Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
r~
- HOECHST AKTIENGESELLSCHAFT ~OE 90/F 093 Dr.WN/PP
De~cription
Substituted purines, processes for their preparation and
their use as antiviral agents
The pre~ent invention relates to derivative~ of purine,
which carry an alkoxymethyl radical in the 7-position, to
processes for the preparation of these compounds and to
their use as antiviral agents.
The invention in particular relates to purine~ ~uch as
adenine, guanine, 6-chloro-2-aminopurine, 2-aminopurine,
6-isopropoxy-2-aminopurine, 2,6-diaminopurine, purine and
thioguanine which carry an unsubstituted or acyl- and/or
alkyl- and/or benzyl-substi~uted 2-hydro~yethoxymethyl
radical or 1,3-dihydro~y-2-propoxymethyl radical or
1~ 2,3-dihydroxy-1-propox~methyl radical in the 7-position.
The invention further relates to the phyQiologically
tolerable ~alts of said compounds.
While the antiviral activity and the prepara~ion of
purine nucleosides which carry an acyclic radical in the
9-position are already l~ng-known (~ee, for example,
DE-OS 2,539,963 or R.g. Ogilvie et al., Can.JOChem. 62,
241 (1~84) or C.g. Chu and S.J. Cutler, J. ~eterocyclic
Chem. 23, 289 (1986)), to date nothing i~ known about a
specific synthesis of a~yclic purines substituted in the
2S 7-position or their antiviral activity.
Only J. R~ellberg et al., J. Heterocyclic Chem. 23, 625
(1986) and J. ~. 8essler et al., Nucleo ide~ + ~ucleo-
tides 8, 431 (1989) de~crlbe a more or le~s selective
method for the preparation of carboacyclic guanines and
2-aminopurines substituted in the 7~po~ition. However,
the compounds prepared in this way were not lnve~tigated
for their antiviral activity or were inactive in in vitro
2 ~
investigations.
In individu l cases, the acyclic purine derivatives
substituted in the 7-po~ition were ~eparated from the
desired acyclic purine deriva~ives substituted in the
9-position and investigated for their antiviral activity
in vitxo (R. ~. Ogilvie et al.~ Can. J. Chem. 62, 2702
(1984), R. ~. Ogilvie et al.~ Can. J. Ch~m. ~2, 241
(1984)) and found to be inactive.
It has now surprisingly been found that certain 7-~ub-
stituted purines and ~heir physiologically tolerablesalts have antiviral prop~rties agsins~ various DNA
~iruses, ~NA viruses and retroviruses.
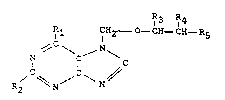
The invention accordingly relates to compounds of the
formula I
R3 ~R~
Rl CH2 _ O- CH I~H ~5
N ~ C ~ ~ ~ (I)
in which
Rl is hydrogen, halo~en, azide, hydroxyl, Cl-C6-alkoxy,
benzyloxy, phenoxy, m~rcapto, Cl-C5-alkylthio,
benzylthio, phenylthio, amino, Cl C6-alkyl~mino,
benzylamino, phenylæmino, C2-Cl2-dialkylamino,
dibenæylamino, cyclic dialkylamino, diphenyl~mino,
C1-C8-acylamino, C2-C16-diacylamino, (N-alkyl-
2-pyrrolidinylidene)amino or C2-C1O-dial~ylamino-
methylideneamino,
R2 is hydrogen, halogen, azide, hydroxyl, mercapto,
~5 amino, Cl-C6-alkylamino, C2-C12-dialkylamino,
benzylamino/ dibenzylamino, cyclic dialkylamino,
~2 ~ 8 ~
-- 3 --
phenyl~nino, diphenylamino, C1-C3-acylamino and
thioacylamino, C2-Cl6-diacylamino or di(thioacyl)-
amino,
R3 is hydrogen, C~C6-alkyl, optionally substituted by
halogen or ~y a hydroxyl, amino, thio, C1~C6-alkoxy,
C1 C6-alkylthio, Cl~C6-alkylamino, benzylo~y,
benzylamino, b~nzylthio, C2-C12-dialkylamino, di-
benzylamino, diphenylamino, C1-Ca-acyloxy,
C1-C~-acylamino, C2-C1~-diacylamino or C1-C~-acylthio
group or a radical R6, where R6 is -P~O~OR~)(OR7),
-o-~Cl-C4-alky~)-P(~)(oRB)(~R7)~
-S ~cl~C4-alkyl)-P(o~(oR6)(
NH (cl-c4-alkyl)-P(~)(OR6)(oR7)~
-N(Cl c6-alkyl)-C,-C4-alkYl-P(O)(OR8)(0R7)~
-P(cl-c6-alkyl~(o)(oR6~,
-o-(Cl-C4-alk~l)-P(Cl-C6-a~ )(O)(OR6)~
-S-(Cl-(:~4-alk~l)-P(Cl-C6-alkyl)(O)(OR6),
-NH-(Cl-C4-alkyl)-P(Cl-C6-~lkyl)(O)(OR6),
-N(Cl-C6-alkyl)-Cl-C~-alkyl-P(Cl~C~-alkyl)(O)(OR6)
in which R6 and R7 are independently of one another
hydrogen or a Cl-C6-alkyl radical or ammonium,
triethylammonium or an alkali metal or alkaline
earth metal ion,
R4 is hydrogen, Cl-C6-~lkyl, hydroxyl, mercapto, amino,
2~ halogen, azide, Cl-C6-alkoxy, Cl-C6 alkylthio,
Cl-C6-alkylamino, C2-Cl2-dialkylamino, benzyloxy,
benzylthio, benzylamino, dibenzylaminol phenylamino,
diphenylamino, phenoxy, phenylthio, Cl-C8-acylo~y,
Cl-C6-acylthio, Cl-C8-acylamino, C2-Cl6-diacyl~ino or
-O-~Cl-C4-alkyl)-P~O)(OR6~(0R7) or -O-(Cl-C4-alkyl)-
p(O)(~R6)(OR7) or -O-(C1-C4-alXyl)-
P(C1-C6-alkyl)(O)(OR~), where the radicals R6 and ~7
are as defined above,
and
R5 is hydrogen, Cl-C6-alkyl, optionally ~ubstituted by
a hydroxyl, thio, amino, C1-C6-B1kOXY,
2~3~
C1C6-a~ thio, c~-c6 alkylamino~ C2-C12-dialkylamino,
C~_CB_aCY10XY, C~_Ca_aCY1thiO~ Cl-CB-acylamino,
C2-C~6~diacylamino, benzyloxy, ben~ylthio,
benzyl~nino, dibenzylamino, phenoxy, phenylthio,
phenylamino or diphenylamino group or a radical R
where R8 is
_p(~)(OR6)(~R7)~
-O-(Cl-~4-alkyl)P(O)(OR~)(OR~,
-S-(C~ C4-alkyl)-P(O)(OR~)(OR7),
-NH-(cl-c4-alkyl)-p(o)(oR6~(oR7)~
-N ( C1_C~_a1kY1~ -C~ C4 alkyl-P(O)(OR6)(OR7),
-P(cl-c6-;~ ) () (OR6) ~
-O-(C~-C4-alkyl)-P(Cl-C~;-alkyl)(O)~OR6),
-S- ( Cl-C4-alkyl ) -P ( Cl-C6-~llkyl ) ( O ) ( OR6 ),
-NH-(Cl-C~-alkyl) P(C~-C6-alkyl~(O)(OR6),
N(C~-C6-alkyl)-Cl-C4 alkyl-P(C1-C6-alkyl~(O)(OR~), in
which R5 and R~ are independently of one another
hydro~en or a Cl-C6-alkyl radical or ammonium,
triethyla~monium or an alkali metal or alkaline
earth metal ion,
and their physiologically tolerable ~alts and obvious
chemical equivalents, with the proviso that, at the s2me
time, Rl is not hydroxyl and R2 is not amino or
R1 is not hydroxyl, R2 i6 not acetamido, R3 is not
benzyloxymethyl, R4 i6 not benzyloxy and R5 i~ not hydro-
gen or
Rl is not chlorine or methoxy, R2 i~ not amino, R3 ~ not
benzyloxymethyl, R4 i~ not benzyloxy and R5 is not
hydrogen or
Rl i~ not hydroxyl, R2 is not acetamido, R3 i8 not
acetoxymethyl, R4 i8 not acetoxy and R5 i.s not hydrogen or
Rl is not methoxy, R2 is not amino, R3 ~. not hydroxy-
methyl, R4 i~ not hydroxyl and Rs i~ not hydrog9n or
Rl is not chlorine or amino, Rz i~ not hydrosen/ R3 i~ not
hydroxymethyl or benzyloxymethyl, R4 i~ not hydroxyl or
~enzyloxy and Rs i~ not hydrogen or
R1 ie not amino, R2 is not mercapto, R3 i8 not benzyloxy-
methyl, R4 is not benzyloxy and Rs is not h~drogen or
; ~
~ ~ ~3 ~ ~ ~ 9
-- 5 --
Rl is not benzyloxy, R2 iS not chlorine, R3 is not
benzyloxymethyl, R4 is not benzyloxy and R5 is not
hydrogen or
R1 is not chlorine, R2 iS not amino, R~ i5 not aceto~y-
methyl, R4 is not acetoxy and R5 is not hydrogen or
Rl i5 not benzyloxy, R2 i8 not chlorine, ~3 i8 not
hydrogen, R4 i8 not ben~yloxy and R5 i~ not ben~ylo~y-
methyl or
Rl and R2 are not chlorine, R3 is not benzyloxymethyl, R4
is not benzyloxy and R5 iS not hydro~en or
Rl is not amino, R2 is not mercapto, R3 and R5 are not
hydrogen and R4 is not acetoxy or
R, is not hydrogen, R2 is ~ot amino, R3 and R5 are not
hydrogen and R4 is not hydroxyl or acetoxy or
R1 and R2 are not chlorine, R3 and R5 are not hydrogen and
R4 is not benzyloxy or
R1 is not iodine, R2 is not chlorine, R3 and R5 are not
hydrogen and R4 is not hydroxyl.
Preferred compounds of the formula I are those in which
Rl is hydrogen, halogen, hydroxyl, benzyloxy, alkoxy
having 1-6 carbon atoms~ amino, C1-C6-alkylamino or
C2-C6-di(alkyl~amino, or Cl~-C6-alkylthio,
R2 is hydrogen, halogen, hydroxyl, amino,
C1-C6-alkylamino, Cz-C6-di~alkyl)amino or
C1-C6~acylamino,
R3 i~ hydrogen, Cl-C6-alkyl, optionally ~ubstituted by
a hydroxyl, amino or Cl-C6-alkoxy group or halogen or
a Cl-C~ acyloxy, Cl-C8-a~ylamino or Cl C6-alkylamino
group or a group R~, where R8 is
-O-(C1-C~-alkyl)-P(O)(OR6)(OR7), -~(O)(ORs)tOR7) or
-P(Cl-C4-alkyl)(O)~OR6), in which R6 and R~ are
independen~ly of one another hydrogen or a
Cl~C6-alkyl radical or an alXali metal or alXaline
earth metal ion,
~3~
~ R4 i~ hydrogen, hydroxyl, amino, mercapto, C1-C6-alkoxy~
C~-C8-acyloxy, C1~C6-alkylamino or an
O-(C,-C4 alkyl)-P(O~(OR6)(OR7) or
-O-(C1-C~-alkyl)~P(C1~C~-alkyl)(O)(OR6)radicalhaving
the meanings R6 and R7 a~ described above
and
R5 i~ hydrogen or C1-C4-alkyl, optionally sub~tituted by
hydroxyl, Cl C8-acylo~y, benzyloxy, C1-C6-alkoxy,
amino, C1-C6-alkylamino or a radical R8, where R8 i6
-P(O)(OR6)(OR7~ or -P(Cl-c4-alkyl)(o)~oR6)~ in which
Rs and R7 are d~fined as de~cribed above.
Particularly preferred compound~ of the formula I are
those in which
Rl is hydrogen, hyd~oxyl, chlorine, mercapto,
ben~yloxy, Cl-C6-alkoxy, Emino, Cl-C3-alkylamino or
C2-C6-dialkylamino,
R2 i~ hydro~en, hydroxyl, ami.no or Cl-C8-acylEmino,
R3 is hydrogen, Cl-C3-alkyl, optionally substituted by
a hydroxyl, Cl-C8-acylo~y or Cl-C6-alkoxy group or a
2- ~- OR6-
OR7
or P~O)(OR6)(OR7) group, where R6 and R7 have the
above meanings,
R4 i~ hydrogen, hydroxyl or Cl-C8-acyloxy sr
Cl-C6alkoxy or CH2-P-OR6 and
~R7
R5 is hydrogen or C1-C4-alkyl, optionally ~ubstituted by
hydroxyl, Cl-C8-acyloxy or Cl-C6-alkoxy or
-P(O)(OR6)(OR7), where R6 and R7 have the above-
mentioned meanings.
Very particularly preferred compounds of the formula I
are those in which
Rl is hydrogen, hydroxyl, chlorine, Cl-C4-alkoxy, amino,
Cl-C3~alkylamino or C2-C6-dialkylamino,
R2 is hydrogen, hydroxyl, amino or Cl-C3-acylamino,
R3 is Cl-C3-alXyl, optionally substituted by hydroxyl or
by C1-C8~acyloxy or by Cl-C6-alkoxy or by
-P(O)~OR6)(0R~), where R6 and R7 ha~e the above-
mentioned meanings,
R4 is hydroxyl ox Cl-Ca acyloxy or Cl-C~ alkoxy a~d
R5 is hydrogen.
Compounds of the formula I furthermore have particular
significance, in which
Rl is hydrogen, chlorine or amino,
R2 is amino or Cl-C3-acylamino,5 R3 is C1-C3-alkyl, optionally substituted by hydroxyl or
by C1-Cs-acyloxy or by C1-C5-alkoxy or by
-P(O)(OR8)(OR7), where R6 and R7 have the above-
mentioned meanings,
R4 is hydxoxyl or Cl-C5 acylo~y or C1-Cs-alkoxy and0 R5 is hydrogen;
and compounds of the formula I have very particular
signific~nce, in which
R1 is hydrogen,
R2 is amino,5 R3 is C1-C3-alkyl, optionally substituted by hydroxyl or
by C1-C4-acyloxy or by Cl-C4-alkoxy,
R4 is hydroxyl or Cl-C4-acyloxy or Cl-C4-aIkoxy and
R5 iB hydrogen,
in particular the compound of the formula I in which
R~ is hydrogen,
Rz is amino,
- 8 -
R3 is hydro~ymethyl,
R4 is hydroxyl and
Rs is hydrogen.
Said alkyl groups as substituents of the aboveme~tioned
formula I can be branched, u~branched or cyclic. Examples
of alkyl groups are the methyl, ethyl, propyl, isopropyl,
bu~yl or isobutyl group. Examples of alkoxy groups are
the methoxy, ethoxy, propoxy, isopropoxy, butoxy or
cyclopentyloxy group.
Examples of cyclic dialkylamino groups are the
pyrrolidino, pipPridino, morpholino, ~methylpiperazino
or 1,2,4 triazolo group.
The preferred halogen substituent is chlorine~ Particu-
larly suitable alkali metal or alkaline earth metal
substituents are sodium and calcium.
The compounds of these inventions are all ~ubstituted
acyclic purine nucleosidesj ~hich carry the acyclic
substituent in the 7-position of the purine ring system.
Salts o~ the compounds according to the invention par-
ticularly suitable for therapeutical purposes are saIts
of physiologically tolerable organic and inorganic acids
such as acetic acid, lactic acid, malic acid, p-toluene-
sulfonic acidl methane6ulfonic acid, isethionic acid,
hydrochloric acid or sulfuric acid.
Obvious chemical equival~nts of the compounds according
to the invention are in particular derivative~ thereof
which can be converted into the compounds according to
the invention without problem, for example under phy~io
logical conditions~
Of the compounds of the formula I according to the
invention 2-amino-7-(lg3-dihydroxy-2 propoxymethyl)purine
= compound of th~ fonmula I in which Rl = H, R2 = NH2~
9 2~$e3~5~
- R3 = CH2-OH, R~ = 3H and Rs = H tExample 6.12.),
2-amino-7 (l-hydroxy 3-i~opropoxy-2-propox~methyl)purine
= compound of the formula I in which R, = h~drogent R2 =
amino, R3 = hydroxymethyl, R4 = i~vpropoxy ~nd R5 =
hydrogen (Example 6.10.
and
2-amino-7-(1,3-bisti~opxopoxy)-2-propoxymethyl)purine =
compound of the formula I in which Rl = ~ R~ = NR2, R3 a
CH2-O-CH(CH3~ 2 ~ R4 - O-CH(CH3)2 and R5 = ~ ~xample 6.7.)
are particularly preferred, in particular because of
their particularly hi~h antiviral activity asainst herpes
viruses.
Other compounds of the formula I where R~ = hydrogen,
R2 = amino and an acyclic side chain whose h.ydroxyl
function or hydroxyl functions is/are e~terified with
Cl-C6-alkyl radicals or esterified with Cl-C6-acyl radicals
show particularly high antiviral activity.
The invention furthermore r~lates to the use of said
cvmpounds as antiviral agents, the compound~ classif~ed
above as excluded not being excluded. The compound~
accordin~ to the inv~ntion are particularly active
against herpe~ simplex viruses type 1 and type 2, cyto-
megaloviruses, varicella zostex viruses, Epstein Barr
viruses and human herpes viruses ~ (HEV6).
~he present invention furthermore relates to proces~e
for the preparation of substituted purine~ of the formula
I or a physiologically ~olerable salt thereof, which
comprise
1) if in the compound of the formula I R4 is hydroxyl,
amino, alkylamino or mercapto, replacing a protact-
ing group (blocking group~ Al in a compound of the
formula II
2~3~9
-- 10 --
~3 ~5
Rl ~--O~Ag
N~ ~ ~C ( I
~ C~ jC '~.. N~
by a hydroxyl, amino, alkylamislo or merc:apto group,
2 ) if in the compound of the formula I R3 is hydroxya
lkyl, aminoalkyl, alk~rlaminoalkyl or thioalkyl,
replacing a protecting group Aa in a compound of the
5formula III
R~ o ~ 5
N ~ \t: ~ ~ C
.4
by a hydroxyl, amino, alkylamino s:r mercapto group,
3 ) if in the compound of the formula I Rs i~ hydro~y-
alkyl, aminoalkyl, morloalkylaminoal3cyl ox thioalkyl,
rep~acing a protecting group A3 in a compolmd of the
10:fonnula IV
R3 ~lkyl- ~3
O~ R4
~C~ ~N~ (~Y)
R2 ~N ~ N
by a hydroxyl, amino, alkylamino or mercapto group,
4 ) if in the compound of the formula I R3 i5 hydro~-
alkyl, aminoalkyl, monoalkylaminoalkyl ox thioallcyl
and/or R4 i5 hydroxyl, ~ninol alkylamino or mercapto
and/or Rs is hydxoxyalkyl, aminoalkyl,
- monoalkylaminoalkyl or thioalkyl, replacing a
protec~ing group A4 and/or A5 and/or ~5 in a compound
of the formul V
~;C ~ ~N ~ Y1~6
N e ~ ~v3
R~ ~aD ~a
by a hydroxyl, ~mino, allcylamino or mercPpto group,
5 ) converting a compound of the fo:rmul2 VI
~3 ~l4
Y C~2~ 5
~N~
R 6 ~VI )
Z f ~ ~ C ~N~
in which Y and Z are precursors of the grollps Rl and
R2 into a compound of the formula I in which Rl and
R2 have tha meanings described above,
6 ) reacting a compound of the fonnula VII
' 3i~.
1~
S~ S
~ ~ ~ ~ ~ N /
with a coml?ound of the formula VIII
~3 ~4
L2--CH2--0- CH~ C~ (VII2)
R!i
in which Lz is a leaving group and ~ hydrogen or
2~3~
- 12
-- a leaving group,
7~ removing a blocking group from a compound of the
formula I in which one or both radicals Rl and R2 are
blocked, and if the product o~ the reaction i8 a
base of the formula I, optionally converting it into
an acid addition product of khi~ base of the formula
I, or if the product of the reaction is a ~al$ of a
base of the foxmula I, optionally con~erting it into
its base or into another sal~ of this ba~e.
In the cases of processes 1) - 4), hydroxyl, mercapto,
amino and mono ubstituted amino $unctions of the acyclic
side chain in the 7 po~ition of the purine ~y~tem, if
present, are modified at the end by a blocki~g group D
and, if appropriate, a further blocking group E, where D
can be identic~l to or different from E.
~hese blocking groups can be ester~ - for example acyloxy
groups - and/or benzyloxy groups - and/or Cl-C6-alkyloxy
groups - for example isopropo~y groups.
In the first case, the acyloxy group can be aliphatic -
for example acetoxy or pivaloyloxy - or aromatic - for
ex~mple benzoyloxy.
Both types of acyl groups can be removed, for e~ample, by
mild basic hydrolysi~; in ~eneral warming with agueou~ or
alcoholic methylamine i~ adequate in order to achieve
removal of the blocking group.
In the ~econd case, the benzylo~y blocking group can be
removed by hydrogenolysis, either catalytically or by
means of hydrog2n and Raney nickel or pallad~u~/carbon
or by means of ammonium formate and palladium/carbon or
39 by means of a transfer hydrogenoly~is with palladium
hydroxide and cyclohexene or cyclohexadiene or chomically
by reaction with boron halides - for example boron
trichloride - at low temperatures - for example at -70
degrees Celsius - or by means of sodium in liquid
3S ammonia, the liquid ammonia being used as solvent.
2~3~
- 13 -
- In the ca~e of catalytic hydrogenolysis, th~ preferred
solvent is an alkanol; however, a series of inert
solvents can also be u ~d i the substrate i8 at least
partially soluble therein. Examples of the~e are benzene,
toluene, tetrahydrofuran or dio~ane.
For chemical reaction by mean~ of boron trichloride 9 in
which a ~olution of boron trichloride in n-hexane or in
dichloromethane or alternatively gaseous boron
trichloride is used, dichlorom2thane i~ the preferred
solvent.
In the third case, deblocking of the Cl~C6-alkyloxy groups
can be achieved if boron rihalides - for example boron
trichloride ~ are reacted with the substrate at tempera-
tures which are not quite so low - for example at -60C
to 0C, preferably at ~40C to -20C. The preferred
solvent for this is dichloromethane and the boron
trichloride can be employed in gaseous iorm, as a
solution in n-hexane or as a solution in dichlorome~hane.
The conversion of a compound of $he formula VI into a
compound of the formula I can be achieved in a ~ery
different manner by process 5). For example, one of the
two radicals R1 or R2 or both the radicals Rl and R2 can be
converted into a halogen by halogenation, into a hydroxyl
group by hydrolysis, into a Cl-C6=~alko~y group by
conversion with a C1-C~-alkanolate, into a mercap~o group
by sulfurization, into a Cl-C8-alkylthio group by reaction
with a Cl-C6-alkylthiolate, into an amino group by
ammonolysis ~ into sn amino group by deblocking a Cl-Ca-
acylamino, Cl-C~-thioacylamino, benzylamino or
Cl-C6-alkylamino group, into a Cl-C~ alkylamino group or
C2-Cl2-dialkylamino group by aminolysis, or into hydrogen
by hydro~enolysis or desulfurization or formation of the
azide.
All these processes are known and can be found, for
example, in: Heterocyclic Compound~ - Fused Pyrimidines
Part II, Purines, editor: D~J. Brown, published by
~:~3~
- 14 -
Wiley-Interscience, 1971.
In process 6), the leaving group L2 f a compound of the
formula VIII is either a reactive radical of an inorganic
acid and can thus be
a~ halogen, preferably chlorin~, or
b) a Cl~C6-alkyl~hio or Cl-C6-alXylsulfin~l or
Cl-C6-alkylsulfonyl group, preferably the ~ethylthio
or the methyl~ulfinyl or the methylsulfo~yl group,
or it is a reac~ive radical of an organic ac1d and
can thu be
c) Cl-C8-acyloxy or benæoyloxy, preferably aceto~y.
In proces~ 6a), the leaving group L1 in a compound of
the formula VII is hydrogen or trialkylsilyl, preferably
trLmethyl~ilyl.
In process 6b), the leaving group L1 in a compound of the
formula VII is a Cl-C8-acylo~ group, preferably the
acetoxy group, or a trialkyl ilyl group, preferably the
trLmethyl~ilyl yroup.
In process 6c), the leaving g:roup Ll in a compound of
the formula VII is a C1-C8-acyloxy group or altern2ti~ely
preferably a trialkylsilyl group, in particular the
trLmethylsilyl group.
The preferred proce6s according to 6a) compri~es the
condensation of a purine with the desired ~ub~titution in
the 2- and 6 position with a C1-C8-acyl- or benzyl- or
C1-C6-alkyl-blocked 1-(halomethoxy)ethanol, for example
1-(chlorome~hoxy)-2-aceto~yethane or
1-(chloromethoxy)-2-benzyloxyethan~ or
1-(chloromethoxy)-2-isopropoxyethane, or
an acyl- andtor arylal~yl- and/or alkyl-blocked
2-(halomethoxy)-1~3-propanediol, ~or example
2-(chloromethoxy)-1,3-bi~(acetoxy)propane or
2-(chloromethoxy)-1,3-~is(benzylo~y)propane or
~3~
- ~5 ~
- 2-(chloromethoxy) l/3-bis(isopropoxy)pxopane, or
l (halomethoxy)-2,3-propanediol blocked by a Cl-C~-acyl
andJor benzyl an~/or Cl-C6-alkyl, for example
1-(chloromethoxy)-2,3-bis(acetoxy~propane or
l~(chloromethoxy)-2,3-bis(benzyloxy)propane or
l-(chloromethoxy)-2,3-bi~ opropoxy)propan~
in a ~trongly polar solvent such as dimethylformamide,
dimethylacetamidel ~-methylpyrrolid-2-one, te~r~methyl-
urea or ~imethyl sulfo~ide/ in the pre3ence of a base,
such as triethylamine, ~-ethylmorpholine or of an alkali
metal carbona~e, such as, for examplep potas~ium
carbonate, at room temperature for 1 - 72 hour~, if L1 in
a compound of the formula VII is hydrogen, or
in aprotic solvents such as benzene, toluene, xylene,
1,2-dichloroethane, chlorob~nzene, 1,2-dimethoxyethane,
dioxane or acetonitrile,
in the pre~ence of a base ~uch as triethyl~mine or
N-ethylmorpholine, at a reaction temperature of 0 to
150C, preferably at room temperature, for 1 - 7~ hours,
if Ll in a compound of the formula VII is trimethyl~ilyl.
It is known that alkylthioalkyl ethers, in particular
methylthiomethyl ethers such a~, for example, comp~unds
of the fonmula VIII where Lz = methylthio, can be reacted
with oxygen nucleophiles and Lewis acids such ~s
mercury~II) chloride (E.J. Corey, ~.G. Bock, ~etrahedron
Letters 197S, 3269 or R. Yamada, ~. Rato, H. Nag2se,
Y. Hirata, ~etrahedron Letters 1976, 65) or ~lkyl-
sulfinylalkyl ethers, in particular methyl~ul~inylmethyl
ether6 ~uch as, for example, compound~ of the formula
VIII where Lz = methylsulfinyl, can be reacted with carbon
nucleophiles and Lewi~ acids ~uch a~ zinc iodide (J.A.
Schwindeman, P.D. Magnus, Tetrahedron ~ettex~ 1981,
4925).
The preferred process according to 6bj comprises the
condensation of a ~urine with the desired ~ubstitution in
the 2- and 6-position with a Cl C8-acyl- or benzyl- or
Cl-C6-al~yl-blocked l-(alkyl~hioalkoxy)ethanol, for
- 16 -
example l-(methylthiometho~y) -2-acetoxyethane or
1-(methylthiomethoxy)-2-benzyloxyethane or
l-(methylthiometho~y)-2-isopropoxyethane or
2-(alkylthioalkoxy3- 1, 3-propanediolorwithaCl-Ca-acyl-
and/or benzyl- and/or C,-C6-alkyl-blocked, for example
2-(methylthiomethoxy)-1,3-bis(acetoxy)propane or
2-tmethylthiomethoxy)-1,3 bis(benzyloxy)propane or
2-(methylthiometho~y)-1,3-bis(isopropoxy)propane,orwith
a Cl-Ca-acyl- and/or benzyl- and/or C,-C6-alkyl- blocked
1-(alkylthioalkoxy)-2,3propanediol, for example
l-(methylthiomethoxy)-2,3-bi6(acetoxy)propane or
1-(methylthiomethoxy)-2,3-bis(benzyloxy)propanP or
l-(methylthiomethoxy~2,3-bis(i~opropoxy3propane,
where in each case instead o~ the alkyl~hioalkoxy group
the alkylsulfinylalkoxy or alkylsulfonylalkoxy group can
advantageously also be employed,
in a strongly polar solv~nt or solvent mixture such as
dLmethylformamide, dimethylacetamide, N-methylpyrrolid-
2-one, tetramethylurea and/or dimethyl ~ulfoxide, in the
presence of a protonic acid or Lewi~ acid, such ~s iron
trichloride, boron trifluoride, ~allium trichloride,
aluminum trichloride, titanium tetrachloride, but pxefer-
ably tin tetrachloride, or iodine or trialkylsilyl
trifluoromethanesulfonate, preferably trimethylsilyl
trifluoromethanesulfonate, at a temperature of -40C to
+lOO~C, preferably between -20C and ~80C, for ~eYeral
hours, if Ll in a compound of the formula VII is
C1_CD_aCY1, pre~erably acetyl, or in a le~s polar solvent
or solvent mixture ~uch as dichloro~ethane or 1,2-
dichloroethane in the pre~ence of a Lewis acid such asiron~III) chloride, boron trifluoride, gallium trichlo-
ride, aluminum trichloride, titanium tetrachloride or tin
tetrachloride or of a trialkylsilyl trifluoromethane-
sulfonate, preferably trimethylsilyl trifluorom~thane-
sulfonate, at a temperature of -40C to ~lOODC,
preferably between -30C and l20CI for 0.5 to 8 hours,
preferably for 1 to 4 hourQ, if Ll in a compound of the
formula VII is trial~ylsilyl, preferably trimethylsilyl
or in a polar aprotic solvent ~uch as acetonitrile in the
~3~
17 -
presence of a Lewis acid such as iron (III) chloridP,
boron trifluoride, gallium trichloride, aluminum
trichloride, titanium tetrachloride, ~ut preferably tin
tetrachloride, at a temperature of -40C ~o + 100C, pre-
ferably between -30C and +20~C, for 0.5 to 8 hour~,
perferably for 1 to 4 hours, if ~1 in a compound of the
formula VII is trial~ylsilyl, preferably tri~thylsilyl.
The preferred process according to 6c) compri~es the
condensation of a purine having the desired sub~titution
in the 2- and 6-position, preferably an expediently
modified 2-amino-6-chloropurine, in particular per-tri~
methylsilylated 2-acetamido-6~chloropurine, with a
Cl-C~-acyl- or benzyl- or C~-C6-alkyl- blocked
1-(C1-C8-acyloxymethoxy)ethanol, for example l-acetoxy-
methoxy-2-acetoxyethane or 1-acetoxymethoxy-2-benzyloxy-
ethane or l-acetoxymethoxy-2-isopropoxyethan~, or with a
Cl-C8-acyl- and/or benzyl- and/or Cl-C6-alkyl-blocked
2-~C~-C8-acyloxymethoxy)-1,3-propan2diol, for example
2-acetoxymethoxy-1,3-bis(acetoxy)propane or 2-acetoxy-
methoxy-1,3-bis(benzyloxy)propane or 2-acetoxy-
methoxy-1,3-bis(isopropoxy)propane,
or
with a C~-C8-acyl- and/or benzyl- and/or Cl-C6-al~yl-
blocked l-(C1-C8-acyloxymethoxy)-2,3-propanediol, for
example 1-acetoxymethoxy-2,3-bis~acetoxy)propane or
1 acetoxymethoxy-2,3-bis(benzyloxy)propane or 1-acetoxy-
methoxy-2,3-bis(isopropoxy)propane, in an aprotic solvent
such a~ benzene, toluene, xylene, acetonitrile, dichloro-
methane or 1,2-dichloroethane or mixtures thereof,
in the presence of an acld, preferably a Lewi~ acid such
as aluminum trichloride, boron trifluoride, lron trichlo-
ride, gallium trichloride, tin tetrachloride or titanium
tetrachloride or in the presence of iodine
or preferably
trialkylsilyl trifluoromethanesulfonate, particularly
trimethylsilyl trifluoromethanesulfonate, the amount~ of
the~e reagents being 0.1 to 10, preferably 0.8 to 7
equivalents, relative to the amount of the acetoxymethoxy
~$~
- 18 -
compound employed in each case,
at tempexa~ures between -70C and +80C, preferably
between -40C and ~30C, for 2 to 24 hours, preferably
for 2 to 6 hours, i Ll in a compound of the formula VII
is trialkylsilyl, par~icularly trimethylsilyl.
This process yields in high regio&ele~tivity, as a rule
~>9sl, preferably the 7-isomer of the respectiv~ acyclic
purine derivative.
If product mixtures are formed by prQce~se6 6a) - 6c),
these are separated into the pure components, if neces-
sary after conversion into anothar purine dexi~ative, ~y
chromatography or by fractional crystallization.
Compounds of the formula VIII where L2 = halogen can be
prepared by reacting an expediently modified and protec-
ted alkanol, for example l-acetoxyethanol or 1,3-bis-
(acetoxy)propan-2-ol or 2,3-bis(acetoxy)propanol or
1-benzyloxyethanol or 1,3-bis(benzylo~y)propan-2-ol or
2,3-bis(benzyloxy)propanol or l-isopropoxyethanol or
1,3-bis(isopropoxy)propan-2-ol or 2,3-bis(isopropoxy)-
propanol with paraformaldehyde and a gaseous hydrogenhalide, for ex2mple hydxogen chl~ride, in sn inert
solvent, for example dichloromethane, at room temperature
or below.
The preparation of halomethyl ethers i~ a generally
utilizable reaction; a detail~ description can be found,
for example, in: Houben-Weyl, ~ethoden der organi~chen
Chemie (Methods of Organic Chemistry), Georg Thieme
Verlag, S~uttgart, 1965, volume VI/3, pp. 125 ~t ~eq.
Compounds of the formula VIII where L2 = methylthio can
be prepared by reacting an expendiently modified and
prot~cted alkanol, for ex~mple l-acetoxyethanol or
1,3-bis(acetoxy)propan-2-ol or 2,3-bis(acetoxy)propanol
or l-benzyloxyethanol or 1,3-bis(benzyloxy)propan-2-ol or
2,3-bis(benzyloxy)propanol or 1 isopropoxyethanol or
- 19 - 2~
ll3~bis(isopropoxy)propan-2-ol or 2,3-bis(isopropoxy)-
propanol with dLmethyl sulfoxide, Cl-C8-acid anhydride and
Cl-C8 carbo~ylic acid in a temperature range between 0C
and +40C, pr~ferably at room tempexature, for several
days, as a rule 2 to 4 days.
A detailed description of the preparation of methyl-
thiomethyl ethers of prLmary, secondary and tertiary
alcohols can be found in P.M. Pojer and S.J. Angyal,
~ust. J. Chem~, 31, 1031 (1978~.
The corresponding methylsulfinylmethyl compound~ and
methylsulfonylmethyl compounds can be obtained in a
sLmple manner by oxidation ~y means of peracids, for
example m-chloroperbenzoic acid or peracetic acid.
Compounds of the formula VIII where L2 = Cl-C~-acyloxy can
be prepared either from a compound of the formula VIII
where L2 = halogen, which i~ accessible as described
above, by reaction with an alkali metal carboxylate,
preferably sodium or potas~i~n acetate, in acetone or
dimethylfoxmamide, or by converting an expediently
modified and protected alkanol into the alkoxyalkyl
ether, preferably the methox~methyl ether, which is then
in turn converted into the Cl-C8-acyloxy compound,
preferably l:he acetoxy compound, by reaction with a
C1-C8-carboxylic acid anhydride, preferably acetic an-
hydride, under protonic or Lewi~ acid cataly.is, preferably boron trifluoride etherate ca~alysis (for both
processes see:
Houben-Weyl, Methoden der organi~chen Chemie (Methods of
Organic Chemistry~, Georg Thieme Verlag, Stuttgart, 1965
volume VI/3, pp. 286 et seq).
However, the compounds of the formula VIII where L2 =
Cl-C8-acyloxy are particularly 8imply and effectively
prepared by combining an expediPntly modified and
protected alkanol, for example l-aceto~yethanol or
1,3-bis(acetoxy)propan-2-ol or ~,3-bis(acetoxy)propanol
'~3~81~
20 ~
or 1 benzyloxyethanol or 1,3-bis~benzyloxy)propan-2-ol or
2,3-bis(benzyloxy)propanol or l-i~opropoxyethanol or
1,3~bis(isopropoxy)propan-2-ol or 2,3-bis~isopropoxy)-
propanol with a carboxylic acid, preferably ace~ic aci~,
and the anhydride pertaining to it, preferably acetic
anhydride, in dimethyl sulfo~ide, preferably about 60 ml
of acid, about 50 ml of anhydride and about 1~0 ml of
dimethyl sulfoxide being used per 0.1 mol of alkanol,
below room temperature, preferably at 0C, and stirring
for several h~urs, preferably 4 to 6 hour~, at elevated
temperature, preferably at 40 to 100 degr~es CQlsiu6.
In proces6 7), the ~ubs~ituents R1 and R2 can be blocked
by, for example, trialkylsilyl groups, preferably
trimethylsilyl groups.
Compounds of this type will be the product of the
condensation of a per-trLmeth~ylsilylated purine and a
compound of the formula VIII a~; in process 6).
These blocking group~ are labile and can be removed by
solvolysis with water, with a~leous or alcoholic ammonia
or ~ith aqueous hydro~en carbonate solution or by al-
coholysis.
A further process com~ines processe~ 1) or 2~ or 3) or 4)
with process 5); in thi~ caseJ deblockin~ can be achieved
by solvolysis at the same time a6 the replacement of a
group leaving the purine system, or e~ample halogen,
such as, for exampl~, by reaction with liquid ammonia. In
this ca6e, in addition to the deblocking of the side
chain, if this was protected by a C,-C8-acyloxy group (see
processes 1) ~ 4)), the leaving group in the purine
system is at the same time replaced by the amino group.
In addition here, a Cl-Ca-acyl blocked group in the purine
sy~tem c~n be deblocked.
The compounds of the formula I according to the invention
can have one or more chiral centers in the acyclic ~ide
- 21
chain. The compounds are as a rule racema~0s; preparation
or i~olation of the pure enantiomers is po~sible. The
invention therefore relates both to the pure enantiomers
and ~o mixtur ~ ~hereof, uch as, for example/ the
respective racemate.
The present invention in addition relates to pharmaceut-
icals containin~ at least one compound ac ording to the
invention.
The pharmaceuticals accordin~ to the invention can be
administered enterally (orally) parenterally
(intravenously), and rectally or locally ~topically).
They can be administered in the form of ~olutions,
powders ~tablets, cap ules including microcapsules~,
ointments tcreams or gel) or suppositories. Suitable
lS auxiliaries for formulations of this type are the phax-
maceutically customary liquid or solid fillers and
extenders, solvents, emul~ifiers, lubricants, flavor
correc~ants, colorants and/or buffer substances.
0.1 - 10, preferably 0.2 - 8 mg/kg of body weight, are
administered as an expedient dlosage. The compounds are
expediently administered in dos,age units which contain at
least the effective daily amount of the compounds accord~
ing to the invention, for example 30 - 300, preferably
50 - 250 mg.
The compounds according to the invention can also be
administered in combination wi~h other anti~iral agenks
and immunostimulants ~ such as interferons.
In vitro testB and results:
The antiviral activity of the compounds according to the
invention was tested in in Yitro te5ts. To do ~his, the
compounds sccording to the in~ention were added in
various dilutions to cell cultures of Vero cells in
microtiter plates. After 3 hours, the cultures were
infected with different viruses. Vero cells were infected
- 22 ~
with various human pathogenic herpes viru~es, HeLa cells
were infected with vaccinia virus and ~DBR cells with
vesicular j~omatitis virus. 48 72 hours after infec-
tion, the result of treatment was determinsd microscop-
ically by the cytopathic effect and photometrically(Finter, N.B., in "Interferon~" (N.B. Finter et a~
North Holland Publi6hing Co., ~m~terdam tl966), by
neutral red ab~orption (color test according to Finter~.
The minimum concentration at which about half the cells
show no cy~opathogenic effect is con~idered as the
minimum inhibitory concentration (~IC). The res~lt~ are
summarized in Table 1.
- ~3
Table 1
Substance T~D (~/ml)
from ~IC (~J~)
Example XSV-1 ~SV-2 Vaccinia VSV
7-tl,3-di-
hydroxy-2- ~400 >400 >400 ~00
isopropoxy >400 ~400 >400 >400
methyl~yuanine
>400 >400 ~400 ~400
6.7 >~00 >400 ~90 ~400
>400 ~400 ~400 >400
6.10. >~00 ~00 ~400 >400
133.3 133.3 44.~ ~400
6.12. 1.65 1.65 4.94 >400
Standard:
9-(1,3-di- ~400 ~400 ~400 >133.3
hydroxy-2- 4.94 1.65 >400 133.3
isopropo~y-
methyl)guanine
HSV-1 = Herpes sLmplex virus 1
HSV 2 = Herpe simplex virus 2
MIC - Minimum inhibitory concentration
TMD = ~olerated maximum dose
VSV = Vesicular stomatitis virus
- 24 ~ $~
In vivo tests and results-
NMRI mice, specifically pathogen-free, with a weight of
about 15 g were infected intraperitoneally ~ith herpe6
sLmplex virus 1 and then treated intraperitoneally or
orally with the compound according to the invention (see
Table 2 or Table 3). Treatment was carried out for the
first tLme 3 hours after inection and wa~ continued
twice daily for 4 days. The re~ul~ of trea~ment was
determined by the course of the di~ease and the ~urvival
rate compared to untreated infection control~. The latter
received physiological ~aline ~olution instead of the
compound according to the invention. The observation
period was two week~O
2~3~
- 25 -
Table 2: Antiviral action again~t HSV 1 in ~MRI mice 9n
intraperitoneal administration
~xample Dosage Mean ~urvival Surviving/
(~mol/kg) time (days) total
~
9 x 10 7.0 0.0 4 / 5
6.7. 3~ - 5 / 5
10~ - 5 / 5
~ x 1~ 1~.0 + 1.~ 3 / 5
6.10. 30 - 5 / 5
100 - 5 / 5
9 x 10 - 5 / 5
6.12. 30 - 5 / 5
10~ ~ 5 / 5
7-(1,3-di-9 x 10 10.0 + 1.4 3 / 5
hydroxy-2- 30 10.0 i O.0 4 / 5
isopropoxy-100 9.3 + 2.1 2 / 5
methyl)guanine
Control 0 8.3 + 2.8 1 / 5
~3~
- 26
Table 3., ~tiviral action agains~ HSV-l in N~I
mice on oral ~dmini~ration
Example Dosage Mean survival Surviving/
(~mol/kg) time ~days) total
9x 10 - 5 / 5
6.7. 3~ - 5 / S
100 - 5 / 5
9~c 101~.0 + 0.0 ~ / 5
6 . 10 . 30 - 5 ~ 5
100 - 5 / 5
9x 108.3 + 1.5 ~ / 5
6 . 12 . 30 - 5 / 5
100 - ~ / 5
7-(1,3-di- 9x 10 6.5 2.1 3 / 5
hydroxy-2- 30 8 . 5 O . 7 3 / 5
isopropoxy- 100 9 . O + 1. 4 3 / 5
methyl ) guanine
Control 0 7 . 7 + 1. 5 2 / 5
, '
~ ~ s,~
27 -
B~a~ple~0
Process according to 6b)s
1. Compound of the formula VIII in which L2 = me~hyl-
thio, R3 = isopropoxymethyl, R4 G isopropoxy ~nd
R5 - hydrogen:
303 ml of anhydrous dimethyl sulfoxide ~re slowly
added dropwise with 6tirring and cooling to about
30~C to a mixture of 180 ml of glacial acetic acid
and 150 ml of acetic anhydride. After the addition
is complete, the mixtur2 is su~seguently ~tirred for
30 min. 52.8 g (O03 mol) of 2,3-bi (isopropoxy)-
propan-2-ol tprepared by reaction of sodium iso-
propylate with 2,3-epo~propyl isopropyl ether in
isopropanol) are then added dropwi~e at about 25C.
The reaction mixture is allowed ~o ~tand at room
temperature for 4 days with periodic stirring. The
reaction mixtuxe is then ~3tirred into about 1 1 of
ice-water and extracted ~everal times by shaking
with diethyl ether or hex,ane. The organic pha~e is
washed several times with water, dxied over sodium
sulfate and evaporated. The oily re~idue is
fractionated in vacuo.
~he yield is 53 . 5 g ( 75 . 5% of theory~ of
1,3-bis(isopropoxy)-2 methylthiomethoxypropane.
Colorless oil of boiling point 68-74C at a preC~ure
of 2 n~n Hg.
2. Comp~und of the formula I in which Rl = hydroxyl,
Rz = scetamido, R3 = isopropoxymethyl, R4 = i~opro-
po~ and R5 = hydrogen:
5.7 g tO.0218 mol) of the methylthiomethyl ether
from Example 1 are combined with 4.9 g of anhydrous
dimethyl ~ulfoxide and 4 . 9 g ( O . 0209 mol ) of 2N, 9N~
diacetylguanine (prepared from guanine and acetic
anhydride in N-methylpyrrolid-2-one) in 20 ml of
~3~9
- ~8 -
anhydrous dLmethylformamide. The mixture is cooled
to ~O~C and 5.5 g (0.0209 mol) cf tin tetrachloride
are added dropwise to the suspension with ~tirring.
After addition i~ complete, the reaction mixture is
~tirred at 80C for 5 hours. It is then allowed to
cool, ~.he reaction mixture i~ treated with dichloro-
methane and ice-water, extracted ~ vQral times with
dichlorometh~ne, and the co~bined organic pha~e~ are
fir~t sh~ken with water, then with a~ueous sodium
h~drogen carbonate ~olution and finally with ~atur-
ated sodium chloride solution. ~he or~anic pha~e is
dried over sodium ~ulfate, filtered ~nd evaporated.
HPLC analysis (RP 18 (~iChro~pher (R) 100 RP 18,
5 ~m, 125-4), wat~r/mekhansl 1:1 ~ 0.1% of
trifluoroacetic acid, ammonium acetate) ~hows a
ratio of 7-isomer/9-i60mer of 47.5:47.7. The crude
yield is 7.5 g (94.4% of theory) of a pale oil. The
isolation of the 7-isomer i~ carr~ed out by means of
column chromatography on nsutral alumina uRing a
mixture of ethyl acetate~methanol 9:1 and yields
3.5 g (44% of theory) ~f 2N~acetyl-7-[1,3-bi~-
(isopropoxy)-2-propoxymethyl]guanine of melting
point 162 - 163C.
lH-NMR (60 MHz, d6-D~SO), ppm: 11.93 ( 8 , broad, 2H),
8.35 (s, 1~), 5.73 (s~ 2H), 3.83 (m, l~), 3.55 -
3.17 (m, 6H), 2.20 (s, 3H), 0.93 (d, 12H~.
3.1. Compound of the fonmula I in which Rl - chlorinef
R2 = ~cetamido, R3 = i~opropoxymethyl, R4
isopropoxy and R5 = hydrogen:
6.9 g (0.033 mol) of 2-acetamido-6-chloropurine (for
preparation ~ee under Sa.) are heated to reflux
under argon for 3 hours with 28 ml of hexamethyl-
disilazane (HMDS) and 0.2 g of ammonium sulfate in
28 ml of dry xylene. The ~olvent and exce~s ~MDS are
then distilled off, the residue i~ dissolved in
85 ml of dry 1,2-dichloroethane and the solution is
~3~
- .29 ~
added at -30C to a solution of ~.3 g (0.024 mol) of
the methylthiomethyl ather from Example 1 in 85 ml
of dxy 1,2-dichloroethane. 5 ml (0.026 mol) of
trimethyl~ilyl tri1uoromethanesulonate are then
added and the mixture i8 stirred at 30~C for
2 hours.
The reaction product i8 poured into 150 ml of ice-
water and filtered, and the re~idue i8 washed with
1/2-dichloroethane. The aqueous phase i~ extracted
by shaking with 1,2-dichloroethane; the combined
organic phases are extracted by shaking with water,
then with dilute sodium hydroge~ carbonate solution,
dried over ~odium 6ulfate and concentra ed. HPLC
analysis (RP lB (LiChrospher 100 ~P 18, 125 x 4)l
water/acetonitrile 3:1 + 0.1~ TEA) shows the
presence of 73~ of 2-acetamido-6-chloro-
-7-[1,3-bis(isopropoxy)-2-propoxymethyl]purine in
addition to 2% of the corresponding 9-isomer.
3.2. The analogous conversion in acetonitrile and u~ing
3.B equivalents of tin tetrachloride gives 73~ of
the 7-isomer and 23~ sf the 9-isomer (HPLC analysis
of the crude product as in the preceding exampl~).
Process according to 6c):
4.1. Compound of the formula VIII in which ~2 = ~cetoxy,
R3 = isopropoxymethyl, R4 = isopropoxy and R5 =
hydrogen:
200 ml of anhydrous dLmethyl ~ulfo~ide are added
dropwise with ætirring to a mixture of 120 ml of
glacial acetic acid and 100 ml of acetic anhydride
in ~uch a way that the temperature of the mixture
does not rise above 35C. The mixture is stirred for
a further 30 minutes before 35.2 g (0.2 mol) of
1,3-bis(isopropoxy)propan-2-ol (prepared as
described above) are added dropwise. A~ter
~3~
- 30 -~
completion of the addition, ~he mixture i6 hea~ed at
90 lOOnC for 7 hours. The cooled reaction mixture
is poured into water and ex~rac~ed with diathyl
ether several ~Lme~ ~y shaking. ~he organic phase i~
then ~ashed with water and sub~equently with agueous
hydrogen carbonate ~olution, dried over ~odium
sulfate and evaporated~ A pale yellow oil remain~,
which is ~ubjected to fractional di~tillation. A
forerun o boiling point 46 - 47C at a pressure of
15 ~m Hg is composed of thiometh~lmethyl acetate.
The reaction product, 2~ace~oxymetho~y-1,3-bis-
(isopropoxy)propane~ boils a~ 87 - 92C at a
pressure of 1 mm Hg. The yield is 27.3 g (55% of
theory).
lH~~nMR (60 MHz, CDCl3), ppm: 5.43 ( , 2~I), 4.0 - 3.33
(m, 7H), 2.12 (~, 3H), 1.33 (d, 12H).
The following were prepared in thi6 m~nner:
4.2. 2-acetoxymethoxy-1,3-bi~(methoxy)propane
4.3. 2-acetoxymethoxy-1,3-bis(ethoxy~propane
4.4. ~-acetoxymethoxy-1,3-bis(propoxy)propane
4.5. 2~acetoxymethoxy 1,3-bi~(benzyloxy)propane
4.6. 2-acetoxymetho~y-1,3-bis(cyclopentyloxy)
propane
4.7. 2-acetoxymetho~y-1~3-bi~(prop-2-en-1-o~y)
propane
4.8. 2-acetoxymethoxy-1-benzyloxy-3-(isopropoxy)
pxopane
4.9. ~-acetoxymetho~y~l-isopropoxy-ethane
4.10. 1-acetoxymethoxy-2-benzylo~y-3-isopropoxy-
propane
4.11. 2-acetoxymethoxy-1-benzylo~y-3-pivaloyloxy-
propane
4.12. 2 acetoxymethoxy 1,3-bis(pivaloyloxy~propane
5. Compound of tha fonmula I in which Rl = chlorinP,
R2 a acetamido, R3 = isopropoxymethyl, R4
-- 31 --
~ iBopropoxy and R5 = hydrogen:
5a. 3.17 g (O.015 mol) of 2-acetamido-6-rhloropurine
(prepared according to E.N. Acton and R.H. Iwamoto
in W.W. Zorbach and ~.S. Tipson (editors) Synthetic
Procedures in Nucleic Acid Chemistry, Volume 1,
Inter~cience Publi~hers, John Wiley ~ Son~, New
York, 1968, pp. 25 et ~eq.) are heated under reflux
in an inert gas atmosphexe for 3-4 hours with
11.3 ml of hexamethyldisilazane and 100 mg of
ammonium sulfate in 13 ml of . nhydrou~ xylene and
thus converted into the bis-trimethylsilyl compound.
After the reaction i~ complete, ~he ~olvent and
excess hexamethyldisila~ane are evaporated in ~acuo.
The residue is dissolved in 10 ml of anhydrous
acetonitrile and added dropwise with _tirring to a
solution of 2.8 g (9.01 mol) of 2-aceto~y~ethoxy-
1,3-bis(isopropoxy)propane in ~0 ml of anhydrous
acetonitrile. 13 g (0.05 mol) of tin tetrachloride
are then added slowly at -20~C and undex an inert
yas atmosphere and the mi.xture is stirred at 20C
for 3 hour~. The reaction mixture i~ ~tirred into a
mixture of ice-water ~md dichloromethane and
filtered. The aqueous phase is extracted ~everal
times with dichloromethane and the combined organic
phases are then washed twice with sodium chloride
solution, dried over sodium ~ulfate, and filtered
and Pvaporated. A pale ~yrup xemains who~e HPLC
analysis (RP 18 (Nucleosil 5 Cl8~ (R), water/-
acetonitrile 3:1 ~ O.1~ TEA) give~ a eontent of 86~
of the 7-isomer and 4% of the 9-i~omer. Chromato-
graphic purification on ~ilic~ gel using ethyl
acetate/methanol 20sl ~ives 1.8 g (45~ of theory) of
2-acetamido~6-chloro~7-[1,3-bis~i~opropoxy)-2
propoxyme~hyl]purine of mel~ing point 73-75C.
lH-NMR (270 MHz, d6-DMS0), ppm: 10.68 ~s, lH), 8.84
(s, lH), 5.81 (s, 2H), 3.71 (m, lH), 3.46 - 3.24 (m,
6H), 2-18 (8, 3H), 0.90 (m, 12H).
- 32 -
- 5b. Reaction procedure as in 5a., with the difference
tha~ the ~ilylated 2-acetamido 6-chloropurine
(0.015 mol~ di~solved in 10 ml of anhydrous
1,2~dichloroethane is added dropwi~e to a solution
o~ the acetoxymetho~y compound (O.01 mol) in 70 ml
of anhydrous I,2-dichloroethane, and 2.67 g
(0.012 mol) of trimethylsilyl trifluorom~thane-
sulfonate are added at -30~C and the reaction
mix~ure is stirred at -30C for 2 hour~. XP~C
analysis of ~he crude p~oduct, carried ou~ as in
Sa., gives an isomer ratio of 7-i~omer/9-i~omer of
90:6. Chromatographic purification over ~ilica gel
using ethyl acetate/methanol 20:1 yields 2.15 g
(53.8~ of theory) of a white powder of melting point
7~ - 74C.
T~e following w~re pr~pared in thi~ ~3nner-
5.1. 2-A~etamido-6-chloro-7-[1,3-bis(ethoxy)-2-propoxy-
methyl]purine of melting point 76 - 78C
5.2. 2-Acetamido-6-chloro-7-C1,3-bis(propoxy)-2-propoxy
me~hyl]purine of melting point 74C
5.3. 2-Acetamido-6-chloro-7-(2-i~opropoxyethoxymethyl)-
purlne of melting point 116 - 118C
5.4. 2-Acetamido-6-chloro-7 (1-benzyloxy-3-isopropoxy-
2-propoxymethyl)purine a a viscous oil
(1~_NMR t270 NHz, d6-D~S03, ppm: 10.70 (B, lH~, 8.86
(s, lH), 7.40 - 7.15 (m, 5H), 5.85 (m, 2H), 4.38 (s,
2H), 3.84 (m, lH), 3.50 - 3.25 (m, 5H), 2.17 (
3H), 0.~7 (m, 6H)),
5.5. 2-Acetamido-6-chloro-7-[1,3-bis(methoxy)-2-propoxy-
methyl]purine of melting point 83 - 84C
5.6. 2-Acetamido-6-chloro-7-~1,3-bi~(prop-2-~n-1-oxy)-
;~ ~ 3 ~
33 -
2-propo~ymethyl~purine of melting point 79C
5.7. 2-Acetamido-6-chloro-7 ll,3-bislcyclopentyloxy)
2-propoxymethyl]purine aæ a YiSCoU8 oil;
1H-NMR (60 NHz, d6-DMSO), ppm: 10.73 (B~ lH), 8.83
(s, lH~, 5.83 (s, 2H), 3.7S ~m, 3H), 3.27 (m~ 4H),
2.18 (~/ 3H), 1.42 (6, broad, 16H).
5.8. 2-Acetamido-6-chloro-7-~2-benzyloxy-3 isopropoxy~
l~propoxymethyl)purine as a viscous oil;
l~_NMR (60 ~Hz, d6-DMS0) ppm: 10~77 (~, lH), ~.92
(s, lH), 7.32 (s, 5H), 5.82 (s, 2~), 4-53 (s, 2~),
3.67 - 3.20 (m, 6H), 2~2 (s, 3H), 0.93 ~dt 6~).
.9. 2-Acetamido-6-chloro-7-[1,3-bis(pivaloyloxy)-
2-propoxymethyl~purine as a glassy foam;
l~_NMR (210 MHz, d6-DMS0), ppm: 10.72 (s, lH), 8.88
(s, lH), 5~79 (s, 2H), 5.04 (m, lH), 4.23 (m, lH),
4.05 (m, lH), 3.63 (d, 21I), 2.18 ~s, 3H), 1.05 (s,
9H), l.01 (~, 9H),
5.10. 2-Acetamido-6-chloro-7-(1-benzyloxy-3-pivaloyloxy-
2-propoxymethyl)purine a~ a vi cous oil;
lH-N~R (270 ~Hz, d6-DNSO), ppm: 10.70 ~6, lH~, 8.89
(s, lH), 7.35 - 7.15 (m, ~H), 5.85 ( ~ 2H), 4.40 (s,
2H), ~.15-3.94 (m, 3H), 3.49 (m, 2H), 2.18 ~8, 3H),
0.97 (s, 9H~.
Proce~s according to 5)-
6.1. Compound of the fo~mula I in which R1 = thio, R2 =
thioacetamido, R3 - isopropoxymethyl, R4 = isopropoxy
and R5 - hydrogen:
3.8 g (0.01 mol) of the ~ompound from Example 2. are
stirred at 80 - 85C under argon for 3 hours with
,
- 34 - 2~3$~
4,4 g (0~011 mol) of 2,~-bis(4-methoxyphenyl)-
1,3 dithia-2,4-dipho~phetane-2,4 di ulfide
(Law~s~on's reagent) in 150ml of ~ toluene.After
completion of the reaction, the mixture i allowed
to cool, the precipitate is filtered off with
suction, the residue is wa~hed with toluene ~nd the
filtrate isevaporated.Ayellow~y~pr~mainswhich
is purifisd by chroma~ography on silica gel u~ing
ethyl acet~e/methanol 20.1. In thi~ way, 2
(48.6 % of theory) of 2 thioacetyl-7-(1,3-bis(iso-
propoxy)~2-propoxymethyl)thioguanine are obtained.
Ths yellowish powder decomposs~ at 220C.
6.2. Compound of the fonmula I in which R~ = thio, R2 =
amino, R3 = i~opropoxymethyl, R4 - i~opropoxy and
Rs = hydrogen.
1.6 g (0.00472 mol) of 7-(1,3-bis(isopropoxy)-
2-propoxymethyl)guanine are heated under reflux for
6 hours with 1.01 g (0.0025 mol) of ~awesson's
reagent in a mixture of !50 ml of dry toluene and
10 ml of dry pyridine unde:r argon. The crude product
of the reaction is purified by column chromatography
on silica gel using a mi~ture of dichloromethane/
methanol 5:1. 1.2 g (71.6~ of theory) of a weakly
yellow powder of m~lting point 232 - 236~ are
obtained.
H-NMR (60 NHz, d6-DM50), ppm: 12.05 ~ lH), 8.37
(s, 1~), 6.55 (~, 2H), 6.10 (s, 2H), 3.93 - 3.22 ~m,
7H), 0.97 ~d, 12H).
~he compound 6.2. can al~o be prepared by heating
0.413 g (0.001 mol) of th~ compound of ~x2mple 6.1.
to reflux with 4 ml of 40% strength aqueou~ methyl-
amine solution and 4 ml of methanol for 3 hours.
Yield: 0.~5 g (70.4% of theory).
6.3. Compound of the formula I in which R~ = methoxy,
- 35
R2 = acetamido, R3 = isopropo~ymethyl, R4 = i~o-
propoxy and R5 = hydrogen:
1.4 g (Q.0035 mol~ of the compound from ~xample 5.
are di solved in 14 ml of methanol with 13 mg
(O.2 mmol~ of potassium cyanide and the mixture is
stirred at room temperature ~or 24 hours. It i6 then
diluted with 20 ml of m~thanol and trsated for
5 minutes in each case with S~rdolit Blue (R)
(Serva) (OH form) and Amberlyst 15 (R) ~Fluka) (H~
form~, the ion exchanger is fil~ered off and the
filtrate is evaporated. ~he remaining ~yrup cry8-
talli~es on addition of diisopropyl ether. 1.05 g
(75O8% of theory) of 2-acetamido-6Dmethoxy-7-(1,3-
bis~isopropoxy)-2-propoxymethyl)purine of melting
point 67 - 63C are obtained.
~_NMR (60 MHz, d6-D~SO), ppm: 10.73 (s, lH), 8.87
(s~ lH), 5.83 (s, 2H), 3.80 - 3.15 (m, 10H), 2.17
(8, 3H), 0.88 (d, 12H).
6.4.1. Compounds of the formu:La I in which Rl = amino,
R2 G acetamido, R3 = isopropoxymethyl, R4
isopropoxy and Rs ~ hydrogen
and
6.4.2. Rl = amino, R2 = amino, R3 = isopropoxymethyl,
R4 = isopxopoxy and R5 = hydrogen:
6.4.1. 10 g (0~025 mol) o~ the compound from Example 2.
are treated with about 150 ml of liquid amMonia
in 100 ml of meth~nol and the mix~ure i8 heated
at 80~C in an autoclave at a pre~sure of 5 bar
for 20 hours. The reaction mixture i~ then
completely evaporated and purified by column
chromatography (silica ~el, dichloromethane-
/methanol 9:1). 0.26 g t2.7% of theo~y~ of
~-acetamido-6-amino-7-~1,3-bis(isopropoxy)-
2-propvxymethyl]purine of melting point
136 - 137C is obtain~d as a first fraction.
2~3~8~
- 36 -
H-N~R (60 N~z, d6~D~S0), ppm: 9.72 (c, lH), 8.30
(~, lH), 6.73 (8, 2H), 5.73 (~l 2H), 3.~3 - 3.2
(m, 7H), 2.20 (s, 3~), 0.97 (d, 12H)o
6.4.2. The ~econd fraetion yields 4.6 g (54.4% of
theory) of 2,6-diamino-7-~1,3-bis(i~opropoxy)-
2-propoxym~thyl]purine of m~l~ing point
228 - 2~9C.
H-NMR (60 ~Hz, d6-DM50), ppm: 8.10 (s, lH3, 6.52
(s, 2~), 5.85 (8, 2H), 5.67 (s, 2H), 3.83 - 3.22
(m, 7H), l.00 (d, 12H~.
6.5.1. Compound~ of the formula I in which Rl = methyl-
amino, R2 = acetamido, R3 = isopropoxymethyl,
R~ = isopropoxy and R5 = hydrogen
and
6.5.2. R~ = methylamino, R2 -- amino, R3 = i30propo~y~
methyl, R4 = isopropoxy and Rs = hydrogen:
6.5.1. 1.4 g (3.5 mmol~ of the compound from Example 2
are heated under reflux with 7 ml of 40~ strength
agueous methylamine solution and 14 ml of
methanol for 2 hours. The reaction 801ution i8
then completely evaporated and the residue is
separated by column chromatography on 8ilica gel
using a mixture of dichloromethane/methanol 10:1.
The first fraction i~ composed o~ 0.45 g (32.6%
of theory) of 2-acetamido-6-methylamino-7-~1,3-
bis(isopropoxy)-2~propox~nnethyl]purineofmelting
point 159C.
H-NNR (60 MH~, d6-DMS0), ppms 9.75 (s, lH), 8.28
(s, lH), 6.67 (q, lH), 5.75 ~8, 2H)l 3.77 - 3.22
(m, 7~), 3.00 (d, 3~), 2.27 ts, 3H), 0.97 (d,
12H).
6.5.2. The second fraction i8 composed o 0.45 g (36.5%
of theory) of 2-amino-6 methylamino-7-~1,3
2~33~9
- 37 -
- bis(isvpropoxy)-2-propoxymethyl]purineofmelting
point 103 - 104~C.
H-NMR (270 MHz, d6-DNSO), ppms 7.99 ~ ), 6.29
(q, lH), 5.65 (s, 2H), 5.63 (3, 2~), 3.65 (m,
lH), 3.45 (ml 2~), 3.36 (m, 4H~, 2.92 (d, 3H~,
1.00 tq, 12H3.
If a larger excess of methylami~e solution i~
u~ed and the reaction time i8 lengthened/ the
d~blocked product 6.4.2. i8 isolated exclu~ively
in g2% yield.
6.6. Compound of the formula I in which Rl = hydrogen,
R2 = acetamido, R3 = isopropoxymethyl, R4 =
isopropoxy and R5 = hyd:rogen:
16 g (O.04 mol) of the compound from Example S
are exhau~ti~ely hydrogenolyzed with hydrogen at
room temperature in a duck-shaped shaking ~es~el
using 3.5 g of palladium on carbon (10~) and
11.06 ml (0.08 mol) o~ triethylamine in 350 ml of
methanol. After completion of hydrogen absorp-
tion, the catalyst i6 filtered off with suction
;~ and the methanolic pha~e i6 evaporated. The
residue is stirred in ethyl acetate, the
precipitate of tri~thylamine hydxochlori~e is
separated off and the ethyl acetste ~olution i~
completely concentrated. The cry~t~lline residue
is purified by column chromatography on ~ilica
gel using ethyl acetate/methanol 9:1 as the
eluent. 14 g (95.9% o theory) 5f 2~ace~amido-
7-[1,3-bi~(isopxopoxy~ propoxymethyl]purine of
meltiny point 94 - 96C are obtained.
H-NMR (270 MHz, d6-DM50), ppm: 10.43 (s, lH),
9.02 (s, lH), 8.71 l8, lH), 5.79 (8, 2~), 3.70
(m, lH), 3.41 (m, 2H), 3.32 (m, 4~), 2.19 (8,
3H), 0.93 (m, 12H~.
2~3~9
-- 38 ~
. 7 . Compouald of formula I in which Rl = hydrogen,
R2 = amino, R3 = isopropoxymethyl, R4 = i~opropoxy
and R5 = hydrogen:
û.178 g (0.5 mmol) of the compound of ~3xample
6 . 2 . are treated with 1. 0 ~ of Raney nickel
washed with absolute ethanol in 20 ml o absolute
ethanol and the ~ixture is heated to reflux for
1 . 5 hours . The reactiosl mla~ture is then cooled,
the Raney niclcel i~ filtered off with ~uction and
tha ethanolic ~olution i8 completely concen-
trated. The residue i8 purified by column chroma-
tography on 6ilica gel using ethyl acet~te-
~metharlol 2 0: 1 as the eluent . O . 1 g ( 61. 7 ~6 of
thec>xy) of 2-amino-7- [ 1, 3 bis ~ isopropoa~y) -
2-propoxymethyl ]purir~e is obtained as white
flakes of melting point 153 - 154C.
NMR (270 MHz, d6-DMS0), ppm: 8.65 (s, lH), 8.38
( s ~ lH) ~ 6 . 22 ( 8 ~ 2H) r 5 . 67 ( E; ~ 2H), 3 . 65 (m,
lH), 3 . 42 (m, 2H~, 3 . 32 (m, 4EI), 1. 00 (m, 12H) .
The compound of Example 6.7. can al80 be prepared
from the compound of Example 6 ~ 6 . by reac~ion
with aqueous methylamine solu~ion in methanc)l.
1.1 g of the compound: of 13xample 6 . 6 . yields
0 . 8 ~ ( 82 . 296 of theory) of the compound of
Example 6 . 7 .
6.8. Compound of th~ formula I in which Rl = hydrogen,
R2 = ~cetamido , R3 = hydrogel, R4 = iBopropoxy and
Rs ~ hydr~gen:
The co~pound of Example 5 . 3 . i~ sub~ected to
hydrogenolysis as in Example 606. and yields
2-acetEmido-7-(2-isopropoxyethoxymethyl)purine of
melking point 152C in 95.6% yield.
6.9. Compound of the formula I in which Rl = hydrogen,
'
2~3~8~
- 39 -
R2 = acetamido, R3 - benzylo~ymethyl, R4 = iso-
propoxy and R5 = hydrog~nO
4.47 g ~0.01 mol) of the compound of ~xample 5.4.
are ~ubjected to hydrogenolysis as in Example
6.6. and yield 3 g (72.6% of theory) of 2-acet-
amido-7-(1 b~nzyloxy-3-iaopropoxy-2-propoxy-
methyl)purine a~ an oil.
H-NNR (270 NNz, d6-DMS0~, ppm. 10.43 (~, lH),
9.03 (3~ lH), 8.72 (5, 1~), 7.36 - 7.15 (~, 5H),
5-~ t~ 2~3; 4.39 (3~ 2H), 3.83 (8, lH), 3.50 -
3.25 (mJ 5H), 2.18 (8, 3H)~ 0.90 (m, 6H~.
6.10. Compound of the formula I in which R1 - hydrogen,
Rz = amino, R3 = hydro.xymethyl, R4 = isopropoxy
and R3 = hydrogen:
1.6 g (4.95 mmol~ of the compound of Example 8.3.
are heated under reflux with 50 ml of 40% aqueous
methylamine ~olu~ion i.n ~0 ml of methanol for
1 hour. After distill.in~ off th~ solYent an oil
remains which is treat~ed with acetone and ~rys-
talli~es after some time. 0.7 g (50.3~ of theory)
of 2 ~mino-7~ h~dro~y-3-isopropoxy-2-propoxy-
methyl)purine of melting point 125 - 130-C are
obtained.
l~_NMR (270 ~Mz, d6-DNSO)t ppm: 8.66 ~8, lH), R.37
(~, lH), 6.23 (~, 2H), 5.67 tm, 2H), 4.70
(t, lH), 3.55 (m, lH), 3.36 - 3.20 (m, S~), 0.95
(m~ 6H)-
6.11. Compound of the formula I in which Rl = hydrogen,
Rz = amino, R3 = hydrogen, R4 = i~opropoxy and
R~ = hydrogen:
2.2 g of the compound of Example 6.8. ar~ treated
a~ in Example 6.10. and yield 1.4 g (74.3% of
~3~8~
- 40 -
theory) of 2 amino-7-(2-i.sopropoxy-2-ethoxy-
methyl)purine of melting point 158C.
6.12. Compound of the ~onmula I in which Rl = hydrogen,
R2 = amino, R3 = hydrox~methyl, R4 = hydroxyl and
R5 = hydrogens
1o6 ~ ~O.0057 mol) of the compound of Example
8.2. are heated to reflux for 2 hour~ with 10 ml
of methanol, 10 ml of 40~ tr~ngth aqueous
methylamine solution and 5 ml of water. Working-
up yields 1 g t73.4% Of theory) of 2-amino-
7~(1,3-dihydroxy propoxymethyl)purine of melting
point 176 - 177C.
H-NMR (27G.~Hz, d6-DMSO), ppm. 8.fi8 (~, ~H), 8.38
~s, lH), 6.23 ~s, 2H), 5.69 (s, 2H), 4.62
(~ 2H)/ 3.38 (ml ~H).
6.13.1. Compound of the ~ormula I in which Rl - hydro~yl,
R2 = hydroxyl, R3 = isop:ropoxymethyl, R4 = isopro-
poxy and R5 = hydrogen
and
6.13.2. Compound of the formula I in which R1 = amino,
R2 = hydroxyl, R3 = isopropo~ymethyl, R4
isopropoxy and R5 = hydrogen.
6.13.1. 1.4 g (0.0041 mol) o~ the compound of ~xample
6.4.2. are dissolved in a mixture of 45 ml of
tetrahydrofuran and 30 ml of water. 1.8 g (0.027
mol) of sodium nitrite and 24 ml of glacial
acetic acid are added, the mixture i8 stirred at
50C for 90 minutes, a further 1.8 g ~f sodium
nitri~e and 9 ml of ~lacial acetic acid are
added, the reaction mixture is completely evapor-
atedl and the residue is treated with a ~ittle
water and neutralized with concentrated ammonia.
An oil precipitates which becomes solid after
2~3~8~1
- 41 -
some time. The precipitate is filtered off,
recrystallized from i~opropanol and 0.3 g (21.3%
of theo~y) of 7-tl,3-bi~(isopropoxy)-~-propoxy-
methyl]xanthine of melti~ point 200 - 2Q1C i~
obtained.
H-NMR (270 N~z, d6-D~O), ppm: 11.60 (8, lH),
10.89 (~, lH), 8.13 (~, lH), 5.63 (8, 2H), 3.83
(m, 1~), 3.46 ~m, 2H), 3.30 ~m, 4H~, 1.01 ~m~
12H).
6.13.2. The a~ueou~ mother liquors are evaporated, taken
up with dichloromethane and a little wa~er and
extracted three times by haking with 100 ml of
dichloromethane. The organic phase i~ dried
(sodium sulfate) and evaporated. The ~yrup thus
obtained is purified by chromatography (silica
gel, dichloromethanefmethanol 9:1). After recry-
stallization from water, 60 mg (4.3% of theory)
of 6-amino-2-hydroxy-7-[1,3-bis(isopropoxy)-
2-propoxymethyl]purine (7-[1,3 ~is~i~opro-
poxy)-2-propoxymethyl].i~oguanine) of melting
p~int 213C are obtaini~d.
H-NMR (270 MHz, d6-DMSO), ppm: ll.lS (s, lH),
: 8.02 (s, lH), 6.88 (s, 2H), 5.64 ~s, 2H~, 3.70
(m, 1~), 3.47 (m, 2H), 3.38 (ml 4H), 1.01 (d,
12H).
6.14. Compound of the foxmula I in which Rl = hydrogen,
R2 = acetamido/ R3 = methoxymethyl, R~ = methoxy
and R5 = hydrogen:
1.6 g (4.7 mmol) of the compound o~ ~xample 5.5.
are ~ub~ected to hydrogenolysis a~ in Example
6.6. and after chromatography on silica gel
(ethyl acetate/methanol 5:1) yield 1.25 g ~86~ of
theory) of 2-acetamido-7-t1,3-biR(methoxy)-2-
propoxymethyl~purine of melting poin~
2~3~
- ~2
102C.
.15. Compound of the formula I in which Rl = hydrogen,
R2 - acetamido, R3 = ethoxymethyl, R4 = ethoxy and
R5 c hydrogen:
3.7 g (O.01 mol) of the c~mpound of Exa~ple 5.1.
are subjected to hydrogenoly~is as in Example
6.6. and after chrom~tographic purification on
silica gel (ethyl acetate/methanol 9sl) yield
2.9 g (86~ of theory) of 2-acetamido-7-~1,3-bi3-
~ethoxy)-2-propox~methyl]purîne of melting point
117 l18C.
6.16. Compound of the formula I in which R1 = hy~rogen,
R2 = ~mino, R3 = prop-2-en-1-oxymethyl, R4 = prop-
2enoxy and R5 = hydrogen:
4~35 g (11 mmol) of compound of Example 5.6. are
heated to reflux in 60~ml of water with 3.84 g of
~inc dust. 1.7 ml of concentrated ammonia are
then added dropwise over a period of 2 hours. The
cooled suspen~ion i8 treated with 50 ml of
methanol and filtered off wi~h suction, and ~he
~:~ residue is wa~hed with methanol. ~he co~bined
: filtrates are concentrated and ~hromatographed
on sili~a gel using ethyl acet~te/methanol 9:1.
2.9 g (82.6% of theory) of 2-amino-7-l1,3-bis~
(prop-2 en-1-oxy)-2-propoxymethyl]purine of
melting point 140 - 143C are obtained.
6.17. Compound of the formula I în which Rl = hydrogen,
R2 = acetamido, R3 = cyclopentyloxymethyl, R4 =
cyclopentyloxy and R3 = hydrogen:
1.1 g (2.44 mmol) of the compound of Example 5.7.
are sub~ected to hydrogenolysis as in Exa~ple
6.6. and aft~r chromatographic purification
(silica gel, ethyl acetate/methanol 9sl) yield
~ 43 -
0.8 g (78.6% of theory) of ~-acet~mido-7-~1,3-
bis(cyclopentyloxy)-2 pxopoxymethyl]purine of
melting point 9BC.
6.18. Compound of the formula T in which R1 - hydrogen,
R2 = acetamido, R3 = hydrDgen, R4 = benzyloxy and
R5 - i~opropoxymethyl:
7.5 g ~16.8 mmol) of the compound of Example 5.8.
are ~ub~ected to hydrog~nolysi6 as in ~xample
6 . 6 . and after chromatographic purification on
silica gel ~ethyl acetate/methanol 9:1) yield
6.3 g (90.6~ of theory) of 2 acetamido-7-(2-ben-
æyloxy-3-i opropoxy-l-propoxymethyl)purine of
melting point 116 - 117C.
6.19. Compound of the formula I in which Rl = hydrogen,
R2 = acetamido, R3 = benzyloxymethyl, R4 = piva-
loyloxy and R5 = hydrogen:
20.5 g (41.9 mmol) of the compound of Example
5.10. are ~ub~ected t;o hydrogenolysis a~ in
Example 6.6. and after chromatography on silica
gel (ethyl acetate/me~thanol 9:1) yield 17 g
(~9.2~ of theory) of 2-acetamido-7 (1-benzyloxy-
3-pivaloyloxy-2-propoxymethyl~purine o~ melting
point 76 - 77C.
H-NNR (270 ~Hz, d6-DNSO), ~ pp~: 10.45 (8, 1~),
~5 9.05 (~, lH), 8.77 ~s, 1~), 7.35 - 7.18 ~m, SH),
5.83 (~, ~H), 4.40 (2, 2~), 4.13 (m, lH), 3.99
(m, 2H), 3.49 (m, 2H~, 2.19 (~, 3H), 0.98 (8,
9H).
6.20. Compound of the formula I in which Rl = hydrogen,
R2 - amino, R3 = methoxymethyl, R4 = metho~y and
R5 = hydrogen:
0.77 g ~2.25 mmol~ of the compound of Exampl~
44 2~3~
6.14. is tr~a~ed wiih methylamine solution a~ in
Example 6.10. and yields 0.54 g (89.9~ of theory)
of 2-amino-7-[1,3-bis(methoxy~-2-propo~ymethyl]-
purine of melting point 148C.
6.21. Compound of the formula I in which Rl - hydrogen,
Rz = amino, R3 = ethox~methyl, R~ = ethoxy and
R5 - hydrogen:
1.35 g (4 mmol) of the compound of Examplæ 6.15.
are treated with methylamine ~olution a~ in
Example 6.10. and yi~ld 0o8 ~ (S7.~% of theory)
of 2-amino-7~[1,3-bis(ethoxy~-2-propox~methyl~-
purine of melting point 151C.
6.22. Compound of the formula I in which Rl = hydrogen,
R2 = amino, R3 = cyclope~tyloxymethyl, R4
cyclopentyloxy and R5 = hydrogen:
0.5 g (1.2 mmol) of the compound of ~xample 6.17.
is treated with methylamine solution as in
Example 6.10. and yielcls 0.3 g (66.7% of theory)
of 2-amino-7-[1,3-bis(cyclopentyloxy) 2-propoxy-
methyl]purine of melting point 158C.
6.23. ~ompound of the formula I in which Rl = hydrogen,
R2 = amino, R3 = hydrogen, R4 = hydroxyl and R5 =
hydroxymethyl:
1. 4 g ( 5 mm51 ) of the compound of Example 8.5.
are treated with methyl~mine ~olution ~s in
Example 6.10. and yield 0.45 g (37.7~ of theory~
of 2-amino-7-(2~3-dihydroxy-1-propoxymethyl)-
purine of melting point 130 - 133C;
lH-NMR (270 ~Hz, d6 D~SO), ppm: 8.67 (8, lH), 8.40
(s, lH), 6.25 (s, 2H), 5.60 (~/ 2H), 4.75 (d,
lH), 4.50 (d~ lH), 3.60-3.25 ~m, SH).
~3~
_ 4~ -
6.24. Compound of the formula I in which Rl = hydrogen,
R2 = amino, R3 = benzyloxymethyl, Rb c hydroxyl
and Rs = hydrogen:
0.66 g ~1.45 mmol) of the compound of ~ample
6.19. iB treated ~ith methylamine ~olut~on as in
~xample 6.10. and yields 0.25 g ~5~.4% ~f theory~
of 2-amino-7~ ben~yloxy-3-hydrQxy-2-prepoxy-
methyl)purine of m~lting point 122DC;
1H-N~R ~270 MXz, d6 - DMSO~, ~ ppm: 8.68 (8, lH),
8.39 ~sl lH), 7.36-7.18 (m, 5~), 6.25 (5~ 2H),
5.71 (s, 2H), 4.73 (t, lH), 4.38 (s, 2~), 3.68
(m, lH), 3.50 3.31 (m, 4H).
6.25~ Compound of the formula I in which Rl = hydrogen,
R2 = acetamido, R3 = benzyloxymethyl, R4 = hydrox-
yl and R5 = hydrogen:
0.5 g (1.1 mmol) of the compound of Example 6.19.
i~ dissolved in 10 ml of methanol, and the
solution is treated with 10 ml of concen~rated
aqueou~ ammonia and stirred at room temperature
: 20 for 24 hours. Working-up yields 0.25 g (61.3~ oftheory) of 2-acetamido~7~ benzyloxy-3-hydroxy-
; 2-propoxymethyl)purine of melting point 149C;
H-NMR (60 MH~, d6-DNSO3, ~ ppm: 10.47 (8, 1~),
9 . 10 ( B, lH3, 8.78 (s, lH~, 7.30 (m, 5H), 5.87
(B, 2H)~ 4.73 (t, lH), 4.40 (8, 2H) t 3.80-3.30
(m~ 5H), 2,17 ~s, 3H).
6.26 C~mpound of the formula I in ~hich Rl a hy~rogen,
R2 = acetamido~ R3 = acetoxymethyl, R4 ~ acetoxy
and R5 = hydrogen:
0.24 g ~1 mmol) of the compound of Example 6.12.
i8 treated with 10 ml of ~cetic anhydride and
30 mg of N,N-dimethylaminopyridine and the
2~8~
~ 4~ -
mixture is ~tirxed at room temperature for
18 hours. After n~utralization o the reaction
mixture and chromatographic purification of the
crude product on 6ilica gel u~ing ethyl acetate/-
methanol 9:1, 0.2 g ~61.9~ of ~heory) of
2-acetamido-7-~1,3-bis~acetoxy)-2~pxopo~ymethyl]-
purine of melting point 141C is obtained.
H-NMR (270 ~Hz, d6-DNSO), ~ pp~: 1~.46 (8, lH),
9.04 (~, lH), 8.77 (8, lH), 5.82 ~s, 2H~, 4.16-
4.09 (m, ~), 4.05-3.95 (m, 3H), 2.19 ( 5 t lH),
1.70 ~8/ 6~).
Combination of the proces~es according to 1) - 4) with
the process according to 5): -
7.1. Compound of the formula I in which R1 = isopro-
poxy, R2 = amino, R3 ~ i~opropo~ymethyl, R4 =
isopropoxy and R5 = hydrogen:
~ g (O.005 mol) of the compound of Example 5 are
dissolved in 25 ml of a:nhydrous i~opropanol, the
601ution is treated with a solution of 0.345 g
~0.015 mol~ of sodium in a~hydrous i~opropanol
and the mixture is heated to reflux for 2 hours.
The cooled 8U . pe~sion i6 treated with ic~-water
and neutralized with 2 N acetic acid. The
precipitate i6 filtered off with suction, washed
with water and dried. 1.5 g (78.6~ of theory) of
2-amino 6-isopropoxy-7- [ 1 / 3-bi (i~opropoxy)~
2-propo~ymethyl]purine of melting point 85 - 87C
are obtained.
1H~NMR (60 ~z, d6-DNSO), ppmO 8.22 (8, lH~, 6.08
(~ 2H), 5.62 ts/ 2H), 5.50 lm, lH)~ 3.87 - 3.17
t~, 7H)~ 1-35 (d, 6H), 0.97 td, 12H).
7.2. Compound of the formula I in which Rl = methoxy,
R2 = amino, R3 = i~opropoxymethyl, R4 = isopropo~y
2~3~
- 47
and R5 = hydrogen~
2 g (0.005 mol) of the compound of Ex~mple 5. are
dissolved in 20 ml of methanol and the 301ution
is added to a solution of sodium ethanolate in
methanol (O.35 g o~ ~odium and 20 ml of
meth~nol). The mixture i~ heated under reflu~ fox
2 hour~. Precipitated ~odium chloridP i6 filtered
off with suction, and the ~ethanolic solu~ion is
completely conoentrated. The resldue iB dis~olved
in a little water ~nd the ~olution i~ neutralized
with acetic acid. The precipitated product is
filtered off with suction, washed with water and
dried. 1.5 g (84.9% of theory) of 2_amino-6~
methoxy7-[1,3-bis(i~opropo~y)-2-propoxymethyl]-
purine of melting point lll~C are obtained.
Pxocesses according to 1) - 4):
8.1. Compound of the formula I in which Rl = hydroxyl;
R2 = acetamido, R3 = hyclroxymethyl, R4 = hydroxyl
and R5 = hydrogen:
3.81 g ~0.01 mol) of t:he compound of Example 2
are dissolved in 150 ml of anhydrous dichloro-
methane and the mixture i~ cooled to -60C with
stirring in an ar~on atmosphere. 60 ml (0.06 mol)
of a 1 mol8r ~olution of boron trichloride in
n-hexane or dichlorQmethsne are then slowly
added, the temperature of the reaction mixture i8
slowly allowed to risQ to -40C to -20C, and the
mixture is ~tirred at thi6 tempera~ure ~or
3 hours and then at 10C for a further hour. The
mixture i8 coolad again to -6a DC ~ 60 ml of
methanol and 60 ml of dichl~romethane are 810wly
added dropwise and a ~olution is obtained which
is treated with 37 ml of triethylamine. The
solution i5 ~ubsequently addi~ionally ~tirred at
room temperature ~or 30 minute~ before the
, ~ ,,
- 48
reactîon mixture i~ comple~ely eYaporated.
Chromatography on ~ilica gel using a mixture of
dichloromethane/methanol 3:1 yields 1.32 g (44~4
of theory~ sf 2-acetyl-7-(1,3-dihydroxy~2-pro~
pox~methyl)guanine of melting point 155 - 158~C
(decompoæition).
H-NMR (270 NHz, d6-DNS0), ppm: 12.15 (6, lH)~
11.61 (~, lH), 8.37 (B, 1~), 5-77 t~ 2~)~ 4-61
(t, 2H), 3.62 (m, lH), 3.35 (m, 4~, 2.17 (s,
3H)~
8.~. Compound of the for~ula I in which Rl = hydrogen,
R2 = acetamido, R3 = hydroxymethyl, R4 = hydroxyl
and R5 = hydrogen:
According ~o the same method, 3065 g ~0.01 mol)
of the compound of Example 6.6. were reac~ed with
O.05 mol of boron trichloride and after cry tal-
lization from methanol yielded 2.2 g (~8.3% of
theory) of 2-acetamido-7-(1,3-dihydroxy~2-pro-
poxymethyl)purine of me;lting point 214 - 215~C.
lH-NMR (270 MHz, d6-DMS0), ppm: 10.43 (~, lH),
9.07 (~, lH), 8.72 (~, lH), 5.81 (s, 2H), 4.65
(t, 2H3, 3.55 - 3.28 (m, 5H), 2.20 (8, 3H).
8.3. Compound of the formula I in which Rl = hydrogen,
: R2 = acetamido, R3 = hydro~ymethyl, R4 = isopro-
poxy and R5 = hydrogen:
3 g of the compound of Examp~e 6.9. are heated
under reflux with 4 g of ammonium formate and 1 g
of palladium/carbon (10%) for 8 hour~ in 75 ml
of me~hanol. The reaction mixture i~ filtered,
concentrated, treatPd with acetone and ~tirred
until it crys~allizes. 0.9 g (57.44 of theory) of
2-acetamido--7-(l-hydroxy~3-i opxopoxy-~-propoxy-
methyl)purine of melting point 170C is obtained.
.. . . . .
~3~
49
H-NMR (270 M~z, d6-DMSO~, ppm: 10.43 (~, lH),
9.04 (s, lH), 8.72 (~, lH), 5.80 (m, 2~), 4.73
(t~ lH), 3.60 ~m, lH), 3.46 - 3.20 (m, 5H), 2.17
(~, 3H), 0.90 (m, 6H).
8.4. Compound of the formula I in which Rl = hydrogen,
R2 = acetamido, R3 - hydrogen~ R4 = hydroxyl and
R5 = hydrogen:
2.93 g (0.01 mol) of the compound from ~xample
6.8. are treated at -60C with boron trichloride
for 6 hours as in Ex~mple 8.1. After chromato-
graphy on silica gel using dichloromethane/-
methanol 5:1, 2.2 g (87.6~ of theory) of
~-acetzmido-7 (2-hydroxyethoxymethyl~purine of
melting point 194C are obtained.
15 8.5. Compound of the formula I in which R~ - hydrogen,
R2 = acetamido, R3 = hydrogen, R4 = hydroxyl and
R~ = hydro~ymethyl:
4.13 ~ (10 mmol) of the compound of Example 6.18.
are treated with boron trichloride as descri~ed
: 20 in Example 8.1. and after chromatographic purifi-
ca~ion on ~ilica gel (dichlorome~hane/methanol
3:1) yield 2.3 g (99.6% of theory) of 2-acet-
amido-7-(2,3-dihydroxy-1-propo~ymethyl)purine of
melting point 167 - 168C.
,
`
- 50 ~ 8 ~ ~ ~
Tabla 4
Example Rl R2 ~3 ~4 R5
2. OHNHC(O)CH3i:H20CH(cH3)2OC~(~H3)2
______ ___ _ ___ _ _______ _________ ~_; ____ __
3 .1.
5.ClNHC(O)CH3 CH2OC~cH3~2 OCH~H3)2
__ ___ _____ ______ _ ___ ______ ______ _ _
5.1. Cl NHC(O3CH3 CH~OCH2CH3 ~ H2~H3 H
___ ____ _ ________ _____ _ ____,____________ ___
5.2. Cl NHC(O)CH3 CEi2O(cH2)2c~3 o(~I2)2CH3
_ _ _ _ _ _ _ _ _ _ _ _ ~ _ _ _ ~ _ _ _ _ _ _
5 . 3 . Cl NHC(O)CH3 OCH(~H3~2 H
_____ ___ _ _ _ ___ ____ _ __ _ _
5 . 4 . Cl NHC ( O ) ~H3 CH20CH2C6H5 QCH ( ~H3 ) 2 H
_ _ _ _ .. _ _ _ _ _ _ _ _ _ _ ,. _ _ _ _
5. 5 Cl NHC(O)CH3CH2OCH3 OCH3 H
__ ..... ._ _ _ __ __ _ _ __
5 . 6. Cl NHC(O)CH3 CH~OCH~CH=CH2 ocH2cH ~H2 H
____ _ _ _ _ _ ___..... .._ _ _ ____ _ ___
5,7 Cl NHC(O)CH3 CH20-cyclopentyl O-cyclop~rltyl H
____~_ _ ________ __ _ _ __ _ _ _ ___ _
5 . 8 C 1 NHC ( ) ~;3 il OCH2c6H6 CH2 OCH ( CH3 ) 2
___ _ _ _____ __ _______ _ _ _ _ ___ __ _____
5.9 Cl (O)CH3 C}I2Oc(O)~(c~3)3 oc(33c(eH3)3 H
___ _ _ _____ __ ______________--__________ ___________ ____
5 .10 I::l NIIC(O)CH3 C~;j!OC~I2t 6~5 oc(o)t:tC:~33)3 ~3
__ ._ _.. ,~.. , __-- _--_------_--_--_--___--_--____________________
6.1. SH NHC(S)CH3 CH2OCH(CH3)~ OCH~CH3~2 H
___ _ ___ _____--___. _--___-- ___--________ __~ ______ __ __
6.2. SH NH2 CH2C)c~(CH3)2 OCX~t H3)2 H
__ _ ___ _ ________ ___ _____ ___________ _________ _
6.3. OCH3 NHC(O)~H3 C:H2OCH(~3)2 OCH(CH3)2
__ ___ _ __ __ _____ ____ ____ __ __
- ",~,
2 ~
-- 51 --
Continu,~tion of Table 4; ~ormula I
E~ample Rl R2 R3 R4 R5
6 . ~ . 1 . NH2 NHC ( O ) ~ H3 CH2 O~ 3 ) 2 OCH ( CH3 ) 2 H
_______________________________ ______________________~________
6 . 4 . 2 . NH2 ~H2 CH20~ H3 ) 2 O~H ( I: H3 ~ 2 H
_____________ _________________________________________________
6 . 5 . 1 . I~HCH3 NH~ ( O ) C~3 ~HZocH ( C~3 ) 2 OCH ( CH3 ) 2 H
_____________ _________________________________________________
6 . 5 . 2 . NHCH3 NH2 CH;~OCH ( CH3 ~ 2 OCH ( CH3 ~ 2 }I
_______ ______________________________.. _____________________,__
~ . 6 . H NHC ( O ) CH3 CH;~ OCH ( ~H3 ) 2 OCH ( CH3 ) 2 H
__ _ ____ ___________________________________________ __.. __
6 . 7 . H NH2 CH20~H ~ CH3 ) .2 OCH ~ C~3 ) 2 H
__ ____ ___________________________ _________________________
6 . 8 . H N~IC~O)CH3 H OCH(CH3)2 H
___ __ _______________________________________________________
6 . 9 . H NHC ( O ) CH3 CH20C H2C6H5 OCH ( CH3 ) 2 H
__ ___ _ ___ _______ ________________________________________
6.10. H NH2, CH20H Ol:~(CH3~2 H
__ __________________________________________________________
6 . 11 . ~I NH2 H OCH ( eH3 ) 2 H
__ __~__ _____________________________________________________
6.12. H NH2 CH20H O~I H
_______________________~._______________________________________
6 . 13 . 1 . OH OH CH20CH ( CH3 ) 2 OC:H ( CH3 ) 2 H
__________________________ ___________~_,______,_______________
6 . 13 . 2 . NH2 t)H CH;~OCH ~ CH3 ) 2 OCH ( CH3 ) 2 H
_____________________________________________~_________________ .
6 . 14 . H ~IHC ( O ) CH3 C:H20CH3 OCH3 H
_______________________________________________________________
6.15. H NH~:(O)CH3 cH2oe2H5 C2H5 H
_______ _______________________~____O__________~.. ______________
2~3~
~ontinuation of Table 4; formula I
Example Rl R2 ~3 ~4 R5
6.16. H N~2 CH20C~2C~=cH2 OCH2CH=CH2 H
____ _______ ____________ ___________________ _________________
6.17. H h~C(O)CH3 CH2O-cyclopentyl O-cyclopentyl H
________________________________ ____________________________
6.18. H MHC(O)CH3 H OcH2c6H5 C~2OC~(CH3)2 2
____________________________________,,_________________________
6.19. H NHC(O)CH3 CH20CH2C6H5 oC(O)C(CH3)3 H
_______ _ ______________________________ ______ __________
6.20. H NH2 CH20CH3 OCH3
_ _ _ _ _ _ _ _ ~:
6.21. X h~2 CH23C~H5 C2H5 H
___ ______ __ ____~______ ___ ________ ___________________
6.22. ~ h~2 CH20-cyclopentyl O-cyclopentyl H
_____ _ __ _______ __________._______________ __________
6.23. .- h-~2 X OH CH20H
___ _ _________ _____ _____ ___ ___________ __ ____
6.~. r. hH2 CH20C~2c6H5 OH
__ _ ____ _ ________ ____ _____ ___ _ ________
6 . 25 . Y. NHC(O)C~3 CX20C~2c6H5 OH H
~ _
5 ,5 H ~r:C(O)cH3 C~2OC(O)CH3 OC(0)CH3
___ _ ___ ____ ______ _ ____ _____ __ ._ ~__ _______
1 _ CH(CH3)~_NH2___ ~__ CH20CH(CX~)2 OCH(CH3)2 H
7.2. OC.-3 NH2 C~2ocH(cH3)2 oCH(CH3)2 H
______ __ __________________ ___________~._____ ___________
8.'. OH NHC(O)C-~3 CX2H OH H
___ ____ _ _ ___ __________ ______ ___
8.2. r. NHC(O)C~3 C~2H OH H
__ _______ _ _____ _ ____ __ __ ________
a.3. r. NHC(O)C~3 C~20C~(C~3)2 ~ ~
_________ __ __ _ ___ _ _ _ _ ____ ___
~ 8.~. H NXC(O)C.i3 H OH H
__ __ ____ _ ___________ __
8.5. L h~C(O)C~3 H 0H CH2~'
.. ______ _ _ _____ __ __ _ _______