Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
J~G~ Jt
/l J ` '
PROCESS FOR THE Q~JASI--CON---lNUOU~ DE:CA~lNATION OF RAW COFFEE
CROSS REFERENCE TO RELATED APPLICATION
This application claims the priority of Application P
40000474.0 filed in the Federal Republic of Germany on
5 January 10th, 1990, and such application is incorporated
herein by reference.
BACKGROUND OF THE INVENTION
The present invention relates to a process for
decaffeinating raw coffee in which the caffeine is
10 selectively separated from the raw coffee with the aid of a
liquid solvent, i.e., a solvent which is liquid under normal
conditions (1 bar and 20C), which is saturated or even
supersaturated with carbon dioxide at a temperature of 20C
to 110C and a pressure of 30 bar to 300 bar so that a low
15 caffeine or caffeine-free raw coffee is obtained which meets
these definitions. On the other hand, the process according
to the invention yields pure crystalline caffeine as a
byproduct which can be utilized in pharmacology and in the
beverage industry.
Many people frequently do not tolerate coffee because
of its caffeine content. Many processes have therefore been
developed for the purposes of extracting the caffeine from
the raw coffee and simultaneously avoiding the removal of
203;~761D
other raw coffee components which are necessary for the
development of the aroma of the coffee during roasting. The
caffeine is extracted from the raw coffee because if roasted
coffee is decaffeinated a loss of aroma cannot be avoided.
In one prior art decaffeination process, the raw
coffee is pre-treated, for example, in that the coffee beans
are hydrolized by means of water vapor at an increased
temperature, the coffee beans are extracted in a liquid-
liquid extraction process by means of solvents, e.g. methyl-
10 ene chloride or ethyl acetate, then the solvent is removed
from the raw coffee by evaporation and thereafter the moist
raw coffee is dried. In this prior art process, some
solvent residues may remain in the coffee and the raw coffee
may be denatured to a certain extent.
Processes have also been proposed which use other
solvents that need not be removed from the decaffeinated raw
coffee. Of practical significance are primarily those
processes which use water, supercritical carbon dioxide,
liquid carbon dioxide or higher organic fatty acids (coffee
20 oil) originating from coffee as solvents. These processes
require long periods of extraction to remove caffeine to a
sufficient degree. The reason for this is that the caffeine
must first diffuse to the surface of the coffee beans in
2033~6C)
order to be taken up by the solvent. This process becomes
slower and slower with decreasing caffeine concentration in
the coffee beans. In many of these prior art processes, the
solvent is circulated during the extraction process, thus
5 causing high investment and energy costs.
DE-OS 3713953Al discloses a process for decaffeinat-
ing raw coffee which essentially avoids the stated
drawbacks. In this process, the raw coffee is moistened to a
high water content of 35 to 50 weight percent and is then
10 kept for a few minutes to several hours at a temperature of
20 to 80C in a gas atmosphere of 75 to 300 bar and stirred,
if required. Thereafter, the pressure is reduced suddenly or
within a few minutes, while avoiding freezing, to between 1
bar and Pc (Pc = critical pressure of the gas employed). To
15 obtain a selective extraction of the caffeine, the raw coffee
is rinsed with water or with the supercritical gas and the
process is repeated several times if necessary. Thereafter,
the raw coffee is pre-dried in a centrifuge and roasted. The
caffeine is recovered from the rinsing agent in a known
20 manner. This process requires a complicated and cost
intensive repeated build-up of the pressure atmosphere.
SUMMARY OF THE INVENTION
It is the object of the invention to provide a simple,
operationally reliable and economical process for the
-- 3 --
2033`760
decaffeination of raw coffee which ensures a high degree of
decaffeination, which avoids denaturing of the further
components of the raw coffee, and which permits recovery of
the caffeine.
According to the present invention the above objects
are achieved by extracting the moist raw coffee beans by
means of a liquid solvent that is saturated with carbon
dioxide, the extraction taking place at a temperature from
20 to 110C and under a pressure of 30 to 300 bar for one to
10 several hours. The pressure atmosphere is reduced abruptly
or within a few minutes to between 1 bar and 10 bar. The
expanded raw coffee beans are rinsed in the liquid solvent
for a time period lasting between several minutes and two
hours. The process of selective caffeine extraction at
15 elevated pressure in the liquid solvent that is saturated
with carbon dioxide and pressure reduction with rinsing of
the coffee beans in the liquid solvent at a lower pressure is
repeated, if necessary. Then the caffeine is selectively
separated from the liquid solvent and is recovered in pure
20 crystalline form.
The cause for the unexpected success of this pressure
changing process is, among others, that a series of effects
occur which supplement one another in an advantageous manner.
For example, the water absorbed into the cells of the raw
~033~60
coffee beans creates a water-caffeine solution which facili-
tates the extraction of the caffeine. The extraction of
other substances, particularly those required for developing
the aroma during roasting, is reduced to a minimum.
The rapid pressure reduction results in a considerable
increase in the volume of the gas previously diffused into
the raw coffee beans, thus creating an expulsion effect for
the caffeine-water solution to the surface of the raw coffee
beans. The caffeine is selectively absorbed by the liquid
10 phase surrounding the raw coffee and is transported away.
The caffeine is selectively absorbed because, due to the
advantageous features of the process, the process conditions
cause the liquid phase to be saturated with the other
components of the raw coffee but to be substantially caf-
15 feine-free.
The present invention differs advantageously from DE-
oS 3713953A1 in that instead of using wet supercritical C02
or a mixture of supercritical C02 and a little water, a
liquid solvent is used, preferably water or an aqueous
20 solution, ~aturated or supersaturated with C02. The aqueous
solution charged with the raw coffee components is
decaffeinated in a subsequently connected apparatus, for
example, in column 7 of the single figure, by treatment with
supercritical C02 and is saturated with the C02. By
~033760
expanding this saturated water-CO2 ~olution to a lower
pressure, a supersaturated water-CO2 solution is created
which is introduced into the pressure vessels filled with raw
coffee beans. Thus, the present invention differs from the
5 prior art disclosed in DE-OS 3713953A1 in that no additional
C2 circulation is required to build up the pressure
atmosphere; rather, only the slight CO2 losses occurring
during the caffeine recovery are replaced. Moreover,
recompression in the present invention is considerably more
10 economical because the compression work is done with a liquid
medium (water saturated with CO2) which is substantially
incompressible. Additionally, the expansion in the raw
coffee extraction vessels is able to be effected considerably
faster than in DE-OS 3713953A1 because here the liquid
15 medium, in contrast to the gaseous medium in DE-OS
3713953A1, makes it impossible for the container contents to
freeze even if the pressure reduction occurs suddenly.
According to the invention, the process can be imple-
mented particularly advantageously if the raw coffee beans
20 are extracted in an aqueous solution that is supersaturated
with CO2 and saturated with the raw coffee components except
for the caffeine at a temperature from 65C to 90C and under
a pressure of 30 bar to 120 bar and are rinsed, after
reduction of the pressure to 1 bar to 5 bar, in the substan-
Z03376~)
tially caffeine-free aqueous solution; the caffeine contain-
ing aqueous solution is decaffeinated in a subsequently
connected column in supercritical Co2 at 65OC to 90C and 160
bar to 300 bar; the supercritical CO2 phase charged with
5 caffeine is regenerated in a further column by washing the
caffeine out with water at 65C to 90C and 160 bar to
300 bar; and the caffeine obtained in the developing caffeine
containing aqueous solution is recovered, for example, by
reverse osmosis.
According to the invention, saturating the liquid
solvent phase with CO2 is particularly suitable. However,
it is also within the scope of this invention to use any
desired gas or gas mixture which behaves in such a manner
with respect to its thermodynamic characteristics that it
15 diffuses in noticeable concentrations into the cells of the
raw beans which are filled with the water-caffeine solution.
Wetting of the raw coffee beans to be decaffeinated can be
omitted if water or an aqueous solution is employed as the
liquid solvent. Otherwise, the coffee beans are wetted to
20 between 20 weight percent and 40 weight percent and enough
water is added to the liquid solvent that the raw coffee
beans will be unable to dry out.
The raw coffee beans are extracted by means of the
liquid solvent that has been saturated with co2 at the
2033760
elevated pressure for one to six hours! depending on the type
of coffee and the deslred degree of decaffelnatlon. The
reduction of the pressure atrnosphere may occur suddenly since
lt ls lmposslble for the coffee beans to freeze. Although
gaseous carbon dioxlde is released durlng this pressure
reduction process, the liquid phase - surprisingly - does not
foam.
In accordance with the present inventlon there is
provlded a process for decaffelnatlng raw coffee beans by
selective extraction of caffeine contained therein by means of
a solvent that is liquid under ambient condltlons, comprlslng
the steps of
(a) extractlng the caffeine from the raw coffee beans
for a period of between one to several hours by means of a
liquid solvent that ls comprlsed of water and that has been
saturated with carbon dioxide at a temperature of between
about 20C and 110C and at a pressure of between about 30 bar
to 300 bar to provide a charged liquid solvent;
(b) abruptly or within a few minutes reducing the
pressure to between about 1 bar and about 10 bar to expand the
raw coffee beans;
(c) rinsing the expanded raw coffee beans ln the liquid
solvent for a period of between a few minutes to two hours;
(d) drying the expanded raw coffee beans to reduce the
water content thereof to that required for a subsequent
roasting process and provlde decaffelnated and pre-dried raw
coffee beans;
(e) roasting the decaffelnated and pre-dried raw coffee
-- 8
24872-27
~033760
beans; and
(f) selectively separatinq the caffeine from the charge
llquld solvent and recoverlng the caffelne ln pure crystalllne
form.
In accordance with the present invention there is
also provided a process for decaffelnatlng raw coffee beans by
selective extraction of caffelne contained thereln by means of
a solvent that ls llquld under amblent condltlons, comprlslng
the steps of:
(a) extracting the caffelne from the raw coffee beans
for a perlod of between one to several hours by means of a
liquid solvent that is comprised of water and that has been
saturated with a gas or gas mixture whlch has such
thermodynamic characterlstics that lt diffuses in slgnlflcant
concentrations into the cells of the raw coffee beans which
are filled with a water and aqueous caffeine solution, said
extractlon belng carrled out at a temperature of between about
20C to 110C and at a pressure of between about 30 bar and
300 bar to provlde a charged llquld phase;
(b) abruptly or withln a few mlnutes reduclng the
pressure to between about 1 bar and about 10 bar to expand the
raw coffee beans;
(c) rinslng the expanded raw coffee beans ln the llquld
solvent for a perlod of between a few minutes to two hours;
(d) drylng the expanded raw coffee beans to reduce the
water content thereof to that requlred for a subsequent
roasting process and provide decaffeinated and pre-dried raw
coffee beans;
- 8a -
24872-27
s
2033760
(e) roastlng the decaffeinated and predried raw coffee
beans; and
(f) selectlvely separating the caffeine from the charged
liquld solvent and recovering the caffeine ln pure crystalllne
form.
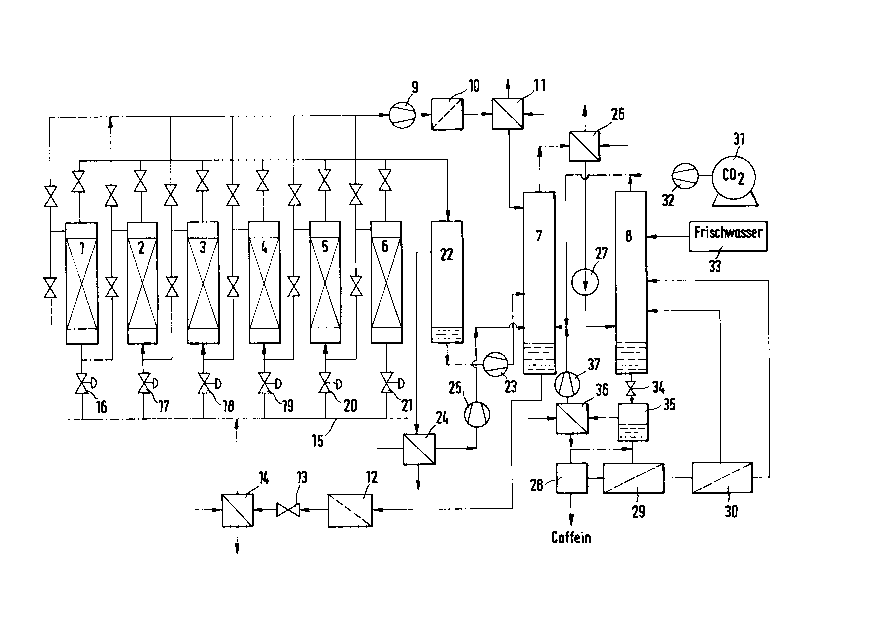
BRIEF DESCRIPTION OF THE DRAWING
The subject matter of the invention will now be
described in greater detail with reference to various
embodirnents thereof with reference to the single figure which
is a schematic representation of the sequence of one variation
of the process for producing decaffeinated raw coffee.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Depending on the type of coffee, the raw coffee
beans are moistened with water vapor to between 20 percent by
weight and 40 percent by weight and are then introduced into
pressure vessels 1 to 6. The pressure vessels shown in the
figure are connected in cascade so that quasi-continuous
operation and thus quasi-continuous decaffeination of raw
coffee becomes possible. This also simplifies the process of
moistening the coffee beans and the subsequent re-drying in
that these process steps can take place substantially
- 8b -
24872-27
,L
2033760
continuously and thus there are no long storage periods for
the moistened coffee beans. Moreover, investment costs for
moistening, intermediate storage and drying are lower than
for a purely discontinuous procedure. The series-connected
5 pressure vessels filled with raw coffee, for example vessels
2 to 5, are charged in such a manner with supersaturated
solvent freed of caffeine that the coffee beans
decaffeinated to the greatest degree are charged first. The
solvent then flows through the individual vessels in the
10 direction of increasing caffeine content of the raw coffee
beans and leaves the last vessel with the maximum possible
caffeine concentration.
The charged solvent phase is then introduced by means
of a pump 9 through a filter stage 10 or a centrifuge (if
15 required) and through a heat exchanger 11 (if required) into
column 7 where selective extraction of the caffeine takes
place at 200 bar to 300 bar and 65C to 110C as well as
renewed saturation of the liquid phase with C02.
The decaffeinated solvent saturated with C02 is ex-
20 tracted at the bottom of column 7, is conducted through afilter stage 12 for the separation of any still existing
solid particles and is expanded in a subsequent expansion
valve 13 to between 30 and 100 bar, thus creating a mixture
f C2 saturated solvent and C02 (supersaturated solution)
203376~)
which is brought to the operating temperature of the pressure
vessels in a subsequently connected heat exchanger 14 and is
introduced into the pressure vessels. A partial stream 15 of
the C02 supersaturated solvent phase, however, is introduced
5 through a control valve 16 to 21 into the base of a pressure
vessel, for example vessel 6, whose pressure has already been
reduced to between 1 bar and 5 bar, so that the caffeine
enriched solvent phase there is replaced by fresh solvent.
The caffeine containing solvent phase expelled, for example,
10 into vessel 6 is fed through a degasification vessel 22 and a
pump 23 into the lower portion of column 7. The C02 released
in degasification vessel 22 is extracted, liquified in a
condenser 24 and newly introduced into column 7 by means of
a liquid gas pump 25. While vessels 2 to 5 are decaf-
15 feinated, as shown as an example in Figure 1, at a pressurebetween 30 and 100 bar and vessel 6 is rinsed at a lower
pressure between 1 and 5 bar, vessel 1, for example, is
emptied and charged with fresh raw coffee. After closing of
vessel 1, the liquid of vessel 6 is conducted to vessel 1 as
20 soon as the rinsing process is completed and at the same time
vessel 1 is ventilated by way of a ventilation valve. For
the sake of clarity, the conduits and valves required for
this purpose are not shown in Figure 1. If necessary, the
decaffeinated raw coffee beans in vessel 6 may be briefly
-- 10 --
~033760
rinsed with fresh water in order to avoid weight losses and
possible technical difficulties during the subsequent drying
process.
The process described above by way of example can be
5 implemented in any other desired sequence or with a smaller
or larger number of vessels, with the quasi-continuity of the
process and thus the economy of the process increasing with
increasing number of vessels. The total number of vessel
reaches its optimum between 4 and 12 vessels.
The caffeine charged C02 leaving the head of column 7
is brought to the operating temperature of column 8 in a heat
exchanger 26 and is introduced by means of a conveying pump
27 into the lower portion of column 8 so as to continue its
circulation. In column 8, the caffeine in the caffeine
15 charged CO2 phase is substantially washed out with fresh
water 33 and with substantially caffeine-free residual water
obtained from the recovery of pure caffeine, for example, by
way of reverse osmosis at 28 to 30. Instead of reverse
osmosis, another manner of recovering the caffeine is also
20 possible, for example, by evaporating the water. From a Co2
tank 31, a pump 32 replenishes the slight C02 losses occurr-
ing during the process in the circulating C02.
Finally, the process according to the invention is
distinguished by the fact that the liquid circulation
~033760
(between column 7 and vessels 1 to 6) which is required for
the decaffeination of the raw coffee and is, moreover,
saturated with the coffee components as well as the water
circulation (between column 8, reverse osmosis 28 to 30 and
5 fresh water 33) required for the recovery of the caffeine are
coupled together by way of a supercritical C02 phase (between
column 7 and column 8). Due to the high selectivity of the
supercritical carbon dioxide for caffeine, there is also no
noticeable displacement and loss of the components important
10 for developing the aroma during roasting of the raw coffee.
Preferably, columns 7 and 8 are operated isobarically
and isothermally. However, with suitable temperature
control, it is also possible to transfer water from one
column to the next. For example, any water losses that my
15 occur in autoclaves 1 to 6 may be compensated by a higher
temperature in column 8. If, however, column 8 is operated
at a lower temperature, the solvent phase in column 7 may be
concentrated. The C02 obtained from the reverse osmosis
during the recovery of caffeine is extracted, after expansion
20 through a valve 34, from the subsequent degasification vessel
35, is liquefied in condenser 36 and is brought to the
pressure of column 7 by means of a pump 37 to be introduced
into column 7.
- 12 -
2033760
For example, 1000 g unroasted coffee beans having a
natural moisture content of 8 weight percent and a caffeine
content of 1.27 weight percent a.d. (a.d. = with reference to
the dry substance) were fed into a 2.2 liter pressure vessel
5 following the process scheme of Figure 1. The coffee beans
were extracted for four hours at a temperature of 850C and
under a pressure of 110 bar, at a solvent flow rate of
4 kg/h by means of a substantially caffeine-free aqueous raw
coffee extract that was supersaturated with C02 and included
lo 22 weight percent dissolved coffee solids. Then the pressure
atmosphere was abruptly reduced to 1 bar and the raw coffee
beans were extracted for another hour by means of the raw
coffee extract, with the flow rate of the substance here
being 2 kg/h. The decaffeinated raw coffee extract which
15 had previously been obtained by repeated leaching of fresh
raw coffee beans at 85OC first with fresh water and then
with an aqueous solution charged with the raw coffee com-
ponents and decaffeinated in a spray column by treatment with
supercritical C02 at 85C and 250 bar to a residual caffeine
20 content of 80 ppm (mg/kg solution) caffeine, was saturated
with Co2 at 85OC and 250 bar in a preceding column, its
pressure was reduced to 110 bar and 1 bar, respectively, thus
producing the supersaturated C02 raw coffee extract solution
which was then introduced into the pressure vessel filled
Z033760
with coffee beans. Thereafter the swelled beans which had a
moisture content of 51.6 weight percent were dried again.
They had a residual caffeine content of 0.08 weight percent
a.d., corresponding to a degree of decaffeination of 93.7
5 percent.
It will be understood that the above description of
the present invention is susceptible to various
modifications, changes and adaptions, and the same are
intended to be comprehended within the meaning and range of
10 equivalents of the appended claims.
- 14 -