents are located at the interior of the
4
2~
1 ~ adsorbent particle. For adsorption to occur, the
2 adsorbate needs to be transported from the
3 external fluid phase to the interior surface of
4 the particle. The transport rate is dominated by
two mass transfer mechanisms in series: (a)
6 interfacial mass transfer - diffusion through the
7 fluid boundary layer surrounding the external
8 surface of the adsorbent particle; and (b)
9 intraparticle mass transfer - diffusion through
the internal pore space (micropores and
11 macropores) of the particle to its interior
12 surface where adsorption takes place. The size
13 of the particle has significant effects on the
14 rates of these two diffusion processes. Small
particles offer large fluid/solid contact areas
16 in the fixed bed for interfacial mass transfer and
17 reduce the path length for the intraparticle
18 diffusion. Hence, small adsorbent particles will
19 increase adsorption rate and result in a narrow
mass transfer zone for fast and efficient
21 operation of adsorption/desorption cycles.
22 The above discussions and analysis show that small
23 adsorbent particles are desirable for efficient adsorption
24 processes, but the minimum particle size is limited by acceptable
hydrodynamic operating conditions of the fixed bed ads,orber.
26 That is, one wants to avoid fluidization and excessive pressure
27 drop. Such a concept also applies to a heterogeneous catalytic
28 reaction process, which involves an adsorption step in the
29 reaction mechanism. The use of small catalyst particles will
5
1 enhance mass transfer between the catalyst and surrounding fluid
2 carrying the reactants, but it will also increase pressure drop
3 through the reactor bed.
4 It would therefore be desirable to provide an adsorber
or catalytic reactor containing adsorbent or catalyst
6 characterized by a relatively small particle size and yet still
7 able to operate with an acceptable pressure drop.
8 At this point, it is appropriate to shortly describe
9 the structure and operation of a known separation device used for
permeation and absorption and referred to as a hollow fiber
11 module. As will become clear below, this module is similar in
12 many respects to a shell and tube heat exchanger. The device is
13 used to separate at least one component (e. g. C02) from a second
14 'carrier' component (e.g. natural gas) with which it forms a feed
mixture. A typical module comprises a cylindrical vessel
16 encapsulating a bundle of small-diameter, elongated, hollow
17 fibers. The fibers are formed of a material having a
18 permeability which, in the case of a permeation module, is
19 selected to allow the component to be extracted to diffuse
therethrough but to substantially reject the carrier component.
21 In the case of an absorption module, the entire feed mixture may
22 readily diffuse through the fiber wall. The fibers are "potted"
23 at their ends in closure means, such as epoxy tube sheets, so
24 that the ends of the fibers project therethrough, leaving their
bores or "lumina" open. The tube sheets function to seal the
26 void space between the fibers at the two ends. The tube sheets
27 further seal or are sealed by means, such as an O-ring, against
28 the inside surface of the vessel. The vessel is provided with
29 a first inlet and first outlet communicating with the ends of the
6
2~~~~
1 ~ fiber lumina. It further has a second inlet and second outlet
2 communicating with the ends of the void space. In operation, the
3 feed mixture of gases is fed through the second inlet into the
4 void space. In the case of an absorption module, absorbent fluid
is fed into the lumina. The absorbate (C02) diffuses through the
6 fiber walls from the void space, is coJ.lected by the absorbent
7 fluid, and exits through the first outlet. The carrier gas,
8 reduced in C02, leaves through the second outlet.
9 With this background in mind, it is now appropriate to
describe the present invention.
11 SUMMARY OF THE INVENTION
12 The present inven tion involves use of a known article,
13 namely a module comprising a bundle of hollow fibers contained
14 in an impermeable casing. The bundle may be used to provide
interaction between minute solid particles and a feed stream
16 component or components. The fibers each have a microporous
17 permeable wall having pore openings in the range of about 0.05
-
18 5 micrometers (known as the "microfiltration range"). The minute
19 solid particles are emplaced
in a first of two passageways,
either the lumina of the
fibers or the void space
between the
21 fibers. The particles are sufficiently densely packed
22 substantially throughout the length and breadth of the
23 passageway) so as to have density equal to or greater than
a the
24 free-standing bulk density of the particles. The particles are
sufficiently small or minut e so as to provide fast mass transfer
26 of the feedstream component or components to the particles where
27 interaction takes place.
They are "free" particles,
not being
28 bonded together by binder or the like. The first passageway
7
1 ,NM~ containing the particles is sealed at its ends, for example by
2 an epoxy tube sheet. The pore openings of the fiber wall are
3 smaller than the particles involved. These openings, however,
4 are large enough to permit the fluid to diffuse therethrough.
The particles are emplaced in the module in a unique
6 fashion. More particularly, a suspension of the particles in a
7 liquid or gas carrier is pumped under pressure into one of the
8 passageways. The carrier filters through the fiber walls into
9 the other passageway and exits the module, leaving the particles
trapped in the original passageway. By this process, a dense
il uniform dispersion of particles is emplaced in the original
12 passageway throughout its length. The particles are individually
13 free but are collectively immobilized in the original passageway
14 due to the completeness of the packing.
The final product, comprising the casing, the hollow
16 fibers, the end closures, and the charge of particles, is
17 hereafter referred to as the "element".
18 As a result of assembling the foregoing, minute solid
19 particles having fast mass transfer rate are immobilized in the
sealed first passageway of the element. Yet feedstream
21 components of a fluid stream that is introduced into the other
22 or second passageway, can still reach and interact with the
23 particles by diffusing through a fiber wall to enter the first
24 passageway.
The pore openings of the fiber wall are sufficiently
26 large to enable the carrier liquid or gas to filter readily
27 therethrough during the fabrication step of emplacing the packing
28 of particles in one of the passageways.
8
_ 2~0
1 ~ In this fashion, it is feasible to fabricate the
2 element without high expense and it is possible to use very small
3 particles having a very high mass transfer rate, in connection
4 with a pressure-driven fluid mixture to be processed, without
having fluidization occur. And the availability of the second
6 passageway, for the passage therethrough of the fluid mixture,
7 has ensured that only a relatively low pressure drop will occur
8 across the element.
9 In one embodiment, the element may be an adsorber. The
feedstream then contains an adsorbate. The particles packed in
11 the first passageway are adapted to adsorb the adsorbate from the
12 fluid stream as it flows through the element. An adsorbate-
13 depleted stream is the result.
14 In another embodiment, the element may be a catalytic
reactor. In this case the feedstream contains reactants. The
16 particles packed in the first passageway are adapted to catalyze
17 reaction between the reactants in the feedstream as it flows
18 through the element. Reaction products in the end stream is the
19 result.
DESCRIPTION OF THE DRAWINGS
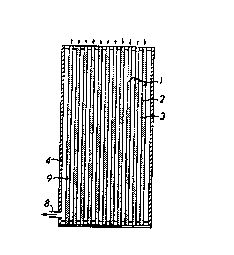
21 Figure 1 is a schematic showing the arrangement used
22 to emplace particles in the lumina of an element;
23 Figure 2 is a schematic showing the arrangement used
24 to emplace particles in the void space between the fibers;
Figure 3 is a schematic showing an element, having the
26 particles in the lumina, being used to provide interaction
27 between the particles and a feedstream; and
9
~~~~
1 ~ Figure 4 is a schematic showing an element, having the
2 particles in the void space between the fibers, being used in
3 conjunction with a vessel to provide interaction between the
4 particles and a feedstream.
nFS~RIpTION OF THE PREFERRED EMBODIMENT
6 The element A can take one of two forms, shown in
7 Figures 3 and 4 (which are not to scale).
8 In Figure 3 the element A is the element itself and
9 comprises a bundle of f fibers 1, each fiber having a bore or lumen
2. The plurality of fibers form a void space 3 between them.
11 An impermeable cylindrical casing 4 contains the bundle. The
12 bundle has top and bottom closures 5, 6 which seal the lumina 2
13 and void space 3. An inlet 7 is provided at one end of the
14 casing 4, for introducing the feed mixture, and an outlet 8 is
provided at the opposite end of the casing for exhausting a
16 stream after interaction with the particles. Particles 9 are
17 packed in the lumina 2. The fiber walls have sub-micron sized
18 pores which enable the feedstream components to diffuse readily
19 therethrough but the pores are smaller than the particles 9. As
a result of providing fiber walls that prevent the particles 9
21 from moving therethrough and sealing the ends of the lumina 2
22 with the closures 5, 6, the particles 9 are immobilized in the
23 lumina 2.
24 In Figure 4, the element B has the particles 9 disposed
in the void space 3 between the fibers 1. Closures 5a, 6a are
26 provided and leave the ends of the lumina 2 open but seal the
27 ends of the void space 3. The element 10 of Figure 4, comprising
28 the bundle of fibers 1, closures 5a, 6a and casing 4, is
2~
1 positioned in a vessel 11 having a top inlet 12 and bottom outlet
2 13. The inlet 12 and outlet 13 communicate with the ends of the
3 lumina 2.
4 From the foregoing, it will be noted that each of the
elements provides a continuous longitudinal flow passageway. In
6 the case of the element A, the passageway is the void space 3.
7 In the case of the element B, the passageway is provided by the
8 lumina 2. For separation of fluid mixtures, the feed is directed
9 to flow through the flow passageway. Since the thin and porous
fiber wall has negligible mass transfer resistance, the fluid is
11 always in intimate and substantially uniform contact with the
12 particles 9. The elements A, B when used as adsorbers are
13 adapted for use with PSA and TSA systems in accordance with known
14 technology.
Typically the hollow fibers will have a lumen diameter
16 less than 2 mm. The fiber wall will typically have pore openings
17 of about 0.5 micrometer in equivalent diameter.
18 The solid particles or crystals (referred to
19 collectively as "particles") can be packed into the lumina 2 or
void space 3 using one of several techniques. More particularly,
21 in the case of non-soluble particles, they are first suspended
22 by agitation in a liquid or gas carrier, such as alcohol, water
23 or air. The suspension is then pumped into the lumina 2 or void
24 space 3, as shown in Figures 1 or 2. The liquid or gas carrier
is able to permeate readily through the microporous fiber wall.
26 In the case of pumping the slurry into the lumina 2 (Figure 1),
27 the top ends of the lumina are open, to receive the feed and the
28 bottom ends are sealed. The particles 9 become trapped in the
29 lumina while the carrier diffuses through the fiber walls and
11
1 p exits through an outlet 8 in the casing 4. In this fashion, a
2 charge of densely packed particles may be accumulated to fill
3 the lumina substantially throughout its length. The top ends of
4 the lumina can then be sealed to immobilize the particles.
Similarly, in the case of pumping the slurry into the void space
6 3 (Figure 2), the top ends of the lumina 2 and the void space 3
7 are closed and the bottom ends of the lumina are left open. The
8 slurry enters the void space, the carrier passes through the
9 fiber walls and exits out the bottom of the lumina, and the
particles 9 remain trapped in the void space 3. In both cases,
11 loading may be facilitated by vibration by immersing the module
12 in an ultrasonic bath.
13 In the case of soluble materials, the element, having
14 fibers that will not be wetted by the solvent, can be packed by
filling a first passageway of the module with the solution and
16 then drying or leaching out the solvent by circulating air or
17 non-solvent through the second passageway of the module.
18 When the element is used as an adsorber, the particles
19 will be adsorbent particles, preferably selected from the group
consisting of molecular sieve zeolites, silica gel, activated
21 alumina, carbon black, and activated carbon. The particle size
22 preferably will be less than 30 microns, most preferably 1 - 30
23 microns. The surface area preferably should be at least about
24 200 m2/g.
Still another class of materials that can be used as
26 the adsorbent are those that can be cast in-situ to form a
27 microporous structure by the sol-gel phase inversion techniques.
28 (See Example 2 and Robert E. Kesting, "Synthetic Polymeric
29 Membrane", 2nd Edition, John Wiley, N.Y., 1985). A typical sol-
12
1 gel process for forming porous structure comprises: preparing
2 a solution of polymeric material, solvent, non-solvent and
3 swelling agent; evaporating or leaching the solvent with non-
4 solvent; and drying the non-solvent.
The present hollow fiber element has certain advantages
6 over conventional packed bed elements, namely:
7 (1) In the hollow fiber element, the fluid pressure
8 drop through the element is independent of the
9 size of the particles, because the fluid flow path
is separated from the particles by the microporous
11 fiber walls;
12 (2) The hollow fiber element can use very fine
13 particles. This will reduce mass transfer
14 resistance, because the use of small particles
increases the fluid/solid interfacial mass
16 transfer areas and reduces the intraparticle
17 diffusion path length. In addition, for adsorbers
18 the binder materials contained in the larger
19 pelletized adsorbents used in conventional
adsorbers is eliminated, resulting in higher
21 adsorptive capacities;
22 (3) The hollow fiber element broadens the choice of
23 materials for the particles. It can use a wide
24 range of powder materials. If the particle size
is small enough, the particles need not be of
26 porous material, because small particles have
27 large external surface areas;
28 (4) The hollow fiber adsorber can use microporous and
29 adsorptive structure that can be cast into either
13
1 ~ the lumina or void space of the module. Many
2 plastic materials can be converted to microporous
3 matrices by the so-called phase inversion
4 technique (see Example 2). The fiber wall
provides a partition between the matrix and the
6 flow passageway in the fiber module;
7 (5) The microporous hollow fibers provide efficient
8 and uniform contact between the particles and the
9 fluid mixture for a wide range of flow rates,
thereby avoiding the channelling problems that can
11 affect conventional elements;
12 (6) The fast mass transfer and low pressure drop of
13 the hollow fiber adsorber enables the PSA process
14 to be operated efficiently at fast cycle and high
feed rates.
16 The invention is illustrated by the following examples
17 ~xamy~le I
18 This example sets forth in detail an embodiment of the
19 best mode presently known to applicants for packing one of the
passageways with a charge of particles. It further describes the
21 character of the charge so emplaced.
22 Three hollow fiber modules were made using microporous
23 polypropylene Celgardl hollow fibers manufactured by the Hoechst
24 Celanese Corporation (Charlotte, N.C.). The physical parameters
of these modules are given in the following Table. Element 1 was
26 packed with molecular sieve zeolite crystals in the fiber lumina
27 (see Figure 1) using cyclohexane as the carrier fluid. Element
28 lTrade-Mark
14
1 '"'" 2 was packed with activated carbon powder in the void space
2 between fibers (see Figure 2) using methanol as the carrier
3 fluid. Both elements were packed using 20 psi slurry solution
4 of adsorbent particles suspended in the carrier fluid, driven by
a diaphragm pump. The slurry pumping operation was then followed
6 by dry nitrogen circulation to dry out the carrier fluid from
7 adsorbent particles. Element 3 was packed with molecular sieve
8 zeolite crystals in the void space between the fibers, using 200
9 psi helium as the carrier fluid. As shown in the Table, the
resulting hollow fiber elements have adsorbent particle packing
11 density considerably greater than the free standing particle bulk
12 density. The packing was uniform throughout the length and
13 breadth of the packing space.
1 TABLE 1
2 Physical Parameters of Hollow ber AdsorberElements
Fi
3 Hollow Fiber Module Element 1 Element 2 Element 3
4 casing, ID, cm .48 .45 .48
fiber type Celgard2 Celgard3 Celgard4
6 X20-400 X20-200 X20-200
7 fiber number 60 132 150
8 active fiber length, cm 65 64 70
9 fiber ID, micrometer 400 200 200
fiber OD, micrometer 460 260 260
11 fiber wall porosity, ~ 40 40 40
12 fiber wall pore opening,
13 micrometer
14 (width x length) .0 65 x .19 .065 x .19 .065 x .19
Adsorbent Packings
16 packing location fiber outside outside
17 lumina fibers fibers
18 adsorbent type Union DarcoS Union
19 Carbide KB Carbide
5A Carbon 5A
21
22 particle size, micrometer <10 <30 <10
23 particle bulk density, g/cc
24 (free standing) .49 .25 .49
packing density, g/cc .53 .40 .64
26 total packing weight, g 2.6 2.3 4.6
27 2Trade-Mark
28 3Trade-Mark
29 4Trade-Mark
STrade-Mark
16
2
1 ~ Example II
2 This example illustrates the use of very fine, non-
3 soluble adsorbent particles in a hollow fiber adsorber for gas
4 separation.
Two hollow fiber modules were made containing
6 microporous polypropylene Celgard5 X10-400 hollow fibers. The
7 fiber had a 400 micron internal diameter lumen and 30 micron
8 thick wall. The fiber wall had 30~ porosity provided by .065 x
9 . 19 microns pore openings . Each of the test modules had 30 open-
ended fibers of 50 cm length encased in a 3/16 inch OD stainless
11 steel tube ( . 375 cm ID) with both ends of the fiber bundle potted
12 in 3 cm long polyurethane tube sheets.
13 The previously described filtration technique was used
14 to pack a type Y zeolite powder (less than 10 micron size) into
the modules. One module was packed with 1.3 g of powder in the
16 fiber lumen, and the other was loaded with 1.7 g of the same
17 powder in the void space between the fibers. The different modes
18 of adsorbent loading were chosen only to demonstrate the
19 workability of each version of the process.
The two modules were plumbed and instrumented to
21 operate as a cyclic pressure swing adsorption (PSA) system in
22 accordance with C. W. Skarstrom, U.S. patent 2,944,627. The
23 cyclic operation was automated with an 8 port valve directing
24 the gas to and from the inlets and outlets of the two adsorbers.
The valve was, in turn, driven by a solenoid controlled by a
26 programmable timer.
27 The PSA system was used to purify a feed stream
28 consisting of helium gas containing 1~ C02. In the first step of
29 5 Trade-Mark
17
s
1 ~ the PSA cycle, the feed gas, at 200 psig and 23°C, was fed to the
2 first adsorber for C02 removal at a rate of 200cc (STP)/min.
3 Simultaneously a portion (25cc/min.) of the purified helium was
4 throttled down to about 6 psig and supplied to the second
adsorber to purge previously adsorbed C02. The remainder, still
6 at high pressure, was taken off as purified helium product.
7 After 3.5 minutes, the timer switched the system into
8 the second step of operation. At the beginning of this step, the
9 first adsorber was de-pressurized to atmospheric pressure and the
second adsorber was pressurized with feed gas. It then started
11 the adsorption and purging operations for the second and first
12 adsorbers, respectively. The duration of the second step was the
13 same as the first step, and the system was alternated between
14 these two steps in cyclic fashion. The gas flow direction in
each adsorber for adsorption and pressurization cycles was
16 countercurrent to that for purging and de-pressurization cycles .
17 A thermal conductivity gas analyzer was used to measure
18 the C02 concentration in helium. The test results showed that
19 the microporous hollow fiber module, packed with minute adsorbent
particles, in both versions, was effective for gas purification
21 by pressure swing adsorption, because no C02 could be detected in
22 the purified effluent helium.
23 Examsle III
24 This example illustrates the use of the sol-gel phase
inversion technique for casting a microporous matrix into the
26 hollow fiber module for use as an adsorbent.
27 A hollow fiber module was made using microporous
28 polypropylene Celgard hollow fibers of 240 micron ID and 30
18
1 '""' micron wall thickness. The fiber wall had 30% porosity with .065
2 x .19 micron pore openings. The module had 60 50-cm long fibers
3 encased in a 3/16 inch OD nylon tube, with both ends of the fiber
4 bundle potted in 3 cm long polyurethane tube sheets.
A microporous cellulose acetate matrix structure was
6 cast into the void space between the fibers by first filling it
7 with a cellulose acetate solution (made of 22 g cellulose
8 acetate, 132 g acetone, 30 g Water and 10 g ZnCl2), and then
9 circulating water through the fiber lumina to leach out the
acetone, followed by dry air circulation to remove water.
11 The element was tested for gas dehydration. The water
12 content in the gas was measured using a hygrometer. An air
13 containing .04% water vapour at 80 psig and 23°C was fed to the
14 module through the lumina at a rate of about 400 cc ( STP ) /min and
dry air, containing only 20 ppm of water, was obtained from the
16 element outlet.
17 The moist air started to break through the element
18 outlet only after about 20 minutes of operation. The water
19 saturated cellulose acetate was able to be regenerated by purging
the element with 6 psig dry air at 100 cc/min for about 20
21 minutes.
22 Example IV
23 This example illustrates the use of non-porous soluble
24 particles as an adsorbent in the hollow fiber adsorber. A hollow
fiber module similar to the one described in Example 2 was packed
26 with CuCl2 powder by filling the void space between the fibers
27 with a 60°C concentrated aqueous CuCl2 solution (67% CuCl2 by
28 weight) followed by dry air circulation through the fiber lumina
19
1 o remove water. The module was tested for air dehydration, as
2 described in Example 2. An air containing .052% water vapour was
3 fed to the module through the fiber lumina at 80 psig, 23°C, and
4 500cc(STP)/min. Dry air containing 110 ppm of water was obtained
from the outlet of the element. The moist air started to break
6 through the element outlet after about 24 hours of operation.
7 The water-saturated CuClZ was regenerated by purging the element
8 with 100 cc/min. dry air at 100°C for 12 hours.
9 Exaingle VV
This example illustrates the efficiency of the hollow
11 fiber adsorber in the fast-cycle pressure swing adsorption
12 process for high feed gas flow rates.
13 A hollow fiber module was made containing polypropylene
14 Celgard hollow fibers. The fiber had a 200 micron ID and 30
micron thick wall. The fiber wall had 40% porosity provided by
16 about .065 x .19 micron pore openings. The module had 132 open-
17 ended fibers of about 70 cm length encased in a 1/4 inch OD nylon
18 tube (0.44 cm ID) with both ends of the fiber bundle potted in
19 3 cm long epoxy tube sheets.
The previously described filtration technique, with
21 the aid of ultrasonic vibration, was used to pack 2.3 g Darco KB6
22 activated carbon powder (particle size less than 30 microns) into
23 the void space between the fibers.
24 6 Trade-Mark
' Trade-Mark 20
i3
2~~~v
1 '~'' The element was plumbed and instrumented as a pressure
2 swing adsorber
operating according
to the following
sequential
3 steps in cycle:
4 ( 1 ) Adsorbing adsorbate from a high pressure feed
gas
for a predetermined time period to obtain purified
6 gas from the adsorber outlet;
7 (2) Depressurizing the gas remaining in the adsorber
8 (after the adsorption step) through its outlet
and
9 into a first gas storage vessel having an internal
volume approximately equal to the internal void
11 volume of the adsorber;
12 (3) Further depressurizing the gas in the adsorber
13 into a second gas storage vessel having the same
14 internal volume;
(4) Venting the remaining gas in the adsorber through
16 its inlet;
17 (5) Purging the adsorber using the gas stored in the
18 second storage vessel; the purge gas flow
19 direction being countercurrent to the feed gas
direction in the adsorption step;
21 (6) Pressurizing the adsorber using the gas stored
in
22 the first storage vessel; the remaining gas in
the
23 storage vessel is then removed as low pressure
24 product;
(7) Further pressurizing the adsorber to feed gas
26 pressure using a portion of the purified high
27 pressure product gas, and thus readying the
28 adsorber for the next adsorption cycle.
21
1 "'"'' The aforementioned hollow fiber adsorber containing
2 2.3g of minute activated carbon particles was used to purify a
3 314 psia hydrogen gas containing about 10% C02 using the above
4 pressure swing adsorption steps. In the tests, we varied the
feed gas flow rate and determined the corresponding maximum
6 permissible adsorption step time without any C02 breakthrough
7 from the adsorber outlet. The following results were obtained:
8 Maximum Permissible
9 Feed Rate (Without
Adsorption Step Time C02 Breakthrough)
il Seconds cc (STP)/min.
12 180 200
13 120 300
14 72 500
36 1,000
16 17 2,000
17 10 3,600
18 It is seen that the maximum permissible feed gas rate
19 is inversely proportional to the adsorption step time. The
corresponding hydrogen recovery for each of these flow rates is
21 virtually identical and equal to about 76%.
22 These test results clearly indicate that the feed gas
23 throughput of a hollow fiber adsorber can be effectively
24 increased without loss of separation efficiency by simply
shortening the PSA cycle time. The high adsorption efficiency
26 at short adsorption cycle time and high feed rate is made
27 possible by the fast mass transfer rate and low gas pressure drop
28 in the hollow fiber adsorber using minute adsorbent particles.
72
2~~
1 ~"" Example VI
2 This example illustrates the use of the hollow fiber
3 module as a catalytic reactor having minute catalyst particles.
4 A hollow fiber module similar to the one described in
Example III was packed according to the method in Example I with
6 1.17 g of minute catalyst particles in the void space between
7 fibers. The catalyst consists of 1% weight of palladium on
8 alumina powder of about 25 micron particle size (AP-4
9 heterogeneous catalyst manufactured by Engelhard Corporation of
Newark, New Jersey, U.S.A.). The module was tested for room
11 temperature deoxygenation process for converting oxygen and
12 hydrogen into water. A hydrogen gas containing about 0.66%
13 oxygen at 10 psig was passed through the fiber lumina of the
14 module. A gas chromatographic instrument was used to measure
oxygen content in the gas. At 700 cc (STP)/min. feed gas flow
16 rate, no oxygen content could be detected from the effluent
17 stream of the module, indicating complete conversion of oxygen
18 to water. This highly efficient deoxygenation process was due
19 mainly to the fast mass transfer between the gas stream and
catalyst resulting from the use of small catalyst particles in
21 the hollow fiber module.
22 The scope of the invention is defined by the claims
23 now following.
23