Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ ~3 ~
. FC 445
-- 1 --
COMBINED USE OF N-IMIDAZOLYL DERIVATIVES OF BICYCLIC
COMPOUNDS AN~ CYCLOSPORINE IN T~ERAPY
The present invention relates to the use of
N-imidazolyl derivatives of bicyclic ccompounds, in
particular in combination with cyclosporin A, in therapy.
Cyclosporin A (CyA) is an effective immunosuppressive
agent that dramatically improves allograft survival in human
transplantation. At the same time, the use of ~yclosporin A
has been applied to various diseases thought to have auto-
immune basis (including for example: multiple sclerosis,
Guillan Barre syndrome, uveitis, myasthenia gravis, ~eymann
nephritis, juvenile diabetes type I, systemic lupus
erythematosus, aplastic anaemia, pure red cell anaemia,
idiopathic thrombocytopaenia, polychondritis, 6clerodoma,
Wegener granulomatosis, dermatomyositis, chronic active
hepatitis, auto-immune male infertility, psoriasis and
psoriatic arthiritis, Steven-Johnson syndrome, idiopathic
sprue, Chrone's disease, s~rcoidosis, glomerulonephritis,
interstit$al lung fibrosis and primary bil~ary cirrhosis)
and to inflammatory conditions, ln particulag inflammatory
conditions with an aetiology lncluding an auto-immune
component such as arthritis and rheumatic diseases.
Report~ and results in vitro, in animal models and in
clinical trials in the above-mentioned diseases are wide-
spread in literature. ~owever, the therapeutic potential of
20.'-3~1132
-- 2 --
this drug is often limited by renal dysfunction
characterized by a dose-dependent decrease in glomerular
filtration rate (GFR) and creatinine clearance and increased
blood urea nitrogen levels, as reported in Ann. Inter. Med.
99, 851 (1983); Lance~ 1, 470 ~1981) and N. Engl. J. Med.
311, 699 (1984).
The mechanism responsible for CyA-induced renal
failure has not been fully elucidated. ~owever, on the
other hand, preservation of tubular function with the
absence of frank tubular necrosis does not support a direct
toxic effect of CyA on tubular cells. Therefore, the
nephrotoxic effect of CyA may be more likely caused by
altered hemodynamics, as it is associated with reduced renal
blood flow and a progressive narrowing of the afferent
arteriolar lumenal diameter, as shown by scanning electron
microscopy. The basis for renal vasoconstriction is
unclear, but it has been suggested that thromboxane (TxA2)
is a potential mediator of altered hemodynamics.
TxA2, a cyclooxygenase metabolite of ~rachidonic
acid, is a potent vasoconstrictor and platelet
proaggregatory agent that has been implicated in many
pathological processes. For instance increased renal TXA2
synthe is has recently been associated with the
immunologically mediated renal injury characteristic of
murine lupus.
Cyclosporin A has been reported to alter arachidonic
~.3~3~
- 3 -
acid (A.~.) metabolism in some in v$tro models of cultured
monocytes and of smooth muscle cells [Lipids, 18, 566 ~1983)
and Transplantation 38, 377 (1984)~. Moreover in vivo
studies have shown that chronic administration of CyA ln the
rat induces a progressive $ncrease in the renal synthesis of
TxA2 ~Am. J. Physiol. 251, F 581-587 (19B6)). Therefore,
means of reducing this side effect would clearly be of major
benefit.
We have now found that cyclosporin A-induced
nephrotoxicity can be limited by concomitant administration
of a therapeutically effective amount of a N-imidazolyl
derivative of formula (I), 8S herein defined, or a
pharmaceutically acceptable salt thereof. Accordingly in
one aspect the present invention relates to a method of
preventing or treating cyclosporin A-induced nephrotoxicity
in mammals by administration of a therapeutically effective
amount of a compound of formula (I), as herein defined, or a
pharmaceutically acceptable salt thereof.
In another aspect the invention provides the use of a
compound of formula lI), as herein defined, or a
pharmaceutically acceptable salt thereof, in the preparation
of a pharmaceutical composition for use in preventing or
treating cyclosporin A-induced nephrosis.
The present invention further provides a method of
treating a disease having an auto-immune basis, which method
comprifies concomitantly admin$stering ~1) a pharmaceutical
composition containing a compound of formula (I), as ~erein
defined, or a pharmaceutically acceptable salt thereof, and
(2) a pharmaceutical composit$on comprising cyclosporin A as
active agent.
The invention also provides products containing a
compound of formula (I) or pharmaceutically acceptable salt
thereof and cyclosporin A as a combined preparation for
simultaneous or sequential use in treating a disease having
an autoimmune basis.
S 0 ~ 2
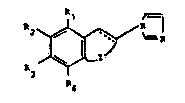
The N- ~ dazolyl der~vatives, accon~ng to the nethod of
prevention or treatment pro~ded by the present inventlon,
are described ln US-A-4,510,149 and ~n GB-B-2,141,705 and
have the following formula (I)
~ (I)
wherein
J 1 R6
(a) JZ is ~ ~ wherein Y completes a single
R5
bond or is oxygen or a -CH2- group and thj symbol --- repre-
sents a single or a double bond or tb) Z ls
and the symbol --- represents a double ~ond;
one of Rl, R2, R3 and R4 is CH20H, C2-C4 acyl,
/ 7 ~ R7
-CON \ , -COOR7, -CH2-COOR7, -CH2-CON \
R8 RB
R17 C9 ~ 7
-CH=C-COOR8 or -CH= -CON ln which e~ch of R7, R8 and
R6
Rg ls independently hydrogen or Cl-C4 ~lkyl~and the others
~ ~, .3 ~ 3 ~
are independently chosen from hydrogen, hydroxy, halogen,
Cl-C4 alkyl, Cl-C4 alkoxy and -COOR7 whereln R7 i8 as de-
flned above; and one Or R5 and R6 ls hydrogen and the other
is hydrogen, Cl-C6 alkyl or phenyl.
Preferred compounds of formula (I) are those whereln Z is
R6
or
R5
one of Rl, R2~ R3 and R4 is -CH20H, C2-C4 acyl,
/ R7 / R7
-CON , -COOR7, -CH2-COOR7, -~2-CON
R8 R8
17 19 / 7
-CH=C-COOR8 or -CH=C-CON ln which each of R7, R8 and
RR
Rg is independently hydrogen or Cl-C4 alkyl,and the others
are hydrogen andR5 and R6 are hydrogen; and the pharmaceu-
tically acceptable salts thereof.
Examples of preferred compounds of formula (I) are the fol-
lowing:
1,2-dihydro-3-(1-imidazolyl)-6-carboxynaphthalene;
1,2-dlhydro-3-(1-lmldazolyl)-6-ethoxycarbonylnaphthalene;
1,2-dihydro-3~ imldazolyl)-6-hydroxymethylnaphthalene;
1,2-dihydro-3-(1-imldazolyl)-7-carboxynaphthalene;
1,2-dihydro-3-(1-lmidazolyl)-6-(2-carboxyvlnyl)-naphthalene;
1,2-dihydro-3-~1-imldszolyl)-6-(2-ethoxycarbonylvinyl)-
naphthalene;
1,2-dihydro-3-(1-imidazolyl)-6-carboxymethylnaphthalene;
1,2,3,4-tetrahydro-2-(1-$midazolyl)-7-carboxynaphthalene;
2-(1-imidazolyl)-7-carboxynaphthalene:
2~ imidazolyl)-6-carboxynaphthalene;
and the pharmaceutically acceptable salts thereof and, when
appropriate, the Cl-C4 alkyl esters thereof.
Pharmaceutically acceptable salts of the compounds of
formula (I) include acid addition salts, with inorganic,
e.g. nitric, hydrochloric, hydrobromic, sulphuric,
perchloric and phosphoric, acids, or organic, e.g. acetic,
propionic, glycolic, lactic, oxalic, malonic, malic, maleic,
tartaric, citric, benzoic, cinnamic, mandelic, and
salicyclic acids, and salts with inorganic, e.g. alkali
metal, especially sodium or potassium, bases or
alkaline-earth metal, especially calcium or magnesium,
bases, or with organic bases, e.g. alkylamines, preferably
triethylamine.
A halogen atom is, for example, fluorine, chlorine or
bromine, preferably chlorine or bromine.
The alkyl and alkoxy groups may be branched or
straight chain groups.
A Cl-C6 alkyl group may be a Cl-C4 alkyl group.
Preferably ~he alkyl group is methyl or ethyl. A Cl-C4
alkoxy group is preferably methoxy or ethoxy.
As stated above, the compounds of formula (I), as
~03~032
- 7 -
well as the preferred ones hereabove specif~cally mentioned,
are already described in US-A-4,510,149 and G2-B-2,141,705.
The details of their preparation are described in the above-
identified US and British patents.
The compound of formula (I) or salt thereof and the
cyclosporin A are typically formulated separately and
presented for use in separate pharmaceutical compositions.
~he separate formulations may be administered simultaneously
or sequentially. By ~concomitant~ and ~combined~
administratior. according to the present invention is
therefore meant both separate and substantially
contemporaneous administrations of cyclosporin A and of a
compound of formula (I), as herein defined, or a
pharamceutically acceptable salt thereof.
The separate treatment with a compound of formula
(I), or a pharmaceutically acceptable salt thereof, can
commence prior to cyclosporin A treatment or subsequent to
the commencement of cyclosporin A treatment. A compound of
formula (I) or one of its salts can therefore be
administered 1 or 2 days in advance of commencing
cyclosporin A treatment, cO as to limit the nephrotoxic
effects of cyclosporin A. Alternati~ely the treatment with
the compound of formula (I) or salt thereof may begin at the
same time as or at any time after commencement of
cyclosporin A treatment, i.e. when the nephrotoxic effect of
cyclosporin A is detected or when it is desired to prevent
it.
Doses of cyclosporin A to be admingstered in
practisinq the invention will of course vary depending upon,
e.g., the mode of administration, the condition to be
treated (e.g. whether treatment is for the purposes of
immunosuppression or otherwise, ~nd if for immunosuppression
whether for use in relation to e.g. organ transplant, bone
n .~ ~
-- 8 --
marrow transplant, or the treatment of auto-immune
disease), as well as the effect desired. In addition,
dosaging will generally require adjustment for individual
patients in order to establish an appropriate long term
drug serum concentration e.g. by administration of an
initial daily starting or "loading" dose with subsequent
dose adjustment (generally dose reduction) in accordance
with serum levels, e.g. as determined by regular
radioimmunoassay (RIA) monitoring.
In general, amounts administered will be of the same
order to those conventionally employed in cyclosporin A
therapy, i.e. required to achieve ~i) immunosuppressive or
(ii) anti-inflammatory effectiveness. Thus, in general,
satisfactory results are obtained on administration in a
dose range of from about 5 or about 10 to about 20
mg/kg/day during the initial phase of therapy, reducing to
a maintenance dose of from about 1 to about 5 to about lO
mg/kg/day administered to the patient orally, once or in
divided doses 1 or 3 times a day. Where i.v.
administration is required, e.g. administration by infusion
(for example in the initial phase of treatment) lower
dosages, e.g. of the order of from about l or about 3 to
about 5 mg/kg/day for an initiating dose, or to about 2.5
mg/kg/day for a maintenance dose, are generally indicated.
The toxicity of the compounds of formula (I) is
negligible, therefore they can be safely used in therapy.
.
~ o~
Mice and rats which had been deprlved of food for nlne
hours were treated orally with slngle admlnlstratiOns of
increaslng doses of compounds of the lnventlon, then hous-
ed and normally fed. ~he orlentative acute toxlclty (LD50)
was assessed on the seventh day after the treatment and
was higher than 800 mg/kg.
In view of their high therapeutlc lndex the compounds of
formula (I) can be safely used in medicine.
The dosage level ~uitable for oral administratlon to adult
humans of the compounds of formula (I), e.g. 1,2-dlhydro-
3-(1-imidazolyl)-6-carboxy-naphthalene, may range ~rom about
100 mg to about 800 mg per dose 1 to 3 times a day, prefer-
ably from about 200 mg to about 400 mg per dose 1 to 3 times
a day. The exact dosage depends on the age, weight, condi-
tion of the patlent and administration route.Cyclosporin A and a compound of formula (I), or a pharma-
ceutically acceptable salt thereof, can be administered in
a variety of dosage forms, e.g., orally, in the ~orm of tab-
lets, capsules, sugar, or film coatcd tablets, llquld solu-
tlons or suspensions; rectally, ln the form of suppositories;parenterally, e.g. intramuscularly; or by lntravenous lnJec-
tlon or lnfuslon.
The present inventlon also provides a klt comprlsing in se-
parate containers (1) a pharmaceutlcal composltlon contain-
ing an ef~ective amount of a compGund of ~ormula (I), as
f3 3 ~
10 .
herein defined, or a pharmaceutlcally accept~ble salt the~o~, as actlve ingredient, and t2) a pharmaceutlcal composl-
tion containing an effectlve amount of cyclosporln A,as
active ingredlent.
The pharmaceutical compositlons ean be usually prepared fol-
lowing conventional methods and administered ln a pharmaceu-
tically suitable form.
For example, the solid oral forms may contain, together wlth
the active compound, diluents, e.g. lactose, dextrose, sac- -
charose~ cellulose, corn starch or potato starch; lubricants,e.g. silica, talc, stearic acid, magnesium or calcium stea-
rate, and/or polyethylene glycols; binding agents, e.g.
starches, arabic gums, gelatin, methylcellulose, carboxy-
methylcellulose or polyvinyl pyrrolidone; dlsaggregating
agents, e.g. a starch, a~ginlc acid, hlglnates or sodium
starch glycolate; effervescing mlxtures; dyestuffs; sweeten-
ers; wetting agents~ such as leclthin, polysorbates, lauryl-
sulphates; and, in general, non-toxic and pharmacologlcally
lnactive substances used ln pharmaceutical formulations.
Sald pharmaceutical preparatlons may be manufactured ln
known manner, for example, by means of mixing, ~ranuiating,
tablettlng, sugar-coatlng processes.
The llquld disperslons for oral admlnistration may be e.g.
~yrups, emulsions, and suspensions. The syrup may contain
as carrier, for example, saccharose or saccharose wlth gly-
~ ~ 3 ~ 2
cerlne and/or mannitol and/or sorbltol.The suspenslons and the emulsions may contaln as carrler,
for example, a natural gum, agar, sodlum alglnate, pectln,
methylcellulose, carboxymethylcellulose, or polyvlnyl al-
5 cohol.
The suspensions or solutlons for lntramuscular lnJections
may contain, together with the active compound, a pharmaceu-
tically acceptable carrier, e.g. sterile water, olive oil,
ethyl oleate, glycols, e.g., propylene glycol, and lf de-
sired, a suitable amount of lidocaine hydrochlorlde.The solu~ions for intravenous inJectlons or infusions may
contain as carriçr, for example, sterile water or preferably
they may be in the form of sterile, aqueous, lsotonic sallne
solutions.
The suppositories may contain together with ~he actlve com-
pound a pharmaceutically acceptable carrier, e.g. cocoa-but-
ter, polyethylene glycol, a polyoxyethylene sorbitan fatty
acid ester surfactant or lecithln.
The following examples illustrate but do not limlt the pre-
sent invention.
;Example_l
The activlty of a representatlve compound accordlng to the
present lnventlon, i.e. 1,2-dlhydro-3-(1-lmldazolyl~-6-
carboxynaphthalene, internal code FCE 22178, was evaluated
by the following method:
~ ~ .
~ ~ ,n.~2
Three groups of male Sprague-Dawley rats with initial
weights of 250-275 g were used in this study. All animals
were housed in a constant-temperature room with a 12-h
light-dark cycle, fed standard rat chow (Altromin-Rieper,
Vandoies, Italy), and had free access to tap water.
Group I received CyA (Sandoz, Basle, Switzerland) orally
by gastric tube for 12 months at the dose of 40 mg/kg every
other day. Thereafter the drug was discontinued and the
animals were monitored for another 2 months without any
treatment. Group 2 received for 12 months an oral dose of
the vehicle, polyoxyethylenated castor oil (Sabo, Bergamo,
Italy), in which CyA (40 mg/kg) was dissolved and was
considered as control group. Group 3 received an oral dose
of CyA of 40 mg/kg every other day, the compound FCE 22178
at the daily dose of 50 mg/kg per os and a further amount
of compound FCE 22178 at the dose of 100 mg/kg dissolved in
drinking water both for 12 months. Compound FCE 22178 was
administered concomitantly to CyA treatment, i.e. either
just before or just after CyA treatment.
Twenty-four-hour urine samples were collected at monthly
intervals using metabolic cages and were immediately frozen
and stored at -20-C until the extraction and the radio-
immunoassay (RIA) for thromboxane (TX) B2, 6-k~to-
prostaglandin Fl~ (PGFl~), and prostaglandin (PG) E2, which
reflects the renal synthesis of TXA2, prostacyclin (PG12),
- and PGE2, respectively, was performed. Blood samples for
determination
2~ rjn32
of 8erum TXB2 concentr~tlon were collected rro~ the tall
veln at monthly lntervals. In addltlon, every month ~lve
rats from each group underwent renal clearance studles.
Arter the clearances, the kldneys were removed and anslyzed
by llght mlcroscopy.
Concomitant administration of compound FCE 22178 markedly
inhibited TXA2 synthesis (measured as TX~2 levels) ln whole
blood and reduced the signli~icant lncrease of TXB2 uri-
nary excretion, creatinine and blood urea nitrogen: i.e.
parameters that are indicative of abnormal renal function.
Therefore the concomitant administration of compound FCE
22178 ameliorated the renal i~unction.
Example 2
Tablets, each weighing 300 mB and containing 100 mg of the
active substance can be manufactured as follows:
Com~ositions (for 10,000 tablets)
1,2-dihydro-3~ imidazolyl)-6-carboxynaphthalene 1000 g
Lactose 1420 g
Corn starch 475 g
Talc powder 75 g
Magnesium stearate 30 g
1,2-dihydro-3~ imidazolyl)-carboxy-naphthalene, lactose
203~ 0~
- 14 -
and a half of the corn starch are mixed; the mixture is
then forced through a sieve of 0.5 mm openings. Corn
starch (36 mg) is suspended in warm water (350 ml). The
resulting paste i8 used to granulate the powder. The
granules are dried, comminuted on a sieve of sieve size
1.4 mm, then the remaining quantity of starch, talc and
magnesium are added, carefully mixed, and processed into
tablets using punches of lO mm diameter.
EXAMPLE 3: Intramuscular in;ection
An injectable pharmaceutical composition can be
manufactured by dissolving 100 mg of 1,2-dihydro-3-(1-
imidazolyl)-6-carboxynaphthalene sodium salt in sterile
water or sterile normal saline solution (1-2 ml).
EXAMPLE 4: Capsules ~100 mg)
15 1,2-dihydro-3-(1-imidazolyl~-6-carboxynaphthalene 100 mg
Lactose 248 mg
Corn starch 50 mg
Magnesium stearate 2 mg
Total 400 mg
Encapsulate in two-piece hard gelatine capsules.
EXAMPLE 5: Suppository (100 mg)
1,2-dihydro-3-(1-imidazolyl)-6-carboxynaphthalene 0.10 g
Lecithin 0.14 g
- 25 Cocoa butter 1.76 g
Total 2.00 g