Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2~~~~~~r~
RAN 4039/57
The present invention is concerned with novel oxetanones, a process for
their manufacture, pharmaceutical preparations which contain such
oxetanones as well as the use of these oxetanones in the manufacture of
pharmaceutical preparations.
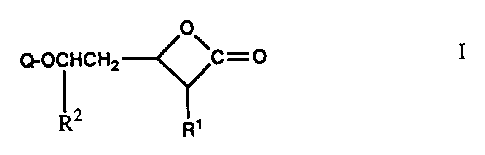
These oxetanones have the formula
O
(~-OCFICHa ~C--.~ I
R2 R'
Q is a group of the formula
(R3,R4)NCO(X)n-CO- Q1
(R3,R4)NCO-X'- Q2
~o or
3
~C~~~~
Il
and
R1 and R2 are alkyl with up to 18 C atoms substituted by 1 to 3 halogen
atoms or alkyl, alkenyl, alkynyl or alkadienyl groups with up to 20 C
atoms optionally interrupted by a 1,4-arylene graup, optionally substitu-
ted by an aryl group in the w-position and optionally substituted by an
aryl-C1-4-alkyl group, whereby R1 can be interrupted by an O or S atom
Me/ 16.1.91
~03~~..~~'~
_2_
or by a sulphinyl or sulphonyl group in a position other than the
a-position to an unsaturated C atom, or R1 is an aryl-NH- or aryl-C1-4-
alkyl-OCONF-i- group,
R3 and R'~ are hydrogen or C1_~-alkyl or together with the N atom to
which they are attached farm a saturated 3- to 6-membered ring
optionally containing an O or S atom in a position other than the a-
pasition to the N atom,
n is the number 1 or 0,
X is an alkylene group which contains up to b C atoms, which is
l0 optionally interrupted by an O or S atom or by a sulphinyl or sulphonyl
group and which is optionally substituted by a hydroxy, mercapto, aryl,
aryloxy, arylthio, aryl-C~-4-alkyl, aryl-C~-4-alkoxy, aryl-C~,-4-alkylthia,
aryl-C1_~-alkylidene, Cg-~-cycloalkylidene or C1-(-alkylidene group or by
one or two C1-~-alkyl, C1-~-alkoxy or C1-6-alkylthio groups, whereby two
C1-6-alkyl, C1_6-alkoxy or C1-6-alkylthio groups on the same C atom or
on two adjacent C atoms can form an optionally mono-unsaturated 3- to
7-membered ring and an optionally present hydroxy or mercapto group
or an optionally present unsaturated C atom must be in a position other
than the a-position to an optionally present O or S atom or to an
zo optionally present sulphinyl or sulphonyl group, or X is a group of the
formula
=CI-IN(R,Ro) or -CHN(R,Ro)CH2-
R and R~ are hydrogen C1-q;-alkyl, C1_~-alkyl(CO or OCO)-, aryl, aryl(CO
or OCO)-, aryl-C1..~-alkyl or aryl-C~_4-alkyl(CO or OCO)- and
X' is an alkylene group containing up to 6 C atoms which can be substi-
tuted by a (~1~-alkoxy, aryl, aryloxy, arylthio, aryl-C1-~-alkyl, aryl-C~_4-
alkoxy or aryl-C~-4-alkylthio group or by one or two C1-f,-alkyl graups,
whereby two C1-s-alkyl groups attached to adjacent C atoms can form a
3- to ~-membered ring.
3o The alkyl, alkenyl and alkadienyl groups can be straight-chain or
branched. Methyl, ethyl, propyl, i-propyl, butyl, i-butyl, pentyl, hexyl,
undecyl
and heptadecyl are examples of alkyl groups.
-3-
"aryl" and "arylene" denote phenyl and, respectively, phenylene or
phenyl and, respectively, phenylene substituted by up to 5 halogen atoms or up
to 3 C1_,~-alkyl, CI_4-alkoxy or nitro groups.
Preferred oxetanones of formula I are those in which Q is a group QI, R1
and R2 are alkyl, alkenyl or alkadienyl groups with up to 20 C atoms
optionally
interrupted by a 1,4-phenylene group, optionally substituted by a phenyl group
in the w-position and optionally subskituted by a phenyl-CI_~-alkyl group,
whereby R1 can be interrupted by an O or S atom in a position other than the
a-position to an unsaturated C atom, X is an alkylene group, which contains
up to 6 C atoms, which is optionally interrupted by an O or S atom and which
is optionally substituted by a hydroxy, mercapto, phenyl, phenoxy, phenylthio,
phenyl-CI_4-alkyl, phenyl-CI_~-alkoxy, phenyl-CI-4-alkylthio, phenyl-CI-4-
alkylidene, C3-~-cycloalkylidene or C1_6-alkylidene group or by one or two CI-
6-alkyl, CI-6-alkoxy or CI-6-alkylthio groups, whereby two CI-g-alkyl, CI-6-
alkoxy or CI_6-alkylthio groups attached to the same C atom can form a 3- to 7
membered ring and an optionally present hydroxy or mercapto group must be
in a position other than the a-position to an optionally present O or S atom,
or
X is a group =CHN(R,R°), R and R° are hydrogen, C1_,~-alkyl,
CI_~-alkyl-(CO or
OCO)-, phenyl or phenyl-(CO or OCO)- and n, R3 and R4 have the significance
given above.
Further preferred oxetanones of formula I, wherein Q is a group QI, are
those in which R1 and R2 are alkyl, alkenyl, alkynyl or alkadienyl groups with
up to 20 C atoms optionally substituted by an aryl group in the c~-position,
whereby RI can be interrupted by a S atom in a position other than the a-
position to an unsaturated C atom, or RI is anilino, alkyl with up to 18 C
atoms substituted by a halogen atom or a phenyl-CI_4-alkyl-OCONH- group,
R3 and R4 are hydrogen or CI_4-alkyl or together with the N atom to which
they are attached form a saturated 6-membered ring containing an 0 or S atom
in a position other than the a-position to the N atom, n is the number I or O,
X is an alkylene group, which contains up to 6 C atoms, which is optionally
interrupted by an O or S atom or by a sulphinyl group and which is optionally
substituted by one or two CI_6-alkyl or CI_~-alkoxy groups, whereby two CI-6-
alkyl or CI_6-alkoxy groups attached to the same C atom or to two adjacent C
atoms can form an optionally mono-unsaturated 3- to 7-membered ring, or X
is a group =CHN(R,Ro) or -CHN(R,Ro)CH2- and R and Ro are hydrogen, C2-5-
alkanoyl or benzyloxycarbonyl.
~~3~~~'~
-4-
There are further preferred the oxetanones of formula I in which Q is a
group Q2, R1 and R2 are C1-20-alkyl. R3 and R4 are hydrogen and X' is an
alkylene group containing up to 6 C atoms which can be substituted by a C1-~-
alkoxy group or by one or two C1-6-alkyl groups, whereby two C1-6-alkyl
groups attached to adjacent C atoms can form a 3- ko 7-membered ring.
The oxetanones or formula I in which Q is a group Q3, R3 is hydrogen
and R1 and R2 are C1_2p-alkyl, especially hexyl or undecyl, are also
preferred.
Especially preferred among the oxetanones of formula I in which Q is a
group Q1 are those in which R1 is methyl, ethyl, propyl, hexyl, 2-butenyl,
Zo 3-methyl-2-butenyl, 2-propynyl, rnethylthio, pentylthio, 5-chloropentyl,
benzyl,
phenylthio, benzylthio, pentafluorobenzyl, anilino or benzyloxycar-
bonylamino, R2 is undecyl, heptadecyl or g,11-heptadecadienyl, R3 and R'1 are
hydrogen, methyl or isopropyl or together with the N atom form a morpho-
lino or thiomorpholino group, n is the number 1 or 0 and X is the group -
(CH2)1-g-, ethylidene, propylidene, isopropylidene, butylidene, isobutylidene,
pentylidene, isopentylidene, t-butylmethylene, dimethylvinylidene, cyclo-
pentylidene, cyclohexylidene, phenethylidene, phenylpropylidene, 1,2-cyclo-
hexylene, cyclohex-3-en-1,6-ylene, acetamidomethylene, benzyloxycar-
bonylaminomethylene, 1-benzyloxycarbonylamino-1,2-ethylene, methylene-
oxymethylene, methylenethiomethylene, methylenesulphinylmethylene,
ethylenethioethylene, ethylenesulphinylethylene, methoxymethylene or
ethylene- or propylenedioxymethylene.
Especially preferred among the oxetanones of formula I in which Q is a
group Q2 are those in which R1 is hexyl, R2 is undecyl and X' is ethylene, 1-
methoxy-1,2-ethylene or 1,2-cyclohexylene.
The following are examples of such compounds:
(S)-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (S)-2-isopropyl-
malonamate,
(S~1-[[(2S,3Sr3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (S or R)-2-
3o carbamoylvalerate,
(all Z,S)-1-[[(2S,3S)-3-ethyl-4-oxo-2-oxetanyl]methyl]-9,12-octadecadienyl
(S)-2-isopropylmalonamate,
2~~~:~~~
-5_
(S)-1-(((2S,3S or 2R,3R)-4-oxo-3-pentylthio-2-oxetanyl]methyl]dodecyl (S)-2-
isopropylmalonamate,
(S)-1-[[(2S,3S) or 2R,3R)-4-oxo-3-pentylthio-2-oxetanyl]methyl]dodecyl
(S:R(2:1 )]-2-isopropylmalonamate,
(S)-1-[[(2S,3S)-3-ethyl-4-oxo-2-oxetanyl]methyl]octadecyl (S or R)-2-t-
butylmalonamate,
(S)-1-[[(2S,3S)-3-ethyl-4-oxo-2-oxetanyl]methyl]octadecyl 1-carbamoylcyclo-
pentanecarboxylate,
(S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (RS)-2-benzyl-
1o malonamate,
(S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl 3-((2-carba-
moylethyl)thio]propionate,
5-oxo-D-proline (S)-1-[[(2S,3S)-3-ethyl-4-oxo-2-oxetanyl]methyl]octadecyl
ester,
15 5-oxo-L-proline (S)-1-([(2S,3S)-3-ethyl-4-oxo-2-oxetanyl]methyl]octadecyl
ester and especially
(S)-1-([(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (S or R)-2-iso-
propylmalonamate,
(S)-1-([(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (RS)-2-carbamoyl-
2o valerate (epimers 1;1),
(all G,S)-1-(((zS,3S)-3-ethyl-4-oxo-2-oxetanyl]methyl]-9,12-octadecadienyl (S
or R)-2-isopropylmalonamate,
(S)-1-([(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (RS)-2-carbamoyl-
4-methylvalerate (epimers 1:1),
25 (S)-1-([(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyll-carbamoylcyclo-
hexanecarboxylate,
(S)-1-[((2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (RS)-2-methyl-
malonamate (epimers 1:1),
-6-
(S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (RS)-2-ethyl-
malonamate (epimers 1:1),
(S)-1-[((2S,3S)-3-hexyl-~4-oxo-2-oxetanyl]methyl]dodecyl (RS)-2-butyl-
rnalonamate (epimers 1:1),
(S)-1-[((2S,3S)-3-ethyl-4-oxo-2-oxetanyl]methyl]octadecyll-carbamoylcyclo-
hexanecarboxylate,
(S)-1-[(2S,3S or 2R,3R)-4-oxo-3-pentylthio-2-oxetanyl]methyl]dodecyl [S:R
or R:S(2:1)]-2-isopropylmalonamate and
(S)-1-[[(2R,3R)-3-benzyl-4-oxo-2-oxetanyl]methyl]dodecyl (S or R)-2-iso-
~o propylmalonamate.
The oxetanones of formula I contain at least three asymmetric C atoms
and can accordingly be present as optically active enantiomers, as mixtures
thereof, e.g. as racemates, or a diastereomers.
The oxetanones of formula I can be manufactured in a manner known
per se by
a) esterifying an alcohol of the formula
o
FI~CHCN2 \C---~ IIa
R2 R'
with an aeid of the formula Qa-OH, wherein Qa is a group of the formula Q1 or
Q3, or
2o b) cyrlizing an acid of the formula
(Q-O,RZ)CHCHZCH(OH)CH(R1 )-COOH llb
or
c) converting the carboxy group in the group T in an acid of the formula
~~3~~~~
Q
T-OCHCHx ~C=O IIc
9
R2 Ri
wherein T is a group of the formula
I ~OCO(X)n-CO- T1
or HOCO-X'- T2,
into an amide group (R3, R4)NCO-, and
d) if desired, separating a mixhxre of epirners of formula I into the
individual epimers.
The esterification a) can be carried out in the presence of triphenylphos-
phine and an azodicarboxylic acid diester such as the di-t-butyl ester of
diiso-
1o propyl ester in a solvent, e.g. an ether such as tetrahydrofuran (THF), at
room
temperature or while cooling, e.g. to 0 to -SoC.
The cyclization b) can be carried out in a solvent such as rnethylene
chloride,, dimethylformamide (DMF) or acetonitrile using a molecular sieve,
e.g. in the presence of 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium
z5 hexafluorophosphate (HBTU) and of a base such as triethylamine at room
temperature or at a temperature up to 50oC.
The amidation c) can be carried out using a solution of ammonia or of an
amine of the f~rmula (R3,R4)NH, e.g. in acetonitrile, in the presence of HBTU
at room temperat<ire or at a temperature up to 40oC.
20 The optional separation of a mixture of epimers of formula I can be
carried out e.g. by chromatography over silica gel with ethyl
acetate,/hexane/methylene chloride as the eluting agenk.
The alcohols of formula lda are known, e.g. from European Patent
Application No. 0 185 359 A2, or can be prepared in analogy to the kmown
25 alcohols of formula IIa or as described in Examples A to I, M and 0 to T.
CA 02035967 2000-11-14
-8-
The and starting materials of formulae IIb and IIc can be prepared in a
manner known per se, e.g. starting from corresponding alcohols of formula IIa
as described hereinafter in Examples J and K (for the acids IIb) and K, L, and
N
(for the acids IIc).
Example A
a) 465 g of methyl acetoacetate and then 458 g of ethyl bromide are added
under nitrogen to 720 g of 30% sodium methylate solution. The reaction
mixture is subsequently boiled at reflux. After distilling off the methanol
the
residue is poured on to ice-water. Then, the mixture is extracted with n-
hexane
l0 and water. The organic phases are combined and dried. After evaporation of
the solvent and distillation there are obtained 328 g of methyl 2-
acetylbutyrate,
b.p. 77-790/15 Torr.
b) 144.17 g of the methyl ester from a) are added under argon at 0-50C to a
suspension of 26.4 g of sodium hydride in 1250 ml of THF. After stirring at 0-
15 50C for 1.5 hours the mixture is cooled to -100C. At this temperature there
are
added 675 ml of 1.56M butyllithium in hexane. After stirring at -100C for 30
minutes a solution of 149.3 g of methyl stearate in 250 ml of THF is added
dropwise. After stirring at -100C for 1.5 hours the reaction solution is added
under argon to 250 ml of 37% hydrochloric acid and 300 g of ice. The mixture
is
20 extracted with hexane and water. The combined organic phases are dried,
filtered and evaporated.
The residue is dissolved in 2500 ml of THF, treated with 76.1 g of 1,8-
diazabicyclo[5.4.0]undec-7-ene(1.5-5) (DBU) and boiled at reflux under argon.
The cooled reaction solution is extracted with 37% hydrochloric acid and then
25 with saturated sodium chloride solution. The combined organic phases are
dried and evaporated. The product is dissoved in ethyl acetate. The solution
is
cooled to room temperature and stirred at 250C overnight. The crystallizate is
filtered off under suction, washed with ethyl acetate and dried. There are
obtained 122.5 g of 3-ethyl-6-heptadecyl-4-hydroxy-2H-pyran-2-one, m.p. 101-
30 1020C.
c) 100 g of Raney-nickel and 2000 ml of THF are added to 100 g of the pyrone
from b). After hydrogenation at 250 for 3 days the catalyst is filtered off
under
suction and washed with THF. The filtrate is evaporated to dryness. The
residue is dissolved in ethyl acetate and stirred at 100 for I7 hours. The
35 crystallizate is filtered off under suction, washed with cold (-100) ethyl
acetate
* Trademark
-9-
and dried at 40a for 17 hours. There axe obtained 90.54 g of rac-(2RS,2RS,5SR)-
2-
ethyl-5-heptadecyl-3-hydraxy-8-valerolactone, m.p. 101-102oC.
d) 138.5 g of benzaic anhydride and subsequently 2.5 ml of 70% perchloric
acid are added to a suspension of 191.3 g of the d-lackone from c) in 1250 ml
of
toluene. After stirring fox 2.5 hours the reaction mixture is extracted in
toluene
with 1N sodium hydroxide solution in 20% sodium chloride solution and
then with saturated sodium chloride solution. The axganic phases are
combined, dried and evaporated. There are obtained 243.4 g of rac-
(2RS,3RS,5SR)-3-benzoyloxy-2-ethyl-5-heptadecyl-8-valerolactone, m.p. 64.5-
66aC.
e) 243 g of the benzoate from d) are dissolved in 450 ml of toluene at 40oC
under argon. 1000 ml of methanol and thereafter 2.5 ml of cone sulphuric acid
are added and the reaction mixture is stirred at 25oC for 20 houxs. After
neutralization of the sulphuric acid with triethylamine the solvent is
evaporated. The xesidue is dissolved in t-butyl methyl ether and washed with
water. The aqueous phase is extracted with t-butyl methyl ether and the
organic phases are combined and dried over sodium sulphate, the drying agent
is filtered off under suction and washed with t-butyl methyl ether and
subsequently evaporated. There are obtained 257 g of methyl rac-
(2RS,3RS,5SR)-3-benzoyloxy-2-ethyl-5-hydroxydocasanoate.
f) 257 g of the hydroxyester from e) in 1250 ml of n-hexane are treated under
argon with 152 g of benzyl 2,2,2-trichloroacetimidate. Then, 3.2 rnl of tri-
fluoromethanesulphoruc acid are added. After stirring for 18 hours the preci-
pitate is filtered off under suction and washed with n-hexane. The filtrate is
extracted with 5% sodium bicarbonate solution and water. The combined
hexane phases are dried, filtered and concentrated. After starring at -20oC
for
20 hours the crystallizate is filtered off under suction, washed with n-hexane
and discarded. The filtrate is evaporated. There are obtained 239.6 g of
methyl
rac(2RS,3RS,5SR)-3-benzoyloxy-5-benzyloxy-2-ethyldocosan oats.
g~ 239.6 g of the benzyl ether from f) are treated under argon with a solution
of 140 g of potassium hydroxide in 1250 ml of 95% (v/v) methanol/water and
stirred at 40oC for 17 hours. Subsequently, the mixture is concentrated at
40aC,
the suspension is taken up in t-butyl methyl ether and washed in sequence
with 10% sodium chloride solution, 1N hydrochloric acid and again with 10%
sodium chloride solution. The organic phase is dried with sodium sulphate,
the drying agent is filtered off under suction and washed with t-butyl methyl
-10-
ether. The filtrate is evaporated. There are obtained 182.1 g of rac-
(2RS,3RS,5SR)-5-benzyloxy-2-ethyl-3-hydroxydocosanoic acid.
h) 33.3 g of (S)-(-)-oc-methylbenzylamine axe added to a solution of 182.1 g
of
the [3-hydroxyadd from g) in 1250 ml of methyl acetate. The solution is seeded
with 50 mg of the phenethylamine salt of (2S,3S,5R)-5-benzyloxy-2-ethyl-3-
hydraxydocosanoic acid and left to stand for 20 hours. The crystallizate is
filtered off under suction, washed with cold (-20aC) methyl acetate and then
dried. Tlus 1st crystallizate is dissolved in hot methyl acetate, cooled to
45aC
and seeded with 50 mg of the pl~enethylarnine salt of (2S,3S,5R)-5-benzyloxy-2-
1o ethyl-3-hydroxydocosanoic acid. The solution is left to stand at roam
tempera-
ture far 20 hours. The crystallizate is filtered off under suction, washed
with
cold (-20oC) methyl acetate and dried. The same procedure as with the 1st
crystallizate is repeated with the 2nd crystallizate. There are obtained 39.4
g of
the phenethylarnine salt of (2S,3S,5R)-5-benzyloxy-2-ethyl-3-hydroxy-
docosanoic acid, m.p. 92-95oC.
i) 39.4 g of the phenethylamine salt from h) are treated with 400 rnl of t-
butyl methyl ether and 80 ml of 1N hydrochloric acid and dissolved while
stirring. The organic phase is washed with water, dried, filtered and concen-
trated. There are obtained 31.4 g of (2S,3S,5R)-5-benzyloxy-2-ethyl-3-hydroxy-
docosanoic acid, m.p. 62-63.5oC.
j ) 17.6 g of benzenesulphonyl chloride are added dropwise at OoC under
argon to a solution of 24.5 g of the ~3-hydroxyacid from i) in 250 ml of
pyridine.
After stirring at OoC for 20 hears 5 ml of water are added dropwise to the
solution. The mixture is stirred at room temperature far 1 hour. The pyridine
is evaporated. The crystal slurry is taken up in t-butyl methyl ether and
washed
in sequence with 2N hydrochloric acid, 5%O sodium bicarbonate solution and
10% sodium chloxide solution. The organic phase is dried over sodium
sulphate and thereafter triturated with active charcoal. The drying agent and
active charcoal are filtered off under suction and the filtrate is evaporated.
3o There are obtained 23.4 g of (3S,4S)-4-[(R)-2-benzyloxynanadecyl]-3-ethyl-2-
oxetanone.
k) A solution of 23.4 g of the oxetanone from j) in 250 ml of THF is treated
with 2.3 g of 10% Pd/C. After hydrogenation fox 5 hours the hydrogenation
solution is suction filtered. After washing with THF the filtrate is
evaporated,
the residue is dissolved in n-hexane and seeded with (3S,4S)-3-ethyl-4-[(R)-2-
hydroxynonadecyl]-2-oxetanane. After 18 hours the crystallizate is filtered
off
-11- _
under suction, washed with hexane and dried. There are obtained 16.1 g of
(3S,4S)-3-ethyl-4-[(R)-2-hydroxynonadecyl]-2-oxetanone, m.p. 66.5-68oC, the
alcohol starting material of Example 1.
Example B
a) 50 g of methyl (R)-3-hydroxytetradecanoate, 35 g of t-butyldimethylchloro-
silane, 6.1 g of 4-dimethylarninopyridine and 29.4 g of triethylamine are
dissolved in 200 ml of methylene chloride and stirred at room temperature for
30 hours as well as under reflux for 16 hours. Thereupon, a further 2 g of t-
butyldimethylchlorosilane are added. After a further 24 hours under reflux the
1o precipitated triethylamine hydrochloride salt is filtered off, washed with
ether
and the filtrate is concentrated. The residue is dissolved in ether and washed
in sequence with water, 0.5M citric and, again with water and saturated
sodium chloride solution, dried, concentrated and subsequently freed from
volatile material at 5oC in a high vacuum for 5 hours. There are obtained
71.8 g of (R)-3-[(1,1-dimethylethyl)dimethylsilyloxy]tetradecanoic acid, IR
(cm-
1): 1745,1254, 895, 776.
b) 18.63 g of the product from a) dissolved in 100 ml of ether are treated
with
65 ml of 1M diisobutylalurninium hydride solution in hexane at a temperature
of -70oC to -75oC and then stirred at this temperature for 1 hour. Thereupon,
2.5 ml of isopropanol, 10 ml of water and 50 ml of 0.5M citric acid solution
are
added dropwise at a max. of lOoC. The ether phase is separated, the aqueous
phase is extracted with ether, the combined ether phases are washed with
brine, dried and concentrated. The residue is chromatographed on silica gel
with pentane/ether (5:1) arid there are obtained 14.47 g of (R)-3-[(1,1~
dimethylethyl)dimethylsilyloxy]tetradecanal, IR (em-1):1728,1254, 836, 775.
c) A solution of 2.55 ml of diisopropylamine in 45 ml of THF is treated at
OoC with 22.5 ml of a solution of 1.6M n-butyllithium in hexane and, after
stirring for 15 minutes, cooled to -75oC. Then, a solution of 2.9 g of
pentylthio-
acetic acid in 9 ml of THF is added dropwise. After stirring for 10 minutes
the
reaction mixture is left to warm to room temperature, stirred for 5 minutes
and again cooled to -75oC. At this temperature there is added dropwise a
solution of 2.4 g of the aldehyde from b) in 9 ml of THF. After stirring for
20 minutes the reaction mixture is poured into saturated ammonium chloride
solution and extracted with hexane. The hexane phase is dried and concen-
trated. There are obtained 3.89 g of (2R/S,3R/S,SR)-5-[(1,1-
-12-
dimethylethyl)dimethylsilyloxy]-3-hydroxy-2-pentylthiohexadecanoic acid as a
mixture of 4 diastereomers.
d) A solution of 3.89 g of the product from c), 3.18 g of 2-(1H-benzotriazol-1-
yl)-1,1,3,3-tetramethyluronium hexafluorophospliate (HBTU), 2 g of 4.~
molecular sieve and 3 rnl of triethylamine is stirred fox 2 hours in a mixture
of
130 ml of metliylene chloride and 6 ml of UMF. Thereupon, the mixture is
filtered, the filtxate is concentrated, the residue is dissolved in
water/methanol
(3:7) and extracted with hexane. The hexane phase is dried and concentrated.
There axe obtained 3.57 g of (3R/S,4R/S)-4-[(R)-2-[(1,1-
1o dimethylethyl)dimethylsilyloxy]tridecyl]-3-pentylthio-2-oxetanone as a
mixture
of 4 diastereomers.
e) A solution of 4.5 g of the product from d) in 200 ml of acetonitrile is
treated with 15 ml of 40°~o hydrofluoric acid and stirred for 18 hours.
Thereupon, sodium bicarbonate solution is added, the mixture is then
extracted with hexane and the hexane phase is dried and concentrated. The
residue is chromatographed on silica gel with 1-5% ether in methylene
chloride. There are obtained 699.9 mg of 3R,4R (or 3S,4S)-4-[(R)-2-hydroxytri-
decyl]-3-pentylthio-2-oxetanone, m.p. 43oC, and 691.2 mg of 3S,4S (or 3R,4R)-4-
[(R)-2-hydroxytridecyl]-3-pentylthio-2-oxetanone, m.p. 7loC, the alcohol
2o starting materials of Examples 5 and 6.
Example C
a) 18 ml of a 1M solution of lithium bis(trimethylsilyl)arnide in THF are
treated with 1.8 ml of ethyl acetate under argon at -75oC, stirred at this
temperature for 30 minutes and subseuently treated with 4.8 g of (R)-3-benzyl-
oxytetradecanal in 15 ml of THF and stirred at -78oC for half an hour. A
solution of 3.8 ml of cone. hydrochloric acid in 6 ml of water is added
dropwise
to the reaction mixture. The solution obtained is extracted with ethyl
acetate,
the combined organic phases are washed with 10% sodium bicarbonate and
water, dried, filtered and concentrated. Thzre is obtained ethyl (3R,5R and
3S,5R)-5-benzyloxy-3-hydroxyhexadecanoate (1:1).
b) A solution of 4 ml of diisopropylamine in 12.5 ml of THF is treated with
17 ml of a 1.6M solution of n-BuLi in n-hexane under argon at OoC. After
stirring for 15 minutes 5 g of the product from a) in 2.5 ml of THF are added
dropwise at -50oC. After 10 minutes at -lOoC the temperature is lowered to -
50oC and, after the dropwise addition of a solution of 3.18 g of benzyl
bromide
-I3-
in 3.1 ml of hexamethylphosphoric acid triamide, the mixture is stirred at -
50oC for 15 minutes. Thereafter, the cooling bath is removed and the reaction
mixture is stirred at room temperature for 3 hours. The reaction mixture is
cooled to OoC and 50 ml of saturated sodium chloride solution are added
thereto, the mixture is extracted with t-butyl methyl ether, the extracts are
dried, filtered and the solvent is evaporated. The residue is chromatographed
on silica gel with n-hexane/ethyl acetate (4:I). 1'he residue is dried. There
is
obtained ethyl (2R,3R,5R and 2S,3S,5R)-5-benzylaxy-2-benzyl-3-hydroxyhexa-
decanoate as 1:I thxeo diastereomers.
1o c) A solution of 3.1 g of the product from b) and 26 ml of 2.5N sodium
hydroxide solution in 37.2 ml of ethanol is heated at reflex for 50 minutes
and
subsequently neutralized at room temperature with 26 ml of 2.5N hydrochloric
acid. The ethanol is distilled off, whereupon the residue is extracted with t-
butyl methyl ether and water. The combined organic phases are dried and
concentrated. A solution of 3 g of the residue in 109 ml of methylene chloride
is stirred under argon and treated with 2.59 g of I-IBTU and 2.74 g of
molecular
sieve. Subsequently, 5.5 ml of DMF and 2.8 ml of triethylamine are added and
the reaction mixture is stirred for I hour, filtered and concentrated. The
residue is taken up in n-hexane, the solution is thereupon extracted with
2o water, dried and concentrated in a vacuum. Chromatography on silica gel
with
methylene chloride gives a Ist traps diastereomer (3S,4S or 3R,4R)-3-benzyl-4-
[(R)-2-benzylaxytridecyl]-2-oxetanane, Rf value: 0.45 (thin-layer chromato-
graphy over silica gel 5-40 m with methylene chloride) and a 2nd traps
diastereomer, (3R,4R or 3S,4S)-3-benzyl-4-[(R)-2-benzyloxytridecyl]-2-
oxetanone, Rf value: 0.50 (thin-layer chromatography over silica gel 5-40 ~.
with methylene chloride).
d) A solution of 646 mg of the 2nd traps diastereamer from c) in 65 ml of
TI-iF is hydrogenated for 1 hour in the presence of 646 mg of 10% Pd/C. The
reaction mixture is filtered and concentrated. There is obtained a traps
diastereomer: (3R,4R or 3S,4S)-3-benzyl-4-[(R)-2-hydroxytridecyl]-2-oxekanone,
the alcohol starting material in Example 7.
e) As described under d), from the Ist traps diastereomer from c) there is
obtained the traps diastereom~r: (3S,4S or 3R,4R)-3-benzyl-4-[(R)-2-hydroxy-
tridecyl]-2-axetanone, the alcohol starting mateial in Example 8.
Exam~Ie D
~0~~~~'~
-14-
A solution of 3.0 ml of diisopropylamine in 50 ml of TI-IF is treated at OoC
with 12.0 ml of a solution of 1.6M n-butyllithium in hexane and, after
stirring,
cooled to -75oC. A solution of 1.26 g of Z-glycine in 10 ml of TlE-~ is then
added
dropwise. Then, the reaction mixture is left to warm to room temperature and
again cooled to -75oC. 0.7 g of (R)-3-(t-butyldimethylsiloxy)tetradecanal in 5
ml
of THF is now added dropwise at -75oC. The reaction mixhare is stirred at -
75oC
for 1 hour and at -40o to -50oC for 1 /2 hour, then warmed to 5oC, again
cooled
to -75oC, poured into dilute potassium hydrogen sulphate solution and
extracted with ether. The ether phase is dried, concentrated and chromato-
1o graphed on silica gel with methylene chloride/methanol. There are obtained
540 mg of (2R/S,3R/S,SR)-2-[1-(benzyloxy)formarnido]-5-(t-butyldimethyl-
siloxy)-3-hydroxyhexadecanoic acid as a mixture of 4 diastereomers.
In analogy to B)d), from the above product there is obtained benzyl 4-[(R)-
2-(t-butyldimethylsiloxy)tridecyl]-2-oxo-3-oxetanecarbamate as a 1:1 mixture
of
the two traps-diastereomexic ~3-lactones, MS: 476 (M+A-C4H9p).
In analogy to B)e), from the above mixture there are obtained (3S,4S or
3R,4R)-benzyl 4-[(R)-2-hydroxytridecyl]-2-oxo-3-oxetanecarbamate, the alcohol
starting material in Example 15, m.p. 122-124°C, and (3R,4R or 3S,4S)-
benzyl-4-
[(R)-2-hydroxytridecyl]-2-oxo-3-oxetanecarbamate, m.p. 98-99°C.
2p Example E
In analogy to Example B, from thiophenoxyacetic acid and (R)-3-
[(1,1-dimethylethyl)dimethylsilyloxy]tetradecanal there is obtained,
via
a) (2R/S,3R/S,5R)-5-(t-butyldimethylsiloxy)-3-hydroxy-2
(phenylthio)hexadecanoic acid (mixture of 4 diastereomers) and
b) (3R/S,4R/S)-4-[(R)-2-(t-butyldimethylsiloxy)tridecyl]-3-(phenylthio)-2-
oxetanone (mixture of 4 diastereomers),
IR (cm-1 ): 2927, 2855, 1833,1254,
(3S,4S)-4-[(R)-2-hydroxytridecyl]-3-(phenylthio)-2-oxetanone rn.p. 79°C
(ether), and (3R,4R)-4-[(R)-2-hydroxytridecyl]-3-(phenylthio)-2-oxetanone,
rn.p.
47°C (ether), the alcohol starting material of Example 16.
Example E
-15-
a) 270 ml of stearoyl chloride are added dropwise at a maximum of 150oC to
a solution of 117 g of Meldrum acid and 131 ml of pyridine in 1.51 of
methylene chloride. After stirring the reaction mixture is washed with 41x1
hydrochloric acid, the aqueous phase is back-extracted with methylene chloride
and the methylene chloride please is dried and concentrated. The residue is
taken up in methanol and stirred unde reflux. After cooling the separated
cryskals are filkered off, dissolved in methylene chloride and chromatographed
on silica gel with mekhylene chloride. There are obtained 175 g of methyl 3-
oxoeicasanoate, m.p. 52-54aC.
1o b) 1.84 mg of acetyl chloride in 1.84 ml of methanol are added to a
solution
of 9.1 mg of [(R)-2,2'-bis(diphenylphasphino)-6,6'-dimethylbi-
phenyl]ruthenium diacetate in 20 ml of methylene chloride. The solution
obtained is hydrogenated at 35 bar of hydrogen and 60oC together with 39.8 g
of
the ketoester from a) and 170 ml of methanol. After the addition of methylene
15 chloride the mixture is evaporated to dryness. Chromatography on silica gel
with ether and recrystallization from n-hexane yields 35.6 g of methyl (R)-3-
hydroxyeicosanoate, m.p. 64-64.5oC.
c) Analogously to Example B, from the product of b) there are obtained, via
methyl (R)-3-(t-butyldimethylsiloxy)eicosanoate, IR (cm-1): 1745, 1255, 836,
z0 (R)-3-(t-butyldimethylsiloxy)eicosanal, IR (crn-1): 1728, 1463, 1255, 1104,
836, 775,
(2R/S,3R/S,5R)-5-(t-butyldimethylsiloxy)-3-hydroxy-2-
(methylthio)docosanoic acid (mixture of 4 diastereomers), MS: 533 (M+H)+,
4-[(R)-2-(t-butyldimethylsilaxy)nonadecyl]-3-(methylthio)-2-oxetanone,
25 (1:1 mixture of two traps diastereomers),
IR (cm-1): 1834,1463, 2256,1106, 836, and
4-[(R)-2-(t-butyldimethylsiloxy)nonadecyl]-3-(methylthio)-2-oxetanone (1:1
mixture of two cis diastereomers), IR (cm-1): 1834, 1463, 1256, 1106, 1066,
836,
the following alcohol starting materials of Example 17:
30 (3S,4R or 3R,4S)-4-[(R)-2-Hydroxynanadecyl]-3-(methylthio)-2-oxetanone,
m.p. 65°C (from methylene chloride)
-16- ~~3~~~'~
(3R,4S or 3S,4R)-4-[(R)-2-hydroxynonadecyl]-3-(methylthio)-2-oxetanone,
m.p. 67°C (from rnethylene chloride)
(3R,4R or 3S,4S)-4-[(R)-2-hydroxynonadecyl]-3-(methylthio)-2-oxetanone,
m.p. 71°C (from ether) and
(3S,4S or 3R,4R)-4-[(R)-2-hydroxynonadecyl]-3-(methylthia)-2-oxetanone,
m.p. 80°C (from ether).
Example G
Analogously to Example B, from (benzylthio)-acetic acid and (R)-
3-[(1,1-dimethylethyl)dimethylsilyloxy]tEtradecanal there is obtained,
to via
(2R/S,3R/S,5R)-2-(benzylthio)-5-[(1,1-dimethylethyl)dimethylsilyloxy]-3-
hydroxyhexadecanoic acid (mixture of 4 diastereomers) and
(3R/S,4R/S)-3-(benzylthio)-4-[(R)-2-(t-butyldimethylsiloxy)tridecyl]-2-
oxetanone (mixture of 4 diastereomers), MS: 506 (M+),
the following alcohol starting materials of Examples 19 and 20:
(3S,4S or 3R,4R)-3-(Benzylthio)-4-((R)-2-hydroxytridecyl]-2-oxetanone, m.p.
65°C (ether),
(3R,4R or 3S,4S)-3-(benzylthio)-4-[(R)-2-hydroxytridecyl]-2-axetanone, MS:
374 (M+~-H20), and
(3R,4S and 3S,4R)-3-(benzylthio)-4-[(R)-2-hydroxytridecyl]-2-oxetanone (1:1
diast.), MS: 374 (M+~-H20).
Example H
a) 104 g of the mother liquor from the 1st crystallization in Example Ah) are
dissolved in water and methylene chloride. The mixture is acidified to pH 1 by
z5 the addition of cone. HCl while cooling with ice, the methylene chloride
phase
is separated, the aqueous phase is extracted with rnethylene chloride and the
methylene chloride phase is washed with water, dried and concentrated. 'There
are obtained 86.7 g of enriched (2R,3R,5S)-5-benzyloxy-2-ethyl-3-hydroxy-
docosanoic acid which are dissolved in 500 ml of ethyl acetate and txeated
with
20.6 g of (R)-(+)-a-methylbenzylamine while cooling. After the addition of
2~3~~G'~
-17-
ethyl acetate the mixture is heated to reflex, filtered and crystallized-out.
The
crystals obtained are recrystallized from ethyl acetate and methyl acetate.
There
are obtained 70.0 g of the phenethylamine salt of (2R,3R,5S)-5-benzyloxy-2-
ethyl-3-hydroxydocosanoic acid, m.p. 88-91°C.
b) Analogously to Example Ai),j),k), from the above salt there is obtained,
via
(2R,3R,5S)-5-benyzloxy-2-ethyl-3-hydroxydocosanoic acid m.p. 61.5-63°C,
and
(3R,4R)-4-[(S)-2-benzyloxynonadecyl]-3-ethyl-2-oxetanone, m.p. 38-40°C,
(3R,4R)-3-ethyl-4-[(S)-2-hydroxynonadecyl]-2-oxetanone, rn.p. 66-68°C.
c) A solution of 14.2 g of the product obtained and 8.65 g of triphenyl-
phospine in 250 ml of THF is treated at +5oC with 1.19 ml of formic acid and
then with a solution of 5.12 g of diethyl azodicarboxylate in 20 ml of THF.
The
mixture is then again treated with 0.4 ml of formic acid, 2.9 g of triphenyl-
phosphine and 1.7 ml of diethyl azodicarboxylate. The xeaction mixture is
concentrated and the residue is chromatographed on silica gel with
hexane/ethyl acetate; there are thus obtained 13.1 g of (R)-1-[[(2R,3R)-3-
ethyl-4-
oxo-2-oxetanyl]methyl]octadecyl formate.
The product obtained is dissolved in 150 ml of methanol and treated at
l5oC with 0.114 g of p-toluenesulphonic acid monohydrate. After stirring the
reaction mixture is concentrated, the residue is partitioned between methylene
chloride and aqueous sodium bicarbonate and extracted with methylene
chloride. The methylene chloride phase is dried and concentrated and the
residue is recrystallized from ethyl acetate. There are obtained 9.5 g of
(3R,4R)-
3-ethyl-4-[(R)-2-hydroxynonadecyl]-2-oxetanone, m.p. 80-82oC, the alcohol
starting material in Example 21.
Example I
a) 187.5 ml of n-butyllithium solution (1.61vi in hexane) are added dropwise
at -20oC to a solution of 42.5 ml of diisopropylamine in 500 ml of THF. After
stirring the solution is added dropwise at a maximum of -65oC to a suspension
of 39.9 g of (S)-(-)-2-hydroxy-1,2,2-triphenylethyl-acetate in 600 ml of THF.
Then, the reaction mixture is warmed to OoC, stirred, cooled to -70oC and
treated with a solution of 51.2 g of (R)-3-[(t-
butyl)dimethyisilyloxy]eicosanal in
-18-
400 ml of THF. After stirring 500 ml of saturated ammonium chloride solution
are added dropwise, the mixture is then warmed to roam temperature and
stirred. The reaction mixture is concentrated, partitioned between water and
ether and extracted with ether, the ether phase is washed with water,
concentrated, taken up in 1 1 of methylene chloride, dried and concentrated.
There are obkained 91.9 g of (S)-2-hydroxy-1,2,2-triphenylethyl
[3R:3S(4:1),5R]-5-
(t-bukyldimetlvylsiloxy)-3-hydroxydacosanoate, IR (cm-1): 3525, 1719, 1448,
1250,
1159, 838, 697.
b) A solution of 90.8 g of the produet obtained above in 1 1 of methanol is
x0 treated with 22.15 ml of 5.4M sodium methylate in methanol. After stirring
the
solution is concentrated, the residue is partitioned between ether and
saturated
ammonium chloride solution and extracted with ether. The ether phase is
dried, concentrated and chromatographed on silica gel with hexane/ ethyl
acetate. There axe thus obtained 42.7 g of methyl (3R,5R)-5-(t-butyldimethyl-
siloxy)-3-hydroxydocosanoate, IR (cm-1): 3521, 3468,1738,1254,1168, 1137,
1105.
c) Analogously to Example Be) and Cb), c), the latter compound is
converted, via methyl (2R,3R,5R)-5-(t-butyldimethylsiloxy)-3-hydroxy-2-
methyldocosanoate, IR (cnn-1): 3522, 1739, 1464, 1254,1066, and (3R,4R)-4-[(R)-
2-
(t-butyldimethylsiloxy)nonodecyl]-3-methyl-2-oxetanone, IR (cnrl): 1830, 1464,
z0 1254, 1129, 1071, into (3R,4R)-4-[(R)-2-hydroxynonadecyl]-3-methyl-2-
oxetanone, m.p. 82.5-84°C (from EtOAc/hexane), the alcohol starting
material
in Exarnpie 22.
Exam le
a) 1.1 g of (3S,4S)-3-hexyl-4-[(R)-2-hydroxytridecyl]-2-oxetanone, 1.6 g of
triphenylphosphine, 0.825 g of salicylamide and 3 g of molecular sieve (4~)
are
treated with 20 ml of THF and cooled to OoC. Thereupon, 1.4 g of di-t-butyl
azadicarboxylate are added. After warming to room temperature and stirring
the reaction mixture is concentrated and the residue is partitioned between
methanol/water (70:30) and hexane and extracted with hexane. The hexane
phase is dried and concentrated and the residue is chr~matagraphed on silica
gel with hexane/ethyl acetate (4:1). There are thus obtained 0.727 g of o-
[((S)-1-
[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl]axy]benzamide, MS: 474
(M+H)+.
b) 972 mg of the product obtained above are dissolved in 12 ml of methanol
and treated with 0.2 g of potassium carbonate. After stirring the reaction
-19-
mixture is concentrated and the residue is partitioned between
methanol/water (7:3) and hexane and extracted with hexane. The hexane
phase is dried and concentrated. There are thus obtained 854 mg of methyl
(2S,3S,5S)-5-(o-carbamoylphenoxy)-2-hexyl-3-hydroxyhexadecanoate, MS: 369
(M+~-(o-carbamoylphenaxy)).
c) 850 mg of the product obtained above are dissolved in 12 ml of
rnethanol/water (98:2) treated,with 800 mg of 5 percent rhodium on
aluminium oxide and hydrogenated at '100oC and 100 bar of hydrogen. The
reaction mixture is filtered, concentrated and chromatographed on silica gel
1o with hexane/ ethyl acetate (1:1). There are thus obtained 213 mg of methyl
(2S,3S,5S)-5-[[(cis)-2-carbamoylcydohexyl]oxy]-2-hexyl-3-hydroxyhexadecanoate
(1st. diast.), MS: 367 [M+~-(H2NCOC6HIpe+H20)], 204 mg of a mixed fraction
and 142 rng of methyl (2S,3S,5S)-5-[[(cis)-2-carbamoylcyclohexyl]oxy]-2-hexyl-
3-
hydroxyhexadecanaate (2nd.diast.), MS: 367 [M+~-(H2NCOC6I-I10~+H20)].
d) 210 rng of the 1st diastereomer obtained above are dissolved in 10 ml of
acetone and treated with 3 ml of 1N potassium hydroxide. After stirring the
reaction mixture is poured into potassium hydrogen sulphate solution and
extracted with ether. The ether phase is dried and evaporated. There are
obtained 277 mg of (2S,3S,5S)-5-[[(cis)-2-carbamaylcyclohexyl]oxy]-2-hexyl-3-
2o hydroxyhexadecanoic acid (1st. diast.), the acid starting material in
Example
23a).
e) As described in d), from the 2nd diastereomer from c) there is obtained
(2S,3S,5S)-5-[[(cis)-2-carbamaylcyclohexyl]oxy]-2-hexyl-3-hydroxyhexadecanoic
arid (2nd. diast.), the acid starting material in Example 23b).
Example K
a) Analogously to Example Hc), from (3S,4S)-3-hexyl-4-[(R)-2-hydroxy-
tridecyl]-2-oxetarzone and formic acid there is obtained, via (S)-1-[[(2S,3S)-
3-
hexyl-4-oxo-2-oxetanyl]methyl]dodecyl formate, IR (cxn-1): 1826, 1725, 1177,
1122, (3S,4S)-3-hexyl-4-[(S)-2-hydroxytridecyl]-2-oxetanone m.p. 63-
64°C (from
hexane).
b) 1.8 g of the hydroxy-b-lactone prepared above, 1.3 g of pyridinium p-
toluenesulphonate and 2 g of molecular sieve (4~) in 10 ml of methyl 3,3-
dimethoxypropionate are stirred at 100oC under argon, the reaction mixture is
2~~~~~~
-zo-
then filtered, the xesidue is concentrated and chromatographed on silica gel
with ether/ methylene chloxide. There are thus obtained
1. 213 mg of methyl (E)-3-[((S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-
oxetanyl]methyl]dodecyl]oxy]a~.-rylate, IR (crn-1): 1827,1714,1643,1622,1192,
and
2. 826 rng of methyl (R/S)-3-[[(S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-
oxetanyl]methyl]dodecyl]oxy]-3-methoxypropionate (1:1 epimer mixture), IR
(crn-1): 1824,1743,1438,1117.
c) 235 mg of the product of b)2. are suspended in 25 ml of 0.02N NaOH and
the reaction mixture is diluted with acetone. After stirring for 24 hours the
1o mixture is acidified with 5 percent potassium hydrogen sulphate solution
and
extracted with ether. The ether phase is dried and concentrated and the
residue
is chromatographed on silica gel with methylene chloride/ methanol. There
are thus obtained 60 mg of (2S,3S,5S)-2-hexyl-3-hydroxy-5-[(R/S)-1-rnethoxy-2-
(methoxycarbonyl)ethoxy]hexadecanoic acid, MS: 337 (M~' ~-(H20+-O-(CH30)-
CH2-COOCH3)).
d) A solution of 58 mg of the above compound in 4 ml of condensed
ammonia is heated at 50oC in an autoclave. Subsequently, the ammonia gas is
allowed to escape and the mixture is then treated with potassium hydrogen
sulphate solution and extracted with rnethylene chloride. The methylene
2o chloride phase is dried and concentrated. 'There are obtained 42.5 mg of
(2S,3S,5S)-5-((R/ S)-2-carbarnoyl-1-rnethoxyethoxy]-2-hexyl-3-hydroxyhexa-
decanoic acid, the acid starting material of Example 24.
Example 1L
A solution of 200 mg of the product of Example IGb)1. in 10 mI of THF is
hydrogenated using 200 mg of Pd/C (10%). Then, the mixture is filtered, the
filtrate is concentrated and the residue is chromatographed on silica gel with
1% ether in methylene chloride. There are obtained 99 mg of methyl 3-[[(S)-1-
[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl]oxy]propionate, MS: 285
(M+~_(C11H23')).
A suspension of 544 mg of this compound in 49 ml of 0.02N sodium
hydroxide is treated with acetonitrile, 'The resulting solution is acidified
with
aqueous potassium hydrogen sulphate, the reaction mixture is extracted with
ether and the ether phase is dried and concentrated. Chromatography on silica
gel with 2% ether in rnethylene chloride and then 5% methanol in methylene
-21-
chloride yields 43.7 mg of 3-[[(S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-
axetanyl]methyl]dodecyl]oxy]propionic acid, L1? (cm-1): 1823,1715,1466,1105,
the acid starting material in Example 25.
Example 1VI
Analogously to Example Cb) and c), methyl (3R,5R)-5-(t-butyldimethyl-
silaxy)-3-hydroxydacosanoate (Example Ib) is reacted with propargyl bromide to
give methyl (2R,3R,5R)-5-(t-butyldimethylsiloxy)-3-hydraxy-2-(2-
propynyl)docosanoate, IR (cm-1): 3310, 2120, 1740, 1255, the latter is
saporu'fied
to give (2R,3R,5R)-5-(t-butyldimethylsiloxy)-3-hydroxy-2-(2-
propinyl)docosanoic aad, IR (cm°1): 3315, 2120, 1715, 1255, and this
acid is
cyclized to give (3R,4R)-4-[(R)-2-(t-butyldimethylsilaxy)nonadecyl]-3-(2-
propynyl)-2-oxetanone, IR (cm-1): 3315, 2130, 1830,1255 .
After cleavage of the protecting group analogously to Example Ee) there is
abtained (3R,4R)-4-[(R)-2-hydroxynonadecyl]-3-(2-propynyl)-2-oxetanone, m.p.
'15 62-63°C (~ram ethyl acetate), the alcohol starting material in
Example 60.
Example N
Analogously to Example 1, from (3S,4S)-3-hexyl-4-[(R)-2-hydroxytridecyl]-
2-oxetanone and monobenzyl malonate there is obtained benzyl (S)-1-[[(2S,3S)-
3-hexyl-4-oxo-2-oxetanyl]methyl]dodecylmalonate, IR (cxn-1): 1824, 1734, 1149,
1125.
A solution of 430 mg of this product in 15 ml of TPIF is treated with
100 mg of Pd/C and then hydrogenated. The reaction mixture is filtered and
the filtrate is concentrated. There are obtained 361 mg of (S)-1-[[(2S,3S)-3-
hexyl-
4-axo-2-axetanyl]methyl]dodecyl hydrogen malonate, IR (cm-1): 1824, 1745, the
aad starting material of Example 26.
Examt~le O
A solution of 1.96 g of the alcohol product of Example IvI in 50 ml of ethyl
acetate is hydrogenated using 0.25 g of 10 percent Pd/C; the reaction mixture
as
then filtered and the residue is chramatographed on silica gel with ethyl
acetate/ hexane. There are thus obtained 1.44 g of (3R,4R)-4-((R)-2-hydroxy-
nonadecyl]-3-propyl-2-oxetanone, an.p. 84-85°C (from ethyl
acetate/hexane), the
alcohol starting material in Example 61.
~03~~~'~
_~- _
Example P
a) Prom (R)-3-(t-butyldimethylsiloxy)tetradecanal (Example Bb) there is
obtained analogously to Example Ca),b),c), via ethyl (3R and 3S,5R)-S-(t-butyl-
dimethylsiloxy)-3-hydroxyhexadecanoate (epimer mixture) and
ethyl (R and S)-2-[(1R and 1S,3R)-3-(t-butyldimethylsiloxy)-1-hydroxy-
tetradecyl]-5-methyl-4-hexenoate (1:1 threo diastereomers),
(3R,4R and 3S,4S)-4-[(R)-2-(t-butyldimethylsiloxy)tridecyl]-3-(3-methyl-2-
butenyl)-2-oxetanone (1:1 trans diastereomers).
b) A solution of 1.87 g of the product of a) in 50 ml of acetonitriie is
treated
with 6.2 ml of 40% hydrofluoric acid. After stirring sodium bicarbonate
solution is added, the mixture is then extracted with methylene chloride and
the methylene chloride phase is dried and concentrated. The residue is
chromatographed on silica gel with ethyl acetate/methylene chloride/n-
hexane (1:4.5:4.5). The chromatography gives the alcohol starting materials
for
Examples 62-65:
A 1st traps diastereomer, (3S,4S)-4-((R)-2-hydroxytridecyl]-3-(3-methyl-2-
butenyl)-2-oxetanone, Rf-value: 0.31, and
a 2nd traps diastereomer, (3R,4R)-4-[(R)-2-hydroxytridecyl]-3-(3-methyl-2-
bukenyl)-2-oxetanone Rf value: 0.26 (thin-layer chromatography over silica gel
5-40 a with ethyl acetate/ methylene chloride/hexane (1:4.5:4.5).
Example Q
From ethyl (3R,5R and 3S,SR)-5-benzyloxy-3-hydroxyhexadecanoate (1:1)
(Example Ca) there are obtained analogously to Example Cb) to e) via ethyl-
(2R,3R,5R and 2S,3S,5R)-5-benzyl-2-(5-chloropentyl)-3-hydroxyhexadecanoate
(threo diastereomers),
a 1st traps diastereomer, (3S,4S or 3R,4R)-4-[(R)-2-(benzyloxy)tridecyl]-3-(S-
chloropentyl)-2-oxetanone, Rf value: 0.47,
and a 2nd traps diastereomer, (3R,4R or 3S,4S)-4-[(R)-2-(benzyloxy)tridecyl]-3-
(5-
chloropentyl)-2-oxetanone, Rf value: 0.28 (thin-layer chromatography over
3o silica gel 5-40 ~ with rnethylene chloride), the alcohol starting materials
for
Examples 66-69:
2~~~~~~
(3R,4R or 3S,4S)-3-(5-chloropentyl)-4-((R)-2-hydroxytridecyl]-2-oxetanone
and
(3S,4S or 3R,4R)-3-(5-chloropentyl)-4-[(R)-2-hydroxytridecyl]-2-oxetanone.
Exazn~le R.
From (R)-3-(t-butyldimethylsiloxy)tetradecanal (Example Bb) there are
obtained analogously to Example Cb) and c) via ethyl (R and S,E)-2-[(1R and
1S,3R)-3-(t-butyldimethylsiloxy)tetradecyl]-4-hexenoate (1:1 threo diastereo-
rners) and
(3R,4R and 3S,4S)-3-((E)-2-butenyl]-4-((R)-2-(t-butyldimethyl-
1o siloxy)tridecyl]-2-oxetanone (1:1 traps diastereomers), the alcohol
starting
materials for Examples 70-73:
A 1st traps diastereomer, (3S,4S or 3R,4R)-3-[(E)-2-butenyl]-4-[(R)-2-
hydroxytridecyl]-2-oxetanone, Rf value: 0.475 and
a 2nd traps diastereomer, (3R,4R or 3S,4S)-3-((E)-2-butenyl]-4-[(R)-2-
15 hydroxytridecyl]-2-oxetanone, Rf value: 0.44 (chromatography and thin-layer
chromatography over silica gel with ethyl acetate/methylene chloride/n
hexane (1:2:2).
Example S
From ethyl (3R,5R and 3S,5R)-5-benzyloxy-3-hydroxyhexadecanoate (1:1)
2o (Example Ca) there are obtained analogously to Example Cb) and c) via ethyl
(2R,3R and 2S,3S,5R)-5-(benzyloxy)-3-hydroxy-2-(2,3,4,5,6-pentafluoro-
benzyl)hexadecanoate (threo diastereomers) and
(3R,4R and 3S,4S)-4-[(R)-2-(banzyloxy)tridecyl]-3-(2,3,4,5,6-
pentafluorobenzyl)-2-oxetanone (traps diastereomers), the alcohol staxting
25 materials of Examples 74-77:
A 1st traps diastereomer, (3S,4S or 3R,4R)-4-[(R)-2-hydroxytridecyl]-3-
(2,3,4,5,6-pentafluorobenzyl)-2-oxetanone, Rf value: 0.43, and
a 2nd traps diastereorner, (3R,4R or 3S,4S)-4-[(R)-2-hydroxytridecyl]-3
(2,3,4,5,6-pentafluorobenzyl)-2-oxetanone, Rf value: 0.39 (chromatography and
3o thin-layer chromatography on siliea gel with ethyl acetate/methylene
chloride/n-hexane (1:4.5:4.5)).
-24-
Example T
a) A solution of 0.5 ml of diisopropylamine in 15 ml of THF is treated at OoC
with 2.0 ml of a solution of 1.6M n-butyllithium in hexane and, after
stirring,
cooled to -75oC. Then, a solution of 765 mg of N-benzyl-N-phenylglycine
methyl ester in 3 ml of TFIF is added. After stirring a solution of 700 mg of
(R)-
3-(t-butyldimethylsiloxy)tetxadecanal (Example Bb) in 5 rnl of THF is added
dropwise. After stirring at -75oC the reaction mixture is poured into aqueous
potassium hydrogen sulphate and extracted with ether. The ether phase is
dried, concentrated, partitioned between hexane and methanol/water (7:3), the
1o hexane phase is dried and concentrated and the residue is chromatographed
on
silica gel with pentane/ether (5:1). There are obtained 96.3 mg of methyl (5R)-
2-
(N-benzylanilino)-5-(t-butyldimethylsiloxy)-3-hydroxyhexadecanoate,
diastereomer A, MS: 540 (M+ ~-C,IHHg ~ ), and 142.8 mg of methyl (5R)-2-(N-
benzylanilino)-5-(t-butyldimethylsiloxy)-3-hydroxyhexadecanoate, diastereo-
mer B, MS: 540 (M+~-C4Hg ~ ), and 313.4 g of a mixture of the above two
diastereomers.
b) 134 mg of diastereomer B are suspended in 3 ml of 0.1N NaOH and
treated with sufficient acetonitrile to farm a clear solution. After stirring
the
mixture is poured into aqueous potassium hydrogen sulphate and extracted
with ether and the ether phase is dried and concentrated. After chromato-
graphy on silica gel with methylene chloride/methanol (9:1) there are obtained
108 mg of (5R)-2-(N-benzylanilino)-5-(t-butyldimethylsiloxy)-3-hydroxyhexa-
decanoic acid, diastereomer 8, MS: 526 (M+~-C4Hg~).
c) Analogously, from diastereomer A from a) there is obtained (5R)-2-(N-
benzylanilino)-5-(t-butyldimethylsiloxy)-3-hydroxyhexadecanoic acid,
diastereomer A, MS: 526 (M+~-C4Hg~).
d) 1.1 g of diastereomer B from b), 1.1 g of HBTU, 0.5 g of triethylamine and
2 g of molecular sieve 4~ are stirred in 50 ml of ac~torutrile. After
filtration
and concentration the product is chromatographed on silica gel with
methylene chloride. There are thus obtained 1.04 g of 3R,4R (or 3S,4S)-3-(N-
benzylanilino)-4-[(R)-2-(t-butyldimethylsiloxy)tridecyl]-2-oxetanone,
diastereomer B, MS: 566 (M+H)+.
e) Analogously, from diastereomer A from c) there is obtained 3S,4S (or
3R,4R)-3-(N-benzylanilino)-4-[(R)-2-(t-butyldimethylsiloxy)tridecyl]-2-
oxetanone, diastereorner A, MS: 566 (M+H)+.
~~~~~6'~
-~- _
f) 1.0 g of diastereomer 8 from d) and 0.8 g of Pd/C (10%) are hydrogenated
in 30 ml of THP. Thereupon, the mixture is filtered and concentrated. There
are thus obtained 834 yng of 3R,4R (or 3S,4S)-3-anilino-4-((R)-2-(t-butyldi-
methylsiloxy)tridecyl]-2-oxetanone, diasteromer B, MS: 475 (M+~).
g) Analogously, from diastereomer A from e) there is obtained 3S,4S (or
3R,4R)-3-anilino-4-[(R)-2-(t-butyldinaethylsiloxy)tridecyl]-2-oxetanone,
diastereomer A, MS: 475 (M~~ ~ ).
h) The products of f) arid g) are converted individually analogously to
Example Be) into 3R,4R (or 3S,4S)-3-anilino-4-[(R)-2-hydroxytridecyl]-2
to oxetanone, diastereomer B, m.p. 104°C, and, respectively
3S,4S (or 3R,4R)-3-anilino-4-[(R)-2-hydroxytridecyl]-2-oxetanone,
diastereomer A, m.p. 60-62°C, the alcohol starting materials for
Example 79.
The acids of the formula Qa-OH axe known or can be prepared in analogy
to the known acids, e.g. by saponifying a corresponding lower alkyl ester in a
solvent such as acetone or methanol with an alkali metal hydroxide such as
potassium hydroxide in an alcohol such as ethanol or methanol. Thus, the
acid starting materials of Examples 2d) and 2e) hereinafter can be prepared as
follows:
A solution of 3.8 g of ethyl 2-propylmalonamidate in 30 ml of acetone is
2o treated with 22 ml of 1N KOH in ethanol and stirred fox 4 hours, then
concentrated, taken up in sodium bicarbonate solution and extracted with
ethyl acetate. The aqueous phase is acidified to pH 2 at OoC with hydrochloric
acid and extracted with ethyl acetate. The ethyl acetate phase is washed with
brine, dried, concentrated and the residue is recrystallized from ethyl
acetate/ether. There are obtained 1.96 g of 2-propylmalonic acid monoamide,
rn.p. 137oC.
The 2-phenethylmalonamic acid, m.p. 141.5oC, the acid starting material
for Examples 58-59, is prepared analogously from ethyl 2-phenethyl-
malonamate.
3o The (+) and (-)-2-isopropylmalonic acid monoamide (the amide starting
material of Example 11) can be prepared as described hereinafter:
5.5 g of rac-2-isopropylmalonic aad monoamide and 12.0 g of quinidine
are dissolved in 100 ml of boiling water, seeded with a few crystals of the
-26-
quinidine salt of (S)-(+)-2-isopropylmalonic acid monoamide and then
crystallized out. The crystallizate is filtered off under suction, washed with
water and ether and dried; there are thus obtained 8.3 g of the quinidine salt
of
(S)-(+)-2-isopopylmalonic acid monoamide. This salt is dissolved in 10 percent
hydrochloric acid and left at 5oC, the separated crystals are filtered off
under
suction, washed with water, dried and recrystallized again from water with the
addition of a few drops of 1N hydrochloric acid. There are thus obtained
720 mg of (S)-(+)-2-isopropylmalonic acid monoamide, m.p. 174°C, (a] a
=
+45.6° (ethanol, c = 1).
The mother liquor resulting in the crystallization of the quinidine salt is
made acid with 10% hydrochloric acid and left at SoC, the separated crystals
are
filtered off under suction, washed with water, dried and again xecrystallized
from water with the addition of a few drops of 1N hydrochloric acid. There are
thus obtained 850 rng of (R)-(-)-2-isopropylmalonic aad rnonoamide, m.p.
176°C, [a]~= -45.6° (ethanol, c =1).
The acid starting material for Example 42 can be prepared as follows:
a) 20.6 g of thiomorpholine are added dropwise to a solution of 13.6 g of
methyl rnalonate monochloride in 100 rnl of methylene chloride. After
stirring the mixture is diluted with 200 ml of methylene chloride, washed with
2o water in a separating funnel, then dried, filtered and evaporated. The
residue
is purified by chromatography on siliea gel with rnethylene chloride and then
methylene chloxide/acetone (1:1). There are obtained 17.6 g of methyl tetra-
hydro-(3-oxo-4H-1,4-thiazine-4-propionate.
b) 85 ml of 1N potassium hydroxide solution are added dropwise to a
solution of 17.3 g of the ester from a) in 170 rnl of acetone: After stirring
and
filtering the mixture is evaporated and the residue is triturated in 200 ml of
acetone and then filtered. The filter cake is washed with acetone and dried.
An
aqueous solution of the resulting potassium salt is chromatographed with
water on a ration exchanger column. The 2luate is concentrated to dryness and
the residue is triturated with ether and filtered off. There are obtained 13 g
of
tetrahydro-(3-oxo-4H-1,4-thiazine-4-propionic acid, m.p. 119-120oC.
The acid starting material fox Example 44 is prepared as follows:
a) 33 ml of 1N potassium hydroxide solution are added dropwise to a
solution of 5.6 g of methyl 1-carbamoylcyclopentanecarboxylate in 66 ml of
~~3~9~'~
-27_
acetone. Afker stirring the mixture is treated with 250 ml of acetone and the
separated potassium salt is filtered off and then washed with acetone and
dried.
b) A solution of the 5.79 g of potassium salt obtained in 35 ml of water is
acidified to pI-I 1 with 4 ml of cone. hydrochloric acid at OoC. The
precipitate is
filtered o.ff and washed with water and then with diethyl ether. 3.5 g of 1-
carbamoylcydopentanecarboxylic acid axe obtained after drying,
The acid starting material for Example 46 can be prepared as follows:
A solution of 10.4 g of monomethyl methoxymalonate in 70 ml of
1o methylene chloride is added dropwise at -lOoC to 26 ml of 25 percent
aqueous
ammonia. After stirring the mixture is evaporated and the residue is dissolved
in water and chromatographed on a canon exchanger with water. The eluate is
concentrated and the residue is triturated with diethyl ether and filtered
off.
The precipitate is washed with water and dried. There are obtained 8.9 g of
15 methoxymalonamic acid, m.p. 128-130oC.
The acid starting material for Example 49 can be prepared as follows:
A solution of 1.79 g of carbamoylmethylthioacetic acid in 42 ml of water is
treated with 3.71 g of monoperoxyphthalic acid magnesium salt hexahydrate.
After stirring the mixture is filtered and the filtrate is concentrated and
20 acidified with 2 ml of cone. hydrochloric acid. After filtration the
filtrate is
percolated over a cation exchanger, eluted with water and the eluate is
evaporated to dryness. The residue is suspended in acetone and filtered off.
It is
washed with acetone and dried. There are obtained 1.65 g of rac-
((carbamoylmethyl)sulphinyl]acetic acid, m.p. 137-138oC.
25 The lower alkyl esters corresponding to the ands of formula Qa-OH are
known or can be prepared in analogy to the known esters, e.g. as described
hereinafter starting from the monoestex of the formula H-(X)h-COOR",
wherein R" is lower-alkyl, via the dicarboxylic acid monoester of the formula
HOCO-(X)n-COOR". Thus, the starting acid of Example 2f) can be prepared as
3o follows:
a) 48 ml of a 1.6M n-butyllithium solution in hexane are added dropwise at -
l5oC to 11 ml of diisopropylamine and 5 g of 4~ molecular sieve in 75 ml of
THF. After 15 minutes the reaction mixture is cooled to -78oC and a solution
of
9.5 g of ethyl 1,3-dioxolane-2-carboxylate in 50 ml of TIFF is added dropwise.
-28-
After stirring for 20 minutes C02 is introduced at a temperature below -70oC.
After saturation the mixture is stirred at -75oC for 20 minutes and then
warmed to room temperature. After volatizadon of the C02 gas the reaction
mixture is concentrated, the residue is treated with saturated bicarbonate
solution and ethyl acetate, the ethyl acetate phase is discarded, the aqueous
phase is acidified to pH 2 with potassium hydrogen sulphate and extracted
with ethyl acetate. The ethyl acetate phase is dried and concentrated.
b) 0.93 ml of isobutyl chloroformate in 5 ml of THF is added dropwise at OoC
to a solution of 1.08 g of the product from a), 1.1 ml of triethylamine and 3
g of
molec~.llar sieve 4th in 30 ml of TI-IF. After stirring for 40 minutes ammonia
gas is introduced for 10 minutes and the reaction mixture is subsequently
stirred overnight. Thereupon, it is filtered, the filtrate is concentrated and
the
residue is chromatagraphed on silica gel with methylene chloride/methanol
(95:5). There are thus obtained X20 mg of ethyl 2-carbamoyl-1,3-dioxolane-2-
carboxylate, m.p. 99-100oC.
c) A solution of 190 mg of the product from b) in 10 ml of methanol is
treated with 1 rnl of 2N ICON in methanol and stixred at room temperature for
90 minutes. Thereupon, a solution of 280 mg of potassium hydrogen sulphate
in 1 ml of water is added, the reaction mixture is suetion filtered and the
filtrate is evaporated. There is thus obtained 2-carbamoyl-1,3-dioxolane-2-
carboxylic acid.
2-Carbamoyl-m-dioxane-2-carboxylic acid (the acid starting material for
Example 3m) is obtained analogously from ethyl m-dioxane-2-carboxylate.
The acids of the formula (R3,R4)NCO(X)n-COON in which X is a group
=CHN(R,Ro) can be prepared starting from the corresponding dicarboxylic acid
monoester of the formula HOCO-X-COOK" via a corresponding succinimide
and the corresponding amide ester of the formula H2NC0-X-COOK", e.g. as
described hereinafter for the acid starting material of Example 9.
a) 4.54 g of dicyclohexylcarbodiimide; 4.16 g of rnonoethyl acetamino-
malonate and 2.53 g of N-hydroxysuccinimide are added to 54 ml of THF at
OaC. After stirring for 1 hour the mixture is left to warm to room temperature
and is stirred overnight. 'Then, it is cooled to OoC and filtered. The
filtrate is
treated with 20 ml of 25% aqueous ammonia solution, left to stand at room
temperature over the day and at 4oC overnight. Then, the solution is
evaporated and the residual aqueous solution is treated with sodium bicar-
2~~~~'~
_29_
bonate. The aqueous phase is separated, the organic phase is washed with
sahirated sodium chloride solution, then dried and concentrated. The residue
is filtered in hexane containing ethyl acetate. The crystals obtained are
washed
with ether and then dried. There are obtained 1.2 g of [D,L]-N-acetyl-2-carba-
moylglycxne ether ester, m.p. 126-128aC.
b) A solution of 5.8 ml of 1N potassium hydroxide is added dxapwise to a
suspension of 1.09 g of the amide ester from a) in 7 ml of acetone. After
stirring
for 3 hours the mixture is concentrated and the residue is dissolved in
aqueous
sodium bicarbonate solution. The solution is extracted with ethyl acetate, the
to aqueous phase is acidified to pH 3 with hydrochloric acid while cooling and
then percolated over an ion exchanger. The eluate is concentrated to dryness
and the residue is triturated v,~ih acetone. There are obtained 500 mg of
[D,L]-N-
acetyl-2-carbamoylglycine, m.p. 120oC (decomposition).
Acid starting materials of the formula (R3,R4)NCO(X)n-COON in which
at least one of R3 and R4 is different from H can be prepared by reacting the
corresponding acid ester of the formula HOC(O)-(X)n-C(O)O-R" with an amine
HN (R3,R4).
Thus, the acid starting material of Example 10d) can be prepared as
follows:
A solution of 3 g of rnonomethyl malonate in 15 ml of 40% aqueous
dimetlhylamine is concentrated after stirring for 18 hours, filtered through a
strongly acidic cation exchanger, concentrated to dryness and crystallized
from
chloroform. Concentration of the mother liquor and crystallization from ether
yield 1.3 g of dimethylcarbamoylacetic acid, m.p. 72-76oC.
The oxetanones of formula I have valuable pharmacological properties.
In particular, they inhibit pancreas lipase and can accordingly be used in the
control or prevention of obesity, hyperlipernia, atherosclerosis and
arterioscle-
rosin.
The inhibition of pancreas lipase by the axetanones of formula I can be
demonstrated experimentally by measuring titrimetrically the oleic acid
liberated in the cleavage of triolein by hog panereas lipase. To an emulsion
which contains 1 rnlvl of taurodeoxycholate, 9 mM of taurocholate, 0.1 mM of
cholesterol, 1 mM of egg lecithin, 15 mg/ml of ESA, 2 mM of Tris HCI,
100 rnIdl of sodium chloride, 1 mM of calcium chloride and triolein as the
-30-
substrate is added the compound of formula I dissolved in ethanol or dimethyl
sulphoxide (10°l° of the emulsion volume) and the reaction is
started by the
addition of 1-3 ~,g of hog pancreas lipase. The pH is held at 8 during the
reaction by the addition of sodium hydroxide solution. The IC50 is calculated
from the consumption of sodium hydroxide solution determined during
minutes. The IC50 is that concentration at which the lipase activity is
inhibited to half of the maximum. The following Table contains the IC50
values determined for the compounds of formula I.
Example1a,11a1b,11b2b,12b 2d 2e 2f 2g1.'13a2g2
IC50 0.0320.025 0.063 0.12 0.0510.47 0.052 0.013
Example3c 3d 3e 3f 3g 3h 3i 3j
~~
IC50 0.39 0.90 1.41 0.0830.294 2.1 0.42 0.16
Example3k 31 4a 6b,14c 7b 10a 10b 10c
IC50 0.12 0.78 0.16 0.056 0.079 0.0830.042 0.1
Example10d 10e 10f 37 43 45 51 78c 78d
ICSp 0.47 0.027 0.32 0.034 0.120.047 0.36 0.28 0.051
The acute to~daty (after single oral administration to mice) amounts to
more than 5000 rng/kg for the products of Examples 3d, 3h, 3i, 31, 4a and 10a,
b,
a and f.
The oxetanones of formula I can be used as medicaments, e.g. in the form
of pharmaceutical preparations. The pharmaceutical preparations can be
administered orally, e.g. in the form of tablets, coated tablets; dragees,
hard and
soft gelatine capsules, solutions, emulsions or suspensions.
z~~~~~~
-31-
For thE~ manufacture of pharmaceutical preparations the products in
accordance with the invention can be processed with pharmaceutically inert,
inorganic ox organic carriers. Lactose, maize starch or derivatives thereof,
talc,
stearic acid or its salts can be used, for example, as such carriexs far
tablets,
coated tablets, dragees and hard gelatine capsules. Suitable carriers far soft
gelatine capsules are, for example, vegetable oils, waxes, fats, semi-solid
and
liquid polyols; depending on the nature of the active ingredient no carriers
axe,
however, generally required in the case of soft gelatine capsules. Suitable
carriers for the manufacture of solutions and syrups are, for example, water,
~o polyols, sacchaxose, invert sugar and glucose.
Moreover, the pharmaceutical preparations can contain preserving
agents, solubilizers, stabilizing agents, wetting agents, emulsifying agents,
sweetening agents, colouring agents, flavouring agents, salts for varying the
osmotic pressure, buffers, coating agents or antioxidants. They can also
contain
15 still other therapeutically valuable substances.
As mentioned earlier, medicaments containing an oxetanone of formula
I are likewise an object of the present invention, as is a process for the
manufacture of such medicaments which comprises bringing an oxetanone of
formula I and, if desired, one or more other therapeutically valuable
2o substances into a galenical administration form. As mentioned; the
compounds of formula I can be used in the control or prevention of illnesses,
especially in the control or prevention of obesity, hyperlipemia,
atherosclerosis
and arteriosclerosis. The dosage can vary within wide limits and will, or
course, be filled to the individual requirements in each particular case. In
25 general, in the case of oral administration a daily dosage of about 0.1 mg
to
100 mg/kg body weight should be appropriate.
'The oxetanones of formula I can also be added to industrially-produced
foodstuffs, whereby fats, oils, butter, margarine, chocolate and other
confectionery goods especially come into consideration. Such industrially-
3o produced foodstuffs, which can contain about 0.1 to 5 wt.% of an oxetanone
or
formula I, and their manufacture are likewise objects of the present
invention.
The following Examples illustxate the present invention in more detail,
but they axe not intended to limit its scope in any manner. All temperatures
are given in degrees Celsius.
35 Example 1
~~~~r ~~~
-32-
A solution of 574 mg of (3S,4S)-3-ethyl-4-[(R)-2-hydroxynonadecyl]-2-
oxetanone, 525 mg of triphenylphosphine, 290 mg of 2-isopropylmalonic acid
monoamide and 2 g of molecular sieve (4.~.) in 10 ml of THF are treated which
skirring at Oo with 0.4 ml of diisoprapyl azodicarboxylate. After stirring at
Oo for
30 minutes and at room temperature for 1 hour the reaction mixture is
filtered, the molecular sieve is washed with ether and the solvent is
evaporated. The residue is dissolved in hexane and extracted with
methanol/water (7:3). The hexane phase is diluted with ether, dried and
evaported. The residue is chromatographed on silica gel with methylene
chloride/ether (9:1). There are obtained
a) 239 mg of (S)-[[(2S,3S)-3-ethyl-4-oxo-2-oxetanyl]methyl]octadecyl (R or S)-
2-
isopropylmalonamate, m.p. 115°, and
b) 266 mg of (S)-[[(2S,3S)-3-ethyl-4-oxo-2-oxetanyl]methyl]octadecyl (S or R)-
2-
isopropylmalonamate, m.p. 118°.
Example 2
Analogously to Example 1, from (3S,4S)-3-hexyl-4-[(R)-2-hydroxytridecyl]-
2-oxetanone and 2-isopropylmaloruc acid monoamide there are obtained
a) (Sr1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (R or S)-2-
isopropylmalonamate, m.p. 136°, and
b) (S~1-[[(2S,3S~3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (S or R)-2-
isopropylmalonamate, m.p. 82°;
c) from (3S,4S)-3-hexyl-4-((R)-2-hydroxytridecyl]-2-oxetanone and
isopropylidenemalonic acid monoamide them is obtained (S)-1-[[(2S,3S)-3-
hexyl-4-oxo-2-oxetanyl]methyl]dodecyl 2-carbamoyl-3-methylcrotonate, m.p.
108-111 °;
d) from (3S,4S)-3-hexyl-4-[(R)-2-hydroxytridecyl]-2-oxetanone and 2-
propylmalonic acid monoamide there is obtained (S)-1-[[{2S,3S)-3-hexyl-4-oxo-
2-oxetanyl]methyl]dodecyl (RS)-2-carbarnoylvalerate (epimers 1:1), m.p. 92-
94°;
e) from (3S,4S)-3-ethyl-4-[(R)-2-hydroxynonadecyl]-2-oxetanone and 2-
propylmalonic acid monoamide there is obtained (S)-1-[[(2S,3S)-3-ethyl-4-oxo-
2-oxetanyl]methyl]octadecyl (RS)-2-carbamoylvalerate (epimers 1:1), m.p. 78-
80°;
-33- ~~~~~~3~
f) from (3S,4S)-ethyl-4-((R)-2-hydroxynonadecyl]-2-oxetanone and 2-
carbamoyl-1,3-dioxolan-2-carboxylic acid there is obtained (S)-1-([(2S,3S)-3-
ethyl-
4-oxo-2-oxetanyl]methyl]oetadecyl 2-carbamoyl-1,3-dioxolane-2-carboxylate,
m.p. 95°;
g) from (3S,4S)-3-ethyl-4-[(R,10Z,13Z)-2-hydroxy-10,13-nonadecadienyl]-2
oxetanone and 2-isopropylmalonic acid monoamide there are obtained
1. (all Z,S)-1-[[(2S,3S)-3-ethyl-4-oxo-2-oxekanyl]methyl]-9,12-octadecadienyl
(R
or S)-2-isopropylmalonamate, m.p. 87-88° (from ether) and
2. (all Z,S)-1-[[(2S,3S)-3-ethyl-4-oxo-2-oxetanyl]methyl]-9,12-octadecadienyl
(S
or R)-2-isopropylmalonamate, IR: 3393, 1840, 1716, 1647, 1185 czW 1.
Exam le 3
The following ester amides are obtained analogously to Example 1 by
reacting (3S,4S)-3-hexyl-4-[(R)-2-hydroxytridecyl]-2-oxetanone with the
following amides:
a) with 4-carbamoylbutyric acid the 4-carbatnoylbutyric acid (S)-1-[((2S,3S)-3-
hexyl-4-oxo-2-oxetanyl]methyl]dodecyl ester, m.p. 67-68°,
b) with 3-carbamoylpropionic and the 3-carbamoylpropionic acid (S)-1-
[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl ester, m.p. 50.5-51°,
c) with 2-carbamoylacetic acid the 2-carbamoylacetic acid (S)-1-[[(2S,3S)-3-
hexyl-4-oxo-2-oxetanyl]methyl]dodecyl ester, m.p. 86.5-87°,
d) with oxalic acid monoamide the (S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-
oxetanyl]methyl]dodecyi oxamate, m.p. 77-78°,
e) with methylcarbamoylacetic acid the (S)-1-[I(2S,3S)3-hexyl-4-oxo-2-
oxetanyl]methyl]dodecyl N-methylmalonamate, m.p. 63-67°,
f) with rac-2-carbamoyl-4-methylvaleric acid the (S)-1-[[(2S,3S)-3-hexyl-4-
oxo-2-oxetanyl]methyl]dodecyl (RS)-2-carbamoyl-4-methylvalerate (epimere
1:1), m.p. 102-104°,
g) with 1-carbamoylcyclohexanecarboxylic acid the (S)-1-[[(2S,3S)-3-hexyl-4-
oxo-2-oxetanyl]methyl]dodecyl 1-carbamoyltyclohexanecarboxylate, m.p. 50-
52°,
-
h) with 2,2-dimethylmalonamidic acid the (S)-1-[[(2S,3S)3-hexyl-4-oxo-2-
oxetanyl]methyl]dodecyl 2,2-dimethylmalonarnate, (a]p = -23.8° (CHC13,
c = 0.9%),
i) with roc-2-methylmalanamidic acid the (S)-1-[[(2S,3S)3-hexyl-4-oxo-2-
oxetanyl]methyl]dodecyl (RS)-2-methylmalonamate (epimers 1:1), m.p.
107-108°,
j) with roc-2-ethylmalonamidic acid the (S)-1-[[(2S,3S)3-hexyl-4-oxo-2-
oxetanyl]methyl]dadecyl (RS)-2-ethylmalanamate (epirners 1:1), m.p. 87-
90°,
lc) with roc-2-butylmalonamidic acid the (S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-
to oxetanyl]methyl]dodecyl (RS)-2-butylmalonamate (epimers 1:1), m.p. 96-
98°,
1) with 2,2-diethylmalonamidic acid the (S)-1-[[(2S,3S)-3-hexyl-4-axo-2-
oxetanyl]methyl]dodecyl 2,2-diethylmalonamate, [a]p =-21.1° (CHCl3, c
=1%),
m) with 2-carbamoyl-m-diaxane-2-carboxylic acid the (S)-1-[[(2S,3S)-3-hexyl-4-
oxo-2-oxetanyl]methyl]dodecyl 2-carbamoyl-m-dioxane-2-carboxylate, m.p.
51°.
15 Example 4
Analogously to Example 1, from (3S,4S)-3-ethyl4-[(R)-2-
hydroxynonadecyl]-2-axetanane and
a) 1-carbamoylcyclohexanecarboxylic aad there is obtained (S)-1-[[(2S,3S)-3-
ethyl-4-axo-2-oxetanyl]methyl]octadecyl 1-carbamoylcyclahexanecarboxylate,
2o m.p. 7&79°, and
b) 2-carbamoyl-m-dioxane-2-earbaxylic acid there is obtained (S)-1-[((2S,3S)-
3-ethyl-4-oxa-2-oxetanyl]methyl]octadecyl 2-carbamoyl-m-dioxane-2-
carbaxylate, m.p. 79°.
Example 5
25 Analogously to Example 1, from (3R,4R or 3S,4S)-4((R)-2-hydroxytridecyl]-
3-pentylthio-2-axetanane and 2-isopropylmalonic acid amide there are
obtained
a) (S)-1-([(2R,3R ar 2S,3S)-4-oxo-3-pentylthio2-oxetanyl]methyl]dodecyl (R or
S)-2-isopropylmalonamate, MS: 354 [Mv ~-(2-isopropylmalonic acid amide)]; IR
30 (cm 1): 3397, 2924,1829,1731,1657,1120, and
~~~~a9~'~
-35-
b) (S)-1-[[(2R,3R or 2S,3S)-4-oxo-3-pentylthio2-oxetanyl]methyl]dodecyl (S or
R)-2-isopropylrnalonamate, m.p. T7-78° (diethyl ether).
Example 6
Analogously to Example 1, from (3S,4S or 3R,4R)-4[(R)-2-hydroxytridecyl]-
3-pentylthio-2-oxetanone and 2-isopropylmalonic acid amide there are
obtained
a) (S)-1-[[(2S,3S or 2R,3R)-4-oxo-3-pentylthio-2-oxetanyl]methyl]dodecyl (R or
S)-2-isopropylmalonamate, m.p. 133° (ethyl acetate), and
b) (S)-1-[[(2S,3S or 2R,3R)-4-oxo-3-pentylthio-2-oxetanyl]methyl]dodecyl [S:R
or R:S(2:1)]-2-isopropylmalonamate, m.p. 102-104° (ethyl acetate).
Example 7
Analogously to Example 1, from 3-benzyl-4-[(R)-2-hydroxytridecyl]-~2-
oxetanone and 2-isopropylmalonic acid monoamide there is obtained an
epimer rnixure which is separated by chromatography on silica gel with ethyl
t5 acetate/hexane/ methylene chloride (1:2:2) into
a) (S~1-[[(2R,3R)-3-benzyl-4-oxo-2-oxetanyl]methyl]dodecyl (R or S)-2-
isopropylmalonamate, m.p. 85-87° (rnethylene chloride), and
b) (S)-1-[[(2R,3R)-3-benzyl-4-oxo-2-oxetanyl]methyl]dodecyl (S or R)-2-
isopropylmalonarnate, m.p. 108-110° (methylene chloride).
Example 8
Analogously to Example 1, from 3-benzyl-4-[(R)-2-hydroxytridecyl]-2-
oxetanone and 2-isopropylmalonic acid monoamide there is obtained an
epimer mixture which is separated by chromatography on silica gel with ethyl
acetate/ hexane/methylene chloride (1:2:2) into
a) (S~1-[[(2S,3S)-3-benzyl-4-oxo-2-oxetanyl]methyl]dodecyl (R or S)-2-
isopropylmalonamate, m.p. 107-108° (methylene chloride); and
b) (S)-1-[[(2S,3S)-3-benzyl-4-oxo-2-oxetanyl]methyl]dodecyl (S or R)-2-
isopropylmalonamate, m.p. 148-14g° (methylene chloride).
Example 9
~~~ >~~
-
1.03 g of di-t-butyl azodicarboxylate are added to a suspension, cooled to -
IOoC, of 1.06 g of (3S,4S)-3-hexyl-4-[(R}-2-hydroxytxidecyl]-2-oxetanone, 480
mg
of [D,L]-N-acetyl-2-carbamoylglycine, 1.1 g of triphenylphospine and 1.2 g of
molecular sieve 4~ in 12 rnl of THF. After stirring at OoC for 1 hour and at
room temperature overnight the reaction mixture is worked-up analogously
to that described in Example 1. There are obtained
a) 190 mg of (S}-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (R or S)-
2-acetamidomalonamate, m.p. 125-126°, and
b) 100 mg of (S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (RS)-2-
acetamidomalonamate (epimers 1:1), m.p. 110-116°, [a]p = -8° (c
= 0.5, CHC13)
Example 10
The following ester amides are obtained analogously to Example 1, but
using the following amides:
a) from oxalic and monoamide the (S)-1-[[(2S,3S)-3-ethyl-4-oxo-2-
oxetanyl]methyl]octadecyl oxamate, m.p. 99-100°,
b) from 2-carbamoylacetic acid the (S)-1-[[(2S,3S)-3-ethyl-4-oxo-2-
oxetanyl]methyl]octadecyl malonamate, m.p. 90.5-91.5°,
c) from methylcarbamoylacedc acid the (S)-1-[[(2S,3S)-3-ethyl-4-oxo-2-
oxetanyl]methyl]octadecyl N-methylmalonamate, m.p. 84-85°,
2o d) from dimethylcarbamoylacetic acid the (S)-1-[[(2S,3S)-3-ethyl-4-oxo-2-
oxetanyl]methyl]octadecyl N,N-dimethylmalonamate, m.p. 66-67°,
e) from rac-2-ethylmalonamidic acid (S)-1-[[(2S,3S-3-ethyl-4-oxo-2-
oxetanyl]methyl]octadecyl (RS)-2-methylmalonamate (epimers 1:1), m.p. 91.5-
92°,
z5 f) from 3-carbamoylpropioruc acid the (J)-1-[[(2S,3S)3-ethyl-4-oxo-2-
oxetanyl]methyl]octadecyl succinamate, m.p. 74.5-75.7°.
Example 11
Analogously to Example 1, but using (S)-(t)- or (~-2-isopropylmalonic
acid monoamide, there are obtained
-37-
a) (S)-[[(2S,3S)-3-ethyl-4-oxo-2-oxetanyl]methyl]octadecyl (R)-2-
isopropylmalonamate, rn.p. 115°, and
b) (S)-[((2S,3S)-3-ethyl-4-axo-2-oxetanyl]methyl]octadecyl (S)-2-
isopropylmalonarnate, m.p. 118°.
Example 12
Analogously to Examples 1,2a),b) and 11, from (3S,4S)-3-hexyl-4-[(R)-2-
hydroxytridecyl]-2-oxetanone and (t)- and (S)-(+)-2-isopropylmalonic acid
rnanoamide there are obtained
a) (S)-1-[((2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (R)-2-
1o isopropylmalonamate, m.p. 136°, and respectively,
b) (S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (S)-2-
isopropylmalonamate, m.p. 82°,
c) from (3S,4S)-3-hexyl-4-[(R)-2-hydroxytridecyl]-2-oxetanone and 2-
propylmalonic acid monoamide, after separation by chromatography on silica
gel with methylene chloride/ acetonitrile (85:15),
1. (S)-1-([(2S,3S)r3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (R or S)-2-
carbamoylvalerate, m.p. 113° (from methanol/water), and
2. (S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (S or R)-2-
carbamoylvalerate, m.p. 85°.
2o Example 13
Analogously to Examples 1, 2g) and 11, from (3S,4S)-3-ethyl-4-
[(R,10Z,13Z)-2-hydroxy-10,13-nonadecadienyl]-2-oxetanone and (~)- and (S)-(+)-
2-isapropylmalonic and monoamide there are obtained
a) (all Z,S)-1-[[(2S,3S)-3-ethyl-4-oxo-2-oxetanyl]methyl]-9,12-octadecadienyl
(R)-2-isopropylmalonamate, m.p. 87-88° (fxom ether), and
b) (all Z,S)-1-[[(2S,3S)-3-ethyl-4-oxo-2-oxetanyl]methyl]-9,12-octadecadienyl
(S)-2-isopropylmalonamate, m.p. 109° (from aqueous methanol).
Example 14
-38-
Analogously to Examples 1, 6 and 11, from (3S,4S or 3R,4R)-4-((R)-2-
hydroxytridecyl]-3-pentylthio-2-oxetanone and (+_)- and (S)-(+)-2-
isopropylmalanarnide there are obtained
a) (S)-1-[[(2S,3S or 2R,3R)-4-oxo-3-pentylthio-2-oxetanyl]methyl]dodecyl (R)-
2-isopropylmalonamate, m.p. 133° (ethyl acetate),
b) (S)-1-([(2S,3S or 2R (S)-2-isopropylmalonamate, rn.p. 93° (diethyl
ether/hexane) and
c) (S)-1-[[(2S,3S or 2R,3R)-4-oxo-3-pentylthio-2-oxetanyl]methyl]dodecyl [S:R
(2:1)]-2-isopropylmalonamate, m.p. 102-104° (ethyl acetate).
1o Example 15
Analogously to Examples 1 and 11, from (3S,4S or 3R,4R)-benzyl 4-[(R)-2-
hydroxytridecyl]-2-oxo-3-oxetanecarbamate and (S)-(+)-2-isopropylmalonic acid
monoamide there is obtained (S)-1-([(2S,3S or 2R,3R)-3-[1-
(benzyloxy)formarnido]-4-oxo-2-oxetanyl)methyl]dodecyl (S)-2-
15 isopropylmalonamate, m.p. 133° (from ether/hexane).
Example 16
Analogously to Examples 1 and 11,
a) from (3R,4R)-4-[(R)-2-hydroxytridecyl]-3-(phenylthio)-2-oxetanone and
(S)-(+)-2-Isopropylmalonic acid monoamide there is obtained (S)-1-[[(2R,3R)-4-
20 oxo-3-(phenylthio)-2-oxetanyl]methyl]dodecyl (S)-2-isopropylmalonamate,
m.p. 88° (ether),
b) from (3S,4S)~-4-[(R)-2-hydroxytridecyl]-3-(phenylthio)-~-oxetanone and 2-
isopropylmalonic acid monoarnide there is obtained (S)-1-[[(2S,3S)-4-oxo-3-
(phenylthio)-2-oxetanyl]methyl]dodecyl (RS)-2-isopropylmalonamate (1:1
25 epimers), m.p. 109° (ether), and
c) from (3R,4R)-4-[(R)-2-hydroxytridecyl]-3-(phenylthio)-2-oxetanone and
2-isopropylmalonic acid monoamide there are obtained the same products as
in a) and (S)-1-([(2R,3R)-4-oxo-3-(phenylthio)-2-oxetanyl]methyl]dodecyl 2-
isopropylmalonamate (R:S = 7:1), m.p. 85° (ether).
30 Exam,~le 17
1~~~.~~~
-39-
Analogously to Examples 1 and 11, .from (S)-(+)-2-isopropylmalonic acid
monoamide and the following alcohols there are obtained the following esters:
a) From (3S,4S or 3R,4R)-4-((R)-2-hydroxynonadecyl]-3-(methylthio)-2-
oxetanone the (S)-1-([(2S,3S or 2R,3R)-3-(methylthio)-4-oxo-2-
oxetanyl]methyl]octadecyl (S)-2-isopropylmalonamate, m.p. 133° (from
ether),
b) from (3R,4R or 3S,4S)-4-[(R)-2-hydroxynonadecyl]-3-(methylthio)-2-
oxetanone the (S)-1-([(2R,3R or 2S,3S)-3-(methylthio)-4-oxo-2-
oxetanyl]methyl]octadecyl (S)-2-isopropylmalonarnate, m.p. 103° (from
ether),
c) from (3S,4R cr 3R,4S)-4-[(R)-2-hydroxynonadecyl]-3-(methylthio)-2-
l0 oxetanone the (S)-1-[[(2R,3S or 2S,3R)-3-(methylthio)-4-oxo-2-
oxetanyl]methyl]octadecyl (S)-2-isopropylmalonamate, mp. 96° (from
ether/hexane), and
d) from (3R,4S or 3S,4R)-4-[(R)-2-hydroxynonadecyl]-3-(methylthio]-2-
oxetanone the (S)-1-([(2S,3R or 2R,3S)-3-(methylthio)-4-oxo-2-
15 oxetanyl]methyl]octadecyl (S)-2-isopropylmalonarnate, m.p. 120°
(from
ether/hexane).
Example 18
Analogusly to Examples 1 and 11, from (R)-(-)-2-isopropylmalonic acid
monoamide and
2o a) (3S,4S or 3R,4R)-4-[(R)-2-hydroxynonadecyl]-3-(methylthio)-2-oxetanone
there is obtained (S)-1-[[(2S,3S or 2R,3R)-3-(methylthio)-4-oxo-2-
oxetanyl]methyl]octadecyl (R)-2-isopropylmalonarnate, m.p. 96° (from
ether),
b) (3R,4R or 3S,4S)-4-[(R)-2-hydroxynonadecyl]-3-(methylthio)-2-oxetanone
there is obtained (S)-1-[[(2R,3R or 2S,3S)-3-(methylthio)-4-oxo-2-
25 oxetanyl]methyl]octadecyl (R)-2-isopropylmalonamate, m.p. 87° (from
ether/hexane).
Example 19
In analogy to Examples 1 and 11, from (S)-(+)-2-isopropylmalonic acid
monoamide and the following alcohols there are obtained the following esters:
2~ ~ ~~~~~
-40-
a) From (3S,4S or 3R,4R)-3-(benzylthio)-4-[(R)-2-hydroxytxidecyl]-2-
oxetanone the (S)-1-[[(2S,3S or 2R,3R)-3-(benzylthio)-4-oxo-2-
oxetanyl]methyl]dodecyl (S)-2-isopropylmalonamate, m.p. 84° (from
pentane),
b) from (3R,4R or 3S,4S)-3-(benzylthio)-4-[(R)-2-hydroxytridecyl]-2-oxetanone
the (S)-1-[[(2R,3R or 25,35)-3-(benzylthio)-4-oxo-2-oxetanyl]methyl]dodecyl-
(S)-
2-isopropylmalonamate, m.p. 65° (from ether/pentane) and
c) from (3S,4R and 3R,4S)-3-(benzylthio)-4-[(R)-2-hydroxytridecyl]-2-
oxetanone
the (S)-1-[[(2S,3R or ZR,3S)-3-(benzylthio)-4-oxo-2-oxetanyl]methyl]dodecyl
(S)-2-isopropylmalonamate, m.p. 69° (from pentane), and
2. the (S)-1-[[(2R,3S or 25,3R)-3,~(benzylthio)-4-oxo-2-
oxetanyl]methyl]dodecyl
(S)-2-isopropylmalonamate, m.p. 106° (from hexane).
Example 20
In analogy to Examples 1 and 11, from (R)-(-)-2-isopropylmalonic acid
monoamide and the following alcohols there are obtained the follawing esters:
a) Frorn (3S,4S or 3R,4R)-3-(benzylthio)-4-[(R)-2-hydroxytridecyl]-2-
oxetanone the (S)-1-[[(2S,3S or 2R,3R)-3-(benzylthio)-4-oxo-2-
oxetanyl]methyl]dodecyl (R)-2-isopropylmalonamate, m.p. 130° (from
ether/pentane),
2o b) from (3R,4R or 3S,4S)-3-(Benzylthio)-4-[(R)-2-hydroxytridecyl]-2-
oxetanone the (S)-1-j[(2R,3R or 2S,3S)-3-(benzylthio)-4-oxa-2-
oxetanyl]methyl]dodecyl (R)-2-isopropylmalonamate, m.p. 119° (from
hexan/pentane), and
c) from (3S,4R and 3R,4S)-3-(benzylthio)-4-[(R)-2-hydroxytridecyl]-2-
oxetanone
1. the (S)-1-[[(2S,3R or 2R,3S)-3-(benzylthia)-4-oxo-2-oxetanyl]methyl]dodecyl
(R)-2-isopropylmalonamate, m.p. 132° (from ether/pentane), and
2. the (S)-1-[[(2R,3S or 2S,3R)-3-(benzylthio)-4-oxo-2-oxetanyl]methyl]dodecyl
(R)-2-isopropylmalonamate, m.p. 102° (from ether/pentane).
Example 21
_41_
Analogously to Examples 1 and 11, by reacting (3R,4R)-3-ethyl-4-[(R)-2-
hydraxynonadecyl]-2-oxetanone
a) with (S)-(+)-2-Isopropylmalonic acid monoamide there is obtained (S)-1-
[[(2R,3R)-3-ethyl-4-oxo-2-oxetanyl]methyl]octadecyl (S)-2-isopropylmalonamate,
m.p. 116-118° (methylene chloride), and
b) wikh 1-carbamoylcyclohexanecarboxylic acid there is obtained (S)-1-
[[(2R,3R)-3-ethyl-4-oxo-2-oxetanyl]methyl]actadecyl 1-
carbamoylcyclohexanecarboxylate, m.p. 71-74°.
Example 22
to In analogy to Examples 1 and 11,
a) from (S)-(+)-2-isopropylmalonic and monoarnide and (3R,4R)-4-[(R)-2-
hydroxynonadecyl]-3-methyl-2-oxetanone there is obtained (S)-1-[[(2R,3R)-3-
rnethyl-4-oxo-2-oxetanyl]methyl]octadecyl (S)-2-isopropylmalonamate, m.p.
124-126° (from ethyl acetate/hexane), and
15 b) from rac-2-t-butylmalonic acid monoamide and (3R,4R)-4-[(R)-2-
hydroxynonadecyl]-3-methyl-2-oxetanane there is obtained (S)-1-[[(2R,3R)-3-
methyl-4-oxo-2-oxetanyl]methyl]octadecyl (RS)-2-t-butylmalonamate (epimers
1:1), m.p. 51-54° (ethyl acetate/hexane).
Example 23
2o A solution of 277 mg of (2S,3S,5S)-5-[j(cis)-2-carbamoylcycloh.exyl]oxy]-2-
hexyl-3-hydroxyhexadecanoic acid (Example Jd) in 24 ml of methylene chloride
and 3 ml of DMF is treated with 2 g of molecular sieve (4~), 240 mg of HBTU
and 240 mg of triethylamine. After stirring 300 rng of HBTU and 300 mg of
triethylamine are added thereto. The mixture is then filtered and the filtrate
is
25 concentrated. The residue is partitioned between methanol/water (7:3) and
hexane and extracted with hexane. The hexane phase is diluted with
methylene chloride, then dried and concentrated and the residue is
recrystallized from ether/hexane. There are thus obtained
a) 96 mg of (1R or S,2S or R)-2-[[(S)-1-[[(2S,3S)-3-hexyl-4-oxo--2-
30 oxetanyl]methyl]dodecyl]oxy]cyclohexanecarboxamide (1st cis diastereomer),
m.p. 102° (from ether/hexane), and
b) analogously from (2S,3S,5S)-5-[[(cis)-2-carbamoylcyclohexyl]oxy]-2-hexyl-3-
hydroxyhexadecanoic acid (2nd diast. acid) in Example Je) there is obtained
(1S
or R,2R or S)-2-[[(S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-
oxetanyl]methyl]dodecyl]oxy]cyclohexanecarboxamide (2nd cis diastereomer),
rn.p. 94° (from ether/hexane).
Example 24
A solukion of 42.5 mg of (2S,3S,5S)-5-[(R/S)-2-carbamoyl-1-
methoxyethoxy]-2-hexyl-3-hydroxydecanoic acid (Example Kd) in 10 ml of
mekhylene chloride/acetonitrile (1:1) is txeated with 1 g of molecular sieve
(4th), 0.1 ml of triethylamine and 50 mg of HBTU. After stirring the reaction
mixture is filtered and concentrated and the residue is partitioned between
hexane and methanol/water (1:1); the aqueous methanolic phase is extracted
with hexane, the hexane phase is dried and concentrated; the residue is
subsequently chromatographed on silica gel with 5 percent methanol in
methylene chloride. There are thus obtained 21 mg of (R/S)-3-[[(S)-1-[[(2S,3S)-
3-
hexyl-4-oxo-2-oxetanyl]methyl]dodecyl]oxy]-3-methoxypropionamide (epimers
3:1 ), m.p. 54°.
Example 25
Ammonia gas is blown into a solution of 40 mg of 3-[[(S)-1-[[(2S,3S)-3-
2o hexyl-4-oxo-2-oxetanyl]methyl]dodecyl]oxy]propionic acid (Example L) in 2.5
ml
of acetonitrile until the solution is saturated and subsequently 50 mg of HBTU
are added. Then, the mixture is filtered and evaporated and the residue is
chromatographed on silica gel with 5 percent methanol in methylene chloride.
There are obtained 32.5 mg of (3S,4S)-4-[(S)-2-(2-carbamoylethoxy)tridecyl]-3-
hexyl-2-oxetanone, m.p. 35°.
Example 26
A solution of 33 5 mg of (S)-1-([(2S,3S)-3-hexyl-4-oxo-2-
oxetanyl]methyl]dodecyl hydrogen malonate (Example N) in 2 ml of
acetonitrile is treated with 60 mg of HBTU and 25 mg of isopropylamine. After
stirring the reaction mixture is filtered, the filtrate is concentrated and
the
residue is chromatographed on silica gel with hexan~/methylene
chloride/ethyl acetate (2:2:1). There are obtained 31.1 mg of (S)-1-[[(2S,3S)-
3-
hexyl-4-oxo-2-oxetanyl]methyl]dodecyl N-isopropylmalonamate, m.p. 59°.
-43-
The following compounds are manufactured in an analogous manner to
Example 1:
Example 27: (S)-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl 5-
carbamoylvalerate, m.p. 50-510.
Example 28: (S)-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]methyl~dodecyl 6-
carbamoylhexanoate, m.p. 52-53°,
Example 29~ (S)-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl 7-
carbamoylheptanoate, m.p. 40-43°,
Example 30: (S)-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl 9-
carbamoylnonanoate, m.p. 29-30°,
Example 31: (S)-1-[[(2S,3S)-3-Ethyl-4-oxo-2-oxetanyl]methyl]octadecyl 4-
carbamoylbutyrate, m.p. 74-75°,
Example 32: (S)-1-[(2S,3S)-3-Ethyl-~f-oxo-2-oxetanyl]methyl]octadecyl
adipamate, m.p. 63.5-64.5°,
Example 33: (S)-1-[[(2S,3S)-3-Ethyl-4-oxo-2-oxetanyl]methyl]octadecyl 6-
carbamoylhexanoate, m.p. 71.5-72.5°,
Example 34: (S)-1-[[(2S,3S)-3-Hexyl-4-oxo-oxetanyl]methyl]dodecyl (R or S)-
2-t-butylmalonamate, m.p. 53-54°,
Example 35: (S)-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl] methyl]dodecyl (S or
R)-2-t-butylmalonamate, [cc]p = -16.4° (c = 0.8, ~H~13),
Example 36: (S)-1-[[(2S,3S)-3-Ethyl-4-oxo-2-oxetanyl]methyl]octadecyl (R or
S)-2-t-butylmalonamate, m.p. 48-49°,
Example 37: (S)-1-[[(2S,3S)-3-Ethyl-~4-oxo-2-oxetanyl]methyl]octadecyl (S or
R)-2-t-butylmalonamate, m.p. 81-82°,
Example 38: (S)-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl-(S)-3-
[2~~(benzyloxy)formamido]succinamate, m.p. 72-73°,
Example 39: (S)-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]met)nyl]dodecyl cis-6-
carbamoyl-3-cyclohexene--1-carboxylate, m.p. 55-59°,
-
Example 40: (S~-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl cis-2-
earbamoylcydohexanecarboxylate, m.p. 76-77°,
Example 41: (S)-1-[[(2S,3S)-3-Hexyl-~4-axo-2-oxetanyl]methyl]dodecyl (3-oxo-
4-morpholinopropionate, m.p. 49-51°,
Exam lp a 42:" (S)-1-[[(2S,3S)-3-I-Iexyl-4-oxa-2-oxetanyl]methyl]dodecyl
tetrahydro-(i-oxo-4hl-1,4-thiazine-4-propionate, m.p. 59-61°,
Example 43: (S)-1-[[(2S,3S)-3-Ethyl-4-oxo-2-oxetanyl]methyl]octadecyl 1-
carbam.oylcydopentanecarboxylate, m.p. 62-63a,
Examt~le 44: (S)-1-[[(2S,2S)-3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl 1-
1o carbamoylcyclopentanecarboxylate, m.p. 40-41°,
Example 45: (S)-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (RS)-
2-benzylmalonomate (epimers 1;1), m.p. 86-92°,
Example 46: (S)-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (RS)-
2-methoxyrnalonamate (epimers 1;1), m.p. 65-67°,
z5 Example 47: (S)-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl
[(carbamoyl)thio]acetate, m.p. 58-60°,
Example 48: (S)-1-[[(2S,3S)-3-Aethyl-4-oxo-2-oxetanyl]methyl]octadecyl
[(caxbaanoylrnethyl)thio]acetate, m.p. 83-84°C,
Example 49: (S)-1-[((2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl [(RS)-
20 (carbamoylmethyl)sulphinyl]acetate (epimers 1:1), m.p. 55-59°,
Example 50: (S)-1-[[(2S,3S)-3-Ethyl-4-oxo-2-oxetanyl]methyl]octadecyl [(RS)-
(earbamoylmethyl)thio]acetate S-oxide, m.p. 80-82°,
Example 51: (S)-1-[[(2S,3S~3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl 3-[(2-
carbamoylethyl)thio]propionate, m.p. 73-74°,
25 Example 52: (S)-1-[[(2S,3S~3-Hexyl-4-oxo-2~-oxetanyl]methyl]dodecyl 3
[(RS)-(2-carbamoylethyl)sulphinyl]propionate (epimers 1:1), m.p. 48-
51°,
Example 53: (S)-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl
(carbamoylmethoxy)acetate, m.p. 52-53°,
?~'~~~~'~
-45-
Example 54: (S)-1-[[(2S,3S)-3-Ethyl-4-oxo-2-oxetanyl]methyl]octadecyl
(carbamoylmethoxy)acetate, m.p. 75-76°,
Example 55: (S)-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (RS)-
2-[1-(benzyloxy)formamido]malonamate (epimers 1:1), m.p. 94-95°,
Example 56: (S)-1-[[(2S,3S)-3-Ethyl-4-oxo-2-oxetanyl]methyl]octadecyl (RS)-
2-isabutylmalonamate (epimers '1:1), m.p. 96-99°,
Example 57a): (S)-1-[[(2S,3S)-3-Ethyl-4-oxo-2-oxetanyl]axiethyl]octadecyl
(RS)-2-benzylmalonamate (epimers 1:1), rn.p. 96-103°,
Example 57b): (S)-1-[[(2S,3S)-3-Ethyl-4-oxo-2-oxetanyl]methyl]octadecyl (R
'l0 or S)-2-benzylmalonarnate, m.p. 118-120°,
Example 58: (S)-1-[[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]methyl]dodecyl (RS)-
2-phenethylmalonamate (epirners 1:1), m.p. 92-93°,
Example 59: (S)-1-[[(2S,3S)-3-Ethyl-4-oxo-2-oxetanyl]methyl]octadecyl (RS)-
2-phenethylmalonamate (epimers 1:1), m.p. 97-98°.
Example 60
Analogously to Examples 1 and 11, from (S)-(+)-2-isopropylrnalonic acid
monoamide and (3R,4R)-4-[(R)-2-hydroxynonadecyl]-3-(2-propynyl)-2-
oxetanone (Example IvI) there is obtained (S)-1-[[(2R,3R)-4-oxo-3-(2-propynyl)-
2-
oxetanyl]methyl]octadecyl (S)-2-isopropylmalonamate, m.p. 92-95°C (from
ethyl acetate/ hexane).
Example G1
Analogously to Examples 1 and 11, from (S)-(+)-2-isopropylmaloruc acid
monoamide and (3R,4R)-4-[(R)-2-hydroxynonadecyl]-3-propyl-2-oxetanone
(Example O) then is obtained (S)-1-[[(2R,3R)-4-~xo-3-propyl-2-
oxetanyl]methyl]octadecyl (S)-2-isopropylrnalonamate, m.p. 90-93° (from
ethyl
acetate/hexane).
The following compounds are manufactured in an analogous manner to
Example 1 and 11:
Example 62: (S)-1-[[(2S,3S)-3-(3-Methyl-2-butenyl)-4-oxo-2-
3o oxetanyl]methyl]dodecyl (R or S)-2-isopropylmalonamate, m.p. 100-
102°,
-
Example 63: (S)-1-([(2S,3S)-3-(3-Methyl-2-butenyl)-4-oxo-2-
oxetanyl]methyl]dodecyl (S or R)-2-isopropylmalonamate, m.p. 120-121°,
Exarn~le 64: (S)-1-[[(2R,3R)-3-(3-Methyl-2-butenyl)-4-oxo-2
oxetanyl]methyl]dodecyl (R or S)-2-isopropylmalonamate, m.p. 60-62°,
Example 65: (S)-1-[[(2R,3R)-3-(3-Methyl-2-butenyl)-4-oxo-2
oxetanyl]methyl]dodecyl (S or R)-2-isopropylmalonamate, m.p. 76-78°,
Exarziple 66: (S)-1-[[(2S,3S or 2R,3R)-3-(5-Chloropentyl)-4-oxo-2-
oxetanyl]methyl]dodecyl (S)-2-isopropylmalonamate, m.p. 66-72°,
Example 67: (S)-1-(((2S,3S or 2R,3R)-3-(5-Chloropentyl)-4-oxo-2-
oxetanyl]methyl]dodecyl (R)-2-isopropylmalonamate, m.p. 130°,
Example 68: (S)-1-[[(2R,3R or 2S,3S)-3-(5-Chloropentyl)-4-oxo-2-
oxetanyl]methyl]dodecyl (S)-2-isopropylmalonamate, m.p. 44-50°,
Exam 1__p a 69: (S)-1-[((2R,3R or 2S,3S)-3-(5-Chloropentyl)-4-oxo-2-
axetanyl]methyl]dodecyl (R)-2-isapropylmalonamate, m.p. 85-87°,
Example 70: (S)-1-(2S,3S or 2R,3R)-[[3-[(E)-2-Butenyl]-4-oxo-2-
oxetanyl]methyl]dodecyl (R or S)-2-isopropylmalonamate, m.p. 105-107°,
Example 71: (S)-1-(2S,3S or 2R,3R)-[[3-((E)-2-Butenyl]-4-oxo-2
oxetanyl]methyl]dodecyl (S or R)-2-isopropylmalonamate, m.p. 96-98°,
Example 72: (S)-1-(2R,3R or 2S,3S~([3-[(E)-2-Butenyl]-4-oxo-2-
oxetanyl]methyl]dodecyl (S)-2-isopropylmalonamate, m.p. 89-90°,
Example 73: (S)-1-(2R,3R or 2S,3S~[[3-[(E)-2-Butenyl]-4-oxo-2-
oxetanyl]methyl]dodecyl (R)-2-isopropylmalonamate, m.p. 60-63°,
Example 74: (S)-1-[[(2R,3R or 2S,3S)-3-(2,3,4,5,6-Pentafluorobenzyl)-2-
oxetanyl]methyl]dodecyl (R)-2-isopropylmalonamate, m.p. 107-109°,
Example 75: (S)-1-[[(2R,3R or 2S,3S)-3-(2,3,4,5,6-Pentafluorobenzyl)-2
oxetanyl]methyl]dodecyl (S)-2-isopropylmalonamate, m.p. 115-118°,
Example 76: (S)-1-[[(2S,3S or 2R,3R)-3-(2,3,4,5,6-Pentafluorobenzyl)-2-
oxetanyl]methyl]dodecyl (R)-2-isopropylmalonamate, m.p. 88-90°, and
-47-
Example 77: (S)-1-(((2S,3S or 2R,3R)-3-(2,3,4,5,6-Pentafluorobenzyl)-2-
oxetanyl]methyl]doderyl (S)-2-isoprapylmalonamate, m.p. 52-55°.
Example 78
Analagously to Examples 1 and 2, but using (R)- or (S)-2-pyrrolidane-5-
carboxylic acid in place of 2-isopropylmalonic acid manoarnide there are
obtained
a) 5-oxo-D-proline (S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl
ester, 1H-1VMR (CDC13): 0.88 (m,6H); 1.26 (m,26H); 1.55-1.9 (m,4H); 1.95-2.55
(m,6H); 3.21 (m,lH); 4.25 (m,lH); 4.31 (m,lH); 5.14 (m,lH); 5.80 (s,lH) ppm,
1o b) 5-oxo-L-proline (S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-axetanyl]methyl]dodecyl
ester, m.p. 51-52°,
c) 5-oxo-D-praline (S)-1~[[(2S,3S)-3-ethyl-4-oxo-2-oxetanyl]methyl]octadecyl
ester, m.p. 55° (diethyl ether), and
d) 5-axo-L-praline (S)-1-[[(2S,3S)-3-ethyl-4-axo-2-oxetanyl]methyl]octadecyl
ester, m.p. 57-58°.
Example 79
Analogously to Examples 1 and 11, from (S)-(+)-2-isopropylmalonic acid
monoamide and
a) 3R,4R (ar 3S,4S)-3-anilino-4-[(R)-2-hydroxytridecyl]-2-oxetanone,
diastereomer B (Example T) there is obtained (S)-1-[[(2R,3R or 2S,3S)-3-
anilino-
4-oxo-2-oxetanyl]methyl]dodecyl (S)-2-isopropylmalonamate (diastereomer B),
m.p. 92°, and
b) from 3S,4S (or 3R,4R)-3-anilino-4-[(R)-2-hydroxytridecyl]-2-axetanone,
diastereomer A (Example T) thexe is obtained (S)-1-[((2S,3S or 2R,3R)-3-
z5 aniline-4-oxo-2-oxetanyl)methyl]dodecyl (S)-2-isopropylrnalonamate
(diastereomer A), m.p. 77°.
Pharmaceutical preparations of the following composition are
manufactured in a manner known per se:
Example A
3o Soft gelatine capsules:
Amount per capsule
An oxetanone of formula I 50 mg
Medium-chain triglyceride 450 yl
Example B
I-Iard geltaine capsules:
An oxetanone of formula I 20.0 mg
Lactose tryst. 37.0 rng
Mieracrystalline cellulose 20.0 mg
Polyvinylpolypyrrolidone 8.5 mg
1o Sodium salt of the carboxymentyl ether of starch 8.5 mg
Talc 4.5 mg
Magnesium stearate 1._ 5 m~
Capsule fill weight 100.0 mg
Example C
Tablets
An oxetanone of formula I 30.0 g
Lactose anhydrous 118.8
Microcrystalline cellulose 30.0
Polyvinylpyrrolidone 10.0
2o Polymer of carboxymethylcellulose 10.0
Magnesium stearate 1.2
Tablet weight 200.0 mg
-4~-
Example D
Tablets with controlled release of
the active ingredient and increased
residence time in the skomach:
An oxetanone of formula I 60.0 mg
Lactose powd. 70.0 mg
Hydroxypropylmethylcellulose 52.5 mg
Polyvinylpyrrolidone 7.5 mg
Talc 8.0 mg
Magnesium stearate 1.0 mg
Colloidal silicic acid 1.0 mg
Nucleus weight 200.0 mg
Hydroxypropylmethylcellulose 2.5 mg
Talc .1.25 mg
Titanium dioxide 1.25 mg
Weight of the film coating 5.0 mg
Exarn_ple EE
Reconstitutable powder:
An oxetanone of formula Z 200.0 mg
Ethylvanillin 10,0 mg
2o Aspartame 30.0 mg
Sprayed skinned milk powder 4,760.0 mg
Total 5,000.0 mg