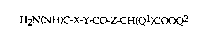Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
RAN 4045~
The present invention relates to new acetic acid derivatives, to
processes for their preparation, to pharmaceutical preparations which contain
such compounds, and to the use of these compounds for the production of
pharmaceutical preparations.
S The invention relates in particular to acetic aeid derivatives of the
formula
H2N(NH)C-X-Y-C~Z~H(Q1)COOQ2 (I)
10 in which
Q1 is hydrogen, methyl or phenyl,
Q~ is hydrogen, phenyl-lower alkyl or lower alkyl which can be cleaved
under physiological conditions,
X is 1,4-phenylene, 1,4-piperidinylene bound via the C atom in the
~position to the group Y, or 2,5- or 3,6-pyridylene
Y is a group of the formula
-~CH2)0 2-CONHCH(Q3)(C~2)1-3- (yl)
~oNHCH2CH(Q4)- (y2)
-(CH2)2NHCOCH2-
-NHCO(CH2)3-
-(C~)o~ C~ (y5)
,CN,~ (R ) (y6)
or
Mé/6.2.91
"~
~r~ (y7)
S Q3 is hydrogen, methyl, phenyl, -COOH, -CO~lower alkyl, -CONH(CH2)2-
COOH or -CONH(CH2)2~OO lower aLkyl,
Q4 is hydrogen, methyl or phenyl,
Z is a 1,4 piperazinylene group, a 1,4-piperidinylene group bound via the
N-atom in the 1-position ts) the CO group, or a group of the formula
-NHCH(R1)- or -NHCH(COR2)-
R1 is hydrogen, methyl, phenyl or -COO-lower alkyl,
R2 is the radical of an oc-aminocarboxylic acid bound via the amino group or
an ester or amide thereof, or a group of the formula -NHCH2CH2-Ar, or
-CO-R2 is an optionally mono- or di-lower-alkylated carbamoyl group or a
15 pyrrolidinoyl or piperidinoyl group,
Ar is phenyl or phenyl which is substituted by lower alkyl, lower alkoxy,
~OOH, ~O~lower aLIcyl, -O(CH2)1~00H, ~(CH2)1 4-COO lower alkyl,
-CONH2, -CONH-lower alkyl, -CON(lower alkyl)2, pyrrolidinoyl or
piperidinoyl,
20 and hydrates or solvates and physiologically utilisable salts thereof.
In the context of the present invention, tBu denotes t-butyl, Boc denotes
t-butoxycarbonyl, Z denotes benzyloxycarbonyl, Val denotes L-valyl, Phe
denotes L-phenylalanyl, Ser denotes L-seryl, Gly denotes glycyl, Ala denotes L-
alanyl, Asp denotes L-a-aspartyl, Leu denotes L-leucine and Tyr denotes L-
25 tyrosine.
The expression "lower" denotes groups having 1-6, preferably 1-4, C
atoms. Examples of lower alkyl groups are methyl, ethyl, propyl, isopropyl and
n-, s- or t-butyl. Examples of lower aLI~yl groups which can be cleaved under
physiological conditions are prinnary and secondary lower alkyl groups.
Examples of ~-aminocarboxylic acid radicals bound via the amino
~ ~ 3 3 ~,, P~ '~
group are Val, Phe, Ser, Leu, Tyr and their corresponding lower alkyl or
phenyl-lower alkyl esters, amides and mono- or di-lower aL~cyl amides.
The compounds of the formula I can be solvated, in particular hydrated.
Hydration can take place in the course of the preparation process or occur
5 gradually as a consequence of hygroscopic properties of an initially anhydrous compound of the formula I.
Examples of physiologically utilisable salts of the compolmds of the
formula I are salts with physiologically tolerable mineral acids, such as
hydrochloric acid, sulphuric acid or phosphoric acid, or with organic acids,
10 such as methanesulphonic acid, ace~c acid, trifluoroacetic acid, citric acid,fumaric acid, succinic acid or salicylic acid. The compounds of the formula I
can also form salts with physiologically tolerable bases. Examples of such saltsare alkali metal, alkaline earth metal, ammonium and alkylammonium salts,
such as the Na, K, Ca or tetramethylammonium salt. The compounds of the
15 formula I contain an amidino group and can therefore be present in the form
of zwitterions.
The compounds of the formula I, which contain one or more
asymmetric C atoms, can be present as enantiomers, as diastereomers or as
mlxtures thereof, for example as racemates.
Preferred compounds of the formula I are those of the formula
H2N(HN)C-X-Ya-CONHCH(Rl 1 )CH2COOQ21 I-A
in which
X is 1,~phenylene or 1,4-piperidinylene bound via the C atom in the
4-position to the group ya,
ya is a group of the formula
-(CH2)0 1-CONHCH(Qa)(CH2)1-2- (yll)
-(CH2)2NHCOCH2- (Y )
-NHCO(CH2)?~-
or
f"~ P ' ''
- 4 -
o
,C,V~~ (y51
J
Qa is hydrogen or phenyl,
5 Rll is hydrogen or -C~R22,
R22 is the radical of an a-aminocarboxylic acid bound via the amino group
or of an ester or amide thereof, and
Q21 is hydrogen or lower alkyl which can be cleaved under physiological
conditions.
In the fonnula I, Y is preferably a group of the formula y1, in particular
~oNH(cH2~2-4
-CH2CONH(CH2)~
~ONHCH(C6H5)CH~-,
-CONHCH(CONHCH2CH2COOH)CH2-,
15 ~ONHCH(COOH)CH2- or
-CONHCH(CH3)CH2- -
In the formula I, Z is preferably a group of the formula -l!~HCH2-,
-NHCH(CH3)-, -NHCH(C6Hs)-, -NHCH(CO~isobutyl)-, -NHCH(C~Val)-,
-NHCH(C~Phe~, -NHCH(CO-Tyr)-, -NHCH(C~Ser{)C2Hs)-, -NHCH(CO-
20 Leu-~isopropyl~,-NHCH(CONHCH2CH2-C6H~CH3)-,
-NHCH(CONHCH2CH2-C6H4-COOH)-, -NHCH(CONHCH2CH2-C6H4-
OCH2COOH)-, -NHCH(CONH~)- or -NHCH(pyrrolidinoyl)-.
Particularly preferred compounds of the formula I are the following:
N-[N-[N-(~amidinobenzoyl)-~-alanyl]-L-a-aspartyl]-3-phenyl-L-alanine,
N-[N-[4-(p-amidinobenzamido)butyryl]-L-a-aspartyl]-L-valine,
N-[N-(p-amidinobenzoyi)-l~-alanyl]-~-alanine,
N-[N-[N-(p-amidinobenzoyl) ~-alanyl]-L-a-aspartyl]-L-leucine isopropyl
ester,
N-[N-[N-(p-amidinobenzoyl)-~-alanyl]-L-a-aspartyl]-L-valine,
N-[N-[N-(p-amidinobenzoyl)-~-alanyl]-L-a-aspartyl]-3-(p-
hydroxyphenyl)-L-alanine,
N-[N-[5-(p-arnidinobenzamido)valeryl]-L-a-aspartyl]-3-phenyl-L-
- 5 -
alanine,
isobutyl N-~5-(p-amidinobenzamido)valeryl]-L-a-aspartate,
N-[N-[N (p-amidinobenzoyl~-~-alanyl]-L-a-aspartyl]-L-serine ethyl ester
and
N-[N-[[(R)-1-(p-amidinobenzoyl)-3-pyrrolidinyl]~arbonyl]-L-a-aspar~yl]-
3-phenyl-L-alanine.
Further examples of compounds of the formula I are the following:
N-[N-[N-(1-amidino-4-piperidinylcarbonyl)-~-alanyl]-L-oc-aspartyl]-3-
phenyl-L-alanine,
N-[N-[N-(p-amidinophenylacetyl)-~-alanyl]-L-oc-aspartyl]-3-phenyl-L-
alanine,
N-[N-[4-(p-amidinophenylcarbamoyl)butyryl]-L-~-aspartyl]-3-phenyl-L-
alanine,
N-[N-[(p-amidinophenylcarbamoyl)acetyl]-L-oc-aspartyl]-3-phenyl-L-
15 alanine,
rac-N-[1-(p-amidinobenzoyl)-3-piperidinyl-carbonyl]-$-alanine,
N-[4-(p-amidinobenzamido)butyryl]-~-alanine,
N-[(DL)-N-(p-amidinobenzoyl)-3-phenyl-~-alanyl]-~-alanine,
N,N'-[[(S)~(p-amidinobenzamido)ethylene]dicarbonyl]di-~-alanine,
2-N-(p-amidinobenzoyl)-4-N-(2-carboxyethyl)-L-asparagine,
N-[5-(p-amidinobenzamido)valeryl]-~-alanine,
rac-N-[[1-[3-(1-amidino-4-piperidinyl)propionyl]-3-piperidinyl]carbonyl]-
~-alanine,
N-[[(S)-l-(p-amidinobenzoyl)-2-pyrrolidinyl]-acetyl]-~-alanine,
~S)-3-[[N-(p-amidinobenzoyl)-B-alanyl]amino-3-[(p-
methoxyphenethyl)carbamoyl]propionic acid,
N-[[(R)-1-(p-amidinobenzoyl)-3-pyrrolidinyl]~carbonyl]-~-alanine,
N-[N-(p-amiclinobenzoyl)-2-methyl-~-alanyl] -L-oc-aspartamide,
N-[N-[(p-amidinophenyl)acetyl]-l~-alanyl]-~-alanine,
- 6 -
rac-N-[[[1-(p-amidinophenyl)acetyl]-3-piperidinyl]-carbonyl]-~-alanine
benzyl ester,
rac-N-[[1-[(p-amidinophenyl)acetyl]-3-piperidinyl]-carbonyl]-~-alanine,
N-[N-[N-[3-(1-amidino-4-piperidinyl)propionyl]-~-alanyl]-L-a-aspartyl]-
5 3-phenyl-L-alanine,
(S)-:~-[[DL-N-(p-amidinobenzoyl)-3-methyl-:~-alanyl]amino]-~- oxo-1-
pyrrolidinebutyric acid,
DL-N-[N-(p-amidinobenzoyl)-~-alanyl] -3-methyl-~-alanine,
N-[DL-N-(p-amidinobenzoyl)-2-phenyl-~-alanyl] -~3-alanine,
DL-N-[N-(p-amidinobenzoyl)-~-alanyl]-2-methyl-~-alanine,
DL-N-[N-(p-amidinobenzoyl)-B-alanyl~-2-phenyl-~-alanine,
p-[2-[[N-[N-(p-amidinobenzoyl~-~-alanylJ-L-o~-aspartyl]-
amino]ethyl~benzoic acid,
DL-N-[N-(p-amidinobenzoyl)-~-alanyl-3-phenyl-~-alanine,
[p-[2-[[N-[N-(p-amidinobenzoyl)-~-alanyl]-L-cc-aspartyl]amino]ethyl]-
phenoxy]acetic acid,
1-[N-(p-amidinobenzoyl)-B-alanyl]-4-piperidineacetic acid,
4-[N-(p-amidinobenzoyl)-~-alanyl]-1-piperazineacetic acid and
N-[N-[N-[(5-amidino-2-pyridyl)carbonyl]-~alanyl]-L-a-aspartyl]-3-
20 phenyl-L-alanine.
The above compounds can be obtained according to the invention by
cleaving at least one protecting group from a compound of the formula
X'-Y-C~Z'~H(Ql)-COO Q5 Il
in which
25 Q1 and Y have the meaning indicated further above,
X'is phenyl or 4-piperidinyl which is substituted in the 4-position by an
optionally protected amidino group, and
Z' and Q5 have the same meaning as indicated further above for Z and Q2,
or
30 b)converting the nitrile group into the amidino group in a nitrile of the
~ , ' ! ,i t- ~
! :., ~ . -,i..
-7-
formula
~- C ~Y- Co-~ -c ~l(Q )-C~O-Q (III)
5 and, if desired, converting a compound of the formula I into a physiologicallytolerable salt or converting a salt of a compound of the formula I into the freeacid or base.
Examples of protecting groups in the compounds of the formula II are
the benzyl or lower alkyl groups, such as t-butyl, contained in the ester groups10 benzyl-OC~ or t-Bu~C~; and amidino protecting groups, such as Z and Boc.
Examples of protected amidino groups are -C(NH)NH-Z, -C(NH)NH-Boc and -
C(N-Boc)-NH~Boc.
Ester groups can be cleaved in a manner known per se, for example by
hydrolysis using a base, such as an alkali metal hydroxide, for example sodium
15 hydroxide, in a solvent, such as methanol. Benzyl esters can be cleaved by
hydrogenation in the presence of a noble metal catalyst, such as palladium on
carbon (Pd/C) in a solvent, such as methanol, ethanol, formic acid or acetic
acid, at a temperature up to about 40C, preferably at room temperature. In thisprocess, an amidino protecting group present in the group X', such as Z, is
20 removed at the same time.
Ester groups, such as t-butyl, or amidino protecting groups, such as Boc,
can be cleaved, for example, using an acid, such as formic acid or
trifluoroacetic acid, if desired in a solvent, such as dichlorome~ane, at a
temperature up to 40C, preferably at room temperature.
The compounds of the formula II are new and likewise a subject of the
present invention. They can be prepared starting from known compounds by
methods which are known per se, for example as described below.
Thus, in a first step an amine of the formula
H-Z'-CH(Q1)COC}Q22 IV
30 is coupled with an acid of the formula
R5-NHCH(Q31)(CH2)1 3-COOH Va
R5-NHCH2CH(Q4)~ooH Vb
~CC)O ~ Vc
" COO H Vd
C !-1, COO 11 ve
or
S ~ - C ~ ( CHZ )n-N~co(cH2)p-cooH VI
in which
Q22 is an easily cleavable alkyl group,
~31 i5 hydrogen, methyl, phenyl, -COO-lower alkyl, or
-CONH(C~2)2-COO-lower alkyl,
R5 is an amino protecting group, such as Z or Boc,
n = 0 and p = 3 or n = 2 and p = 1,
with the formation of an amide group by the methods known
per se from peptide chemiætry.
Thus, the coupling of IV with Va, Vb or VI can be
carried out, for example, in tetrahydrofuran ~THF) at
-10C to room temperature under argon in the presence of
O-benzotriazo:L-1-yl-N,N,N',N'-tetramethyluronium hexa-
fluorophosphate (HBTU). The coupling of IV with Vc, Vd or
Ve i~ effected, for example, by initially activating the
acid Vc, Vd or Ve in THF using chlorodLmethoxytriazine
and N-methylmorpholine and then reacting the product with
the p-toluenesulphonate of the amine IV and N-methyl-
morpholine.
From the reaction product thus obtained, an amino
protecting group R5/ for example Z or Boc, can then be
selectively removed as descrihed above by catalytic
hydroyenation or by means of trifluoroacetic acid.
An amine of the formula
H2NCH(Q31)(CH2)l3-Co-Z~-CH(Ql)Coo-Q22 VIII
-- 9 ~ p~ f~ --~
obtained in this manner, for example starting from IV and
Va, can then be coupled with 4~cyanoben~oic acld or
4-cyanophenylacetic acid to give a nitrile, for example
one of the ormula III, for example as described above
for the coupling of IV with Va.
A nitrile obtained in this way or a nitrile
obtained by coupling IV and VI can be converted into a
compound II in which X~ contains a free amidino group,
for example by reaction with hydrogen sulphide and
triethylamine in pyridine to give the thioamide, methyla-
tion with methyl iodide in acetone and subsequent reac-
tion with ammonium acetate in methanol. A nitrile III chn
be converted analogously into the corresponding compound
of the formula I.
An amine of the above formula VIII can also be
reacted, for example, with l-amidino-4-piperidinecar-
boxylic acid, p-amidinophenacetyl chloride or p-amidino-
benzoyl chloride to give a compound II in which X' is
l-amidino-4-piperidinyl or p-amidinophenyl.
An amine of the formula VIII or an amine of the
formulae Va to Ve obtained starting from a compound of
the formula IV and an acid can furthermore be xeacted in
methylene chloride/aqueous sodium bicarbonate solution
with p-amidinobenzoyl chloride and subsequently with
benzyl chloroformate or with di-t-butyl dicarbonate in
the presence of sodium carbonate to give a compound of
the formula II in which X' is p-amidinophenyl protected
by Z or Boc.
The compounds of the formulae IV-VI are known or
can be prepared in analogy to the Xnown compounds or as
descxibed in the examples below.
Thus, a nitrile VI can be prepared by coupling
the appropriate amine of the formula NCC6~4(CH2)n-N~2 with
the appropriate acid of the formula ~OOC(C~2)p-COOQ22, for
example analogously to the coupling of an amine IV with
an acid V, followed by removal of the ester group Q22.
To prepare a compound of the formula II in which
X' is 4-piperidinyl substituted in the 4-position by a
protected amidino group, a compound of the formula
-- 10 --
~C~ C~ COOH IX
can first be reacted, for example, with N,N'-bi3-
tt-butoxycarbonyl)-S-methylisothiourea, in t-butanol and
sodium hydroxide solution to give a compound of the
formula
BOC-N= C(l~l~ S30C) ~CI~,C H ~,- COO~I x
and the latter can then be coupled, for example, with a
compound of the formula
H2NCH(Q3l)(CH2)13-CoNHCH2CH2CooCH2C6H5 XIa
H2NCH2CH(Q4 ) CONHCH2CH2COOCH2c6H5 XIb
,CON,' ,C !~,C L!, COOC~? C6 ~5 XIc
H N ~ .. Co~c~c~cc~ C6~S XId
or
H ~ -C.~CO~J~C.U~C~CCX~C6!~5 XIe
in which
Q31 iS hydrogen, methyl, phenyl, -COO-lower alkyl or
-CONH(CH2)2-COO-lower alkyl.
A compound of the formula II in which X' is a
phenyl or 4-piperidinyl which is substituted in the 4-position by a protected
amidino group, can addi-tionally be prepared by coupling of an acid of the
formula X'-Y-COOH with an amine of the above formula IV.
The compounds of the formula I, their solvates and their salts inhibit
5 both the binding of fibrinogen, fibronectin and the Willebrand factor to the
fibrinogen receptor of blood platelets (glycoprotein IIb/IIIa) and the binding
thereof and of other adhesive proteins, such as vitronectine, collagen and
laminine, to the corresponding receptors on the surface of different cell types.The said cornpounds thus influence cell-cell and cell-matrix interactions. They
10 prevent in particular the formation of blood platelet thrombi and can be usedin the control or prevention of diseases such as thrombosis, cerebral infarct,
myocardial infarct, inflammation and arteriosclerosis. These compounds also
have an effec~ on tumour cells in that they inhibit metastasis formation
thereof. They can thus also be employed as anti-tumour agents. They can
15 further accelerate the healing of wounds.
The inhibition of fibrinogen formation on the fibrinogen receptor,
glycoprotein IIb/ma, can be detected as follows:
Glycoprotein rtb/ma is obtained from Triton X-100 extracts of human
blood platelets and purified by lectin affinity chromatography (Analytical
20 Biochemistry 151,1985,169-177) and chromatography on an Arg~ly-Asp-Ser
affinity column (Science 231,1986, 1559-62). The receptor protein thus obtained
is bound to microtiter plates. The specific binding of fibrinogen to the
immobilised receptor is determined with the aid of an ELISA system
("enzyme-linked imnnunosorbent assay"). I~e ICso values below correspond
25 to the concentration of the test substance which is required in order to inhibit
the binding of fibrinogen to the immobilised receptor by 50%:
- 12 ~
_
Product from
E~ple: 1 2 3 4 5 6 7
_
IC~ (mM) 0.0003 0~13 0.045 0.035 0.01 0.01 o.o~
_ _ . . . _
Product from
E~ple:
10 8 9 lO 11 18 25 28 31 38 39
_ _
IC~ (mM)
0.10 0.005 0.022 0.0025 0.010 0.0067 0.005 0.0128 0.0036 0.0012
As mPntioned at the beginning, the present
invention likewise relates to medicaments containing a
compound of the formula I, a solvate thereof or a salt
thereof, and furthermore also to a process for the
production of medicaments of this type, which is charac-
terised in that one or more of the said compounds and, if
desired, one or more other therapeutically useful sub-
stances are brouqht into a pharmaceutical administration
form. The medicaments can be administered enterally, for
example orally in the form of tablets, film tablets,
coated tablets, hard and soft gelatin capsules, solu-
tions, emulsions or suspensions, or rectally, for example
in th~ form of suppositories, or as a spray.
Adminstration, however, can also be carried out paren-
terally, for example in the form of injection solutions
or as an infusion.
To prepare tabiets, film tablets, coated tablets
and hard gelatin capsules, the active compound can be
mixed with pharmaceutically inert, inorganic or organic
excipients. Lactose, cornflour or derivatives thereof,
talc, stearic acid or its salts, for example, can be used
as excipients of this type for tablets, coated tablets
and hard gelatin capsules. Vegetable oils, waxes, fats,
semi-solid and liquid polyol~, for example, are suitable
- 13 - b`,` :~; f ~ 1 J'' J
as excipients for soft gelatin capsules; depending on the
nature of the active compound, however, no excipients at
all are necessary in the case of soft gelatin capsules.
Water, polyols, sucrose, invert sugar ~nd glucose, for
example, are suitable as excipients for the production of
solutions and syrups, water, alcohols, polyols, glycerol
and vegetable oils, for example, are suitable for injec-
tion solutions, and natural or hardened oils, waxes, fats
and semi-solid or liquid polyols, for example, are
suitable for suppositories. The pharmaceutical prepara-
tions can in ddition al~o contain preservatives, solubi-
lisers, stabiliser , wetting agents, emulsifiers, sweete-
ners, colorants, flavourings, salts for changing the
osmotic pressure, buffers, coatings or antioxidants.
For the control or prevention of the diseases
mentioned further above, the dosage of the active com-
pound can vary within wide limits and is naturally to be
suited to the individual conditions in each individual
case. In general, on oral administration a dose of about
0.1 to 20 mg/kg, preferably of about 0.5 to 4 mg/kg, per
day may be suitable for adults, it also being possible,
however, to exceed the upper limit just indicated if this
should prove appropriate.
Example 1
15 ml of trifluoroacetic acid are added to a
suspension of 100 mg of N-[N-[N-(p-amidinobenzoyl)-~-
alanyl]-3-(t-butoxycarbonyl)-L-alanyl]-3-phenyl-L-alanine
t-butyl e~ter hydroiodide in 10 ml of dichloromethane.
After 3 hour~ at room temperature, the solvents are
evaporated and the residue is crystallised using ether.
After recrystallisation from methanol/ethyl acetate,
32 mg of the trifluoroacetate of N-[N-[N-(p-amidino-
benzoyl)-~-alanyl]-L-~-aspartyl~-3-phenyl-L-alanine, m.p.
216-220C (decomposition), are obtained.
The starting material can be prepared as follows:
a) 834 mg of HBTU and 0.24 ml of N-methylmorpholine are
added at 0C under argon to a solution of 446 mg of
Z-~-Ala-OH and 785 mg of H-Asp(O-tBu)-Phe-O-tBu (obtained
from the condensation of Z-Asp(O-tBu) OH with H-Phe-O-tBu
J
~ 14 ~
followed by hydrogenolysis). After 5 hours, the mixture
is concentrated and the residue is partitioned in ethyl
acetate/5% NaHC03. The organic phase is washed with water
and lM RHS04. The organic extxacts are dried, filtered and
concentrated. The residue is recrystallised from ethyl
acetate/diisopropyl ether, 5~2 mg of Z-~-Ala-Asp(0-tBu)-
Phe-0-tBu, m.p. 138-139C, being obtained.
b) By catalytic hydrogenation of the precursor ~550 mg)
in ethanol in the presence of 10% Pd/C, 417 mg of
~ Ala-Asp~0-tBu)-Phe-0-tBu, MS: 464 (M+H)', are obtained
after chromatography on silica gel using ethyl
acetate/methanol 4~
c) By coupling 350 mg of the product from b) with
125 mg of 4-cyanobenzoic acid in the manner described in
Example la), 293 mg of N-[3-(t-butoxycarbonyl)-N-[N-(p-
cyanobenzoyl)-~-alanyl]-L-alanyl]-3-phenyl-L-alanine
t-butyl ester, m.p. 74-76C, are isolated after chromato-
graphy on silica gel using ethyl acetate.
d) A solution of 270 mg of the precursor in pyridine/
triethylamine 15:1 is saturated with hydrogen sulphide.
The solvents are removed after 24 hours and the residue
is partitioned in ethyl acetate/5% NaHC03. The organic
extracts are washed with water and with lM potassium
hydrogen sulphate solution, dried and concentrated. After
chromatography of the residue on silica gel using ethyl
acetate followed by recrystallisation from hexane, 230 mg
of N-[3-(t-butoxycarbonyl)-N-[N-[p-thiocarbamoylbenzoyl]-
~-alanyl]-L-alanyl]-3-phenyl-L-alanine t-butyl ester,
m.p. 101-103C, are obtained.
e) 2 ml of methyl iodide are added to a solution of
200 mg of the precursor in 15 ml of acetone. After
3 hours at boiling temperature, the mixture i3 allowed to
cool to room temperature and the product is precipitated
by addition of diethyl ether. 226 mg of N-[3-(t-butoxy-
carbonyl)-N-[N-[p-[l-(methylthio)formimidoyl3benzoyl]-~-
alanyl]-L-alanyl]-3-phenyl-L-alanine t-butyl ester
hydroiodide, m.p~ 139-140C, are obtained.
f) 32 mg of ammonium acetate are added to a solution of
160 mg of the precursor in 10 ml of methanol. The
- 15 -
reaction mixture is kept at boiling temperature for
4 hours. After cooling to room temperature, the solution
is filtered, concentrated and treated with diethyl ether.
The precipitated product is filtered off and dried.
135 mg of hydroiodide of N-[N-[N-(p-amidinobenzoyl)-~-
alanyl]-3-(t-butoxycarbonyl)-L-alanyl]-3-phenyl-L-alanine
t-butyl ester, m.p. 162-163C, are obtained.
Example 2
By treatment with trifluoroacetic acid in methy-
lene chloride as described in Example 1 and after crystallisation from methanol/diethyl ether, 26 mg of N-[N-
[N-(l-amidino-4-piperidinylcarbonyl)-~-alanyl]-L-~-
aspartyl3-3-phenyl-L-alanine trifluoroacetat~ (2:3),
m.p. 127-130C, are obtained from 60 mg of N-[N-[N-
(1-amidino-4-piperidinylcarbonyl)-~-alanyl] 3-(t-butoxy-
carbonyl ? -L-alanyl]-3-phenyl-L-alanine t-butyl ester.
The starting material is prepared as follows:
Coupling 86 mg of 1-amidino-4-piperidine-
carboxylic acid (Belg. Pat. 893,282, Nippon Chemiphar Co.
Ltd.) with 119 mg of H-~-Ala-Asp(O-tBu)-Phe-O-tBu
(Example lb) in dioxane in the presence of 58 mg of
pyridinium hydrochloride in the manner described in
Example la) yields 71 mg of N-[N-~N-(1-amidino-4-piperi-
dinylcarbonyl)-~-alanyl]-3-(t-butoxycarbonyl)-L-alanyl]-
3-phenyl-L-alanyl t-butyl ester, MS: 617 (M+H)t, after
chromatography on silica gel using methylene
chloride/methanol 1:1.
Example 3
By treatment with trifluoroacetic acid as des-
cribed in Ex~mple 1, 36 mg of the trifluoroacetate ofN-[N-[N-(p-amidinophenylacetyl)-~-alanyl]-L-~-aspartyl]-
3-phenyl-L-alanine, m.p. 21~-220C (from diethyl ether),
are obtained from 50 mg of N-[N-[N-(p-amidinophenyl-
acetyl)-~-alanyl~-3-(t-butoxycarbonyl)-L-alanyl]-
3-phenyl-L-alanine t-butyl ester hydroiodide.
The starting material can be prepared as follows:
a) By coupling 142 mg of 4-cyanophenylacetic acid with
371 mg of H-~-Ala-Asp(O-tBu)-Phe-O-tBu (~xample lb),
250 mg of N-[N-~N-(p-cyanophenylacetyl)-~-alanyl]-3 t-
- 16 ~
butoxycarbonyl-L-alanyl]-3-phenyl-L-alanine t-butyl
ester, m.p. 93-94C, are obtained in the manner described
in Example la) after chromatography on silica gel using
ethyl acetate/methanol.
b) Thionation of 230 mg of the precursor as described
in Example ld) yields 160 mg of N-[N-[N-(p-thiocar~amoyl-
phenylacetyl)-~-alanyl]-3-t-butoxycarbonyl-L-alanyl]-
3-phenyl-L-alanine t-butyl ester, MS: 607 (M+H)+, after
chromatography on ~ilica gel using ethyl acetate.
c~ Methylation of 120 mg of the precursor analogously
to Example le~ gives 100 mg of N-[N-[N-(p-methylthio-
formimidoyl)phenyl)acetyl]-~-alanyl]-3-t-butoxycarbonyl-
L-alanyl]-3-phenyl-L-alanine t-butyl ester hydroiodide,
m.p. 103 104C tacetone/diethyl ether), after crystal-
lisation.d) Reaction of 90 mg of th~ precursor with ammonium
acetate as described in Example lf) gives 62 mg of N-[N-
[N-(p-amidinophenylacetyl)-~-alanyl]-3-t-butoxycarbonyl-
L-alanyl] 3-phenyl-L-alanine t-butyl ester hydroiodide,0 m.p. 152C (methanol/diethyl ether).
Example 4
By treatment of 55 mg of N-~N-[4-(p-amidinophenyl-
carbamoyl)butyryl]-3-(t-butoxycarbonyl)-L-alanyl]-3-phenyl-
L-alanine t-butyl ester hydroiodide with trifluoroacetic
acid as described in Example l, 31 mg of N-[N-[4-~p-
amidinophenylcarbamoyl)butyryl]-L-~-aspartyl]-3-phenyl-L-
alanine trifluoroacetate (5:4), m.p. 159-161C, are
obtained after crystallisation from ethanol/diethyl ether.
The starting material can be prepared as follows:
a) By coupling 1.18 g of 4-aminobenzonitrile with
1.25 ml of monomethyl glutarate as described in Example
la), 1.83 g of methyl 4-[(p-cyanoph~nyl)carbamoyl]-
butyrate, m.p. 126-127C, are obtained after recrystal-
lisation from ethyl acetate.
b) 6 ml of lN sodium hydroxide solution is added to a
solution of 1 g of the precursor in 10 ml of methanol.
After 7 hours, the reaction mixture is concentrated, the
residue is extracted with ethyl acetate and the extract
i6 then neutralised using lN hydrochloric acid. The
' ' ?;' , ' , j
,) .,: ': l ; . . ' ` '
- 17 -
resulting precipitate ls filtered off, washed with water
and dried. 575 mg of ~-[(p-cyanophenyl)carb~moyl]butyric
acid, m.p. 200-201C, are obtained.
c) By coupling 464 mg of the precursor with 863 mg of
H-Asp(O-tBu)-Phe-O-tBu as described in Example la),
701 mg of N-[3-(t-butoxycarbonyl)-N-[4-(p-cyanophenyl-
carbamoyl)butyryl]-L-alanyl]-3-phenyl L~alanine t-butyl
ester, m.p. 64-66C, are obtained after chromatography on
silica gel using ethyl acetate and subsequent stirring in
hexane.
d) Reaction of 350 mg of the precursor with hydrogen
sulphide in analogy to Example ld) yields 304 mg of
N-[3-(t-butoxycarbonyl)-N-[4-(p-thiocarbamoylphenyl)-
butyryl]-L-alanyl]-3-phenyl-L-alanine t-butyl ester,
m.p. 178-179C, after r~crystallisation from ethyl
acetate.
e) Methylation of 150 mg of the precursor in analogy to
Example le) gives 175 mg of N-[3-(t-butoxycarbonyl)-N-
[4-[[p~ (methylthio)formimidoyl]phenyl]carbamoyl]-
butyryl]-L-alanyl]-3-phenyl-L-alanine t-butyl ester
hydroiodide (1:1), m.p. 127-128C, after crystallisation
using diethyl ether.
f) 60 mgofN-~N-[4-(p-amidinophenylcarbamoyl)butyryl]-
3-(t-butoxycarbonyl)-L alanyl]-3-phenyl-L-alanine t-butyl
ester hydroiodide, m.p. 130-132C, are obtained from
90 mg of the precursor in ~nalogy to Example lf).
Example 5
Reactlon of 100 mg of N-[N-t(p-amidinophenethyl-
carbamoyl)acetyl]-3-(t-butoxycarbonyl)-L-alanyl]-3-phenyl-
L-alanine t-butyl ester hydroiodide with trifluoroacetic
acid as described in Example 1 gives 69 mg of N-[N-[(p-
amidinophenethylcarbamoyl)acetyl]-L-Q-aspartyl]-3-phenyl-
L-alanine trifluoroacetate, m.p. 141-143C, after crystal-
lisation using diethyl etherO
The startin~ material can be prepared as follows:
a) 370 mg of p-(2-aminoethyl)benzonitrile,
MS: 147 tM+H~, are obtained from 876 mg of p-cyanohydro-
cinnamic acid (Pharmazie 26, 1973, 724) by a known reaction
sequence (Organic Synthesis 51, 1971, 48).
18 ,
b) A solution of 0.59 ml of methyl malonyl chloride in
5 ml of THF i9 added dropwise at -10C to a solution of
731 mg of p (2-aminoethyl)benzonitrile and 1.39 ml of
triethylamine in 10 ml of T~F. The mixture is then
allowed to warm to room temperature, and is poured into
ice-water and adjusted to pH 2 with lN hydrochloric acid.
The THF is evaporated and the aqueous extracts are
extracted with ethyl acetate. After drying and concen-
trating, 380 mg of methyl (p-cyanophenethyl)malonamate,
m.p. 125C, are obtained.
c) Analogously to Example 4b), hydrolysis of 750 mg of
the product from b) gives 365 mg of N-(p-cyanophenethyl)-
malonamic acid, m.p. 137-139C.
d) Analogou ly to Example la), 352 mg of N-[3-(t-
butoxycarbonyl)-N-[(p-cyanophenethylcarbamoyl)acetyl]-L-
alanyl]-3-phenyl-L-alanine t-butyl ester, MS: 607 (M+~)+,
is obtained by coupling 300 mg of the precursor with
507 mg of H-Asp~O-tBu)-Phe-O-tBu after chromatography on
silica gel using ethyl acetate.
e) Analogously to Examples ld), e) and f), the same
reaction sequence using 3fl0 mg of the product from d)
yields 152 mg of N-[N-[(p-amidinophenethylcarbamoyl)-
acetyl]-3-(t-butoxycarbonyl)-L-alanyl]-3-phenyl-L-alanine
t-butyl ester hydroiodide, m~p. 91-93C (decomposition),
after crystallisation using diisopropyl ether.
Example 6
119 mg of the trifluoroacetate of N-[N-[4-(p-
amidinobenzamido)butyryl]-L-~-aspartyl]-~-valine, m.p.
174C (decomposition), are obtained in analogy to
Example 1 from 200 mg of N-[N-[4-(p-amidinobenzamido)-
butyryl~-3-(t-butoxycarbonyl)-L-alanyl]-L-valine t-butyl
ester hydroiodide after recrystalli~ation from ethanol/
diethyl ether.
The starting material can be prephred as follows:
a) By coupling 783 mg of Z-4-aminobutyric acid with 1 g
of ~-Asp(O-tBu)-Val-O-tBu (obtained from the condensation
of Z-Asp(O-tBu)-OH with H-Val-O-tBu followed by hydro-
genolysis) as described in Example la), 1.17 g of
N-[N-[4-[1-(benzyloxy)formamido]butyramido]-3-(t-butoxy-
-- 19 --
carhonyl)-L-alanyl]-L-valine t-butyl ester, m.p. 64-65C,
are obtained afte- chromatography on silica gel using
ethyl acetate and crystallisation using hexane.
b) Hydrogenolysis of 1.1 g of the precursor analogously
to Example lb) gives 1 g of N-[N-(4-aminobutyryl)-
3-(t-butoxycarbonyl)-L-alanyl]-L-valine t-butyl ester,
m.p. 99-100C.
c) A solution of 859 mg of the product from b~ is
added, after 2 hours at 0C, to a solution of 324 mg of
4-cyanobenzoic acid, 352 mg of 2 chloro-4,6-dimethoxy-
1,3,5-triazine and 0.24 ml of N-methylmorpholine in 10 ml
of dimethylformamide. The mixture is allowed to warm to
room temperature and 772 mg of N-[N-r4-(p-cyanobenz-
amido)butyryl]-3-(t-butoxycarbonyl)-L-alanyl]-L-valine
t-butyl ester, MS: 559 (M+~)+, are obtained after working
up as in Example ld) and after chromatography on silica
gel using ethyl acetate.
d) If 760 mg of the product from c) are subjected to
the same reaction sequence as described in Examples ld),
e) and f), 444 mg of N-[N-[4-(p-amidinobenzamido)-
butyryl]-3-(t-butoxycarbonyl)-L-alanyl-L-valine t-butyl
ester hydroiodide, m.p. 105-107C (decomposition) are
obtained after crystallisation using dii~opropyl ether.
Exam~le 7
1.09 g of rac-N-l-[[[p-[N-~benzyloxycarbonyl)-
amidino]benzoy:L]-3-piperidinyl]carbonyl]-~-alanine benzyl
ester and 0.25 g of Pd/C are stirred in 20 ml of acetic
acid under hydrogen. The catalyst i9 filtered off and the
filtrate is evaporated. The residue is taken up in water
and the solution i8 evaporated. The precipitate is
suspended in methanol, adjusted to pH ~ with ammonia and
st.irred, then filtered with suction, washed with methanol
and dried. 530 mg of rac-N-[[l-(p-amidinobenzoyl)-
3-piperidinyl]carbonyl]-~-alanine are obtained in the
form of the hydrate (2sl), m.p. >265C, MS: 347
(96, M~H).
The starting material can be prepared as follows:
a) rac-N-(t-butoxycarbonyl)piperidine-3-carboxylic acid
(Can. J. Physiol. Pharmacol. 57, 1979, 763) in THF is
- 20 ~ r
activated with chlorodimethoxytriazine and N-methyl-
morpholine and then coupled with ~-alanine benzyl ester
p-toluenesulphonate (J. Org. Chem. 17, 1952, 1564) and
N-methylmorpholine to give rac-N-[[l-(t-butoxycarbonyl)-
3-piperidinyl]carbonyl]-~-alanine benzyl ester,
m.p. 57-59C.
b) By cleavage with trifluoroacetic acid, the tri-
fluoroacetate of rac-(3-piperidinylcarbonyl)-~-alanine
benzyl ester is obtained therefrom.
c) This is reacted in methylene chloride/aqueous sodium
bicarbonate solution with p amidinobenzoyl chloride and
then with benzyl chloroformate in the presence of sodium
carbonate to give rac-N-l-[[[p-[N-(benzyloxycarbonyl)-
amidino]benzoyl]-3-piperidinyl]carbonyl]-~-alanine benzyl
ester, MS: 571 (10, M~H).
Example 8
512 mgofN-[4-[p-[N-(benzyloxycarbonyl)amidino]-
benzamido]butyryl]-~-alanine benzyl ester and 170 mg of
Pd/C in 10 ml of acetic acid are stirred under hydrogen.
The catalyst is f iltered off and the filtrate is evapora-
ted. The residue is dissolved in water and the solution
is evaporated. The residue is s~spended in water, adjus~
ted to pH 7 using ammonia, filtered with suction, washed
with water and dried. 239 mg of N-[4-(p-amidinobenz-
amido)butyryl]-~-alanine, m.p~ >250C, MS: 321 (12, M+H),
are obtained.
The starting material can be prepared as follows:
a) 4-(t-Butoxycarbonylamino)butyric acid in THF is
activated wit:h chlorodimethoxytriazine and N-methyl-
morpholine and reacted with ~-alanine benzyl ester p-
toluenesulphonate in the presence of N-methylmorpholine
to give N-[4-~1-t-butoxyformamido)butyryl]-~-alanine
benzyl ester, m.p. 54-55C.
b) From this in trifluoroacetic acid, the trifluoro-
acetate of N-(4-aminobutyryl)-~-alanine benzyl ester is
obtained.
c) The latter is reacted in methylene chloride/water/
sodium hydrogen carbonate with p-amidinobenzoyl chloride
and then with benzyl chloroformate in the presence of
- 21 ~J ,? 3 ,~
sodium carbonate to give N-[4-[p-[N-(benzyloxycarbonyl)-
amidino]benzamido]butyryl]-~-alanine benzyl ester,
m.p. 173-183C.
Example 9
S Analogously to Example 8, N-[N-(p-amidino-
benzoyl)-~-alanyl]-~ alanine, m.p. >250C, MS: 307
(6, M+H), is obtained in the form of the hydrate (2:1)
from N-[N-[p-[N-(benzyloxycarbonyl)amidino]benzoyl~
alanyl~-~-alanine benzyl ester.
The starting material can be prepared as follows:
a) N-(t-butoxycarbonyl)-~-alanine and ~-alanine benzyl
ester are coupled analogously to th~ above examples to
give N-[N-(t-butoxycarbonyl)-~-alanyl]-~-alanine benzyl
ester, mp. 84-85C.
b) The trifluoroacetate of (~-alanyl)-~-alanine benzyl
ester is obtained therefrom in trifluoroacetic acid.
c) This is reacted with p-amidinobenzoyl chloride and
subsequently with benzyl chloroformate to give N-[N-[p-
[N-~benzyloxycarbonyl)amidino~benzoyl]-~-alanyl]-~-
alanine benzyl ester, m.p. 165-166C.
Example 10
In analogy to Example 8, N-[(DL)-N-(p-amidino-
benzoyl)-3-phenyl-~-alanyl]-~-alanine is obtained as the
hydrate ~3:1), m.p. >250C, MS: 383 (62, M+H), from
N-[(DL)-N-[p [N-(benzyloxycarbonyl)amidino]benzoyl]-
3-phenyl-~-alanyl]-~-alanine benzyl ester.
The starting material can be prepared as follows:
a) DL-N-(t-butoxycarbonyl)-3-phenyl-~-alanine and
~-alanine benzyl ester are coupled analogously to the above
examples to give N-[(DL)-N-(t-butoxycarbonyl)-3-phenyl-~-
alanyl]-~-alanine benzyl est~r, m.p. 143-144~C.
b) The trifluoroacetate of [(DL)-3-phenyl~-alanyl]-~-
alanine benzyl ester is obtained therefrom in trifluoro-
acetic acid.
c) This is reacted with p-amidinobenzoyl chloride and
subsequently with benzyl chloroformate to give N-[(DL)-
N-[p-[N-(benzyloxyc~rbonyl~amidino]benzoyl]-3-phenyl-~-
alanyl-~-alanine benzyl ester, mOp. 187-189C.
- 22 -
Example 11
443 mg of N-[3-[(benzyloxy)carbonyl~-N-[5-[p-[N-
[(benzyloxy)carbonyl]amidino]benzamido]valeryl]-L-alanyl]-
3-phenyl-L-alanine benzyl ester and lll mg of Pd/C in 9 ml
of acetic acid are stirred under hydrogen gas for 3~ hours.
The solution is filtered and evaporated, the residue is
dissolved in water and the solution is again evaporated.
The residue is stirred in water, filtered off with suction
and dried. 246 mg of N-[N-[5-(p-amidinobenzamido)valeryl]-
L-~-aspartyl]-3-phenyl-L-alanine, m.p. 241C, are obtained
as the hydrate (1:2).
The starting ester, m.p. 169-171C, can be
prepared as follows:
a) N-(t-butoxycarbonyl)-L-aspartic acid 4-benzyl ester
is coupled with 3-phenyl-L-alanine benzyl ester to give
N-[3-[(benzyloxy)carbonyl]-N-(t-butoxycarbonyl)-L-
alanyl]-3-phenyl-L-alanine benzyl ester, m.p. 93-94C.
b) This is deprotected using trifluoroa~etic acid and
coupled with 5-(1-t-butoxyformamido)valeric acid to give
N-[3-[(benzyloxy)carbonyl]-N-[5-(1-t-butoxyformamido)-
valeryl]-L-alanyl]-3-phenyl-L-alanine benzyl ester,
m.p. 119.5-120.5C.
c) The latter is freed of the t-butoxycarbonyl protec-
ting group in trifluoroacetic acid and then converted
into the starting material in methylene chloride/water/
sodium bicarbonate with p-amidinobenzoyl chloride and
subsequently with benzyl chloroformate.
Example 12
Analogously to Example 11, N,N'-[[(S)-tp-amidino-
benzamido)ethylene]dicarbonyl]di-~-alanine, m.p. ~250C~
is obtained from N,N'-[[(S)-[p-[N-[(benzyloxy)carbonyl]-
amidino]benzamido]ethylene]dicarbonyl]-di-~-alanine
dibenzyl diester after evaporating with water and stir-
ring with methanol.
The starting ester, m.p. 171-172C, is obtained
as follows:
a) N-(t butoxycarbonyl)-L-aspartic acid is coupled with
two equivalents of ~-alanine benzyl ester to give N,N'-
[[(S)-(l~t-butoxyformamido)ethylene]dicarbonyl]di-po-
- 23 - ~, 5 C? ~ J ,~
alanine dibenzyl ester, m.p. 109-110C.
b) After c]eavage of the t-butoxycarbonyl group with
trifluoroacetic acid, the product is reacted in methylene
chloride/aqueous sodium bicarbonate with p-amidinobenzoyl
chloride and subsequently with benzyl chloroformate to
give the starting ester.
Example 13
Analogously to Example ll, 2-N-(p-amidino-
benzoyl)-4-N-(2-carboxyethyl)-L-asparagine, m.p. 212C
(dec.) i8 obtained from N-[(S)-N-[p-[N-[(benzyloxy~-
carbonyl]amidino]benzoyl]-3-[(benzyloxy)carbonyl3-~-
alanyl]-~-alanine benzyl ester.
The starting ester is obtained as follows:
a) N-(t-butoxycarbonyl)-L~aspartic acid l-benzyl ester
is coupled with ~-alanine benzyl ester to give
3-[[2-[(benzyloxy)carbonyl]ethyl]carbamoyl]-N-(t-butoxy-
carbonyl)-L-alanine benzyl ester, m.p. 77-78C.
b) After cleavage of the t-butoxycarbonyl protecting
group in trifluoroacetic acid, the product is coupled in
methylene chloride/water/sodium bicarbonate with
p-amidinobenzoyl chloride and subsequently reacted with
benzyl chloroformate to give the ~tarting ester,
m.p. 122-123C.
Example 14
Analogously to Example 8, N-[5-(p-amidino-
benzamido)valeryl]-~-alaninel m.p. >280C, is obtained
from N-[5-[p-[N-[~benzyloxy)carbonyl]amidino]benzamido]-
valeryl]-~-alanine benzyl e~ter.
The s1:arting ester can be prepared as follows:
a) 5-(1-t-butoxyformamido)valeric acid i8 coupled with
~-alanine benzyl ester to give N-[5-(1-t-butoxy-
formamido)valeryl]-~-alanine benzyl ester, m.p. 69-70C.
b) The trifluoroacetate ofN (5-aminovaleryl)-~-alanine
benzyl ester i8 obtained therefrom in trifluoroacetic
acid~
c3 This is reacted in methylene chloride/water in the
presence of sodium bicarbonate with p-amidinobenzoyl
chloride and subsequently with benzyl chloroformate to
give the starting ester, m.p. 161-161.5C.
- 24 -
Exam~le 15 ~ J -'
704 mg of raC-N-[[1-[3-[1-[(E/Z))-N,N'-biS-
(t-benzyl ester and 176 mg of Pd/C in 14.1 ml of formic
acid are stirred under hydrogen for 18 hours. The cata-
lyst i5 filtered off and washed with 1:1 formic acid/water. The filtrate is evaporated, the residue is dis-
solved in water and the æolution is again evaporated. The
residue is chromatographed using ethanol/methanol on
silica gel. 254 mg of rac-N-t[1-[3~(1-amidino-4-piper-
idinyl)propionyl]-3-piperidinyl]carbonyl]-~-alanine,
MS: 382 (100, M+~), are obtained.
The starting ester can be prepared as follows:
a) 4-Piperidinopropionic acid is reacted with N,N'-
bis(t-butoxycarbonyl)-S-methylisothiourea in t-butanol
and 2N sodium hydroxide solution to give 3-[1-[(E/Z)-
N,N'-bis(t-butoxycarbonyl)amidino]-4-piperidinyl]-
propionic acid.
b) This is coupled with rac-N-(3-piperidinylcarbonyl)-
~-alanine benzyl ester to give the starting ester,
MS: 672 ~12, M+H).
Example 16
115 mg of N-[[(S)-l-[p-[N-[(benzyloxy)carbonyl]-
amidino]benzoyl]-2-pyrrolidinyl]acetyl]-~-alanine and
50 mg of Pd/C are stirred under hydrogen for 4 hours in
2.5 ml of acetic acid. The catalyst i~ filtered off and
the solution i8 evaporated. The residue is dissolved in
water, evaporated again and chromatographed on silica gel
using methanol. 46 mg of N-[[(S)-l-(p-amidinobenzoyl)-
2-pyrrolidinyl]acetyl]-~-alanine, m.p. >250C, are
3~ obtained.
The starting ester can be prep~red as follows:
a) (S)-1-[(Benzyloxy)carbonyl]-2~pyrrolidinoacetic acid
is coupled with ~-alanine t-butyl ester to give ~-~[(S)-
1-[(benzyloxy)carbonyl]-2-pyrrolidinyl]acetyl]-~-alanine
t-butyl ester, MS: ~91 (69, M+R).
b) The acetate of N-[(2-pyrrolidinyl)acetyl~ alanine
t-butyl ester is obtained therefrom by hydrogenation in
acetic acid.
c) This is reacted in methylene chloride/water in the
presence of sodium bicarbonate with p-amidinobenzoyl
chloride and subsequently with benzyl chloroformate to
give N-[[(S)-1-[p-[N-[(benzyloxy)carbonyl~amidino]~
benzoyl]-2-pyrrolidinyl]acetyl]-~-alanine t-butyl ester,
m.p. 127-128C.
d) The starting ester, MS: 481 (100, M+~), is obtained
therefrom in formic acid.
Example 17
300 mg of ben~yl-(S)-3-[[N-[p~N-(t-butoxy-
carbonyl)amidino]benzoyl]-~-alanyl]amino]-3-[(p-methoxy-
phenethyl)carbamoyl3propionate and 75 mg of Pd/C are
stirred under hydrogen for 4~ hours in 6 ml of formlc acid.
~he catalyst is filter~d off, the filtrate is evaporated,
the residue i~ taken up in water and the solution is
evaporated again. The crystalline substance iB suspended in
water, adjusted to pH 8 while stirring with ammonia and
then filtered with suction. 151 mg of (S)-3-[[N-(p-amidino-
benzoyl)-~-alanyl]amino-3-[(p-methoxyphenethyl)carbamoyl]-
propionic acid is obtained as the hydrate (1
m.p. 217C.
The starting ester can be obtained as follows:
a) ~-Alanine benzyl ester is reacted in methylene
chloride/water sodium bicarbonate with p-amidinobenzoyl
chloride an~ sub3eguently with di-t-butyl dicarbonate and
sodium carbonate to give N-[p-[N-(t-butoxycarbonyl)-
amidino]benzoyl]-~-alanine benzyl ester, m.p. 127-128C.
b) N-[p-[N-(t-butoxycarbonyl)amidino]benzoyl]-~-
alanine, MS: 336 (21, M~), is obtained therefrom by
catalytic hydrogenation.
c) N-(t-butoxycarbonyl)aspartic acid 4-benzyl ester is
coupled with 2-(4-methoxyphenyl)ethylamine to t~butyl-
[(S)-2 ~(benzyloxy)carbonyl]-l-[(p-methoxyphenethyl)-
carbamoyl]ethyl]carbamate, m.p. 103-104C.
d) From this in trifluoroacetic acid is obtained the
trifluoroacetate of 3-[(p methoxyphenethyl)carbamoyl]-~-
alanine benzyl ester.
e) The latter is coupled with the product described in
b) to give the starting ester, m.p. 150C (dec.).
- 26 ;j !
Example_18
Analogously to Example 8, N-[N~[N-(p-amidino-
benzoyl)-~-alanyl]-L-~-aspartyl]-L-leucine isopropyl
ester is obtained as the hydrate (1:1.3), m.p. 234~C
(dec.), from N-[3-[(benzyloxycarbonyl]-N-[N-[p-[N-
[(benzyloxy)carbonyl]amidino]benzoyl]~-alanyl]-L-
alanyl]-L-leucine isopropyl ester.
The starting ester can be obtained in the fol-
lowing way:
a) N-(t-butoxycarbonyl)-L-leucine is con~erted into
N-(t-butoxycarbonyl)-L-leucine isopropyl ester,
[~]D = -33 (MeOH, c = 0.5), u ing dicyclohexylcarbo-
diimide, p-toluenesulphonic acid and isopropanol in
pyridine.
b) The trifluoroacetate of L-leucinr isopropyl ester is
obtained therefrom in trifluoroacetic acid.
c) This is coupled with N-(t-butoxycarbonyl~-L-aspartic
acid 4-benzyl ester to give N-[3-[tbenzyloxy)carbonyl]-
N-(t-butoxycarbonyl)-L-alanyl]-L-leucineisopropylester,
MS: 479 (25, M+~).
d) After cleavage of the t-butoxycarbonyl protecting
group and coupling with N-(t-butoxycarbonyl)-~-alanine,
N-[3-[(benzyloxy)carbonyl]-N-[N-(t-butoxycarbonyl)-~-
alanyl]-L-a~anyl]-L-leucine isopropyl ester,
m.p. 103-104C, is obtained therefrom.
e) This i8 freed of the t-butoxycarbonyl protecting
~roup in trifluoroacetic acid and then converted into the
starting ester in methylene chloride/water/sodium bicar-
bonate with p-amidinobenzoyl chloride and subsequently
usinq benzyl chloroformate, m.p. 176-177C.
~xample_l9
Analogously to Example 8, N-[~(R)-1-(p-amidino-
benzoyl)-~-pyrrolidinyl]carbonyl]-~-alanine i5 obtained
as the hydrate (4:3), m.p. >250C, [~]D = -4.13 (lN ~Cl,
c = 0.46%), from N-[[(R)-l-[p-[N~[(benzyloxy)carbonyl]-
amidino]benzoyl]-3-pyrrolidinyl]carbonyl]-~-alanine
benzyl ester.
The starting ester c~n be prepared in the fol-
lowing manner:
- 27 ~
a) (R)-l-[(R)-~-methylhenzyl]-3-pyrrolidinomethanol,
di-t-butyl dicarbonate and PdiC in ethanol are stirred
under hydrogen for 20 hours. t-Butyl (R~-3-(hydroxy-
methyl)-1-pyrrolidinocarboxylate, m.p. 35C,
[~]D - +19.5 (methanol, c = 1.0), is obtained.
b) From this, (R)-l-(t-butoxycarbonyl)-3-pyrrolidino-
carboxylic acid, m.p. 135-138C, ~]D = -15 (MeOH,
c = 1.O), is obtained using pyridini~m dichromate in DMF.
c) This is coupled with ~-alanine benzyl ester to give
N-[[(R)-l-(t-butoxycarbonyl)-3-pyrrolidinyl]carbonyl]-~-
alanine benzyl ester, mrp. 83-84C, [~]D = -3.4 (MeOH,
c = 1.0).
d) The trifluoroacetate of N-[[(R)-3-pyrrolidinyl]-
carbonyl]-~-alanine benzyl e ter is obtained therefrom in
trifluoroacetic acid.
e) This is reacted in methylene chloride/water/sodium
bicarbonate with p-amidinobenzoyl chloride and subse-
quently with benæyl chloroformate to give the starting
ester, [~]D = +2.2 (MeOH, c - 0.5).
Example 20
Analogously to Example 16, N-[N-(p-amidino-
benzoyl)-2-methyl-~-alanyl]~L-~-aspartamide (2:1
epimers), m.p. 280C, is obtained from benzyl (S)-
3-[[D/L-N-[p-[N-[(benzyloxy)carbonyl]amidino]benzoyl]-
2-methyl-~-alanyl]amino]æuccinamate.
The starting ester can be prepared as follows:
a) Benzyl (S)-3-(1-t-butoxyformamido)succinamate is
deprotected using trifluoroacetic acid and then coupled
with DL-N-(t-butoxycarbonyl)-2-methyl-~-alanine. t-Butyl
[(RS)-2-[[(S)-~-[(benzyloxy)carbonyl]-l-carbamoylethyl]-
carbamoyl]propyl]carbamate, m.p. 135-136C, is obtained.
b) After cleavage of the t-butoxycarbonyl protecting
group in trifluoroacetic a~id, the product is coupled in
methylene chloride/water/sodium bicarbonate with
p-amidinobenzoyl chloride and finally reacted with benzyl
chloroformate to give the starting ester (2:1 epimers~,
m.pO 178~5-179~5Co
Example 21
548 mg of N-[N-[p-[N-(t-butoxycarbonyl)amidino]-
- 28 -
phenyl]acetyl]-~-alanyl]-~ alanine benzyl ester are
allowed to stand in 11 ml of formic acid for 18 hours.
After addition of 137 mg of Pd/C, the mixture is stirred
under hydrogen for 4 hours. The catalyst is filtered off
and the filtrate is evaporated. The residue is dissolved
in water and the solution is evaporated again. The
residue is ~uspended in water and adjusted to p~ 8 using
ammonia, then filtered with suction and dried. 290 mg of
N~[N-[(p-amidinophenyl)acetyl]-~-alanyl]-~-alanine are
obtained as the hydrate (1:1), m.p. 286C ~dec.).
The starting material, m.p. 262C (dec.), is
obtained by reaction of N-(~-alanyl)-~-alanine benzyl
ester in DMF/triethylamine with p-amidinophenylacetyl
chloride and subsequent rea~tion with di-t-butyl dicar-
bonate.
Example 22
535 mg of rac-N-[[l-[p-[N-(t-butoxycarbonyl)-
amidino]phenylacetyl]-3-piperidinyl]carbonyl]-~-alanine
benzyl ester are allowed to stand in 11 ml of formic acid
for 19 hours. The solvent is evaporated, and the residue
is evaporated with water and recrystallised from aceto-
nitrile~ 340 mg of rac-N-[~[l-(p-amidinophenyl)acetyl]-
3-piperidinyl]carbonyl]-~-alanine benzyl ester formate
(1:1), m.p. 97-98C, are obtained.
The starting ester, MS: 551 (9, M+H), is obtained
by coupling rac-N-[(3-piperidinyl)carbonyl]-~-alanine
benzyl ester in DMF/triethylamine with p-amidinophenyl-
acetyl chloride and subsequent reaction with di-t-butyl
dicarbonate.
Example 23
535 my of rac-N-[[l-[p-[N-(t-butoxycarbonyl)-
amidino~phenylacetyl]-3-piperidinyl]c~rbonyl]-~-alanine
benzyl ester are allowed to stand in 11 ml of formic acid
for 19 hours, and the mixture is treated with 134 mg of
Pd/C and stirred under hydrogen for 4 hours. The solution
is filtered and evaporated, the residue is dissolved in
water and the solution is evaporated again. The product
is stirred in acetonitrile, filtered with suction and
dried. 272 mg of rac-N-[[l-~(p-amidinophenyl)acetyl]-
- 29 ~ s,~,
3-piperidinyl]carbonyl]-~-alanine, MS: 361 (41, M+H), are
obtained.
Exam~le 24
1127 mg of N-[3-[(benzyloxy)carbonyl~-N-[N-
[3-~l-[(E or Z)-N,N'-bis(t-butoxycarbonyl)amidino]-
4-piperidinyl]propionyl]-~-alanyl]-L-alanyl]-3-phenyl-L-
alanine benzyl ester are allowed to stand in 22.5 ml of
formic acid for 21 hours~ After addition of 282 mg of
Pd/C, the mixture is stirred under hydrogen for 5 hours.
The solution is filtered and evaporated, the residue is
dissolved in water and the solution i8 evaporated again.
The residue is stirred in water, filtered with suction
and dried. 543 mg of N-[N-[N-[3-(1-amidino-
4-piperidinyl)propionyl]-~-alanyl]-L-~-aspartyl]-
3-phenyl-L-alanine, m.p. 246C (dec.), are obtained.
The starting ester can be prepared as follows:
a) N-(t-butoxycarbonyl)-~-alanine is coupled with
N-[3-[~benzyloxy)carbonyl]-L-alanyl]-3-phenyl-L-alanine
benzyl ester to give N-[3-[(benzyloxy)carhonyl]-N-[N-
(t-butoxycarbonyl)-~-alanyl]-L alanyl]-3-phenyl-L-alanine
benzyl ester, m.p. 124-125C.
b) After cleavage of the t-butoxvcarbonyl protecting
group, the product is coupled with 3-[1-[N,N'-bis-
(t-butoxycarbonyl)amidino]-4-piperidinyl]propionic acid
to give the starting ester, 1:1 ethyl acetate solvate,
m.p. 100C (dec.).
Example 25
1.3 g of 3-[(~enzyloxy)carbonyl]-N-[5-[p-~N-
[(benzyloxy)carbonyl]amidino]benzamido]valeryl]-L-alanine
isobutyl ester and 325 mg of Pd/C in 25 ml of acetic acid
are stirred under hydrogen for 5~ hours. The ~olution is
filtered and evaporated and the residue i~ evaporated
successively with water, methanol and ethanol. The
product is adjusted to pH 8 in acetonitrile using
ammonia, stirred and filtered with suction. 596 mg of
isobutyl N-[5-(p-amidinobenzamido)valeryl]-L-~-aspartate
are obtained a the hydrate (1:1), m.p. 162-166C.
The starting ester, m.p. 127.5-129.5C, can be
prepared as follows:
3 0 - ~ . f 7: . . .
a) Benzyl N-(t-butoxycarbonyl)-L-~-aspartate is con-
verted into the N-(t-butoxycarbonyl)-3-[(benzyloxy)-
carbonyl]-L-alanine isobutyl ester, MS: 323 (8, M~C~H8),
using dicyclohexylcarbodiimide/ p-toluenesulphonic acid
and isobutyl alcohol in pyridine.
b) After clea~age of the t-butoxycarbonyl group in
trifluoroacetic acid, the product is coupled with 5-(1-t-
butoxyformamido)valeric acid to give 3-[(benzyloxy)-
carbonyl]-N-[5-(1-t-butoxyformamido)valeryl~-L-alanine
isobutyl ester, m.p. 64-66C.
c) This is deprotected uRing trifluoroacetic acid and
then reacted in methylene chloride/wat~r/sodium bicar-
bonate with p-amidinobenzoyl chloride and subse~uently
with benzyl chloroformate to give the starting ester.
Example 26
562 mg of benzyl (S)-~-[[DL N-[p-[N-[(benzyloxy)-
carbonyl]amidino]benzoyl]-3-methyl-~-alanyl]amino]-~-oxo-
l-pyrrolidinobutyrate and 140 mg of Pd/C in 11 ml of
acetic acid are stirred under hydrogen for 2 hours. The
filtered solution is evaporated and the residue is
evaporated successively with water, methanol and ethanol.
The residue is finally adjusted to pH 8 in ethanol using
ammonia, stirred and filtered off with suction. 289 mg of
(S)-~-[[DL-N-(p-amidinobenzoyl)-3-methyl-~-alanyl]amino]-
~-oxo-1-pyrrolidinobutyric acid are obtained as the
hydrate (2:3), m.p. 222-224C.
The st:arting ester can be prepared in the follow-
ing way:
aJ Benzyl tS)-~-tl-t-butoxyformamido)-y-oxo-l-
pyrrolidinobutyrate is deprotected using trifluoroacetic
acid and coupled with tRS)-3-tl t~butoxyformamido)butyric
acid to give benzyl tS)-~-[~RS)o3-(1-t-butoxyformamido)-
butyramido]-y-oxo-l-pyrrolidinobutyrate, m.p. 104-105C.
b) From this, the starting e~ter, MS: 642 tlO0, M+~),
is obtained after cleavage of the t-butoxycarbonyl group
in trifluoroacetic acid and reaction with p-amidino-
benzoyl chloride and subsequently with benzyl chloro-
formate in methylene chloride/water/sodium bicarbonate.
- 31 -
Exam~le 27
Analogously to Example 8, DL-N-[N-(p-amidino-
benzoyl)-~alanyl]-3-methyl-~-alanine is obtained as the
hydrate (3:1) m.p. 291C (dec.), from DL-N-[N-[p-[N-
[(benzyloxy)carbonyl]amidino]benzoyl]-~-alanyl]-3-methyl-
~-alanine benzyl ester.
The starting ester, m.p. 179-180C, can be
prepared as follows:
2 ) N-(t-butoxycarbonyl)-~-alanine is coupled with
~0 DL-3-aminobutyric acid benzyl ester to give DL-N-[N-
(t-butoxycarbonyl)-~-alanyl~3-methyl-~-alanine benzyl
ester, m.p. 70-72C.
b) DL-N-(~-alanyl)-3-methyl-~-alanine benzyl ester is
obtained therefrom using trifluoroacetic acid.
c) This is reacted in methylene chloride~water/sodium
bicarbonate with p-amidinobenzoyl chloride with p-amidino-
benzoyl chloride and subsequently with benzyl chloroformate
to give the starting ester.
Example 28
Analogously to Example 8, N-[N-[N-(p-amidino-
benzoyl)-~-alanyl]-L-~-aspartyl]-L-serine ethyl ester is
obtained a~ the hydrate (2:7), m.p. 201-203C, from N-[3-
[(benzyloxy)carbonyl]-N-[N-[p-[N [(benzyloxy)carbonyl]-
amidino]benzoyl]-~-alanyl]-L-serine ~thyl ester.
The starting ester, m.p. 177-179C, can be
prepared as follows:
a) Benzyl N--(t-butoxycarbonyl)-L-~-aspartate iscoupled
with L-serine ethyl ester to give N-[3-[(benzyloxy)car-
bonyl]-N-(t-butoxycarbonyl)-L-alanyl]-L-serine ethyl
ester, m.p. 96-97C.
b) After cleavage of the t-butoxycarbonyl group, the
product i8 coupled with N-(t-butoxycarbonyl)-~-alanine to
give N-[3-[(benzyloxy)carbonyl]-N-[N-(t-butoxycarbonyl)-
~-alanyl3-L-alanyl~-L-serine ethyl ester, m.p. 132-134C.
c) This i~ deprotected in trifluoroacetic acid and the
product is subsequently reacted in methylene chloride/-
water/sodium bicarbonate with p-amidinobenzoyl chloride
and finally with benzyl chloroformate to give the start-
ing ester.
Exam~le 29
Analogously to Example 8, N-[DL-N-(p-amidino-
benzoyl)-2 phenyl-~-alanyl]-~-alanine is obtained as the
hydrate (4:1), m.p. 276C, from N-[N-[p-[DL-N-[~benzyl-
oxy)carbonyl]amidino]benzoyl]-2-phenyl-~-alanyl]-~-
alanine benzyl ester~
The starting ester, m.p 173-174C, is prepared
as follows:
a) DL 2-phenyl-~-alanine is reacted with di-t-butyl
dicarbonate in t-butanol and sodium hydroxide solution to
give DL-N-~t-butoxycarbonyl)-2-phenyl-~-alanine,
m.p. 147-148C.
b) This is co~pled with ~-alanine benzyl ester to give
N-[DL-N-(t-butoxycarbonyl)-2-phenyl-~-alanyl]-~-alanine
benzyl ester, m.p. 115-116C.
c) After cleavage of the protecting group, the product
is reacted in methylene chloride/water/sodium bicarbonate
with p-amidinobenzoyl chloride and subsequently with
benzyl chloroformate to give the starting ester.
Example 30
Analogously to Example 8, DL-N-[N-(p-amidino-
benzoyl)-~-alanyl]-2-methyl-~-alanine is obtained as the
hydrate (3:1), m.p. ~300C, from N-~N-[p-[N-[(benzyl-
oxy)carbonyl]amidino]benzoyl]-~-alanyl]-DL-2-methyl-~-
alanine benzyl ester.
The starting ester, m.p. 159-160C, is obtained
as follows:
a) N-(t-butoxycarbonyl)-~-alanine is coupled with
tDL-2-methyl-~-alanine benzyl e~ter to give DL-N-[N-
(t-butoxycarbonyl) ~-alanyl]-2-methyl-~-alanine benzyl
ester, MS: 365 (47, M+H).
b) After cleavage of the protecting group, the product
is reacted in methylene chloride/water/sodium bicarbonate
with p-amidinobenzoyl chloride and subsequently with
benzyl chloroformate to give the starting ester.
Example 31
Analogously to Example 11, N-[N-[[(R)-l-(p-
amidinobenzoyl)-3-pyrrolidinyl]carbonyl~-L-~-aspartyl]-
3-phenyl-L-alanine acetate (2~1) is obtained as the
33 ,~
hydrate (3:2), m.p. 215C, from N-[N-[ [ (R)-l-[p-[N-
[(benzyloxy)carbonyl]amidino]benzoyl]-3-pyrrolidinyl]-
carbonyl]-3-[(benzyloxy)carbonyl]-L-alanyl]-3-phenyl-L-
alanine benzyl ester after evaporating with water and
stirring in ethanol.
The starting ester is obtained as follows:
a) (R)-l-(t-butoxycarbonyl)-3-pyxrolidinocarboxylic
acid is coupled with N-[3-[(benzyloxy)carbonyl]-L-
alanyl]-3-phenyl-L-alanine benæyl ester to give N-[N-
[~(R)-1-(t-butoxycarbonyl)-3-pyrrolidinyl]carbonyl]-3-
[(benzyloxy)carbonyl]-L-alanyl]-3-phenyl-L-alanine benzyl
ester, m.p. 84-85C.
b) After removal of the t-butoxycarbonyl group in
trifluoroacetic acid, the product is reacted in methylene
chloride/water/sodium bicarbonate with p-amidinobenzoyl
chloride and subsequently with benzyl chloroformate to
give the starting ester, m.p. 144-145C.
Example 32
Analogously to Example 7, DL-N-[N-(p-amidino-
benzoyl1 ~-alanyl]-2-phenyl-~-alanine is obtained as the
hydrate (l:1), m.p. 243-2459C, from N-[N-[p-[N-[(benzyl-
oxy)carbonyl]amidino]benzoyl]-~-alanyl]-DL-2-phenyl-~-
alanine benzyl ester.
The starting ester can be obtained as follows:
a) N-(t-butoxycarbonyl)-2-phenyl-~-alanine is heated to
reflux in acetone with benzyl bromide and potassium
carbonate for 17 hours~ DL-N-(t-butoxycarbonyl)-2-phenyl-
~-alanine benzyl ester, m.p. 58-59C, is obtained.
b) After cleavage of the t-butoxycarbonyl group, the
product i6 coupled with N-(t-butoxycarbonyl)-~-alanine
to give DL-N-[N-(t-butoxycarbonyl)-~-alanyl]-2-phenyl-~-
alanine benzyl ester, m.p. 84.5-86C.
c) This is converted into the trifluoroacetate of the
-(~-alanyl)-2-phenyl-~-alanine benzyl ester in trifluoro-
acetic acid.d) The latter is reacted in methylene chloride/water/-
sodium bicarbonate with p-amidinobenzoyl chloride and
subsequently with benzyl chloroformate to give the
starting ester, m.p. 165-166C.
- 34 ~
,, ,,,., ",
Example 33
Analogously to Example 11, p-[2-[[N-[N-(p-
amidinobenzoyl)-~-alanyl]~L-~-aspartyl]amino]ethyl]-benzoic
acid is obtained as the hydrate (3:7), m.p. 194-196C
(methanol/water), from benzyl p-[2-[[3-[~benzyloxy)car-
bonyl]-N-[N-[p-[N-[(ben yloxy)carbonyl]amidino] benzoyl]-
~-alanyl]-L-alanyl]amino]ethyl]benzoate.
The starting ester, m.p. 162-163C, can be
prepared as follows:
a) p-(2-chloroethyl)benzoyl chloride is converted into
benzyl p-(2-chloroethyl)benzoate, MS: 274 (8, M), using
benzyl alcohol and pyridine in methylene chloridec
b) Benzyl p-(2-azidoethyl)benzoate, MS: 281 (2, M) is
obtained therefrom using sodium azide in DMSO.
c ) This is reacted in pyridine with triphenylphosphine
and subsequently with conc. ammonia to give benzyl p-(2-
aminoethyl)-benzoate, MS: 226 (16, M-CH2NH).
d) Benzyl p-[2-[[3-[(benzyloxy)carbonyl]-N-(t-butoxy-
carbonyl)-L-alanyl]amino]-ethyl]benzoate, m.p. 99-100C,
is sbtained therefrom by coupling with benzyl N-(t-
butoxycarbonyl)-L-~-aspartate.
e) This is deprotected in trifluoroacetic acid and
coupled with N-(t-butoxycarbonyl)-~-alanlne to give p-[2-
[[3-~(benzyloxy)-carbonyl]-N-[N-~t-butoxycarbonyl)-~-
alanyl]-L-alanyl]amino]-ethyl]benzoate, m.p. 138-139C.
f) After cleavage of the t-butoxycarbonyl group in
trifluoroacetic acid, the product is reacted in methylene
chloride/water/~odium bicarbonate with p-amidinobenzoyl
chloride and ~ubsequently with benzyl chloroformate to
give the starting ester.
Example ~4
Analogously to Example 7, [DL-N-[~-(p-amidino-
benzoyl)-~-alanyl-3-phenyl-~-alanine is obtained as the
hydrate (3:1), m.p. 220C (dec.~, from DL-N-[N-[p-[N-
[(benzyloxy)carbonyl]amidino]benzoyl]-~-alanyl]-3-phenyl-
~-alanine benzyl ester.
The starting ester, m.p. 208C, is obtained as
follows:
a) N-(t butoxycarbonyl)-~-alanine is reacted with
- 35 -
Dl,-3-phenyl-~-alanine benæyl ester to give DL-N-[N-(t-
butoxycarbonyl)-~-alanyl]-3-phenyl-~-alanine benzyl
ester, m.p. 124.5-126C.
b) The trifluoroacetate of DL-N-(~-alanyl)-3-phenyl-~-
S alanine benzyl ester is obtained therefrom in trifluoro-
acetic acid.
c) This is reacted in methylene chloride/water/sodium
bicarbonate with p-amidinobenæoyl chloride and subse-
quently with benzyl chloroformate to give the starting
ester.
Example 35
Analogously to Example 11, ~p-[2-[[N-[N-(p-
amidinobenzoyl)-~-alanyl]-L-~-aspartyl]-amino]ethyl]-
phenoxy]acetic acid is obtained as the hydrate (2:7),
m.p. 210-213C, from benzyl (S)-3-[[N-[p-~N-[(benzyloxy)-
carbonyl]amidino]benzoyl]-~-alanyl]amino]-N-[p-[~(benzyl-
oxy)carbonyl]methoxy]phenethyl]succinamate.
The starting ester, m.p. 172-174C, can be
prepared as follows:
a) t-Butyl [2-(4-hydroxyphenyl)ethyl]carbamate, benzyl
bromoacetate and potassium carbonate are heated in
acetone. t-Butyl [p-[~(benzyloxy)carbonyl]-methoxy]-
phenethyl]carbamate, MS: 385 (0.5, M), is obtained.
b) After cleavage of the t-butoxycarbonyl protecting
group, the product is coupled with N-(t-butoxycarbonyl)-
aspartic acid-4-benzyl ester to give benzyl (S)-N-[p-
[[(benzyloxy)carbonyl]methoxy]phenethyl]-3-[l-t-butoxy-
formamido]succ:inamate, m.p. 178.5-180.5C.
c) This i8 deprotected in trifluoroacetic acid and
coupled with N-(t-butoxycarbonyl)-~-alanine to give
t-butyl [2-[[~S)-2-[(benzyloxy)carbonyl]-1-[[p-[[~benzyl-
oxy)carbonyl]methoxv]-phenethyl]carbamoyl]ethyl]-
carbamoyl]ethyl]carbamate, m.p. 123-124C.
d) After cleavage of the t-butoxycarbonyl protecting
group, the product is reacted in methylene chloride/-
water/sodium bicarbonate with p-amidinobenzoyl chloride
and subsequently with benzyl chloroformate to give the
starting ester.
- 36 ~
Example 36
a) A solution of 280 mg of 1-[N-(p-cyanobenzoyl)-~-
alanyl]-4-piperidinoacetic acid and 1 ml of triethylamine
in 15 ml of pyridine is saturated with hydrogen sulphide.
After 36 hours, the solution is evaporated and the
residue is suspended in ethyl acetate/water. Filtration
and drying of the in601uble material gives 255 mg of 1-
[N-[p-(thiocarbamoyl)benzoyl]-~-al nyl]-4-piperidino-
acetic acid.
b) A solution of 150 mg of the precursor in 15 ml of
a~etone i~ heated to boiling temperature with 1 ml of
methyl iodide for 3 hours. After cooling the solution to
room temperature, 130 mg of l-[N-~p-~l-(methylthio)-
formimidoyl~benzoyl3-~-alanyl]-4-piperidinoacetic acid
hydroiodide ~1:1), m.p. 206-207C, precipitate.
c) 100 mg of 1-[N-[p-[1-(methylthio)formimidoyl]-
benzoyl]-~-alanyl]-4-piperidinoacetic acid and 30 mg
ammonium acetate in 10 ml of methanol are kept at boiling
temperature for 3 hours. After cooling to room tempera-
ture, the solution is filtered, concentrated and treated
with diethyl ether. The precipitated oil is chromato-
graphed on silica gel RP 18 using water/methanol (10:1)
after decanting of the solvent. 24 mg of 1-[N-(p-amidino-
benzoyl)-~-alanyl]-4-piperidinoacetic acid hydroiodide
(10:1), m.p. 206C, are obtained.
The starting nitrile can be obtained as follows:
a) 4.96 g of 4-cyanobenzoyl chloride and 2.67 g of
~-alanine are stirred at room temperature for 4 h~urs in
450 ml of ~odium bicarbonate solution (2%) and the mixture
is acidified using concentrated sulphuric acid (pH 6). The
solution is evaporated and extracted using ethyl acetate.
Drying and e~aporation of the organic phase gives a residue
which, with diisopropyl ether, leads to 4.69 g of N-(p-
cyanobenzoyl)-~-alanine~ m.p. 155-157C.
b) Coupling of 635 mg of N-(p-cyanobenzoyl)-~-alanine
with 540 mg of 4-piperidinoacetic acid gives, after
chromatography on silica gel RP 18 using THF/water
(85:15) 300 mg of 1-[N-(p-cyanobenzoyl)-~-alanyl~-4-
piperidinoacetic acid, MS: 344 (M+~
~ 37 ~
Example 37
400 mg of t-butyl 4-[N-tp-amidinobenzoyl)-~-
alanyl]-l-piperazinoacetate are stirred in 15 ml of
methylene chloride and 15 ml of trifluoroacetic acid. The
solution is evaporated, the residue is suspended in 5 ml
of ethanol and the undissolved material iR filtered off.
The filtrate is treated with ethyl acetate and the
precipitate is filtered with suction and dried. Chromato-
graphy of the crude product on silica gel RP 18 using
water leads to 38 mg of 4-~N-(p amidinobenzoyl)-~-
alanyl]-1-piperazinoacetic acid trifluoroacetate (5:8),
m.p. 157-158C.
The starting material can be prepared as follows:
a) 2.23 g of N-benzyloxycarbonyl-~-alanine are coupled
with 2.58 g of piperazine. The evaporation residue is
suspended in T~F, the undissolved material is filtered
off and the filtrate is evaporated~ Chromatography of the
residue on silica gel RP 18 using water/methanol (2-5%)
gives 2.51 g of benzyl [2-(4-piperazinylcarbonyl)ethyl]-
carbamate, MS: 291 (M+-).
b) 600 mg of the precursor, 0.3 ml of t-butyl bromo-
acetate and 25 mg of tetrabutylammonium hydrogen sulphate
are dissolved in 10 ml of toluene and stirred with 10 ml
of 50 % strength sodium hydroxide solution for 1 hour.
The organic phase is washed with water and evaporated.
Chromato~raphy of the residue on silica gel using ethyl
acetate/methanol (9:1) gives 480 mg of t-butyl 4-[N-
[(benzyloxy)carbonyl]-~-alanyl]-l-piperazinoacetate,
MS: 406 tM+H)~.
c) The precursor is hydrogenated in ethanol for 1 hour
in the presence of 200 mg of Pd/C. The catalyst is
filtered off and the filtrate is çvaporated. 290 mg of
t-butyl 4-~-alanyl-1-piperazinoacetate, MS: 271 (M+), are
obtained.
d) The precursor and 341 mg of p-amidinobenzoyl
chloride are stirred in 20 ml of methylene chloride and
10 ml of saturated sodium bicarbonateO The organic phase
is separated off and evaporated, and the residue is
suspended in ethyl acetate. Filtrat~on with suction and
38 - ,
drying of the crystals gives 400 mg of t-butyl 4-[N-(p-
amidinobenzoyl)-~-alanyl]-l-piperazinoacetate, MS: 418
(M+H)~.
Example 38
Analogously to Example 37, 1.6 g of N-[N-[N-(p-
amidinobenzoyl)-g-alanyl]-3-(t-butoxycarbonyl)-L-alanyl]-
L-valine t-butyl ester are deprotected. The crude product
is chromatographed on silica gel RP 18 using water/THF
(95:5). 867 mg of N-[N-[N-(p-amidinobenzoyl)-~-alanyl]-
L-~-aspartyl]-L-valine trifluoroacetate, m.p. 162-163C,
are obtained.
The starting ester ~an be prepared as follows:
a) t-Butyl N-benzyloxycarbonyl-L-~-aspartate and
L-valine t-butyl ester hydrochloride are coupled to give
N-[N-[(benzyloxy)carbonyl]-3-(t-butoxycarbonyl)-L-
alanyl]-L-valine t-butyl ester, m.p. 75C (d).
b) This i~ deprotected analogously to ~xample 37c.
N-[3-t-butoxycarbonyl)-L-alanyl]-L-valine t-butyl ester,
m.p. 71C, is obtained.
c) 2.8 g of the precursor are coupled with 1.78 g of
N-~(benzyloxy)carbonyl]-~-alanine and the crude product
is chromatographed on silica gel using ethyl acetate.
2.24 g of N-[N-[N-[(benzyloxy)carbonyl3-~-alanyl]-3-(t-
butoxycarbonyl)-L-alanyl]-L-valine t-butyl ester, m.p.
126C are obtained.
d~ Deprotection of 2.2 g of the precursor analogously
to Example 37c) leads to 1.61 g of N-~N-~-alanyl-3-(t-
butoxycarbonyl)-L-alanyl]-L-valine t-butyl ester.
e) Reaction of 1 ~ of p-amidinobenzoyl chloride with
1.61 g of the precursor according to procedure 37d gives
1.62 g of starting ester.
Example 39
Analogously to Example 37, 1.4 g of N-[N-[N-(p-
amidinobenzoyl)-~-alanyl]-3-(t-butoxycarbonyl)-L-alanyl]-
3-(p-t-butoxyphenyl)-L-alanine t-butyl ester are depro-
tected. The produ~t is suspended in ethanol, insoluble
material is filtered off and the filtrate is treated with
ether. Filtration with suction and washing of the preci-
pitate with 20 ml of isopropanol/ethanol (1:1) gives
~ ;y ~
-39 -
801 mg of N-[N-[N-(p-amidinoberzoyl)-~-alanylj-L-o~-aspartyl]-3-(p-
hydroxyphenyl)-L-alanine trifluoroacetate (2:5), m.p. 202-204C.
The starting ester can be prepared as follows:
a) N-[(9H-fluoren-9-yloxy)carbonyl]-3-tt-butoxycarbonyl)-L-alanine and 3-
5 (p-t-butoxyphenyl)-L-alanine t-butyl ester are coupled to give N-[N-[(9H-
fluoren-9-yloxy)carbonyl]-3-(t-butoxycarbonyl)-L-alanyl]-3-(p-t-butoxyphenyl)-
L-alanine t-butyl ester and 5 ml of piperidine and the reaction solution is
evaporated. The residue is suspended in methanol and insoluble material is
filtered off. The filtrate is evaporated and chromatographed on silica gel using10 ethyl acetate. 2 g of 3-(t-butoxyphenyl)-N-[3-(p-t-butoxy-carbonyl)-L-alanyl~-L-
alanine t-butyl ester are obtained.
c) The precursor is coupled with 0.96 g of N-benzyloxycarbonyl-~-alanine
and the crude product is chromatographed on silica gel using ethyl acetate.
2.23 g of N-[N-[N-[(benzyloxy)carbonyl]-~-alanyl]-3-(t-butoxycarbonyl)-L-
15 alanyl]-3-(p-t-butoxyphenyl)-L-alanine t-butyl ester, MS: 670 (M+H)+, are
obtained.
d) Deprotection of 2.1 g of the precursor as in Example 37c) gives 1.67 g of
N-[N-~-alanyl]-3-(t-butoxycarbonyl)-L-alanyl]-3-(p-t-butoxyphenyl)-L-alanine t-
butyl ester, MS: 536 (M+H)+.
20 e) Coupling of 1.5 g of the precursor with 0.6 g of p-amidinobenzoyl
chloride as in Example 37d) gives 1.6 g of starting ester~ MS: 682 (M~H)+.
Example 40
Analogously to Example 24 there is obtained N-[N-[N-[(5-amidino-2-
pyridyl)carbonyl]-,B alanyl]-L-a-aspartyl]-3-phenyl-L-alanine, m.p. 222-223C
25 (dec.) from the corresponding ester.
The starting ester can be prepared as follows:
a) N-~3-[(Benzyloxy)carbonyl]-N-[N-(t-butoxycarbonyl)-~alanyl]-L-alanyl]-
3-phenyl-L-alanine benzyl ester is deprotected and coupled with 5-cyano-2-
pyridinecarboxylic acid to give N-[3-[(benzyloxy)carbonyl]-N-[N-[(5-cyano-2-
30 pyridyl)carbonyl]-,B-alanyl]-L-alanyl~-3-phenyl-L-alanine benzyl ester, m.p. 157-
158C.
b) The precursor is converted as in Example 36a) into N-[3-[(benzyloxy)-
carbonyl]-N-[N-[[5-(~iocarbamoyl)-2-pyridyl]carbonyl]-,B alanyl]-L-alanyl]-3-
phenyl-L-alanine benzyl ester, m.p. 131-132C.
- 40-
c) The precursor is converted as in Example 36b) and c) into N-[3-
[(benzyloxy)carbonyl]-N-[N-[[5-[N-(t-butoxycarbonyl)amidino]-2-pyridyl]-
carbonyl]-,B-alanyl]-L-alanyl]-3-phenyl-L-alanine benzyl ester, MS: 779
(1 I,M+H).
Example A
A compound of the formula I can be used in a manner known per se as
an active compound for the production of tablets of the following
composition:
per tablet
Active compound 200 mg
microcrystalline cellulose 155 mg
cornflour 25 mg
talc 25 mg
hydroxypropylmethylcellulose 20 mq
425 mg
Example B
A compound of the formula I can be used in a
manner known per se as an active compound for the produc-
tion of capsules of the following composition: -
per capsule
Active compound 100.0 mg
cornflour 20.0 mg
lactose 95.0 mg
talc 4.5 mg
magnesium stearate 0.5 mg
220.0 mg