Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2 ~ 2 ~
PS/5- 17889/1 +2/+
Triazolvlsulfonamides
The present invention relates to novel herbicidally active and plant growth regulating
N-phenyl-triazolopyrimidinylsulfonamides, to processes for their preparation, tocompositions containing them as active ingredients, and to their use for controlling weeds,
especially selectively in crops of useful plants, or for regulating and inhibiting plant
growth.
Triazolylsulfonamides having herbicidal activity are known from European Patent
Application No. 0 142 ~52. However, the compounds disclosed therein are not always
able to satisfy requirements as regards potency, selectivity and persistence. There is
therefore a need for compounds having better activity and greater selectivity.
Novel triazolylsulfonamides having improved herbicidal and plant growth regulating
activity have now been found.
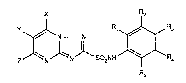
The N-phenyl-tIiazolopyrimidinylsulfonamides according to the invention have theformula I
X R2
Z ~NlN~SO2NH ~R,
wherein
R1 is halogen, phenyl, O-phenyl, Cl-C4alkyl, Cl-C4haloalkyl, -OR8, -ORg, nitro, hydroxy,
cyano, -SR6, -SOR6, -SO2R6, -SR8, -SOR8, -SO2R8, -SO3R8, -SRg, -SORg, -SO2Rg,
-So2NRlo(Rlo)~-so3R9~-cooR7~-coNHR9~-l ONRg(R9), formyl, -~R8, -~Rg~ NH
-NHRg or -NRg(Rg);
R2 and R4 independently of one another are hydrogen, halogen, Cl-(:4alkyl, -COOR7 or
C~ ~ C~ 'jt ~
-ORg;
R3 is hydrogen or halogen;
R5 is hydrogen, halogen, phenyl, O-phenyl, C1-C4alkyl, C1-C4haloalkyl, -OR8, -ORg,
nitro, hydroxy, cyano, -SR6, -SOR6, -SO2R6, -SR8, -SORg, -SO3R8, -SRg, -SOR9, -SO2R9,
-SO2NRlo(Rlo), -SO3R9, -COOR7, -CONHRg, -CONR(,(R~), formyl, -~Rg, - ICIRg, -NH2,
-NHR9 or-NRg(Rg);
R6 is hydrogen, phenyl, benzyl, C2-C4alkenyl, C2-C4alkynyl, or Cl-C4alkyl substituted by
-ORg;
R7 is C1-C4alkyl, C2-C4alkenyl, C2-C4alkynyl, phenyl or benzyl;
R8 is Cl-C4haloalkyl;
Rg is C1-C4alkyl;
Rlo is hydrogen or Cl-C4alkyl;
X, Y and Z independently of one another are hydrogen, C1-C4alkyl, Cl-C4haloalkyl,
phenylthio, benzylthio, hydroxy, halogen, -OR8, -ORg, Cl-C4alkyl substituted by -ORg,
Cl-C4alkyl substituted by -OR8, or -NH2, -NHRg, -NRg(Rg), C3-C6cycloalkyl,
C3-C6cycloalkyl substituted by halogen, -OR8, -ORg, -SR8, -SRg or by C1-C4alkyl, or X
and Y together or Y and Z together form a C2-C3alkylene bridge which may be interrupted
by oxygen or sulfur;
with the proviso that at least one of the substituents X, Y and Z is C3-C6cycloalkyl or
C3-C6cycloalkyl substituted by halogen, -OR8, -ORg, -SR8, -SRg or by Cl-C4alkyl, and the
salts of those compounds.
In the definitions of the substituents, C1-C4alkyl is to be understood as being
straight-chained or branched alkyl, for example methyl, ethyl, n-propyl, isopropyl, n-butyl,
sec.-butyl, isobutyl and tert.-butyl. The alkyl groups present as substituents or in the
substituents preferably have 1 or 2 carbon atoms. Methyl is especially preferred.
The C2-C4alkenyl radicals in the substituents R6 and R7 may be straight-chained or
branched. Alkenyl radicals having a chain length of two or three carbon atoms are
preferred. Examples of C2-C4alkenyl radicals are: vinyl, allyl, methallyl, 1-methylvinyl,
but-2-en-1-yl. Vinyl and allyl are preferred.
The C2-C4alkynyl radicals in the definitions of the substituents R6 and R7 may be
straight-chained or branched. Alkynyl radicals having a chain length of 2 or 3 carbon
atoms are preferred. C2-C4alkynyl radicals are, for example, ethynyl, propargyl,
r~t ~}
-3~
1-propynyl, 3-butynyl or l-methylpropargyl, ethynyl and propargyl being especially
preferred.
Halogen by itself and as part of a substituent such as haloalkyl is to be understood as being
fluorine, chlorine and bromine, but preferably fluorine and chlorine. ~Ialoalkyl is
generally chloromethyl, fluoromethyl, difluoromethyl, trifluoromethyl, 2-chloroethyl,
2,2,2-trifluoroethyl, 1,1,2,2-tetrafluoroethyl, pentafluoroethyl, l,1,2-trifluoro-2-chloro-
ethyl, 2,2,2-trifluoro-l,l-dichloroethyl, pentachloroethyl, 3,3,3-trifluoropropyl,
2,3-dichloropropyl, 1,1,2,3,3,3-hexafluoropropyl, but especially fluoromethyl,
chloromethyl, difluoromethyl and trifluoromethyl.
The C3-C6cycloalkyl groups in the definitions of the substituents X, Y and Z may be
substituted or unsubstituted and include, for example, cyclopropyl, 2-fluorocyclopropyl,
2,4-difluorocyclopropyl, 2-chlorocyclopropyl, 2,3-dichlorocyclopropyl,
2-methylcyclopropyl, 2-methylthiocyclopropyl, 2,3-dimethylcyclopropyl,
2-methoxycyclopropyl, cyclobutyl, cyclopentyl, 3-methylcyclopentyl,
3,4-dimethoxycyclopentyl, cyclohexyl, 3-fluorocyclohexyl, 4-methylcyclohexyl and4-methylthiocyclohexyl.
The invention relates also to the salts that the compounds of formula I are able to form
with amines, alkali metal bases and alkaline earth metal bases.
Of the alkali metal and alkaline earth metal hydroxides that are suitable as salt formers,
special mention should be made of the hydroxides of lithium, sodium, potassium,
magnesium and calcium, but especially those of sodium and potassium.
Examples of amines suitable for salt formation are primary, secondary and tertiary
aliphatic and aromatic amines, such as methylamine, ethylamine, propylamine, iso-
propylamine, the four isomers of butylamine, dimethylamine, diethylamine, di-
ethanolamine, dipropylamine, diisopropylamine, di-n-butylamine, pyrrolidine, piperidine,
morpholine, trimethylamine, triethylamine, tripropylamine, quinuclidine, pyridine,
quinoline and isoquinoline, but especially ethyl-, propyl-, diethyl- and triethyl-amine, and
more especially isopropylamine and diethanolamine.
Of the compounds of formula I, preference is given to those wherein
Rl is halogen, nitro, Cl-C4alkyl, Cl-C4haloalkyl, -OR8, -OR9, hydroxy, cyano or -COOR7;
c~
Rs is hydrogen, halogen, nitro, Cl-C4alkyl, Cl-C4haloalkyl, -OR8, -ORg, hydroxy, cyano
or-COOR7; and
X, Y and Z independently of one another are hydrogen, Cl-C4alkyl, Cl-C4haloalkyl,
Cl-C4alkyl substituted by -OR9, Cl-C4alkyl substituted by -OR8, or halogen,
C3-C6cycloalkyl or C3-C6cycloalkyl substituted by halogen, -OR8, -ORg, -SR8, -SR9 or by
Cl-C4alkyl.
In an especially preferred group of compounds of formula I,
R1 is halogen, nitro, Cl-C2alkyl, Cl-C2haloalkyl, -OR8, -ORg, hydroxy, cyano or -COOR7;
R2 and R4 independently of one another are hydrogen, halogen, Cl-C2alkyl, -COOR7 or
-ORg;
Rs is hydrogen, halogen, nitro, Cl-C2alkyl, Cl-C2haloalkyl, -OR8, -ORg, hydroxy, cyano
or -COOR7;
R7 is Cl-C2alkyl, C2-C3alkenyl, C2-C3alkynyl, phenyl or benzyl;
R8 is Cl-C2haloalkyl;
Rg is Cl-C2alkyl; and
X, Y and Z independently of one another are hydrogen, Cl-C2alkyl, Cl-C2haloalkyl,
halogen, Cl-C4alkyl substituted by -ORg, Cl-C4alkyl substituted by -OR8,
C3-C6cycloalkyl or C3-C6cycloalkyl substituted by halogen, -OR8, -ORg or by Cl-C2alkyl.
Of this preferred group, special mention should be made of those compounds wherein
Rl is fluorine, chlorine, methyl, trifluoromethyl or methoxy;
R2 and R4 independently of one another are hydrogen, fluorine, chlorine, methyl or
methoxy;
R3 is hydrogen;
Rs is hydrogen, fluorine, chlorine, methyl, trifluoromethyl or methoxy; and
X, Y and Z independently of one another are hydrogen, methyl, cyclopropyl, 2-fluoro-
cyclopropyl, 2,3-difluorocyclopropyl, 2-chlorocyclopropyl, 2,3-dichlorocyclopropyl,
1-methylcyclopropyl, 2-methylcyclopropyl, 2,3-dimethylcyclopropyl, cyclopentyl,
fluorine, chlorine, trifluoromethyl or methoxy.
Prominent among the compounds of formula I on account of their good activity are espe-
cially those compounds wherein X is C3-C6cycloalkyl or C3-C6cycloalkyl substituted by
halogen, -OR8, -ORg, -SR8, -SRg or by Cl-C4alkyl, preferably cyclopropyl,
1-methylcyclopropyl, 2-fluorocyclopropyl, 2,3-difluorocyclopropyl, 2-chlorocyclopropyl,
2,3-dichlorocyclopropyl, 2-methylcyclopropyl or 2,3-dimethylcyclopropyl, but especially
$
cyclopropyl.
A preferred sub-group of compounds of formula I according to the invention consists of
the compounds of formula Ia
f ~ !\1~N J~ SO N ~ ~ (Ia)
wherein
R1 is halogen, phenyl, O-phenyl, C1-C4alkyl, C1-C4haloalkyl, -OR8, -ORg, nitro, hydroxy,
cyano, -SR6, -SOR6, -S02R6, -SR8, -SOR8, -SO2R8, -SO3R8, -SRg, -SOR9, -SO2R9,
-SO2NRlo(Rlo), -SO3R9, -COOR7, -CONHR9, -CONR9(Rg), formyl, - IClRg, - IClR9, -NH2,
-NHR9 or-NR9(Rg);
Rs is hydrogen, halogen, phenyl, O-phenyl, C1-C4alkyl, C1-C4haloalkyl, -OR8, -OR9,
nitro, hydroxy, cyano, -SR6, -SOR6, -SO2R6, -SR8, -SOR8, -SO3R8, -SR9, -SOR9, -SO2R9,
-SO2NRlo(Rlo), -SO3R9, -COOR7, -CONHR9, -CONR9(Rg), formyl, - IClR8, - IClR9, -NH2,
-NHR9 or-NR9(R9);
R6 is hydrogen, phenyl, benzyl, C2-C4alkenyl, C2-C4alkynyl, or C1-C4alkyl substituted by
-ORg;
R7 is C1-C4alkyl, C2-C4alkenyl, C2-C4alkynyl, phenyl or benzyl;
R8 is Cl-C4haloalkyl;
Rg is C1-C4alkyl;
Rlo is hydrogen or C1-C4alkyl;
X and Z independently of one another are hydrogen, C1-C4alkyl, C1-C4haloalkyl, phenyl-
thio, benzylthio, hydroxy, halogen, -OR8, -ORg, C1-C4alkyl substituted by -ORg, Cl-C4-
alkyl substituted by -OR8, or -NH2, -NHRg, -NRg(Rg), C3-C6cycloalkyl or
C3-C6cycloalkyl substituted by halogen, -OR8, -ORg, -SRg, -SRg or by C1-C4alkyl;with the proviso that at least one of the substituents X and Z is C3-C6cycloalkyl or
C3-C6cycloalkyl substituted by halogen, -OR8, -ORg, -SR8, -SRg or by C1-C4alkyl.
Of the compounds of formula Ia, preference is given to those wherein
Rl is halogen, nitro, Cl-C4alkyl, Cl-C4haloalkyl, -OR8, -ORg, hydroxy, cyano or -COOR7;
Rs is hydrogen, halogen, nitro, Cl-C4alkyl, Cl-C4haloalkyl, -OR8, -ORg, hydroxy, cyano
or-COOR7; and
X and Z independently of one another are hydrogen, Cl-C4alkyl, Cl-C4haloalkyl,
Cl-C4alkyl substituted by -ORg, Cl-C4alkyl substituted by -OR8, or halogen, C3-C6-
cycloalkyl or C3-C6cycloalkyl substituted by halogen, -OR8, -ORg, -SR8, -SR9 or by
Cl-C4alkyl.
Prominent on account of their good biological activity are especially those compounds of
formula Ia wherein
Rl is halogen, nitro, Cl-C4alkyl, Cl-C4haloalkyl, -OR8, -OR9, hydroxy, cyano or -COOR7;
Rs is hydrogen, halogen, nitro, Cl-C4alkyl, Cl-C4haloalkyl, -OR8, -ORg, hydroxy, cyano
or-COOR7; and
X and Z independently of one another are hydrogen, Cl-C4alkyl, Cl-C4haloalkyl, halogen,
C3-C6cycloalkyl or C3-C6cycloalkyl substituted by halogen, -OR8, -ORg, -SR8, -SRg or by
Cl-C4alkyl.
Of this group, preference is given to those compounds of formula Ia wherein
Rl is halogen, nitro, Cl-C2alkyl, Cl-C2haloalkyl, -OR8, -ORg, hydroxy, cyano or -COOR7;
Rs is hydrogen, halogen, nitro, Cl-C2alkyl, Cl-C2haloalkyl, -OR8, -ORg, hydroxy, cyano
or -COOR7;
R7 is Cl-C2alkyl, C2-C3alkenyl, C2-C3alkynyl, phenyl or benzyl;
R8 is Cl-C2haloalkyl;
R9 is Cl-C2alkyl; and
X and Z independently of one another are hydrogen, Cl-C2alkyl, Cl-C2haloalkyl, halogen,
C3-C6cycloalkyl or C3-C6cycloalkyl substituted by halogen, -OR8, -OR9 or by Cl-C2alkyl.
In a further sub-group of compounds of formula Ia deserving special mention,
Rl is fluorine, chlorine, methyl, trifluoromethyl or methoxy;
Rs is hydrogen, fluorine, chlorine, methyl, trifluoromethyl or methoxy; and
X and Z independently of one another are hydrogen, methyl, cyclopropyl, l-methylcyclo-
propyl, 2-fluorocyclopropyl, 2,3-difluorocyclopropyl, 2-chlorocyclopropyl, 2,3-dichloro-
cyclopropyl, 2-methylcyclopropyl, 2,3-dimethylcyclopropyl, cyclopentyl, fluorine,
chlorine or methoxy.
Most especially prominent groups of compounds of formula Ia are those wherein
.3 2
Rl is fluorine, chlorine, methyl or trifluoromethyl;
Rs is hydrogen, chlorine, methyl or trifluoromethyl; ancl
X and Z independently of one another are hydrogen, cyclopropyl, l-methylcyclopropyl,
2-fluorocyclopropyl, 2,3-difluorocyclopropyl, 2-chlorocyclopropyl, 2,3-dichlorocyclo-
propyl, 2-methylcyclopropyl, 2,3-dimethylcyclopropyl, methyl, methoxy, fluorine or
chlorine.
Prominent among this preferred sub-group on account of their good biological activity are
those compounds wherein X is cyclopropyl.
The following may be mentioned as preferred individual compounds within the scope of
formula I:
S-methyl-7-cyclopropyl-N-(2,6-dichlorophenyl)- 1 ,2,4-triazolo[l ,S-a]pyrimidine-2-sulfon-
amide,
S-methyl-7-cyclopropyl-N-(2,6-difluorophenyl)- 1 ,2,4-triazolo[ 1 ,S-a]pyrimidine-2-sulfon-
amide,
7-methyl-S-cyclopropyl-N-(2,6-dichlorophenyl)- 1 ,2,4-triazolo[ 1 ,S-a]pyrimidine-2-sulfon-
amide,
5-cyclopropyl-7-trifluoromethyl-N-(2,6-dichlorophenyl)- 1 ,2,4-
triazolo[l,S-a]pyrimidine-2-sulfonamide, and
5-methyl-7-cyclobutyl-N-(2,6-dichlorophenyl)-1,2,4-triazolo[l,S-a]pyrimidine-2-
sulfonamide.
The compounds of formula I are prepared by reacting a primary amine of formula II
Rl~ R3
H2N ~ R4
wherein Rl, R2, R3, R4 and Rs are as defined under formula I, in the presence of a base,
with a triazolopyrimidinylsulfonyl chloride of formula III
2~'3
N ``N/~
N--~N~lZ (III),
wherein X, Y and Z are as defined under formula I.
The process according to the invention is advantageously carried out in an inert solvent at
a temperature of from -20~C to the boiling point of the reaction mixture. The
temperatures are usually from +15C to +120C, preferably from t-20C to +80C.
Suitable solvents are chlorinated hydrocarbons, such as methylene chloride, chloroform,
carbon tetrachloride, trichloroethane, tetrachloroethane~ chlorobenzene or
dichlorobenzene; aromatic hydrocarbons, such as benzene, toluene or xylene; ethers, such
as diethyl ether, diisopropyl ether, tetrahydrofuran, ethylene glycol dimethyl ether or
dioxane; nitriles, such as acetonitrile or propionitrile; cyclohexane or pyridine. The
reactions are generally slightly exothermic and can be carried out at room temperature.
Suitable bases are especially tertiary amines, such as trimethylarnine, triethylamine,
N-methylmorpholine, quinuclidine, pyridine, 1,4-diazabicyclo[2.2.2]octane,
1,5-diazabicyclo[4.3.0]non-5-ene or 1,8-diazabicyclo[5.4.0]undec-7-ene.
The end products of formula I can be isolated by concentration and/or evaporation of the
solvent and purified by recrystallisation or trituration of the solid residue in solvents in
which they are not readily soluble, such as ethers, aromatic hydrocarbons or chlorinated
hydrocarbons.
The primary amines of formula II can be obtained by reduction processes from thecorresponding nitro compounds of formula IV
R2
R1~ R3
1~ 11 (IV),
02N~ ~R4
Rs
wherein Rl, R2, R3, R4 and Rs are as defined under formula I.
All the customary processes described in the literature can be used for the reduction of the
nitro compoul1ds of formllla IV to the amino compounds of formula Il. For example, the
reduction can advantageously be carried out in an aqueous medium in the presence of iron,
tin or zinc and hydrochloric acid. Further suitable methods are reduction processes using
complex hydrides, such as lithium aluminium hydride, or catalytic reduction withhydrogen with the aid of platinum, palladium or nickel catalysts.
The nitro compounds of formula IV can be prepared by electrophilic substitution in
nitration processes customary for aromatic compounds. The intermediates of formula II
are known or they can be prepared analogously to known compounds.
The intermediates of formula III are novel compounds, and the present invention also
includes them. The intermediates of formula III are obtainable by treating a compound of
formula V
N--N~
--<N~ (V)~
wherein X, Y and Z are as defined under forrnula I and A is hydrogen, isopropyl or benzyl,
with chlorine in an aqueous acidic medium. The acid used is preferably acetic acid or an
inorganic acid, especially hydrochloric acid. The treatment with chlorine is
advantageously carried out at temperatures in the range of from -25C to 20C, preferably
at from -15C to 0C. The treatment with chlorine is preferably carried out with the
addition of dichloromethane to the medium.
The intermediates of formula V are novel compounds, and the present invention relates
also to them. The intermediates of formula V can be prepared by reacting a compound of
formula VI
N N
H (VI),
wherein A is hydrogen or benzyl, with a compound of formula VII
i9 ~
- 10-
X-C-CHY-e-Z (VII),
wherein X, Y and Z are as defined under formula I.
The compounds of formula I can also be prepared by reacting a compound of formula VIII
HN N R~ R~
ll 1 (VIII),
/~N ~ SO2-NH ~ ~s ~ R4
wherein Rl, R2, R3, R4 and Rs are as defined under formula I, with a compound offormula VII
X-e-CHY-e-Z (VII),
wherein X, Y and Z are as defined under formula I. Such processes are described, for
example, in USP 4,734,123.
The intermediates of formulae VI and VII are known or tney can be prepared analogously
to known methods. The compound of formula VI wherein A is benzyl can be prepared,
for example, in a manner known ~ se by reacting the compound of formula VI wherein
A is hydrogen with benzyl chloride.
The reaction of a compound of formula VI or VIII with a compound of formula VII is
advantageously carried out by first dissolving a compound of formula VI or VIII in a small
amount of glacial acetic acid, with heating, and, after the addition of the compound of
formula VII, heating the reaction mixture to reflux temperature.
The compounds of formula I can also be prepared by reacting a
triazolopyrimidinylsulfonyl chloride of formula III
N--N~
N ~ N~l Z (III),
wherein X, Y and Z are as defined under fornnula I, with a compound of formula IX
R1~ (~)
(C~3~3 Si - H N~ R4
Rs
wherein Rl, R2, R3, R4 and Rs are as defined under formula I. Such processes aredescribed, for example, in EP-A-O 343 752.
The compounds of formula I are generally used successfully at rates of application of from
0.001 to 5 kg/ha, especially from 0.005 to 3 kg/ha. The concentration required to achieve
the desired effect can be determined by experiment. It is dependent on the type of action,
the stage of development of the cultivated plant and of the weed, and also on the applica-
tion (place, time, method? and, in dependence on those parameters, can vary within wide
limits.
When used at relatively low rates of application, the compounds of formula I aredistinguished by growth inhibiting and herbicidal properties, which render them
excellently suitable for use in crops of useful plants, especially in cereals, cotton,
soybeans, rape, maize and rice, their use in maize crops being very especially preferred.
The invention relates also to herbicidal and plant growth regulating compositions
containing a novel compound of formula I, and to methods of inhibiting plant growth.
Plant growth regulators are substances tha~ bring about agronomically desirable
biochemical and/or physiological and/or morphological changes in/to the plant.
The active ingredients contained in the compositions according to the invention influence
~, r~ C~ f~
plant growth in different ways depending on the time of application, the concentration, the
type of application and the environmental conditions. Plant growth regulators of formula I
can, for example, inhibit the vegetative growth of plants. This type of action is valuable in
the case of lawn areas, in the cultivation of ornamentals, in fruit plantations, in the case of
roadside embankments and in sports fields and industrial sites, but also in the specific
inhibition of side-shoots, as in the case of tobacco. In agriculture, inhibition of the
vegetative growth of cereals leads, owing to strengthening of the stalk, to reduced lodging,
and a similar agronomic effect is achieved in rape, sunflowers, maize and other cultivated
plants. Moreover, by inhibiting the vegetative growth it is possible to increase the number
of plants per unit area. Another field of application of growth inhibitors is the selective
control of cover plants in plantations or widely spaced crops by greatly inhibiting the
growth of the cover crops without killing them, so that competition with the main crop is
eliminated but the agronomically positive effects, such as erosion prevention, fi~;ing of
nitrogen and loose soil structure, are preserved.
A method of inhibiting plant growth is to be understood as being a method of controlling a
plant's natural development without changing its life-cycle, as determined by genetic
characteristics, in the sense of mutation. The method of regulating growth is applied at a
time in the plant's development that has to be determined for each individual case. The
compounds of formula I can be applied pre- or postemergence, for example to the seeds or
seedlings, to roots, tubers, stalks, leaves, blossoms or other parts of the plant. This can be
done, for example, by applying the compound as such or in the form of a composition to
the plants, and/or by treating the plant's nutrient medium (soil).
Various methods and techniques are sui~able for the use of the compounds of formula I or
of compositions containing them for regulating plant growth, for example the following:
i) Seed dressin~
a) Dressing the seeds with an active ingredient formulated as a wettable powder, by
shaking in a container until the formulation is uniformly distributed over the surface of the
seeds (dry dressing). Up to 4 g of compound of formula I (in the case of a 50 %
formulation: up to 8.0 g of wettable powder) are used per 1 kg of seed.
b) Dressing the seeds with an emulsifiable concentrate of the active ingredient or with an
aqueous solution of the compound of formula I formulated as a wettable powder according
to method a) (wet dressing).
~'~'t~
- 13 -
c) Dressing by soaking the seeds for a period of from I to 72 hours in a liquor containing
up to 1000 ppm of compound of formula I and, if desired, subsequently drying the seeds
(seed soaking).
Seed dressing or treatment of the germinated seedling are naturally the preferred methods
of application because the treatment with the active ingredient is then directed wholly at
the target crop. From 4.0 g to 0.001 g of active ingredient are normally used per 1 kg of
seed, although, depending on the method employed, which also allows the addition of
other active ingredients or micronutrients, amounts that exceed or fall short of the
specified concentration limits may be employed (repeat dressing).
ii) Controlled release of active in~redient
A solution of the active ingredient is applied to mineral granulate carriers or polymerised
granulates (urea/formaldehyde) and allowed to dry. If requ*ed, a coating may be applied
(coated granulates), which allows the active ingredient to be released in metered amounts
over a specific period of time.
The compounds of formula I are used in unmodified form or, preferably, as compositions
together with the adjuvants conventionally employed in the art of formulation, and are
therefore formulated in known manner e.g. into emulsifiable concentrates, directly
sprayable or dilutable solutions, dilute emulsions, wettable powders, soluble powders,
dusts, granulates, and also encapsulations in e.g. polymer substances. As with the nature
of the compositions, the methods of application, such as spraying, atomising, dusting,
scattering or pouring, are chosen in accordance with the intended objectives and the
prevailing circumstances.
The formulations, i.e. the compositions, preparations or mixtures containing the compound
(active ingredient) of formula I and, where appropriate, a solid or liquid adjuvant, are
prepared in known manner, e.g. by homogeneously mixing and/or grinding the active
ingredients with extenders, e.g. solvents, solid carriers and, where appropriate, surface-
active compounds (surfactants).
Suitable solvents are: aromatic hydrocarbons, preferably the fractions containing 8 to 12
carbon atoms, e.g. xylene mixtures or substituted naphthalenes, phthalates such as dibutyl
phthalate or dioctyl phthalate, aliphatic hydrocarbons such as cyclohexane or paraffins,
- 14-
alcohols and glycols and their ethers and esters, such as ethanol, ethylene glycol, ethylene
glycol monomethyl or monoethyl ether, ketones such as cyclohexanone, strongly polar
solvents such as N-methyl-2-pyrrolidone, dimethyl sulfoxide or dimethylformamide, as
well as vegetable oils or epoxidised vegetable oils, such as epoxidised coconut oil or
soybean oil; or water.
The solid carriers used, e.g. for dusts and dispersible powders, are normally natural
mineral fillers such as calcite, talcum, kaolin, montmorillonite or attapulgite. In order to
improve the physical properties it is also possible to add highly dispersed silicic acid or
highly dispersed absorbent polymers. Suitable granulated adsorptive carriers are porous
types, for example pumice, broken brick, sepiolite or bentonite; and suitable nonsorbent
carriers are, for example, calcite or sand. In addition, a great number of pregranulated
materials of inorganic or organic nature can be used, e.g. especially dolomite or pulverised
plant residues.
Depending on the nature of the compound of formula I to be formulated, suitable
surface-active compounds are non-ionic, cationic and/or anionic surfactants having good
emulsifying, dispersing and wetting properties. The term "surfactants" will also be
understood as comprising mixtures of surfactants.
Both so-called water-soluble soaps and water-soluble synthetic surface-active compounds
are suitable anionic surfactants.
Suitable soaps are the alkali metal salts, alkaline earth metal salts or unsubstituted or
substituted ammonium salts of higher fatty acids (C10-C22), e.g. the sodium or potassium
salts of oleic or stearic acid, or of natural fatty acid mixtures which can be obtained e.g.
from coconut oil or tallow oil. Mention may also be made of fatty acid methyltaurin salts.
More frequently, however, so-called synthetic surfactants are used, especially fatty
sulfonates, fatty sulfates, sulfonated benzimidazole derivatives or alkylarylsulfonates.
The fatty sulfates or sulfonates are usually in the forrn of alkali metal salts, alkaline earth
metal salts or unsubstituted or substituted ammonium salts and contain a C8-C22alkyl
radical, which also includes the alkyl moiety of acyl radicals, e.g. the sodium or calcium
salt of lignosulfonic acid, of dodecyl sulfate or of a mixture of fatty alcohol sulfates
obtained from natural fatty acids. These compounds also comprise the salts of sulfated
~;~ A ~ Ç3 1, ~
and sulfonated fatty alcoho]/ethylene oxide adducts. The sulfonated benzimidazole
derivatives preferably contain 2 sulfonic acid groups and one fatty acid radical containing
8 to 22 carbon atoms. Examples of alkylarylsulfonates are the sodium, calcium ortriethanolamine salts of dodecylbenzenesulfonic acid, dibutylnaphthalenesulfonic acid, or
of a condensate of naphthalenesulfonic acid and formalclehyde.
Also suitable are corresponding phosphates, e.g. salts of the phosphoric acid ester of an
adduct of p-nonylphenol with 4 to 14 moles of ethylene oxide, or phospholipids.
Non-ionic surfactants are preferably polyglycol ether derivatives of aliphatic or
cycloaliphatic alcohols, saturated or unsaturated fatty acids and alkylphenols, said
derivatives containing 3 to lO glycol ether groups and 8 to 20 carbon atoms in the
(aliphatic) hydrocarbon moiety and 6 to 18 carbon atoms in the alkyl moiety of the
alkylphenols.
Further suitable non-ionic surfactants are the water-soluble adducts of polyethylene oxide
with polypropylene glycol, ethylenediaminopolypropylene glycol and alkylpolypropylene
glycol containing l to lO carbon atoms in the alkyl chain, which adducts contain 20 to 250
ethylene glycol ether groups and 10 to 100 propylene glycol ether groups. These
compounds usually contain l to 5 ethylene glycol units per propylene glycol unit.
Representative examples of non-ionic surfactants are nonylphenolpolyethoxyethanols,
castor oil polyethylene glycol ethers, polypropylene/polyethylene oxide adducts, tributyl-
phenoxypolyethoxyethanol, polyethylene glycol and octylphenoxypolyethoxyethanol.
Fatty acid esters of polyoxyethylene sorbitan, e.g. polyoxyethylene sorbitan trioleate, are
also suitable non-ionic surfactants.
Cationic surfactants are preferably quaternary ammonium salts which contain, as
N-substituent, at least one C8-C22alkyl radical and, as further substituents, unsubstituted or
halogenated lower alkyl, benzyl or hydroxy-lower alkyl radicals. The salts are preferably
in the form of halides, methyl sulfates or ethyl sulfates, e.g. stearyltrimethylammonium
chloride or benzyldi(2-chloroethyl)ethylammonium bromide.
The surfactants customarily employed in the art of formulation are described inter alia in
the following publications:
f.i i ~ ~ $
- 16-
"McCutcheon's Detergents and Emulsifiers Annual", MC Publishing Corp., Ridgewood,
New Jersey, 1981;
H~ Stache, "Tensid-Taschenbuch", 2nd edition, C. Hanser Verlag, Munich, Vienna, 1981;
M. and J. Ash, "Encyclopedia of Surfactants", Vol. I-III, Chemical Publishing Co.,
New York, 1980-1981.
The agrochemical compositions generally contain 0.1 to 95 %, preferably 0.1 to 80 %, of a
compound of formula I, 1 to 99.9 % of a solid or liquid adjuvant and 0 to 25 %, preferably
0.1 to 25 %, of a surfactant.
Preferred formulations are composed in particular of the following constituents
(% = percentage by weight):
Emulsifiable concentrates:
active ingredient: 1 to 20 %, preferably 5 to 10 %
surfactant: 5 to 30 %, preferably 10 to 20 %
liquid carrier: 50 to 94 %, preferably 70 to 85 %
Dusts:
active ingredient: 0.1 to 10 %, preferably 0.1 to 1 %
solid carrier: 99.9 to 90 %, preferably 99.9 to 99 %
Suspension concentrates:
active ingredient: 5 to 75 %, preferably 10 to 50 %
water: 94 to 24 %, preferably 88 to 30 %
surfactant: 1 to 40 %, preferably 2 to 30 %
Wettable powder:
active ingredient: 0.5 to 90 %, preferably 1 to 80 %
surfactant: 0.5 to 20 %, preferably 1 to 15 %
solid carrier: 5 to 99 %, preferably 15 to 90 %
Granulates:
active ingredient: 0.5 to 30 %, preferably 3 to 15 %
solid carrier: 99.5 to 70 %, preferably 97 to 85 %.
C,~ , ?~ ~3 f, 3 ,~
- 17 -
Whereas commercial products will preferably be formulated as concentrates, the end user
will normally employ dilute formulations. The formulations can be diluted to a concen-
tration as low as 0.001 % active ingredient. The rates of application are normally from
0.001 to 5 kg a.i./ha, preferably from O.OOS to 3 kg a.i./lla.
The compositions may also contain further auxiliaries such as stabilisers, antifoams,
viscosity regulators, binders, tackifiers as well as fertilisers or other active ingredients for
obtaining special effects.
Formulation Examples:
Example F1: Formulation Examples for active in~redients of formula I (throu~hout,
percenta~es are by wei~ht)
a) Wettable powders a ) b ) c )
compoundno. 1.1 20 % 50 % 0.5 %
sodiumlignosulfonate S % 5 % 4 %
sodium laurylsulfate 3 % - -
sodium diisobutylnaphthalene-
sulfonate - 6 % 6 %
octylphenol polyethylene glycol ether
(7-8 moles of ethylene oxide) - 2 % 2 %
highly dispersed silicic acid 5 % 2 7 % 2 7 %
kaolin 67 % 10 %
sodium chloride - - 5 9 . 5 %
The active ingredient is thoroughly mixed with the adjuvants and the mixture is
thoroughly ground in a suitable mill, affording wettable powders which can be diluted
with water to give suspensions of the desired concentration.
b) Emulsifiableconcentrate a) b)
compoundno. 1.1 10 % 1 %
octylphenol polyethylene glycol ether
(4-5 moles of ethylene oxide) 3 % 3 %
calcium dodecylbenzenesulfonate 3 % 3 %
castor oil polyglycol ether
, r~ 3
- 18-
(36 moles of ethylene oxide) 4 % 4 %
cyclohexanone 3 0 % 10 %
xylene mixture 5 0 % 7 9 %
Emulsions of any required concentration can be obtained from this concentrate by dilution
with water.
c) Dusts a) b)
compound no. 1.2 0.1 % 1 %
talcum g9 9 %
kaolin - 9 9 96
~eady-for-use dusts are obtained by mixing the active ingredient with the carrier and
grinding the mixture in a suitable mill.
d) Extruder ~ranulate a) b)
compoundno. 1.1 10 % 1 %
sodium lignosulfonate 2 % 2 %
carboxymethylcellulose 1 % 1 %
kaolin 8 7 % 9 6 %
The active ingredient is mixed and ground with the adjuvants, and the mixture ismoistened with water. The mixture is extruded and then dried in a stream of air.
e) Coated ~ranulate
compound no. 1.1 3 %
polyethylene glycol (mol. wt. 200) 3 %
kaolin 94 ~
The finely ground active ingredient is uniformly applied, in a mixer, to the kaolin
moistened with polyethylene glycol. Non-dusty coated granulates are obtained in this
manner.
f) Suspensionconcentrate a) b)
compoundno. 1.2 40 % 5 %
ethylene glycol 10 % 10 %
- 19-
nonylphenol polyethylel-e glycol
ether (15 moles of ethylene oxide) 6 % 1 %
sodium lignosulfonate 10 % 5 %
carboxymethylcellulose 1 % 1 %
37 % aqueous fonnaldehyde
solution 0.2 % 0.2 %
silicone oil in the form of a
75 % aqueous emulsion 0.8 % 0.8 %
water 3 2 % 7 7 %
The finely ground active ingredient is intimately mixed with the adjuvants, giving a
suspension concentrate from which suspensions of any desired concentration can be
obtained by dilution with water.
g) Salt solution
compound no. 1.2 5 %
isopropylamine 1 %
octylphenol polyethylene glycol
ether (78 moles of ethylene oxide) 3 %
water 91 %
eparation Examples:
Example P1: Preparation of 2-benzylthio-5-methvl-7-cvclopropvl-1,2,4-triazolo-
~1,5-alpvrimidine (compound no. 2.1) and 2-benzylthio-5-cyclo-
_oPvl-7-methyl-1,2,4-triazolo~1,5-alpvrimidine (compound no. 2.2)
y CH3
,~\ N _ N ~\ N N
(2.2)
(2.1)
41.5 g of 3-amino-5-benzylthio-1,2,4-triazole are dissolved in a small amount of hot
glacial acetic acid and heated under reflux together with 30 g of
2 ~I ., 3
- 20-
l-cyclopropyl-1,3-butanedione. After about one hour, the reaction solution is
concentrated in vacuo and water is added to the residue. The resulting dark resin is taken
up in ethyl acetate and the ethyl acetate phase is separated off, dried over sodium sulfate
and concentrated by evaporation. The residue is purified on a silica gel column (eluant:
ethyl acetate/hexane 1:1), yielding the title compounds A and B:
1st fraction 20 g m.p. +101 to +103C (compound no. 2.2)
2nd fraction 39 g m.p. +84 to +86C (compound no. 2.1)
xample P2: Preparation of 2-benzylthio-7-cyclopropyl- 1~2.4-triazolo~ 1~5-al-
pvrimidine (compound no. 2.20)
~--N (2.20)
20 g of 3-amino-5-benzylthio-1,2,4-triazole together with 17 g of the potassium salt of
1-cyclopropyl-1,3-butanedione (obtained from the condensation reaction of acetylcyclo-
propane with formic acid ethyl ester in diethyl ether and potassium tert.-butanolate) and
0.05 mol of sodium methanolate are heated at boiling point for 3 hours in 300 ml of
absolute ethanol. The reaction mixture is then concentrated in vacuo and water is added to
the residue. After acidification with concentrated acetic acid, the resulting product is
separated off, dlied and recrystallised from methylene chloride/n-hexane, yielding 7 g of
2-benzylthio-7-cyclopropyl-1,2,4-triazolo[1,5-a]pyrimidine (compound no. 2.20) having a
melting point of from +112 to +114C.
Example P3: Preparation of 5-cYcloPropyl-7-methyl-1~2~4-triazolol 1~5-alpyrimidin-
2-vl-sulfochloride (compound no. 3.2)
CH3
~N N
ll (3 2)
V~ N ~N SO2CI
14 g of 2-benzylthio-5-cyclopropyl-7-methyl-1,2,4-triazolo[1,5-a]pyrimidine in 200 ml of
dichloromethane and 200 ml of water are mixed with 10 ml of concentrated hydrochloric
acid and stirred vigorously. 13.5 g of chlorine gas are introduced at from 0C to -3C
within a period of about 20 minutes. After subsequently stirring for about half an hour
without cooling, the organic phase is separated off, washed with water and dried over
sodium sulfate. Concentration by evaporation in vacuo yields a dark oil, which is stirred
thoroughly several times with petroleum ether. The petroleum ether is decanted off and
the resulting viscous residue is dried. 11.5 g of 5-cyclopropyl-7-methyl- 1,2,4-triazolo-
[1,5-a]pyrimidin-2-yl-sulfochloride (compound no. 3.2) are obtained in the form of a dark
oil (crude product), which is suitable for the further reactions. The proton resonance
spectrum confirms the constitution of the product obtained.
Example P4: Preparation of 5-methYl-7-cYcloPropvl-1.2,4-triazolo~1.5-alpvrimidin-
2-vl-sulfochloride (comPound no. 3.1)
~N N (3-1)
CH3 N N SO2CI
35 g of 2-benzylthio-5-methyl-7-cyclopropyl-1,2,4-triazolo[1,5-a]pyrimidine in 200 ml of
dichloromethane are stirred with 300 ml of water and 20 ml of concentrated hydrochloric
acid. 33.5 g of chlorine gas are introduced into the mixture at from 0C to -3C. After
about 30 minutes, the introduction is complete and the reaction mixture is then stirred ~or
about 20 minutes without cooling. After dilution with water, the organic phase is
separated off, dried over sodium sulfate and concentrated by evaporation in vacuo. The
resulting oil is washed with several portions of petroleum ether. Drying yields
5-methyl-7-cyclopropyl-1,2,4-triazolo[1,5-a]pyrimidin-2-yl-sulfochloride (compound no.
3.1) in the form of a crude product, which is suitable for the further reactions. Yield: 28 g
of dark oil.
Example P5: Preparation of 5-cvclopropvl-7-methvl-N-(2.6-
dichlorophenyl)-1.2.4-tliazolol 1.5-alpvlimidine-2-sulfonamide
(compound no. 1.2)
CH3
V/\NlN So2~H~3 (1.2)
Cl
5.75 g of 5-cyclopropyl-7-methyl-1,2,4-triazolo[1,5-a]pyrimidin-2-yl-sulfochloride are
added to a solution of 3.5 g of 2,6~dichloroaniline in lS ml of anhydrous pyridine, and the
mixture is then stirred at room temperature for 20 hours. After the addition of 200 ml of
water and 20 ml of ethyl acetate, the pH of the reaction mixture is adjusted to pH 3 with
concentrated hydrochloric acid. The resulting product is filtered off and the filtration
residue is washed with water and n-hexane. Drying yields 6 g of 5-cyclopropyl-7-methyl-N-(2,6-dichlorophenyl)-1,2,4-triazolo[1,5-a]pyrimidine-2-sulfonamide (compound
no. 1.2) having a melting point of +255C (decomp.).
xample P6: Preparation of S-methY1-7-cvclobutvl-N-(2~6-dichlorophenYI)-1~2.4-
triazolo~ 1~5-alPvrimidine-2-sulfonamide (compound no. 1.25)
CH Nl~l SO~IH~ (1.25)
With the exclusion of moisture, 4.9 g of 5-methyl-7-cyclo-
butyl-1,2,4-triazolo[1,5-a]pyrimidin-2-yl-sulfochloride are dissolved in S0 ml of
acetonitrile and stirred with 4.2 g of N-trimethylsilyl-2,6-dichloroaniline and 0.2 ml of
dimethyl sulfoxide until the reaction is complete. After filtering and washing with
acetonitrile, the filtrate is concentrated by evaporation and then triturated with water,
yielding 1.8 g of S-methyl-7-cyclobutyl-N-(2,6-dichlorophenyl)-1,2,4-
triaæolo[l,S-a]pyrimidine-2-sulfonamide (compound no. 1.25) having a melting point of
+260C.
~6`,4~
- 23 -
Example P7 Preparation of 5-methyl-7-cyclopentyl-N-(2,6-dichlorophenyl)-1,2~4-
triazolo~1~5-alpyrimidine-2-sulfonamide (compound no. 1.85)
~NlNJ~502N;~ 5)
4 g of N-2,6-dichlorophenyl-S-amino-1,2,4-triazolo-3-yl-sulfonamide are s~irred for
10 hours at room temperature and for one hour at +100C in 10 ml of dimethyl sulfoxide
and 15 ml of glacial acetic acid with 4 g of 1-cyclopentylbutane-1,3-dione. Precipitation
of the product by the addition of water and recrystallisation from glacial acetic acid yield
4 g of 5-methyl-7-cyclopentyl-N-(2,6-dichlorophenyl)-1,2,4-triazolo-
[ 1 ,S-a]pyrimidine-2-sulfonamide (compound no. 1.85) having a melting point of from
+245 to +246C.
The compounds of formula I and the intermediates of formulae III and V listed in the
following Tables are prepared analogously.
~ ~3 ~/3 r3 ~
- 24 -
Table 1:
XR2
~N--N ~R3 (I)
Z NJ;~N SO2N H/~R4
2~- ~$2~
o ~ , ~ ~
C~ ~ o ~ oo
o ~ o ~ ~ o o
~, o o
+ + + ~ + + +
'~ ~ o V V C
.
_ X ~ C X X :C
æ x ~c ~ x ~ ~ ~ x ~ c
X ~ ~ ~ X X :~ ~C X :~
~: ~ U
~ x~yyy~ ~ ~yyyy
~ x ~ x x $ ~ ~ ~
x ~ x~ y ~ y y y ~
C~ ~ O ~ oo C1~ ~ --'
~4 ~ ~
~2u2~
- 26-
~, ~
O V
X ' X ~
X ~:: X ~C
X
~ ~ V ~
~ ~ ~ Y ~
~ ~ ~ X ~ ~ X ~
c- ~ ~ o x o
- ~ ~ ~ ~ u~ ~ ~ oo o~ o ~
D E ~ ,, ~ '`' c~l
2~3~(OJ2
~: ~ v
~ ~ x ~ x
x x ~ c x ~
~ x ~ ~ x x ~ ~ x
_ a a a c a a a
a '' ~
X X
a K
~ ~ ~ ~ ~ v ~ oo a~
_ 9
2 ~
- 28 -
P~ ~ L, V ~ V ~
X o
X ~ X ~ V
C X ~ :~ X
v v V v v ~:3 v ~C ~, m
~ ~7 ~ V~ X X
V X ~ X
.
~i x ~ ~7y~ 7 v ~ x~
l ~ o ~ oo ~ o
D¦ ~J ~ ~ ~ ~
~ 3t'~
- 29 -
~ ~ ~ 8 ' ~ ~ o o ~
~ ~ X ~ C X
Co,
x ~ m :C ~ x x :c
X X ~ ~ ~ 3
X ~ :C X X
- - -
~ ~ v~ ~ ~7 m ~ ~ ~ X
C
.5 X :C V X ~ X X ~C X ~ ~
o
~ C
~ . ~ ~ o
O ~< ~ ~ ~t ~ ~ ~t ~ ~ ~ Vt
~ ~
2~ .
- 30-
_ ___
P~ Z z I _ O z; m
_
X :C X ~ X $
X ~ ~ X ~ X
_
~ ~ X X
.
V
0l
~ ~7 y ~ y ~ 7
~ ~ X X
5~ ~ ~ X
_, ~
. ~ ~ ~ ~ ~ ~ Oo a~
U~
_ ~
2J~
L~ x ~ ~ ~ x c ~ v
'D~ X ~ X
~ x x ~ $ x ~ cx ~c
-
~ ~: ~c x ~: x ~ ~ :c x ~~ x
r ~ 0 ; z ~ z ~ v
_ ~ X~ X ~ ~7
O ~ ~ X
.c X ~
_~ ~ o ~ o a' 1-
r'l ~ 2
~ 32-
~ o '~ ~ ~
v ~ v) O+~ + +l
O O, O O O ~ rO o O
O ~ ~ O
+~ + O+ + + +
:~ ~ V V V V V V V ~,L,
~ rV V
P~ X $ $ ~ $ ~ ~ $ $ ~C
$ $ $ ~ $ $ X ~ $ $
P~ ~ ~ V V V ~ C~ V V
~1 X~ C~ $~ y <~ ~ $ $
$ $ X X X $ $ $ $ $
c ~ > ~ I
C ~ ~ ~ U ~ l` ~o ~ O -~
~ s:~ ~ ~ oo oo
D ~_) ~ ~ ~
- 33 -
~ ~ ~ e ~5 ~ n n
~ 8 o o
~ g o ~~ ~. ~ o ~
+ + + + $ A ~ + A A
V ~ V C~
X ~ ~ X ~ C X ~ ~
æ~ x X ~ x ~ ~ x
X X X ~ X
_
_ y ~ X~ ~ y X~ y
~ ~ X
C ;X~ ~y ~ ~ o ~ I y
~ ~ ~ O
~ 00oo 00 00 0000 00 X C~
E- ~
~ ~ t.`~ 2 ~3
- 34 -
~ ~ o~
Eo 'r ~ ~ o~ _
+ y ~+ ~+ ~+ +
_
m v
C~ ~ :C X ~ ~ ~ O
I
~ ~ X X :C ~ V C~
C~ ~ ~ ~ X X
_
cY: m c~ c~ u o
_ ~ X ~ ~ ~
X ~ ~ :C X
C X ''~
.
C
D ~ ) c~ ,
2s3~ 2
Table 2:
J~ Y
A - S--</ J~ ~ (V)
N Z
- 36-
Comp. n . X Y Z A m.p. [C]
2.1 ~ HCH3 Benzyl +84 to +86
2.2 CH3 H <I Benzyl +101 to +103
2.3 ~ H <1 Benzyl +96 to +98
2.4 CF3 H ~ Benzyl +123 to +124
2.5 <I H H Benzyl +82 to +86
2.6 ~1 HCF3 Benzyl
2.7 <I HCH2CH3 Benzyl +112 to +114
2.8 CF3 H <I H
2.9 CH2CH3 H ~1 Benzyl +84 to +85
2.10 C3H7(n) H <1 Benzyl
2.11 C3H7(i) H <I Benzyl
2.12 C4Hg(tert.) H ~ Benzyl +111 to +113
2.13 CH2-OCH3 H <1 Benzyl
2.14 Cl H ~ Benzyl
2.15 OCH3F H<1 Benzyl
2.16 F H H Benzyl
~320~
- 37 -
Table 2: (continuation)
Ccmp. n( . X Z A m.p. [C]
2.17 CF3 H{~::1Benzyl
2.18 -OC2H5 H <1 Benzyl
2.19 OH H ~ Benzyl
2.20 H H <I Benzyl +112 to +114
2.21 -COOCH3 H <1 Benzyl
2.22 CH3 H CH2CH3 Benzyl
2.23 ~ H CH3 Benzyl
2.24 OcH2cHcl2 H ~1 Benzyl
2.25 CH3 H CH3 Benzyl
2.26 CH3 HCH3 ClBenzyl
2.27 CH3 H Cl Benzyl
2.28 NH2 H <I Benzyl
2.29 NHCH3 H <I Benzyl
2 30 N(c2Hs)2 H ~1 Benzyl
2 '~ 3 ~ ~
- 38 -
Table 2: (contin~lation)
Comp n IX ~ Y Z j, m p [CI
2.31 <I H CH3 H
2.32 <I H CF3 H
2.33 ~<1 H SCH3 H
2.34 <I H Cl H
2.35 <I C2H5 CH3 H
2.36 <I CH3 CH3 H
2.37 <1 Cl CH3 H
2.38 CH3 H <1 H
2.39 CH3 C3H7(i) ~<1 H
2.40 CH3 OCH2CH3 <I H
2.41 CH3 CH3 ~1 H
2.42 CH3 CF3 <I H
2.43 CH3 OCH3 <I H
2.44 CH3 ~ ~ H
2.45 CH3 Br --<¦ H
2.46 -CH2-CH -CH2- ~ Benzyl +93 to +94
2.47 <1 ¦ -CH2-CH !-CH2- Benzyl +113 to +114
2 ~ 2 ~
- 39 -
Table 2: (continuation)
Comp. n . X Y Z A rn.p. [C]
2.48 CH3 HCl1, Br +76 to +77
2.49 CH~ HCH3CH3 Br +97 to +100
2.50 CH3 H ~ Br +100 to +102
2.51 <I H Cl Br +108 to ~110
2.52 { ~ H CH3 Br +97 to +98
2.53 <1 HCH2-OCH3 Br +65
2.54 CH2-OCH3 H <1 Br +113 to +116
2.55 C~1, H H Br +62 to +66
2.56 CH3 H ~ Br +76 to +77
CH3
2.57 OCH3 H {~ Br +151 to +154
2.58 OCH3 H <I Br +130
2.59 OH H ~ Br >+260
2 ~
- 40-
Table 3:
x
Cl-S2 ~/ ~/~ (III)
2 ~ 2 ~
- 41 -
Comp nc X _ Z m.p. LCI
3.1 _ HCH3 oil
3.2 CH3 H ~1 oil
3.3 <I H <I +127 to +130
3.4 CF3 H ~1 +78 to +81
3.5 <I H H +100 to +103
3.6 <I HCF3
3.7 <I HCH2CH3 +62 to +63
3.8 H H ~1 +124 to +129
3.9 CH2CH3 H ~1 +83 to +86
3.10 C3H7(n) H <I
3.11 C3H7(i) H <I +121
3.12 C4Hg(tert.) H ~ +123
3.13 CH2-OCH3 H <I
3.14 C1 H <I +82
3.15 OCH3 H <I +125to+130
3.16 F H H
2~3~
- 42 -
Table 3: (continuation)
Comp nc:~ _ z m p [CI
3.17 CF3 H
3.18 -OC2H5 H <
3.19 OH H <
3.20 H H
3.21 -COOCH3 H <I
3.22 CH3 HCH2CH3
3.23 ~ H CH3
3.24 OCH2CHcl2 H
~ t3
3.28NH2 H <
3.29NHCH3 H <1
3 .30N(C2Hs)2 H <I _
- 43 -
Table 3:(continuation)
Comp.llc . X _ Z m.p.[C]
3.31 <I H SCH3
3.32 <I CH3 CH3
3.33 ~1 Cl CH3
3 34 CH3 CF3 <
3 35 CH3 OCH
3.36 CH3 ~<1 <
3.36 CH3 Br <I
3.37 -CH2-CH2-CH2- <I +152 to +153
3.38 ~ -CH -CH2-CH2-
3.39 ~ H H +151 to +156
CH3 CH3
3~40 ~ H CH3 oil
3.41 CH3 H ~ +91 to +95
3.42 <I H CH2-OCH3 +90 to +95
3.43 ~ H CH3 +68 to +75
3.44 CH2-OCH3 H <I +73 to +77
3.45 CH~ H CH3
3.46 OCH3 H {~
f~J ~ J ~ ~
- 44 -
Biological Examples
Example B 1: Preemer ence herbicidal action
In a greenhouse, immediately after the test plants have been sown in seed trays, the
surface of the soil is treated with an aqueous spray mixture in an amownt corresponding to
a rate of application of 4 kg of test compound/hectare. The seed trays are kept in the
greenhouse at 22-25C and 50-70 % relative humidity.
After 3 weeks, the herbicidal action is evaluated according to a scale of nine ratings (1 =
total damage, 9 = no action) in comparison with an untreated control group.
Ratings of from 1 to 4 (especially from 1 to 3) indicate good to very good herbicidal
action. Ratings of from 6 to 9 (especially from 7 to 9) indicate good tolerance (especially
in cultivated plants).
The compounds of Table 1 exhibit pronounced herbicidal activity in this test.
Example B2: Postemer~ence herbicidal action (contact herbicide)
A number of weeds, both mono- and dicotyledonous, are sprayed postemergence (in the 4-
to 6-leaf stage) with an aqueous active ingredient dispersion at a rate of 4 kg of test
compound per hectare and kept at 24-26C and 45-60 % relative humidity. The test is
evaluated 15 days after the treatment. In this test too, the compounds of Table 1 exhibit
good herbicidal activity.
Example B3: Herbicidal action in wild rice (paddy rice)
The weeds Echinochloa crus galli and Monocharia vag., which occur in water, are sown in
plastic beakers (surface: 60 cm2; volume: 500 ml). After sowing, the beakers are filled
with water up to the surface of the soil. 3 days after sowing, the water level is increased to
slightly above the soil surface (3-5 mm). Application is effected 3 days after sowing by
spraying the beakers with the test compounds. The rate of application corresponds to a
concentration of 4 kg of active ingredient per hectare. The beakers are then kept in the
greenhouse under optimum growth conditions for rice weeds, i.e. at 25-30C and at high
humidity.
The evaluation of the tests takes place 3 weeks after application. The compounds of
Table 1 damage the weeds but not the rice.
J ~ J i~.1
- 45 -
Example B4: _rowth inhibition of tropical cover crops
The test plants Centrosema pubescens and Psophocarpus palustris are propagated by
means of cuttings in 4 cm peat pots containing earth (45 %), peat (45 %) and Zonolite
(10 %). The cuttings are raised in a greenhouse at a day temperature of 27C and a night
temperature of 23C. The plants are illuminated for at least 14 hours/day with an intensity
of at least 7000 lux.
About 50 days after the cuttings were taken, they are transplanted into 13 cm pots, 4-5
plantslpot. After a further 60 days, the plants are cut back to a height of about 15 cm and
treated by spraying with an aqueous spray mixture at a concentration of 0.1 to 300 g of
active ingredient/ha (usually as a 25 % formulation)~ The amount of water applied is
about 200 I/ha.
4 weeks after application, the weight of the new growth is determined aud expressed as a
percentage of the average of the untreated controls. The necrotic damage is given as a
percentage of the total leaf area.
The new growth on the treated plants is markedly less than that on the untreated controls.
Example BS: Growth regulation of sovbeans
Test plants of the Williams variety are sown in 11 cm clay pots containing earth (45 %),
peat (45 %) and Zonolite (10 %) and are raised in a climatic chamber at a day temperature
of 24C and a night temperature of 19C. The plants are illuminated for 16 hours per day
with an intensity of about 350 micro-einsteins.
About 24 days after sowing, the plants are transplanted into 18 cm pots, 2 plants/pot.
After a further 12 days, when the plants are in the 5-6 trefoil leaf stage, the test compound
is applied at a concentration of 0.1 to 300 g of active ingredient/ha, usually as a 25 %
formulation and in an aqueous spray mixture. The amount of water applied is about
200 I/ha.
Evaluation is made about 4 weeks after application. The height of the new growth is
measured and expressed as a percentage of the average of the untreated controls. The
necrotic damage is given as a percentage of the total leaf area.
` 2 ~
- 46 -
The treated plants exhibit markedly less new growth than do the untreated controls.
Example B6: Growth inhibition of cereals
Test plan~s (summer barley of the Iban variety) are sown in 15 cm plastic pots containing
sterile earth and raised in a climatic chamber at a day temperature of 10-15C and a night
temperature of 5-10C. The plants are illuminated for 13.5 hours per day with an intensity
of about 25000 lux.
About 34 days after sowing, and after the plants have been thinned out to 4 plants/pot, the
test compound is applied at a concentration of 0.1 to 300 g of active ingredientlha, usually
as a 25 % formulation and in an aqueous spray mixture. The amount of water applied is
about 500 l/ha. After application, the plants are placed in a greenhouse at a day
temperature of at least 10C. They are illuminated for at least 13.5 hours/day.
Evaluation is made about 28 days after the treatment. The height of the new growth is
expressed as a percentage of the average of the untreated controls. The necrotic damage is
given as a percentage of the total leaf area.
The treated plants exhibit a reduction in new growth in comparison with untreated
controls.
Example B7: Growth inhibition of grasses
A mixture of grasses (e.g. Poa, Festuca, Lolium, Bromus, Cynosurus) and clover
(Trifolium pratense/repens) is sown in 15 cm plastic pots containing sterile earth and the
plants are raised in a greenhouse at a day temperature of 21C and a night temperature of
17C. The plants are illuminated for 13.5 hours/day with an intensity of at least 7000 lux.
The emergent plants are cut back weekly to a height of about 6 cm. About 42 days after
sowing and 1 day after the last cut, the test compound is applied at a concentration of 0.1
to 300 g of active ingredient/ha, usually as a 25 % formulation and in an aqueous spray
mixture. The amount of water applied is about 500 I/ha.
Evaluation is made about 3 weeks after treatment. The height of the new growth is
measured and expressed as a percentage of the average of the untreated controls. The
necrotic damage is given as a percentage of the total leaf area.
The tested compounds of Table 1 effect a reduction in new growth in comparison with
2 ~ 8
i
- 47 -
untreated con~ols.