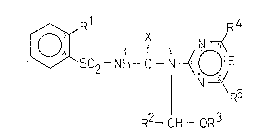Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2~353~9
HERBICIDALLY ACTIVE UREA DERIVATIVES AND A PROCESS FOR THE PREPARATION
THEREOF
This inven-tion relates to novel, herbicidally active substituted
sulfonylurea derivatives of t~le general formula (I)
o ~502~NH-C- j~E
R2--CH--CtR3
15 wberein
Rl stands for hydrogen, halogen, Cl_4alkoxy, halo-Cl_4alkoxy,
Cl_3alkylsulfonyl or a group of the formula COR6;
R2 means hydrogen, Cl_3alkyl or phenyl;
R3 Cl_6alkyl, C3_6alkenyl, Cl 3alkoxyalkyl group;
C2_4alkyl substituted by one or more halogen(s); or benzyl group;
R4 and R5 mean independently of each ot~ler a Cl_4alkyl, Cl_~alkoxy
group; halogen; Cl 3alkylamino or di(Cl_3alkyl)amino; or Cl 3alkylthio ;
R6 stands for a Cl_4alkoxy, C3_6alkenyloxy, C3 6alkoxyalkyl, Cl 3alkyl-
amino, di(Cl 3alkyl)amino, piperazinyl or morpholinyl group;
X means oxygen or sulfur; and
E stands for a methine group or nitrogen,
their salts formed with alkaline metals, alkaline-earth metals, amines
or quaternary ammonium bases. The invention is further directed to herbicide
compositions containing such compounds optionally together with an antidote.
The invention further relates to a process for the preparation of
novel compounds of t~le general formula (I), wherein Rl, R2, R3, R4, R5,
R6, X and E are as defined above.
In the above definitions (throughout the specification):
- "alkyl group" is meant to include a straight or branched chain alkyl
group, such as methyl, etbyl, propyl, isopropyl, as well as any of t~le
isomeric butyl groups;
- "alkoxy group" usually means met~loxy, ethoxy, propoxy, isopropoxy or
any of the four isomeric butoxy groups, particularly met~loxy, ethoxy,
or propoxy group;
A 4716-8D3 KY
~353~9
- 2 -
- "alkenyl group" may be e.g. allyl, isopropenyl, l-propenyl, l-butenyl,
2-butenyl, 3-butenyl, l-isobutenyl or a pentenyl group, particularly
allyl or 4-pentenyl group;
- "balogen" in itself or ~lalogen of a baloalkoxy group may be fluorine,
chlorine or bromine, particularly cblorine.
Suitable alkaline metal or alkaline-earth metal hydroxides forming
salts wit~l the compounds of the general formula (I) are potassium, sodium,
magnesium or calcium ~lydroxide, mainly sodium and potassium bydroxide.
Useful amines for sal-t formation include primary, secondary or
tertiary amines, such as metbylamine, ethylamine, propylamine, isopropyl-
amine, any of the four isomeric butylamines, dimethylamine, diethylamine,
isopropylamine, diisopropylamine, pyrrolidine, piperidine, morpholine,
trimethylamine, pyridine, quinoline or isoquinoline, particularly iso-
propylamine and diet~lanolamine.
Quaternary ammonium bases used for salt formation are e.g. tetra-
ethylammonium, triethylbenzylammonium, trimethylbenzylammonium balide or
any kind of tetrabutylammonium balides.
Herbicidally active sulfonylurea derivatives are comrnonly known
compounds.
The known sulfonylureas are characterized thereby that the nitrogen
in 3-position, substituted by a heterocyclic group, bears hydrogen or a
C1 4alkyl group, preferably a met~lyl group; or a C1 4alkoxy group, pre-
ferably a methoxy group; or a C2 8alkenyl, C2 4alkynyl group or an aralkyl
group. 0-ther substituents ~lave been published in a surprisingly low
number or patent specifications.
Sulfonylureas of suc~l type are e.g. discribed in t~le European
Patent Specification No. 152,378 and in the published Japanese Patent
Applications Nos. J5 8126872 and J6 0078981.
Sulfonylureas exert an excellent herbicidal effect against a
3C number of mono- and dicotyledonous weeds, c~liefly broad-leaf and grass
weeds / J.M. Green et al.: Proc. S. Weed Sci. Soc. 34, 214 (1981)7,
particularly in wheat and barley cultures. Tbeir advantage consists in
t~le very low effective doses (usually 5 to 50 g/~lectare). Their disadvantage
appears therein t~lat upon tbe use thereof cultivated plants are damaged
to a bigher or lower degree. It bas -turned out in tbe practice of their
use that t~leir antidotation is advisable in cereals and essential in
maize cultures. In tbe European Patent Application No. 127,469 antidotes
3 - 2~)3S3~9
are published w~licb can be used after sowing in the preemergent period
and are capable to protect wheat and millet from tbe harmful effec-t of
sulfonylurea-type berbicides. Metbyl 2-L (aminocarbonyl)aminosulfonyl7-
benzoate and its ammonium salt as well as met~yl 3-/ (aminocarbonyl)amino-
sulfonyl7-2-t~iophenecarboxylate were found to be most favourable.
According to the United States Patent Specification No. 4 343 649
1 8-naphthalic acid anhydride ~ -(cyanomethoxyimino)benzeneacetonitrile
or N N-diallyl-dicbloroace-tamide may be used to give a favourable result
in the increase of selec-tivity of tbe berbicidally active 2-cbloro-N-
-/ (4-methoxy-6-methyl-1,3,5-triazin-2-yl)aminocarbonyl7benzenesulfoll-
amide 2 5-dicbloro-N-/ (4 6-dimetboxypyridin-2-yl)aminocarbonyl7benzene-
sulfonamide or 2-carbomethoxy-N-/ (4 6-dimethylpyrimidin-2-yl)aminocarbonyl7-
benzenesulfonamide.
Herbicide compositions containing a sulfonylurea derivative as
active ingredient together witb glycine derivatives as antidotes are
published in tbe Hungarian Patent Specification No. 201 445.
The aim of tlle present invention was to develop berbicide composi-
tions containing novel active ingredientsj which possess more favourable
physical and chemical properties higher selectivity and lower persistency
in comparison to the known sulfonylureas.
In the course of our investigations compositions containing
novel sulfonylurea derivatives of the general formula (I) bave been deve-
loped which are cbaracterized in that the N3 atom (nitrogen in 3-position)
of the sulfonylurea group subs-tituted by a heterocyclic group bears an
R2-CH-OR3 group wherein R2 and R3 are as defined above.
It has been found tha-t compositions containing the novel compounds
of the general formula (I) are particularly useful for weed control
purposes since they show an excellent herbicidal effect in a suitable
low dose are appropriately selective against cultivated plants or can
be made selective by using known antidotes and simultaneously they are
easily decomposed in the soil.
According to another aspect of the invention tbere is provided
a process for tbe preparation of the new compounds of general Formula (I)
in inert organic solvents or a mixture tbereof. Tbis process comprises
a) reacting an isocyanate or isotbiocyanate of tbe general Formula (IV)
- 4 - ~() 353~L9
~R.l
S2 - NCX
wherein Rl is as defined above with an aminopyrimidine or aminotriazine
derivative of the general formula (I:[I)
R4
N ~
0 HN ~O E
¦ N ~ 5 (III)
2 I R
R -- CH--oR3
wherein R2, R3 R4 R5 and E are as defined for formula (I) optionally
in the presence of a base as catalyst at a temperature of 0 to 50 C
preferably between 20 C and 30C; or
b) reacting a sulfonylcarbamate derivative of the general formula (V)
~SO2--NH COOR
wherein Rl is as defined for formula (I) and R stands for phenyl
group with an aminopyrimidine or aminotriazine derivative of the
general formula (III) wherein R2 R3 R4 R5 and E are as defined
above at a temperature of 25 to 120 C preferably between 60C
and 90C; or
c) reacting a carbamoyl c~loride of the general formula (VI)
R4
E O ~ N - C O Cl (VI)
~N
R2- CH--oR3
- 5 - 23305-1180
wberein R2 R3 R4 R5 and E are as defined above
wi-th an a:Lk~line metal salt oE a sulFc7namide oE the general
formula (II)
(II)
S2- ~2
w~lerein Rl is as defined for formula (I) in the presence of a base
as catalyst a-t a temperature between -lO C and 50 C perferably
between 0 C and 25 C; or
d) reacting a N-pyrimidinyl- or N-triazinylcarbamate of t~le general
formula (VII)
R4
~N
E~ ~ N - COOR (VII)
R2- H~oR3
w~lerein R2 R3 R4 R5 and E are as defined above and R means pllenyl
group, witt~ a sulfonamide of the general formula (II) wherein Rl is
as defined for formula (I) in t~e presence of a catalytic amount of
a base at a temperature of 20 to 80 C preferably between 2û C and
40 C; or
e) reacting a sulfonyl cbloride of the general formula (VIII)
....
~SO2 - Cl (VIII)
w~lerein Rl is as defined for formula (I) with an aminopyrimidine
or aminotriaz3ne derivative of tbe general formula (III) wberein
the su~stituents are as defined above and alkaline metal cyanates
at a temperature of 20 C to 120 C preferably between O0 C and
90 C.
. ~ "
~'
- 6 - 20~531~
If desired, tbe compounds of general formula (I) prepared by
using the above process variants may be converted to their salts
with alkaline metal or alkaline-earth metal bydroxides, amines or
quaternary ammoniuln t~alides, e.g. by reacting the desired compound
with tbe appropriate base in a suitable solvent, which is t~len evaporated.
In the preparation of compounds of t~le general formula (I) methylene
cllloride, ctlloroform, carbon tetrach:Loride, dichloroetbane, tric~lloro-
etbane, tetrahydrofuran, ace-tonltrile, dioxane, benzene, toluene,
xylene, chlorobenzene, dimethylformamide, nitromethane, nitroetbane,
N-methylpyrrolidone, dimetboxyethane, diet~lyl ether, diisopropyl ether,
dibutyl etber, bexa;le, petroleum etber, etbyl acetate, butyl ace-tate,
dibutyl pbtbalate as well as mineral and vegetable oils may be used as
inert organic solvents but useful solvents are not restricted to those
listed above.
In the above process variants the reac-tion temperature may be
varied in a wide range usually from -20 C up to the boiling of the
solvent used; however, it is preferred to react the components at a
temperature between 0 C and 90 C, particularly at 15 to 45 C since
this temperature range is useful to prepare also the heat-sensitive
derivatives of the general formula (I). The reactions are suitably
carried out under atmospheric pressure, however an elevated or reduced
pressure may also be used; the reactions can further be performed in
the presence of air or under an inert gas e.g. nitrogen.
For shortening the reaction time or for completing the reaction
a few drops of a base should be used as catalyst.
As catalytically active bases particularly tertiary amines such
as triethylamine, tributylamine, N,N-dimethylaniline as well as nitrogen-
containing heterocyclic compounds, e.g. pyridine or 1,4-diazabicyclo-
-_2.2.27Octane (DABC0) may be empleyed although alkaline metal hydroxides
or alkaline metal alkoxides such as sodium hydroxide or sodium methoxide
are also useful. The catalysts being useful in the practice of tbe
process variants are not restricted to those listed above.
The products prepared by using the above process variants can be
separated by the complete or partial evaporation of ttle solvent and
recrystallization; or by triturating an evaporation residue with a
solvent or solvent mixture weakly dissolving the product. If necessary,
the compounds obtained can be purified by cbromatograplly on a suitable
load, sucb as aluminium oxide, silica gel and the like.
- 7 - ~0~153~3
-r~e berbicide compositions according to tbe invention usually
contain tbe active ingredient of general formula (I) in an amount
of 0.01 to 95 % by weig~lt preferably 2 to 80 % by weight in the
form of known formulations e.g. solutions emulsions dusting
powders suspensions we-ttable powders pastes soluble powders
granules suspension concentrates or emulsifiable concentrates
natural or synthetic materials impregnated with t~le active ingredient
or compositions encapsulated in polymeric substances.
The compositions are prepared by mixiny and/or triturating
the active ingredients with binding agents solvents and/or carriers
optionally by -the simultaneous use of surfactants and/or dispersing
as well as adhesion-promoting agents.
Useful solvents are: aromatic hydrocarbons preferably C7 12
fractions e.g. toluene xylene mixtures or substituted naphthalenes;
chlorinated aliphatic or aromatic hydrocarbons preferably chloro-
ethylene methylene chloride or chlorobenzene; aliphatic hydrocarbons
sucb as cyclohexane paraffins or mineral oil fractions; mineral or
vegetable oils; alcohols e.g. e-thanol or ethylene glycol; or t~eir
ethers such as ethylene glycol monomethyl ether ethyl ether; ketones
suc~ as acetone methyl ethyl ketone metbyl isobutyl ketone cyclo-
hexanone; strongly polar solvents e.g. N-methylpyrrolidone dimethyl-
formamide dimethylacetamide dimetbyl sulfoxide; phthalic acid es-ters
e.g. dibutyl phthalate or dioleyl p~thalate; as well as epoxidized
vegatable oils suc~ as epoxidized cocus oil or soybean oil; or water.
Suitable carriers are: inorganic powders such as kaolin calcite
talc chalk quartz attapulgite montmorillonite diatomaceous earth
perlite amorphous silicon dioxide aluminium oxide highly disperse
silicic acid pumice brick powder sepiolite bentonite or sand;
organic powders e.g. flour-like powders prepared from plant parts
such as corn-stalk or coconut-s~ell as well as corn flour; various
types of starch processed starch; sugar e.g. glucose; powdered or
ground syn-thetic resins e.g. phenol or urea resins In addi-tion a
bigh number of inorganic or organic pre-granulated materials may
be used.
Nonionic cationic and/or anionic -tensides may be employed as
surfactants wbic~ possess favourable emulsifying dispersing and
wetting properties. Mixtures of tensides may also be used. Tbe anionic
tensides are water-soluble soaps e.g. sodium or potassium salt of
- ~ - 23305-1180
oleic or stearic acicl a, well as the scl:L~s of natural fatty aeid
mixtures; however, it is more suitable to use syn~hetic tensides,
particularly fat1y alcohol sulfonates, fatty alcohol sulfates,
sulfonate--henzimldazole deriva-tives o~ alkylarylsulfonates. The
calc~ium or triethanolamine sal~,5 of liyninsulfonic aeid,
dodecylsulfurie acid esters, sulfonic acids prepared from natural
fatty acids as well as sulfuric acid esters and sulfonic acids of
fatty alcohol-ethylene oxide adducts, dodeeylbenzenesulfonie aeid
or tributylnaphthalenesulfon:ie aeid may be mentioned as examples.
In addition, phosphates, e.g. salts of the phosphate ester of p-
nonylphenol-(4-14)ethylene oxide adduet are also suitable.
As eationie tensides quaternary ammonium salts may be
taken in eonsideration whieh contain lower alkyl groups
substituted by halogen or hydroxyl group and/or a benzyl group
bound to the quaternary nitrogen in addition to a C8 20alkyl
group. Examples of tensides of this type are e.g.
stearyltrimethyl-ammonium ehloride or benzyl-bis(2-
chloroethyl)ethyl-ammonium bromide.
The nonionie tensides are mainly polyoxyethylene glyeol
ethers, polyoxyethylene glyeol esters as well as esters of
polyvalent aleohols and their eondensation produets, nonylphenol
polyethoxyethanols, eastor oil polyglyeol ethers and
polypropylene-polyethylene oxide adducts.
Ligninsulfonate, sulfite liquor and methyleellulose are
e.g. useful dispersing agents.
The eompositions may eontain adhesion-promoting agents,
e.g. earboxymethyleellulose, natural and artifieial, powder-like,
granular or latex-like polymers, e.g. polyvinyl aleohol, polyvinyl
- 8a -- 23305-l:L80
acetate as well as native phospholipids such as cephalin, lecithin
or synthetic phospholipids.
In addition, the compositic,ns accord.ing to the invention
may contain other additives such as stabilizers, antifoam agents,
viscosity-recJ~Ilating substances, preserving agents as well as
inorganic or organic pigments.
Activatinq, .synergistic, antidoting substances may also
be incorporated to the compositions according to the invention.
The herbicide compositions containing known antidotes,
lQ such as DKA-24 (N~dichloroacetyl-N-allylglycine N'-allylamide),
AD-67 (N-dichloroacetyl-1-oxa-4-azasprio[4,5]decane), R-15788
(N,N-diallyl-dichloroacetamide), MG-191 (2-dichloromethyl-2-
methyl-1,3-dioxolane), TI-35 or CGA-92194 are also within the
scope of the invention. (TI-35 is N-~dichloroacetyl)-
hexamethylene imine, CGA-92194 is N-(1,3-dioxolan-2-ylmethoxy)-
imino-benzeneacetonitrile.)
;,~i'
X03S3~9
If desi~ed t~le compounds of tbe general formula (I) according
to tbe invention and the compositions containing sarne can be used in
combination witb ot~er agrochemicals whic~ may be herbicides fungicides,
~ insecticides miticides nematocides antiviral agents p~ant ~x~rthreoJulating
agents or attractants.
Tbe compositions according to the invention preferably contain
0 01 to 95 % more preferably 2 -to 80 % by weig~lt of active ingredient
of t~le yeneral formula (I) or a total of active ingredient and antidote
5 to 99.9 % by weigilt of liquid or solid carrier and 0 to 30 preferably
0.1 -to 25 % by weigh-t of surfactant and otber additives. In the compositions
containing also an antidote t~e ratio of tbe antido-te to the active in-
gredient may be 1:1 to 50:1.
Compositions with the components listed hereinafter are particularly
Freferred (percentages are throughout r~iven by weight):
1. Solutions
Active ingredient 1 to 30 % preferably 5 to 25 %
Solvent 1 -to 90 % preferably 0 to 85 %
Surfactant 0 to 99 % preferably 0 to 95 %
2. Emulsifiable concentrate
Active ingredient 1 to 20 % preferably 5 to 10 %
Surfactant 1 to 30 % preferably 5 to 20 %
Liquid carrier 50 to 94 % preferably 70 -to 85 %
3. ~ettable powder
Active ingredient 0.5 to 90 % preferably 20 -to 80 %
Surfactant 0.5 to 20 % preferably 5 to 15 %
Solid carrier 5 to 95 % preferably 15 -to 90 %
4. Suspension concentrate
Active ingredient 5 to 75 % preferably 10 to 50 %
Water 25 to 95 % preferably 30 to 80 %
Surfactant 1 to 40 % preferably 5 to 20 %
5. Powder
Active ingredient 0.5 to 10 % preferably 0.5 to 5 %
Solid carrier 90 to 99.5 % preferably 95 to 99 %
6. Cranules
Active ingredient 0.5 to 30 % preferably 1 to 15 %
Solid carrier 70 to 99.5 % preferably 85 to 95 %
- lo - 20353~9
Tlle compositions according to tbe invention can be diluted to an
active ingredient concentration of 0.01 % by weight with water before
-tbe use. Tbe compositions may be applied onto tbe locus to be treated
after or without dilution by using tbe common methods e.g. spraying
dusting atomization spreading sprinkling or tbe ultra low volume
metbod. The compositions containing tbe novel sulfonylureas of -the general
formula (I) according -to the inven-tion possess an excellent herbicidal
effect agains-t both monocotyledonous and dicotyledonous annual as well
as perennial weeds. Tbe compositions according to tbe invention can
be applied before sowing or both in tbe preemergent or postemergent
period.
When carrying out -tbe treatment before emergence of the weeds
on tbe soil witbout plants the sprouting plants shoot up and develop
up to the cotyledon (seed-leaf) phase then tbeir growth stops and they
perish partially or totally after 3 to 6 weeks. When carrying out tbe
treatment after shooting up (emergence) of the weeds tbe growt~ of the
weeds stops after the treatment and t~e weeds perisb partially or totally
after 1 to 4 weeks. It is characteristic of tbe compositions according
to the invention that t~ey already show effec-t in an extraordinarily low
applied amount. Depending on the weeds treated t~e ~erbicidal effect
is very good in doses of as low as 0.01 to 2.0 kg/hectare. The important
large-scale cultures e.g. wheat maize barley rice and soy sorts
were found to be resistant; tbus the compositions according to the
invention are excellently useful for the control of both mono- and di-
cotyledonous annual and perennial weeds which are difficult to kill
or cannot be killed at all in the above cultures. Cultivated plants are
not restricted to those mentioned above. Depending on t~e active in-
gredient employed 0.05 to 2.0 kg/hectare doses of compositions according
to tbe invention can be used for a total weed control too.
A particular advantage of compositions containing sulfonylureas
of tbe general formula (I) as active ingredient appears therein that
after Qetting to t~e soil, they are rapidly decomposed therefore when
the compositions are postemergently used the field area employed can
be sown with other cultivated plants immediately after harvesting the
bost-plant or after a shorter period in comparison to the compositions
containing known commercially available sulfonylureas.
Tbe preparation and use of tbe active ingredients /compounds
of tbe general formula (I)/ and compositions according to the invention
are illustrated in detail in tne following, non-limiting Examples.
Example 1
Preparation of 3-ethoxymethyl-1-(2-meti~oxycarbonylpnenylsulfonyl)-3-
(4-methoxy-6-methyl-1,3,5-triazin-2-yl)urea (compound No. 8)
Af-ter adding 0.01 9 of DABC0 catalyst to a solution containing
3.96 9 (20 mmol) of 2-etnoxymetrlylamino-4-metrloxy-6-metnyl-1,3,5-triazine
in 50 ml of anhydrous dietnyl etber, 5.3 9 (22 mmol) of 2-methoxycarbonyl-
pnenylsulfonyl isocyanate dissolved in 20 ml of anhydrolls dietnyl etber
are dropped to tne above solution at room temperature under stirring.
The reaction mixture is stirred for 6 nours while precipitation of the
product begins. Next day tne crystalline precipitate is filtered, wasned
with a little diethyl etner and tnen dried at room temperature to give
6.5 9 (74 % yield) of tbe title product, m.p.: 105-106 C.
Example 2
Preparation of 3-ethoxymethyl-1-(2-crlloroprlenylsulfonyl)-3-(4-methoxy-
-6-methyl-1,3,5-triazin-2-yl)urea (compound No. 4)
3.96 9 (20 mmol) of 2-(ethoxymethyl)amino-4-metrloxy-6-methyl-
1,3,5-triazine are added to the solution of 6.23 9 (20 mmol) of pnenyl-
-N-(2-cnlorophenylsulfonyl)carbamate in 120 ml of anbydrous benzene.
Tbe mix-ture is stirred at 75 to 80 C for 8 hours, then evaporated to
dryness. After recrystallizing tne residue from diethyl etner 5.06 9
(61 %) of the title compound are obtained, m.p.: 123-123 C.
Example 3
Preparation of 3-(4,6-dimetnylpyrimidin-2-yl)-1-(2-cnloroprlenylsulfonyl)-
-3-(ethoxymethyl)urea (compound No. 2)
4.87 9 (20 mmol) of N-(4,6-dimet~lylpyrimidin-2-yl)-N-(ethoxymethyl)--
carbamoyl chloride dissolved in 10 ml of dimetbylformamide are dropwise
added to a solution containing 4.27 9 (20 mmol) of 2-cblorobenzenesulfon-
amide sodium salt in 25 ml of annydrous, redistilled dimetnylformamideat 5 to 10 C wnile stirring. After the addition, the mixture is stirred
at room temperature for 3 hours, tben poured onto 250 ml of icewater.
Tbe precipitate is filtered, wasbed wit~ a little water, dried and then
- 12 ~ 3~3~3
recrystallized from a mixture of etbyl acetate and die-thyl ether to
obtain the title product in a yield of 3.34 9 (42 %), m.p.: 118-119 C.
Example 4
Preparation of 3-(4,6-dimet~lylpyrimidin-2-yl)-3-ethoxymethyl-1-(2-methoxy-
carbonylpllenylsulfonyl)urea (compound No. 6)
Af-ter adding 3.1 9 (lû mrnol) of phenyl-N-(4,6-dimethylpyrimidin-
-2-yl)-N-(ethoxymethyl)carbamate to the solution of 2.15 9 (10 mmol)
of 2-met~loxycarbonylbenzenesulfonamide and 1.52 9 (lû mmol) of 1,8-diaza-
bicycloL 5.4.0~ undec-7-ene in 25 ml of anbydrous dioxane, tile reaction
mixture is stirred at 3û to 35 C for lû hours, -tben poured into 200 ml
of water. After adjusting -tbe pH value to 6.5 tbe precipitate is filtered,
dried and t~len recrystallized from a mixture of chloroform and hexane to
give 1.59 9 (37.6 %) of the title compound, m.p.: 124 to 126 C.
Example 5
Preparation of 3-(4,6-dimethylpyrimidin-2-yl)-3-methoxymethyl-1-(2-methoxy-
carbonylp~leny].sulfonyl)urea (compound No. 5)
A mixture containing 4.3 9 (20 mmol) of 2-methoxycarbonylbenzene-
sulfonyl chloride, 3.67 9 (22 mmol) of 4,6-dimethyl-2-(methoxymethyl)-
aminopyrimidine and 3.24 9 (40 mmol) of potassium cyanate in 30 ml of
anhydrous acetonitrile is heated a-t 81 C under vigorous stirring for
2 hours. After filtering off the precipitated inorganic salt, the filtrate
is evaporated, the residue is resuspended in 3û ml of water, t~len the
precipitate filtered off is dried and recrystallized from a mixture of
ethyl acetate and diethyl ether to give the title compound in a yield
of 5.4 9 (66.2 %), m.p.: 133-135 C.
3û The compounds listed in Table I are prepared similarly as described
in t~le above Examples.
- 13 - 23305-11aO
Table I
Compounds of tbe general formula (I), wherein X = oxygen
Compound Rl R2 R3 R4 R5 o
No. C
Cl ~I C~-~3 CH3 C~3 CH 126-B
2 Cl 1-1 C~H5 C113 CH3 CH llB-9
3 Cl H C~13 C~-13 CH30 N 130-5
4 Cl H C2H5 C~13 C~130 N 123-4
COOCH3 H CH3 Cil3 CH3 CH 133-5
6 COOCH3 H C2H5 C~3 CH3 Ul 124-6
7 COOCH3 H CH3 CH3 CH30 N 112-5
8 COOCH3 H C2H5 CH3 CH30 N 105-6
9 COOCH3 H C4H9 CH3 CH30 N 100-2
15 10 COOCH H i-C H9 CH3 ~H30 N 103-4
11 H H C 3 3 CH30 N 125-7
Example 6
Preparation of a wettable powder (10 WP)
Components weigbed in: g
Active ingredient prepared according to Example 2 20
Silicate carrier (Zeolex 444) BO
Siliceous eartb (diatomaceous eart~) BO
25 Sodium alipbatic sulfonate wetting agent (Netzer IS) 4
Cresol formaldebyde sulfonate dispersing agent
(Oispergiermittel 1494 ) 6
Sulfite liquor powder (Borresperse NA) 10
The powder mixture is crusbed in a laboratory ball mill for 30
30 minutes, tben ground to fine particles in an /~lpine 63 C-type laboratory
contraplex mill at a speed degree of 70. Tbe tbus obtained wettable
powder composition contains tl~e compound No. 4 of Table I as active
ingredient in an amoun-t of 10 % by weigl~t.
Floatability (ln 1 % concentration): ~6.5 %.
Wet sieve residue (on a sieve of DIN lû): 0.27 %.
Example 7
Preparation of a wettable powder (50 WP)
~,,`
~'
- l4 - 23305-1180
Components weig~ed in: ~
Active ingredient prepared according to Example 3 100
Synt~etic si]ica-te carrier (Zeolex 444) 30
Siliceous eart~ 50
Sodium alipbalic sulfonate (Ne-tzer IS) 4
Cresol-formaldellydesulfona-te 6
Sulfite liquor powder lO
T~e process described in Example 6 is followed.
The tbus obtained wettable powoer composition contains tbr
compound No. 2 of Table I as active ingredient in an amount of 5û %
by weigbt.
Floatability (in l % concentration): 82.9 %.
Wet sieve resioue (on a sieve of DIN lO0): 0.43 %.
Example 8
Preparation of a wettable powder (85 l~P)
Components weig~led in: g
Active ingredient prepared according to Example 4 17û
Syntbetic silica carrier (Sipr rnat 50 S) 20
Sodium oleyl met~lyl laurate (Arkopon T plv.) 4
Cresol-formalde~lydesulfonate 6
Tbe process described in Example 6 is followed.
Tbe tbus obtained wettable powder composition contains tbe
compound No. 6 of Table I as active ingredient in an amount of 85 %
by weig~t.
Floatability (in l % concentration): 82.9 %.
Wet sieve residue (o~ a sieve of DIN lOû): 0.4~ %.
Example 9
Preparation of an emulsifiable concentrate (5 EC)
Componen-ts weiglled in:
Active ingredient prepared according to Example l 5
Xylene 70
Cyclo~lexanone 15
Calcium dodecylbenzenesulfonate (Emulsogen IP 400)
Fatty acid polyglycol ester (Emulsogen EL 40û) 2
,~ ' ,~ .
... .
- 15 ~ 0~353~9
The active ingredient is dissolved in the mixture of xylene
and cyclohexanone under stirring and 8 q of Emulsogen IP 400 as well
as 2 9 of Emulsogen EL 400 emulsifying agents are added. After homogeni-
zation the mixture is filtered.
T~e thus obtained emulsifiable concentrate contains the compound
No. 8 of Table I as active ingredient in an amount of 5 ~ by weig~t.
Emulsion stability (1 % concentrate in CIPAC A and CIPAC D water):
stable after 2 bours.
A reversible creamification is observed after 24 hours.
Example 10
Preparation of dusting powder (5 D)
Components weighed in: g
Talc 20
Silicate (Sipernat 50 S) 30
Active ingredient prepared according to Example 5 50
Limestone grist (M 10) 900
The mixture of the active ingredient prepared according to
Example 5 t~e synthetic silicate carrier and talc are crushed in a
laboratory ball mill then ground to fine particles in an Alpine 63
C-type laboratory contraplex mill. The grist is homooenized with lime-
stone grist in a laboratory powder mixer.
The thus obtained dusting powder contains the compound No. 5
of Table I as active ingredient in an amount of 5 % by weig~t.
Sieve residue (on a sieve of DIN 100): 0.12 %.
Example 11
Preparation of a suspension concentrate (5 FW)
Components weighed in: q
Active ingredient prepared according to Example 2 lû
Sunflower oil 168
Organophilic bentonite (Ivegel) 2
Polyet~ylene alkyl ether polyoxyethylene castor oil
et~oxylated fatty acids and sodium sulfosuccinate
(Sorpol 3815) 20
Z~-~13~3~9
T~e ac-tive ingredient sunflnwe3 oil organop~ilic bentonite
(Ivegel) and Sorpol 3815 emulsifying agent are weigbed into a laboratory
bead-mill. T~le suspension concentrate is ground toge-tber witb 65 % by
volume of ylass-bead load of 1.û to 1.5 mm in diameter at 775 rpm for
30 minutes.
The tbus obtained suspension concentrate contains -tbe compound
No. 4 of Table I as active ingredient in an amount of 5 % by weight.
Stability (1 % concentra-te in CIPAC and CIPAC D water): stable
after 30 minutes.
Example 12
Preparation of suspension concentrate (40 FW)
Components weigbed in: g
Active ingredient prepared according to Example 1 80
Synthetic silicate (Zeolex 444) 6
Sodium oleyl methyl tauride (Arkopon T plv.) 4
Nonylphenyl polyglycol e-ther (Arkopal N 100) 10
2 % Xanthan gum (Kelzen S) solution 10
Water 80
80 9 of the active ingredient prepared according to Example 1
synthetic silicate (Zeolex 444) sodium oleyl met~lyl tauride (Arkopon T)
and nonylphenyl polyglycol ether (Arkopal N 100) wetting dispersing
agent as well as 80 9 of wa-ter are weigbed in a laboratory bead-mill.
Aftcr homogeinization t~le suspension concentrate is ground together
with 70 % by volume of glass-bead load of 1.0 to 1.5 mm in diameter for
30 minutes. After grinding and separating from the load tbe mixture is
honlogenized with 20 9 of 2 % xanthan gum (Kelzen S) solution in an Ultra
turrax equipment under vigorous stirring.
The thus prepared suspension concentrate contains t~le compound
No. 8 of Table I as active ingredient in an amount of 40 % by weigbt.
F oatability (in 1 % concentration): 97.3 %
Particle size (below 10 /u): 91.6 %
~353~
- 17 -
Example 13
Preparation of water-dispersible granules (75 WDG)
Components weigbed in: g
Compound No. 3 of Table I as active ingredient 375
Synt~letic silicate carrier 55
Polyvinylpyrrolidone (K 30 product of BASF) 20
Alkylpbenol ether pbosphate (Atlox 533û product of ICI) 30
Mixture of polyalkylene glycol etber and polyme-thylene
alkylaryl ether (Atlox 4896 product of ICI) 20
Water 180
375 9 of compound No. 3 of Table I as active ingredient are
pre-crushed (pre-ground) with 55 9 of synthetic silicate carrier in
a laboratory ball mill tben the mixture is ground to fine particles
in an Alpine 63 C-type con-traplex mill at a speed degree of 80. The
granulating liquid is prepared by dissolving 20 9 of polyvinylpyrrolidone
30 9 of alkylphenol ether pbosphate and 20 9 of a mixture of polyalkylene
glycol ether and polymethylene alkylaryl ether in 18n ml of water. The
ground powder mixture is introduced to an FPG 0.5 type batch-operation
(periodically operated) granulating apparatus with fluidizing atomization
and brought into a fluidized state by regulating the air stream entering
with a temperature of 60 to 65 C. W~en the inlet temperature reacbes
30 C tbe evaporation of the granulating liquid is started under a
pressure of 1.5 bar and t~e volume of t~e air flow is increased depending
on the particle growth. After introducing tbe granulating liquid the
drying is continued until the outlet temperature reacbes 36 to 3R C.
T~e tbus prepared granules contain tbe compound No. 3 OL ï able I
as active ingredient in an amount of 75 % by weight.
Floatability (in 1 % concentration after 30 minutes): 93.7 %
Particle size between 0.2 and 1 mm: at least 75 %
Exaple 14
Preparation of water-dispersible granules (25 WDG)
- 18 - ~035~19
Cornponents weighed in: ~
Compound No. 7 of Table I as active ingredient 125
Syntbetic silicate carrier 250
Maltodextrin binding agent 75
Ligninsulfonate (Sorpol 9D 47 K product of Toko) 30
Polyoxyethylene polyall<ylaryl phenyl et~er sulfate (Sorpol
5096 product of Toko) 20
The compound No. 7 of Table I as active ingredient syntnetic
ln silicate carrier maltodextrin binding agent as well as ligninsulfonate
(Sorpol 90 47 K product of Toko) and polyoxyetllylene polyalkylaryl pbenyl
sulfate (Sorpol 5096 product of Toko) tenside are weighed in a laboratory
ball mill. After grinding for 30 minutes the powder mixture is ground
to fine particles in an Alpine 63 C-type contraplex mill. The grist is
introduced into an FPG 0.5-type batch-operation (periodically operated)
fluidization apparatus with fluidizing atomization and brought into a
fluidized state by regulating the air stream baving an inlet temperature
of about 60 C.
The granulation is carried out by atomized water when the outlet
temperature reacbes 28 to 30 C. After obtaining the desired particle
size of about 0.5 mm the atomization of the water is stopped and the
drying is continued until the outlet temperature reaches 36 to 38 C.
The thus prepared granules contain t~le compound No. 7 of the
Table I as active ingredient in an amount of 25 % by weight.
Floatability (in 1 % concentration after 30 minutes): 91.85 %
Particle size between 0.2 and 1.0 mm: at least 95 %.
Example 15
Preparation of a suspension concentrate (14.5 FW)
Components weighed in: g
Compound No. 8 of Table I as active ingredient 2
DKA-24 (N-dichloroacetyl-N-allylglycine N -allylamide)
as antidote 12.5
Zeolex 444 carrier 11.6
Monoethylene glycol 15
Arkopon T wetting agent 3
Arkopol N-090 (nonylphen~l polyglyGol etber)
dispersing agent 6
- 19 ~ 353~9
~later 39 9 9
2 % by weight xantban gum solution as tbickening
agen-t 10
Tbe suspension concentra-te containing the active ingredient
(compound No. 8 of Table I) and t~le DKA-24 antidote in a total amount
of 14.5 ~ by weight in a weig~t ratio of 1:6.25 is prepared as described
in Example 12.
Example 16
Preparation of a suspension concentra-te (27.0 FW)
Components weighed in: g
Compouno No. 8 of Table I as active ingredient 2
R-25788 (N N-diallyl-dichloroacetamide) as antidote 25
Zeolex 444 carrier 5
Monoethyleneglycol 10
Arkopon T wetting agent 2.5
Arkopol N-090 dispersing agent 5.5
~ater 42
2 % by weight xanthan gum solu-tion thickening agent 8
The process described in Example 12 is followed to obtain a
suspension concentrate con-taining the active ingredient (compound No. a
of Table I) and the R-25788 antidote in a total amount of 27 % by weight
in a weight ratio of 1:12.5.
Example 17
Preparation of a wettable powder (20.25 ~P)
Components weig~ed in: g _
Compound No. 4 of Table I as active ingredient 1.5
AD-67 (N-dic~loroacetyl-l-oxa-4-azaspiro/4.57decane)
as antidote 18.75
Zeolex 444 carrier 34.75
Siliceous (diatomaceous) eart~ 33.0
Netzer IS wetting agent 3
Dispergiermittel 1494 dispersing agent 4
~3~33~1L9
- 2U -
Sulfite liquor powder clispersing 2gent 5 9
Tbe prncess described in Example 6 is followed to obtain a wettable
powder composition containing tbe active ingredient (No. 4 of Table I)
and tbe AD-67 antidote in a total amount of 20.25 % by weight in a weight
ratio of 1:12.5.
Example lS
Preparation of water-dispersible granules (13.5 WDG)
Components weig~ed in: g
Compound No. 6 of Table I as active in~redient
CGA-92194 LN-(1 3-dioxolan-2-ylmethoxy)imino-benzene-
acetonitrile7 as antidote 12.5
Synthetic silicate carrier 61.5
Maltodextrin binding agent 15
Ligninsulfonate (Sorpol 90 47 K) 6
Polyoxyethylene polyalkylaryl phenyl ether sulfate
(Sorpol 5096) 4
Tl~e process described in Example 14 is followed to obtain water-
dispersible granules containing -the active ingredient (compound No. 6
of Table I) and t~e antidote (CGA-92194) in a total amount of 13.5 %
by weight in a weight ratio of 1:12.5.
Example 19
Preparation of a wettable powoer (51.2 WP)
Components weiyhed in:
Compound No. 2 of Table I as active ingredient 20
DKA-24 as antidote 31.2
Zeolex 444 carrier 14.4
Siliceous eart~ carrier 24.4
Netzer IS wetting agent 2
Cresol-formaldehydesulfonate 3
Sulfite liquor powder dispersing agent 5
Tl~e process described in Example 6 is followed to obtain a wettable
powder composition containing the active ingredient (compound No. 2 of
- 21 - ~d~3~3~3
rable I) and -tbe antidote (DKA-24) in a total amount of 51.2 % by weig~t
in a weigllt ratio of 1:1.56.
Example 20
Preparation of a wettable powder (24.75 WP)
Components weig~ed in: g
Compound No. 2 of Table 1 as active ingredient 6
MG-191 (2--dic~loromethyl-2-met~yl-1 3-dioxolane) as
antidote lB.75
Zeolex 444 carrier 33
Siliceous earth carrier 32.25
Netzer IS wetting ayent 2
Dispergiermi-ttel 1494 dispersing agent 3
Sulfite liquor powder dispersing agent 5
The process described in Example 6 is followed to give a wettable
powder composition containing the active ingredient (compound No. 2 of
Table I) and the antidote (MG-191) in a total amount of 24.75 % by weight
in a weight ratio of 1:3.125.
Example 21
Preparation of a dusting powder (5.2 D)
Components weighed in: __~__
Compound No. 5 of Table I as active ingredient 2
AD-67 as antidote 50
Talc 19
Synt~letic silicate carrier (Sipernat 50 S)29
Limestone grist (M 10-type) 900
The process described in Example 10 is followed to give a dusting
powder containing the ac-tive ingredient (compound No. 5 of Table I) and
the antidote (AD-67) in a total amount of 5.2 % by weight in a weigh-t
ratio of 1:25.
- 22 - ~0353~9
Example 22
Preparation of a we-ttable powder (21 WF)
Components weighed in: g
Compound No. 6 of Table I as active ingredient
nKA-24 as antidote 20
Zeolex 444 carrier 30
Siliceous ear-t~ carrier 39.5
Dispergiermittel 1494 dispersiny agent 3
Netzer IS wetting agent 2
Su1fite liquor powder 4.5
The process described in Example 6 is followed to obtain a wettable
powder containing the active ingredient (compound No. 6 of Table I) and
15 the antidote (DKA-24) in a total amount of 21 % by weight in a weight
ratio of 1:20.
Example 23
Preparation of a dus-ting powder (6 D)
Components weighed in: g
Compound No. 4 of Table I as active ingredien-t 10
DKA-24 as antidote 50
Talc 15
Synthetic silicate carrier (Sipernat 50 S) 25
Limestone grist (M 10-type) 900
The process described in Example 10 is followed to give a dusting
powder composition containing -t~le active ingredient (compound No. 4 of
Table I) and the antidote (DKA-24) in a total amount of 6 % by weight
in a weight ratio of 1:5.
Example 24
Preparation of a suspension concentrate (22 FW)
- 23 - 23305-1180
Components weigbed in: g
Compound No. 5 of Table I as ac-tive in~redient 2
DKA-24 as antidote 20
Zeolex 444 carrier 6
Arkopon T wetting agen-t 4
Arlopol N-lO0 wetting agent 10
2 % by weigl~-t xantban gum solution as t~ickening
agent lû
Water 4
Tl~e process describrd in Example 12 is followed to give a
suspension concentrate containinr~ tbe active ingredient (compound No. 5
of Table I) and tbe an-tido-te (DKA-24) in a -total amount of 22 % by weig~t
in a weigbt ratio of l:lû.
Example 25
Greenhouse tes-t of tne postemergent effect (effect on emerged plants)
Tbe seeds of tlle test plants were sowed into a mixture containing
1/3 part of sand l/3 part of clay and 1/3 part of floresca cllernozem
soil placed in 200 ml plastic bottles containing 4 ~loles at tlleir bottom.
After sowing tbe soil and tbe smalT~ plants respectively emerged
were daily sprinl<led at a temperature of 20 to 26 C under natural illumi-
nation supplemented Wit~l an illumination of 4 bours. Tne lû WP composition
prepared frorn tne compounds Nos. 3 5 6 7 and ~ respectively of Table I
according to Example 6 were sprayed by using lûOû litres per nectare of
water under a pressure of 4 bar onto tbe 2 to 4-leaf plants by using a
greenbouse sprayer.
T~le damages (injuries) of t~e test plants were evaluated in tl\e
2nd and 4tl~ weeks following t~e spraying. T~le damages of tne various
test plants are given as percentape; û % means no damage and lûû % means
total perisi~ment.
Tl~e results are summarized in Table II. It can be seen t~at
tl~e compositions according to tl~e invention possess a good berbicidal
effect and a favourable selectivity against tl~e cultivated plants. Tbe
compounds Nos. 3 7 and 0 proved to be particularly effective.
In tbe first column of Table II and t~e following Tables t~e
Roman numeral I refers to Tablr- I and tl~e .~rabic numeral refers to tbe
.
- 24 ~ 353~9
number of the compound in Table I.
Table II
Postemeroent effect
Compound No. Dose Peris~lment of the test plants (%)
(in Table I) g/~la ORYS STEM ECHC GALA MATI ZEAM HELA GLYM TRIE
-
I/3 270 30 10080 100 100 20 100 80 0
0 10060 80 100 0 100 30 0
0 100 0 40 90 0 40 30 0
0 100 0 30 30 0 0 0 0
I/5 270 100 100100 80 100100 100 100 100
I/6 270 90 100100 35 70 100 100 80 100
100100 30 50 90 100 50 90
I/7 270 10 100100 60 100 10 100 70 0
100100 50 100 10 100 60 0
0 10020 25 90 0 40 0 0
0 100 0 0 30 0 40 0 0
I/8 270 20 100100 70 100100 100 90 0
10090 50 100 20 100 60 0
0 10030 40 90 10 100 40 0
0 100 0 30 90 0 70 10 0
25 Abbreviations in Table II:
ORYS Oryza sativa STEM Stellaria media
ECHC Echinochloa c.g. GALA Galiurn aparine
MATI Matricana inodor ZEAM Zea mays
HELA Helianthus annuus GLYM Glycine max
TRIE Triticum aesticum
- 25 - ~03~3~9
_xample 26
Greenhouse test of tbe preemergent effect
Tbe seeds of tbe test plan-ts were sowed in 3 repetitions into
a medium-impermeable forest soil placed in a bottom-perforated paper
box of 7 cm in ~leig~t wit~l a footin~q area of 200 cm2. T~le boxes of t~le
separate treatmen-ts were placed on plas-tic trays equipped with a wetting
clotb. After sowing before tbe emergence of tbe plants the 10 WP composi-
tion prepared from compound No. 7 of Table I according to Example 6 was
applied with a water amount corresponding to 1200 litres per hectare onto
tbe soil surface in -the boxes by using a greenbouse microsprayer. The
supplementation wi-th water was ensured by maintaining -the wetting cloth
in a wet state. The experiment was carried ou-t at a temperature of 20 to
28 C under natural illumination supplemented with an illumination of 4
bours. The herbicidal effect of the compound No. 7 of Table I was evaluated
in the 4th and 6th weeks after spraying. The results are summarized in
Table III.
Table III
20Preemergent effect
Compound No. Dose Perishment of test plants (%)
(in Table I) g/~a Solanum Digitaria ECHC STEM TRIE
mlgrum adans
I/7 250 70 50 20 100 0
~51000 100 60 40 100 0
Example 27
Investigation of the effect on cultivated plants in -tbe plant-cultivating
chamber
400 9 of field top soil each (witb a pH value of 6.5 permeability
of 50 and bumus content of 1.4 %) were weighed in plastic cul-tivating
bottles of 0.8 dm2 surface each lined witb PVC foil. Onto tbis soil 10
seeds of Pi-3737 maize each 100 seeds of streaked sunflower "IREG" each.
50 seeds of SA-114 sorghum each and 1 9 of red millet eac~ were sowed.
After covering t~e seeds with 100 9 of soil each the treatments
were carried out. In tbe course of these treatments 10 20 40 60 160
--2~-- ~03S3~9
or 320 respec-tively 9 per bectare doses of water-dispersible granules
(75 ~JDG) prepared according to Example 13 from the compounds Nos. 2 4
6 and 8 respectively as well as of Ally Tell Granstar Glean
Logran and SL-950 respectively control. compositions were applied in
four repe-titions. Tbe treated soil surface was again covered witb
additional lO0 9 of soil in eacb bo-ttle.
T~e bottles were placed in plant-cultivating c~lambers, sprinkled
daily up to t~eir water capacity and cultivated under HGMF/D 400 suns~line-
subsituting lamps with a daily illumunaion period of 16 hnurs. T~le ex-
periment was evaluated on -t~le lOt~l day after sowing wben t~e green weight
of sunflower sorg~lum and millet as well as the green weig~lt and shoot
lengtb of maize were determined. The results obtained are summarized in
Table IV with the designation Sowing I .
Tbe seeds of maize sunflower and millet were repeatedly sowed
6 times into the soil of the experiment evaluated. The results of evalua-
tion of these latter experiments are summarized in Tables V to IX witb
the designations Sowing II, III IV V, VI .
From sowing I up to the evaluation of sowing VI 76 days elapsed.
~Q353~9
- 27 -
O C~ O `~ o o
o ~
o ~ c~ o ~c~ O OCO
~O
o
3 0 C1~ ~ O ~ (-
4-~ O
~ ~0 ~ O O
~, ~ ~~ r~ ~~
Ll~ ~ ~1 ~ r-- c~ ~ o Ico u~ ~~8 ~ O
O 3 H r--I.--I r-l .--I
~ O ~ o o ~u~ OLr~
3 ~ ~ ~1
O 1
C
U~ ~ +~
~ C C
~O -- i! ~Cr~ Lr~ ~ ~ Or--~ ~O
~C ~ )-I O I ~C~
H O~ O C 1
~1 tl) Q)
O t~ ~
E ~ ~ ~t ~I-'\ ~C~ ~~--I ~ G~ ~~
r- c 1~ o
~ O r~
r~ ~ 4~
r~ ~ O
~1 t~ U~l O C~ O O ~O ~ O ~ OC~
O C~ I--t
> N
. ~ ~ E O a~ 3C~ 0
~ tH ~ 1~ J r~r~~
C t-~
C
1-1 E _ ~ r-l O1~ ~ r~
co +~., ~ C~l ~r~ o o ~-- 1~ oco ~ ~D
~Q) 3 r~
~O Q~ O C~ ~ ~ ~ o ~o
o _ ~1 ~1 IJ~ ~ ~ OCO ~ C;~
c
E IJ) rJ
r C C O~
> ~ >~ C~ . ~ U~ (,1
C~ r ~t ~ L) rC l) I
I-- ~I 1--1H H H H ~ ~J ~n ~
20353~L~
- 2~3 -
Il) O ;~ ,u~I r-l N~0 r-l 1~ r-l CD
C O ~ ~D r-lI ~ I r ~_ O
a H r-l
O ~ r~
tD ~ ;a`(~ r-l ~ r~
~ ~1 r-l H H r~l r~
> o3
r 1~
C ~ I U~ ~D~ O r-l ~) H O Lr~ H
~ r-l r~ I r-l r-l r~
tl) CH
+J O r~ r~
. r-l r-lr-l r-1 r-l
r~
Q~ 3 1~ O a~ ~ ~
+~ C O C~ ~\ ~\ r-l~I CD r-l ~
4-1 Q) r-l r-lr-1 r-l r~l r-l r-l r-1 r I
~ r~
t,
H4-1 H Cl~
r-l ~ +~
_O C C C
O O r1 O ~\ ~ ~~ U~ ~O H r-l
~I r-~ r-i~ ~ r~
tL) ~\
O E ~ ~
C C ~1:) C
r1 ~1 ~ r1 O~C) C~ C~l ~1 ~ ~O C~ U~ I~ r-l
+~ O ~ Q) ri Nr-l ~O ~1 ~O 1~ ~ IJ~ O~ ~~
U~ C r~
C ~ ~
.rt C tl~ O
O ~ ~ O 1`-- Nr-- O C~ U~ ~ ;_ ~ C~
r-l O O CO ~O ~ O~ ~t ~ 1~\ ~o U~
r~l ~)
E O ~Ll~ C~ ~)rlU~ 1--
4-1 O ~O Cl:) r-l ~O~C~ C~ U~
~ ~1)
Cl~ ~
.~ .ri
O ~O r~l~I r-1 ~r~ O ~ C~
C ~ ~-- N.'\ ~O ~O~O ~:) r-l ~ 1-- r~
tL) O
.) o c~ o c~ c~ ~ o r~ C~
tl~ r-l ~I ~O r-1~O1~ ~ ~O O~ C~J ~\
+~ 3
q~ C
O
C ~ U)
r-l E L
t~ >~ t~r-lG~ C
~ O r-l C~ r~l I
O I_ r-l ~ ~~ ~ `~ r-1~) J L l
C~ H Hi--1 H O ~ U) t_ ) J
- 29 - ~0353~9
O1-- L~~\1 O ~I C~ D L~ t
r~ o a~ o~o r~
C L~ 1-- .--I O C~ L'~
~0
L~ O 1.'~ Lr~ ~ O ~D ~S)
_ ~~ 1-- 0 ~ ~ L'`\ ~ 1
C
[~ ' OC:lL'~ ~ O ~O ~ ~ ~ 1-- 0 a~
~3 L'~ ~CD .--I .--I ~I ~_1 1:`1 ~ IV`\ ~\
C ~ ,~
1_1 t~ OL^~ G~ O ~ CZ) C~ G~ ~ O
C .C ~D ~ O ~ 1 0 0 r~l
'3
~1) o -I O O ~ r~ I O 1- C~
I_ O O L~O G~
o~ or~ ~ O r~ O ~ 1-- r-
a:~ c~ ~ O ~ o .~
E
~Ho L'~ l Q I-- ~ O O
O ~ o o a ~ O ~
.~
3 Or~ ~
C ~1 ~ (~I o o o~ O
O~
U3 C O +~
C >~ ~ ~ ~ C~ C
E ~I CO ~ l L'~
r-l ~ ~ ~ ~ ~ r-l a) o ~J
- 30 - ~353~L9
o r~ o ~ 0 1 o c~
~ ,~ O O O\ ~ I ~D ~ ~
~ ,~
O r~ ~C~ O ~ ~J ~ C~ L
E ~o r1 1~~ ~ C ~
_
t~n
~ o ~ u~
o ~ ~ r~
_
.
O ~ ~Lr~ ~O O ~ Lr~ ;:1 . 1~ r~
O.) ::t ~O C ) G~
tO O I~~ Lr~ r-l ~ r-l ~O ~ ~D ;~ I~
_ C~ ~)C~ Lr~ O (D t~
1,' C
C ~ O Lr~ ~ ~ I~ CO O ~ r~
r~ ~r~l O C~ G~ O I CD I ~
r~ r~
O ~H
O ~~ L~ C I Lr~
3 0 ~ 1~r Lr~ 1 ~ ; `~O ~D Lr~ G~
0~
U~ O N I ~1 O G~ r~t ¢l ~ ~ ~ L''\
~ 1 ~ C~l1-- I-- L'~ Lr~ L~ 1`-- O ~
.~
3 0 ) C~
O L^~ ~~J r--l ~ ~ ~ 1-- O~ CO Lr~
Q) r~ 1 L'~Lr~ r~ ~ Lr~ G~
~1 ,-1
C L'~
~1 r~ ~~0 t~l L'~ G) r-J CO I n~
l_ , ~ O ~
- 3i -- 2~353~L~
E
o o ~ ~ ~ r~
~ r ~ r~ ~ I o L'~ r~
4o
~7 0 L"\ ~ ~O ~ r~ o r~ o r-
_ ~ ~ r-
3 o
~ ~o r- ~ ~ C3r~ ~ ~o L~
~1 0 CS) ~ ~ L'`\ O L^~ ~ ~-1 ~D C~l CO
c~ ~ r~ co ~D ~~o c~
O L'\ L'~ L'~ r~ ~~ ~~
~ c~ o r~
h O O~ O ~I O ~ C~ O ~ ~t
~1 ~ O~ )O G~ r-l L--O O
O
~ ~ o ~ ~ ~o 0 ~ ~ r-~ ~ ~ ~
U~ ~ ~I ~D ~.0 r-- ~ c~ ~ ~ L--~ ~ ~O L'~
C~
4~ ~
~ O O ~ r~ ~o ~ ~~ ~o ~ r-
~ C ~ ~ L'^\ ~ L'~ O L' \ L'`~ L'~ L^~
H_ ~ r~l ,_1
~ ~
c3 O~ r~ ~o r~ o r- ,~ G~ o r~ r~ O
. ~1 C C ~:1 ~L^~ O L''\ ~ r-- ~o O H
H O ~ 1 r-l H ~--1
Q~ ~ h ~ L~ :~ r- o~o ~ r- ~ ~ O
,o o ~ ~ r~ or~ L'~
o ~3 r~ r~ ~ ~ r~ ~ o
a)
tY U) O G~ ~ ~O ~ r~ ~ ~ ~ ~ r~ r-
Il) O ~ C:) O O\ O o L^~ ~I Lr~ C~ L'~
E
r~ L'~
3 r- ~o ~ L^~ I~ r- ~ L~ r-
C ~ r~ r- L~ L~ r-
~ c~ m ~ r~ OO~
~I ~ O ~ O L~ O r~ L~ ~ Cl~ O
~ O L'~ r- ~ ~ r~ Lr~ 0 0 o
C C O +.
~) .~ L
Z0~53~L9
~ 32 -
r o ~ o~ ~ ~ ~ r ~ ~ c~ r
t-l O ~D O ~ N O r~ r-- o
u~ r~
~> C~L'~ ~ r o r~
r~
O~ O G~ D O O
I~ U~ o r-- o
o ~ o r" ~ : ~ ,~ ~ ~ ~ ,~
~I C~ O C~ O ~ G~ 0
(~ ~ ~ r~
I Lr~ o ~ o ~ ca r~ c~
r- o ~ 1~ I o c~
~ .~
Q) ~ I l'~ o u~ ~ O O O C~
~ ~ I ~ L~
4~ I
~ ~ ~ I o
H Ul ~ ~ r~ ~ L--l ~ LJ~ O
Hr1 O ~ I ~ O 0r~ r--H~1 ) ~0 O H O
~ 3 ~ ~f ¦ ~ L~~0 L'~C~1-- 1~ ~ L'~
~o 4~ 1~ ~ ¦r-- L'\~IL'~ r~ r~ r-- ~D
m o 3 -,~ I ~ L'~ I_ L~r ~ r- ~D L" L-~ r- 1-
,~ ~ r.~ ~ or~ o ~ r-- ~ L~
~ Ll 4--1~1 ~J ~ 1L'`~ ) L'~
U`~ C~ O
Q~ U~ O ~ ~ L'~, L~L'~ r~ r~`J ~ ~ ~ ) ~
o ~ ~2 c~~ r--L~ L~ r-- ~ L~ r--
r~ ~ ~ ~ ~ I r-- I ~ ~
E r~~ r-- ~ r-- ~ c~ ~ , r--
~ ~D~ ~O ~OO 1--,~ L" O 11 0
r~ ~ ~ L'~ ~ ~ ~ r-- L'~ O C::~
3 0 ~ ~,o Q L'~r--r'~ ~
Q~ r~ r~
;~ o r~ ~ r-- r-- rr~ r~ ~ c~ L'~ ~ r
r~l ~ r-- o ~ ~ r-- ~ r~
~_~ Lr~ L~r~ ~ ~ ~ L~ C~ r-
C C o ~
~ C') ~ ~1 L'~ ro r m Lr\ ~n
(1~ ~1 ~~ ~ ~ ~r~ o J ~
I_ Cl~ H1~1~ J t,r) t~)
203~3~9
E o c~ ~ r-- .
L'~ o o~
r~
t~
~n
4~ ~D O ~ ~ L~ 0 N Co~
~I r~
O) o ~ ~ ~ o ~ r~ ~ ~L~ ~o
CDC~ o ~r~ ~ c~ o r~ o~
3 ~ ~ ~ ~ ~
C o o ~ o r- O r- r- rO r~ r-
Q~
t~ ~ o r- ~~o o r~ ~ ~ r~
_ N ~ ~ Q ~I OL~ r- 3 r- a~
o ~ ,~ ~o o ~ ~r,~ r-
o r- ~ ~ ~ r r~ ~ ~
I
~ ~ o
3 ~ ~ ~ ~ ~ ~ ~ r- ~ ~ r~ ~ ~
O ~ r~ ~ r- r- ~ ~ ~ ~ ~ r- o
O) ~ ~
H ~ C ~1 0 ~D ~t ~`D L'~ C~l ~J r-- o ~ O
H r~n U~ rD ~ ~ r- ~ r- o r~ ~ r~ r- O ~
~ .~ ~ .> ~ ~ ~ ~
D O O ~ O r,~ o ~ O~ ,~ ~rJ~ rD
O U) ~ ~ ~ O ~ r ~ r~ ~ r- ~ r,~
,~ rD O ~O O O ~ ~ ~,~ r- r~ r~ O r~
rD ~ ~ ~ r,~ r~ ~ ~ ~ r~ r r- n~
rD O ~ r,~ ~ ~ ~ r~r~ ~ O
U~ rD rD ~ r- r~ ~ r,~ o O r,~ r r-- r~ r-
~ ~ U~
r t~ O
O r,~ ~D r- ~ rJ~ r- O ~ r~
~ 1-- r~ r,^~ ~ r~ O rD r- r~
~ ~1
O
rD r.~l ~ ~ ~ ~ ~ ~ r~ r.~l ~ r-
N 1~ 1~ ~D r~ ~ rJ~ ~ ~ r,~ r~ o~ r~
E O
~D O r- ~ r- r,~ r~ r,~ ~ ~ r- r~
O ~ r~ ~ ~ r~ r~ r.~l o o ~ r~
O ~ r- ~ r.~l r- r- r~ ~o O
~ ~ r- r,~ r,~ r~ r~ r~ u~ C~ o O
.,
r3 o ~O ~ ~ ~ r- r- r~ r~ ~ r~ rD
r- r~ G~ o ~\ o r- rD o r,~r.~
Q~ ~ ~
rD r~l r- r~ r- ~ rD rJ~ rD o r ~ O
O ~o r,~ r~ rJ~
U~ r- o ~ r~ r~ ~ rD rr~ r,~ c~ r~
ro
D ~ r ~~o r r ~ ~ r~ c
C H H H H H ~ ~ J V~ t~
;~0353~
- 34 -
E o r~ O ~ ~
O o ~ c~ o ~ ~ m ~D
O co ~ r~ O G~ O ~ O
o
~ O ~ ~ o ~u~ o
. o c~ c~ co ~ ( ~ ~ o 1- co
3 C~ O ~ C\ u~
~1) ~ O r~ I O 1~ O~ O
r~ o ~ r~ c~
O ~ O ~ ~ C~ ~ O C~
O ~ Ir~ O~ O ~ U~ O
r-l ~ O ~ O O ~ 1~1 0 0 ~ ~1
H ~1 ~
:~ tL) O N ~1 ~ O G~ ~ H
tO 3 ~ ~ U~ C~ ~ ) CO
X '3 C 'D
H O O~1) 0, ~--I 2~ H C~ O
2~ U~U~ ~D C~ ~ c~ 0
~ 4~ ~ C
5 O O.~1
I_ ~ ~ C~ ~ D
.~) ~ O
U~ ~C~ ~ ~ o ~ v~ ~ ~ u~ ~ r~ ~ ~
3 1-- o o ~ ~ ~ ~ o o
Q~ ' O ~ ~ ~ 0 1
r~ o r~
q~
o
~ ~ o ~ ~ c~ ~ ~ o ~ ~
c~ O ~ ~
o u~ ~ ~ u~ ~ c~ ~ c~ ~ 1--
u~ c~ m v~ ~ ~ ~ ~ O O
N ~1 ,~
E~D ,--I ~0 .-1 Q r~ 0 O
4~ \0 ~ ~ C~ ~ ~ ~ ~ cr~
O ~ ~ ~
o ~ ~ ~ r~
c: r-- o ~ c~ ~ o ~ o~
3 o c~ o o o
~ o ~ o ~ ~ r~\ ~ o ~
t~ ~ ~ ~ G~ O O ~ O C~ C~ O O
~c ~ O cr~ I~ 1- U~
E ~
c o ~
I_ C 1--~ H I--I H H ~ I-- O ~r) ~
- 35 - ~305-1180
Ex~Qle 2.~
Text of the herbicldal act1vl~y in a fie~d experiment carried out
in a winter-wheat culture
A postemergent weed control experiment was performed on
winter-wheat in parcels of 10 m2 each near -to the river Sebes-
Koros.
The test was carried out on a meadow-clay soil type
having pH value of 6.3 contailling 3.4 % of oryanic material.
Winter-wheat was used as yreen crop. The seeds were sowed on 24th
October in a well-processed dry seed-bed to a depth of 6 to 7 cm
with a row distance formed by direct sowing.
The test was performed postemergently on 20th April.
The weed species and their development stage at the time of
treatment were: burweed (Galium aparine, abbreviated GALAP) in 4
to 6-leaf-whorl stage; red blindnettle (Lamium purpureum, LAMPU)
in flowering stage; sericaceous blindnettle (Lamium amplexicaule,
LAMAM) meadow-veronica (Veronica arvensis, VERA~) in buddiny
period at the beginning of flowering; medicinal earth-gall
(Fumaria officinalis, FUMOF) in the 6-leaf staye; chickweed
(Stellaria media, STEME) in the flowering state; eastern larkspur
(Consolida orientalis, CONOR) of 10 cm in height, in the stem-
forming period; white goosefoot (Chenopodium album, CHEAL) in 2 to
6-leaf stage; fallow bindweed (Bilderdyckia convolvulus, BILCO) in
a 2 to 3-leaf stage. On establishiny the experiment a parcel-
spraying machine was used with propane-butane gas pressure. Doses
of 10 to 80 g of active ingredient per hectare of the compounds
Nos. 4, 5, 6 and 8r respectively, of Table I were applied in 500
litres per hectare of spray liquid in the form of 5 FW suspension
35cl -- 23305~ Q
concentrates formul.ated according to E~cample 11. The same doses
of I.ogran 75 DF and Granstar 75 L~E', respectively, were used for
comparisoll .
rrhe herbiciclal an(l phytotoxic effects of the treatments
carried out within these e~caminations were evaluated by usiny a
score (scale) from 0 (inactive) up to 100 (total perishment).
These evaluations were performed 4 times (on ~7th April, 4th May,
25th May and 13th June) in the vegetative period following the
treatment by measuring the number of spikes (piece/m2), spike-
length (cm) and the weight of 1000 grains (g). The results, which
are the average values of 4 repetitions, are summarized in Tables
X to XVI.
~...;~
~ - 36 ~ 3 3~
X O~n o Q O OO O O O ~ ~ O O C~ O O
~ .
~ t~ ~1 ~ ~1 1~ ~) Q O~ D ~ O ~ O O~ O
a ~ c~ Or~ ~) ~ o
~ ~ o u~ O u~ o r~ o
I ~1 u~ cr~ Ot~ ~ O
C~ .
Z O O O o o c~ o o ~ o ~ o ~ co r~ u~
u~ ~ I
o c~ o o o ~ ~ r~ o u~ r- o o o c~
, ~! ~ ~ ~ N N ~ C~l U~
'~ .
<5 ~ ~ ~ ~ ~ ~ ~ C~ ~ ~ ~ ~ O
O~ cr~ ~ ~ ~ ~ ~ r~ ~ ~ ~ ~ o~ o
X ~
1~1 ~ ~ o ~ o ~ o ~ o r~ ~o o
.~ J ~ G~ o
I ~
C O O ~ O O V~ U~ OO ~ ~ ~ O ~ ~ O
3 ~ o
~ ~ ~ ~ ~ o o o ~o~ c ) ~ L~ r~ c ~ ~ ~
C r~ `J ~ I~ I~~ L~
'O
' O ~ i~i H H i H H H j H 1--1 H i 1--~ H H ~
e H ~ ~ ~ H 1--1 H
E
~. c~
I_ ~ H H ~/
'~ - 3' - ~(33 533L9
X~\o o o o o o o o o U~ o o o ~,~ o U~ ~
o
~o
Y ~ ~ ~`I o ~ ~ ~ o cr~ o c~ o
_ ~ C~ cr~ _ ~ ~ o ~ o ~ U~ o
CD _ ~ ~
I ~ o ~ o ~D ~ ~ O
O
Z O O O O O o ,~ o o o u~
S O U~ ~ I 0 1-- O~ I O U~ O
I,LI O U~ l ~~. O
U) .~
\~ O U~ ~ o O ~ o ~o o ~ ~ U~ O U~
a~ .
~1 C~ ~ OO~ ~ Q ~ ~ ~ o
X ~
~ n
n o ~o ~D ~ o ~ ~ ~ o o u~ o u~ o
S ~ co ~ r~ ~ ~ ~ O ~ 0~ O
~: o u~ ~ ~o o ~ ~ o o~ ~ o o r~ r-- O
Cl: ~ O
C t~ ~ ~ o U~ t~l O ~ O~ ~-- O
C
4~ 0
~ H H H i H H H i H H H i H H H
i-- t~ H H H H 1--( H H H H H
~ ~ O O C~
O ~J
C . .
E
~ ~1 ~ ~ 1
~ 3~
~ ( ~ 3 5 ;3 ~3L 9
r ~ ~ u~ O O ~ ~ ~ ~? ~ O ~D ~u~ ~D O~
~ ~ o ou~ o
T U~ O .~ ~ O ~O ~`J ~O IJ~ ~O O ~ 1~ 1/'~ O~
~[ O O O O O O O O O O O O O O O O
O\o o ~ u~ ~ o ~ m ~ o ~ c~ ~ o u~ o
D :~: C Ir~ O O ~ u~ ~ O O ~1 ~ ~ ~ O
1~ t~ O O O O O ~ r~ O ~D O v~ t~
H ¦ I ~
~ 1 C ~ o o o o o ~ o
~: o o o o o ~ o o o ~ c~ o o r~
5
CI: O r~ o o o ~ O L~
O '~
t~ H ~ H ~ H H I ~ ~ H :~ H H H
E H 1--1 H H H )--~ ~ H H i--~ H
a) t~ o o o o
O~ ~ ~ ~ C~
E ~) ~ O
O H H H H
.," _ 39 . ~0353 `.
Y~ u~ o ~ ~~ r~ ~ ~o~ ~ O ~ ~ ~
~c ~ ~
~o
o~ o ~ ov~ ~ ~ o
U~ ~ ~ O ~ ~ ~ _
~-J ~ ~ ~ ~~ r~ o ~ o u~ O
I _ ~ u~ ~ ~ _ ~ ~ ~ r~ 1 t~l ~ O
O O O O O O O O O O O O O O O O O
I~J
o\ ~ O O O I O O O ~ O o O~ I O U~ ~ I
0~). .
~ ~o ~ o o ~ o ~ ~ ~ r~ ~ ~
H ¦--~ ~ ~~ ~ 1~ ~
XD C~O O O Oo "~ O o O ~ a:) ~ ~1 0 u~ O
D
o o o o o o o o o u~ o
O C~ O O o U~ o o o U~ - O O U~ ~ ~
J
J O O ~ O u~ O r~ o ~ ~ o ~ r~
C _ ~
c~ ~ H H H i H H H i H ~ H > H H H
O tll H H H ~I H H ~ H H ) I H H
E t~l
O t~ O o o o
O
O ~)
E H H H :~
Q)
~1 -- ~ O ~ ~633 5319
'X .~ o o o C~ o o o o o o o o o o o o
~,,
,~,
O ~ r~ ~O ~ CD r~\ ~o o~ O~ O~ O~ O ~ ~ `
~ ~ .~ C~ ~ ~ o~ o~ _ ~ o~ o ~ ~ o~ o
.
ct ~ O o o r~ ~o o o o ~ o o ~ o o o
.
Zo o o o o o o o o o o o o o U~ o o
O ~O ~o I o 0 r~ Iu~ u~ c~ I H c~ c~ I
I_ ~ o ~IQ ~ o~ o~
o\ ~,
._, ~0 o o o oo U~ ~ ~o ~ r~ C~ o U~ ~ o~ ~
Q~ ~ ~ ~~ ~ ~o u~
.~C
~ ~Y ~ ~ v~ O O~ O ~O ~O r` o ~ ~ o~ O
~~ ~ r~ o~ o~ oc~ O~ O
X i~
O o ~ ~o ~ ~ U~ o~ ~ _; o~ o ~ ~ O~ O
s ~ co o~ o~ . J o~ O ~ o~ O
~: o r~ o o ~ o ~o o o~ C~ o u~ u~ o~ o
~ ~ ,_ o~ ~ o~ O` ~ ` ~ `
C O~~ O O~ O ~ ~O~ C~ O~ O O rr\ o~ O
r~ o~ O~~ ~J o~ o r~ ~ o~ O
0~
1~ ~ H ~ ~; j H H ~ > I_ H ) ~ I i
.r~ ~D I ~ 1 H H ~ H l ~ ~ H H )
~ ~D O O O O
C~ D~
L~
E O o o O
~D C C C C
J J . J J
~ !; 1 ~S3~3
1 '' ' ~ ~uoo
Cl O
~ ~ O0~
cS ~O O C~ o o ~ 00 0
w o d o o o o o o o u~ o~ oo ~ o o
~ oo~ I r~
~ o U~ o ~ o U~ ~ ~o o ~ : oo C~ o
~ o ~ o ~o~l ~ o ~ o ~ O ~
~ ~I ~ o ~ c~ o o U~ ~ ~ o o ~ ~
3~ o ~o ~ ~ ~o C~ o
~ ~ ~ $ ~ ~ ~ o~
E ~ b b b b
U~ ~ ~o o
o~ o~.
. ~o o ~o ~o
~ 4
;~0353~9
- l~2
Table XVI
Results of examinations of the spikes
Treatment Dose Number of Spike-length ~lumber of Weight ofg/~la spikes/m2in cln grains/spike lOOO grains(g)
-
Untreated 5 ' 19,4 39,4
I/8 10 607 7,7 21,~ 4~,~
590 7,9 19,~ 41,4
620 8,2 22,0 40,9
BO 60q 7,B 20,9 40,0
I/4 . 10 620 7,B 21,6 40,0
609 7,9 20,6 41,2
619 7,~ 21,4 42,0
630 7,B 22,0 40,6
I/6 10 424 7,5 14,0 42,1
432 3,8 13,4 42,B
301 3,4 7,2 44,1
266 3,4 6,0 ~4,2
I/5 10 399 5,9 15,4 43,7
250 3,2 10,~ 44,4
: 40 248 2,8 7,0 43,9
. BO 261 3,4 6,1 44,a
Logran 75 WG 10 614 7,8 20,G 42,3
Logran 75 ~YG 20 645 7,9 21,4 40,U
Logran 75 WG 40 63B 8,3 21,4 42,4
Logran 75 IYG 80 641 7,9 22,0 41,3
Granstar 75 OF 10 600 7,8 20,B 40,0
Granstar 75 OF 20 59B ~4 , 3'3
Granstar 75 DF 48o 6629907~0D 21,0 41,3
_
- 43 - 2C)353~3
From -the results illustrated in l~ablesX to XVI it can clearly be
seen that a difference appeared between t~le effectivity of tbe compositions
according -to the invention and that of t~le commercially available composi-
tions used for comparison. Compositions containlng compounds Nos. 4 and
~ of Table I proved to be bighly effective by late postemergent treatments
on developed weeds in extremely strong infections. The selectivity against
cultivated plants of both compounds is also favourable. Cue to their hig~
phytotoxicity as well as weak even-tually unuseful weed control action
berbicide compositions containing tbe compounds Nos. 5 and 6 of Table I
are not suitable for weed control in a win-ter-wbeat culture.
Example 29
Investigation of tbe effect of antidoted compositions on cultivated
plants in a plant-cultiva-ting chamber
40 9 of field top soil each (with a pH value of 6.5 permeability
of 50 and humus content of 1.4 ~) were weighed in plastic cultivating
bottles of 0.8 dm2 surface each lined with PVC foil. Onto this soil 10
seeds of Pi-3737 maize each and 10 seeds of streaked sunflower IREG
each were sowed. The seeds were covered by 100 9 of soil then -the treat-
ments were carried out in 4 repetitions. In the course of these treatments
20 to 160 9 per hectare doses of active ingredients Nos. 2 4 5 6 or 8
respectively of Table I together with 250 to 500 9 per hectare of the
antidotes DKA-24 AD-67 R-25788 MG-191 or CGA-92194 respectively were
applied in the form of composi-tions formulated according to Examples 15
to 21. The treated soil surface was covered with additional 100 9 of
soil in each bottle.
The bottles were placed in a plant-cultivating chamber and
cultivated with a daily illumination period of 16 hours. The plants
were daily sprinkled up to their water capacity.
The experiment was evaluated by determining (measuring) tbe
shoot-length and green weight on tbe 13th day following the treatment.
Tbe results are summarized in Tables XVII to XIX.
-- /' 4 -- 2~3353~9
C~ o U~ CO ~ ~ ~ U~
~ o ~ ~ ~ a~
. ~ U~
¢ O ~D ~ ~ O~ ~ t-
c~ u~ ~
o ~ o ~
o ~ ~ ~ o ~ t_
U~
l ~
~: ~ CO ~ ~ ~ ~D
L) O U~ O O
~ ~J O .
c o I I I O ~ ~ a~
L~ r.~;
~1 ~
r- ~ O ~D ~ O ~ 0~ ~
~ u~ c I ~ a~ u~ o
c~ C 4~ ~D
~ 4~ ~ ~ ~ ~
x o $, c~ a~ a~ ~ ~ ~D co
~ ~ ~ C
~ 3 - ~ .
C ~o U) o L~ ~ ~ O
o~ ~L~ ~ ~ 0
. ~ . ,.
q~ q~ U~ ~ ~ ~ ~1 ~
~ ~, a~ 0 u~
C) ,0)
E 3 a~ .
c~ a~ ~ ~ 0
O C~ ~
0`~ O O O O O O
~ ~ N ~ CU
U~\
e H ~--I ~ H ~ O
.. /I J _ .
~(~3S3~9
o ~,L ~
~0 ~ 0 L~ ~
N .
~ 0~ r~ ~ t~ GL r~ C-
t)Lr~ ~_ 0 ~O 0 1~ .
b~
$ 0 ~o u~ co ~ G
~ o a ~ Lr Cl~
o~ ~ Ir~ ~ ~D N ~O
a~
lC~ I~ b~ . . .
Q~ O C-- O ~ O t~
~ c~ o a~ D 0
E c N
r- q_, ~ boO 0 ~0 ~ ~ C~ ~
~-- N 0 0 N ~ ~ 0
.~ ul t:n
H ~ ~ Na~ ~1 ~ N O
X O ~~ OC~ 0 GL G~ ~D C{~
u~
~D I~ ~ ~ o ~1 . ~ ~ ~ ~ ~o
,L_ ~~ N N ~ 0 N 0 L
U~ E
ObD 0(~ r--I C\l r~ Gl'
o 0 0 ~ C~ L'~ ~
4--1 cn ~_L
~ t~
c ~ I 0 r-- GL cr~ GL ~
~ Lr~
c~ > o3 N G~ GL
t~ ~
O ~ GGL C~ ~ N d-
c~C~ .
I ~ ~ ~ ~ ~}
~ H ~_~ ~ ~ ~ h
- 46 - 20353~L9
t a~ N
I O U~ C- ~ 0
. O ,~r~ ~ ~ ~ O
O , ~ Lr~ 0
o
~ N L~ L'~ C~ O
I--t h r-l O O L~`\ ~ tO Lr~
~1 1 0 U~ ~D ~J ~ G~ L~
3 c ~ N ~D a ~D o ~D u~
O Q)
C ~ 0 o ~ ~ t- t- o ~
Ul 0~ 0 O ~) 0 0 . ~ ~ L'~
CCl N
(~1 E I O t~ 0 lr~ Lr~ 0 r-l
x ~ otr:J N t-- 0 L~ 0 ~ ~)
X q-t+~ t-l
o o o O O N 0 t-- G~ O '.'
1_ ~ ~ 4-t ~ O t- 0 ~ 0
~H ~ ~5)
O 4-t ~! ~1 0 0 0 t-- t_
10 q-t ~
tl) ~ ,-
3 ~ Otl:) O U~ 0
o c~ ~ O , ~1 .
E g N ;0~, ~ 0 If~ ~ ~1
0~ ~
a) ~ c
> 4-t rt 0 a~ 0 N 0 ~1
o o ~ ~
C~,C o~
O O O O O O
~r N ~D N `'-
~ .,
~ ` ~ ~ U~
Q~ H l-t~ I-t
~_ , 1~1
- 47 - ~ ~353~9
Example _
Test of t~le effect of antidoted compositions on cultivated plants in a
plant-cultivating charnber.
400 9 of field top soil eac~ (wit~ a pH value of 6.5 permeability
of 50 and l~umus content of 1.4 %) were weighed in plastic cultivating
bottles of 0.8 dm2 surface each lined wit~l PVC foil. Onto t~is soil 10
seeds of Pi-3747 maize eacl~ and 50 seeds of SA-114 sorghum each were
sowed. T~le seeds were covered by 100 9 of soil t~en t~e treatments were
carried out in 4 repetitions. In -the course of these treatments 20 40
or 80 respectively 9 per hectare dose of tl~e compounds Nos. 2 4 5 6
or 8 respectively of Table I toge-ther with 400 9 per hectare of DKA-24
antidote were applied in the form of compositions formulated according
to Examples 22 to 24. The same doses of Glean Granstar Ally or Tell
respectively were applied together with CKA-24 antidote for comparison.
T~e sprayed soil surface was again covered with additional 100 9 of soil
in each bottle.
The bottles were placed in a plant-cultivating c~amber and
cultivated with a daily illumination period of 16 hours. The plants were
daily sprinkled up to their water capacity.
The experiment was evaluated by determining (measuring) t~e shoot-
lengtll and green weight on the 12th day following t~e treatment. The results
obtained are summarized in Table XX.
- 48 - ~ ~3533i 9
E t
-- O
o ~J--~ tO O a~ ~ o t`- ~ ~ ~.~ o ~ ~ N lS~
4~ `~ N G~ N r~l r ~ r-l r-l O C~ O ~ N C-- t--
+. +
-
o o t-- 0 ~ ~ o o ~ ~o 1
~ I O O 1~ .t r-l ~1 0 U~
C r-l r-l r~l r~l r~ ~1
a~
CJ)
.~ ~
E ~1 0 ~ ~) l~ t~ O G~ 0~ D O C~J ~\1` O r-J r-l
4~ 1 0 t-- ~ ~D 0 CO ~ ~ ~ C~ O O O;~
o ~q I ~ r~
+~ ~
.~ ~
Q)
3 ~1 a~ ~ ~f) ~ O a~ ~ O t~ ~ ~ 0 0
Q) ~
c3
'-
N
~D N t-- O r~ 0 L~ 0 d~ ~ O r-l O O
O 0 0 ~ 0 ~ 1 ~ O a~ t~
a~ ~
._I
E ~ .
o-~l O ~ ~ rl C~ a~ .-1 0 o 0 1~ t~ 0 O a~ N I
.~. c-- U~ 1 G~ O O ~ L~ N ~I r~l
_ t~ +~ r~ r~
~ ~ C
CO~
o o ~
O O O O O O O O O o O O O O O O O O
o o c o) N d~ 0 N ~ 0 N ~t 0 N d- 0 C~ 0 N ~t 0
OO ~
U~
~ ~ .
E
~_ . .' ' , : ~ .
3531~
- 49 -
~ I o o oC:~rr~ ~ I I I
~ c o
U~ + ~
o
~ ~ U~ C~ ~ ~ ~V~Lr~ ~
. ~ ~ o o~
C
E
~ ~ `
o ~ ~ ~'1 ~ r~ r~ ~ I I I
+, C O t~ c~
_ ~0
~ ~ o~
C 3 +
O ~ o~ l O O O~
X t~ ~1~
~O 1- ~ o~ ~ ~ I I I
~) I~ I~ ~
O ~ O
N
. +
E
O ~ O ~ co ~ O
.~ ~ c~ Q
+~
~ ~ C
C
~ ~o i~
I a) --
+' ~ ~
O O C O o o o o o o - o o
- 0-~
U~
+C
L~
~`