Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
203~~~3
-1-
APPLICATION FOR PATENT
INVENTOR: Hector M. Lizama and Bruce M. Sankey
TITLE: BIOLOGICAL PROCESS FOR CONVERSION OF
HYDROGEN SULPHIDE
SPECIFICATION
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates to the desulphurization of
gases and, more specifically, relates to a micro-
s biological method for desulphurizing a gas stream
containing hydrogen sulphide.
2. Description of the Prior Art
Natural gas removed from a well frequently
contains undesirable levels of hydrogen sulphide.
Hydrogen sulphide is a toxic gas which must be removed
from the natural gas prior to marketing or combustion.
After removal, the toxic hydrogen sulphide must be
converted to a non-toxic product. Generally, this is
accomplished by the Claus reaction conversion of
hydrogen sulphide to elemental sulphur. However,
treatment of hydrogen sulphide in a Claus Plant is only
economical on a large scale. For treatment of natural
gas streams with low concentrations of hydrogen
sulphide, alternative technology is needed.
Natural microbes which oxidize hydrogen sulphide
exist. Some bacteria of the group Thiobacillus, such
as Thiobacillus thiooxidans and Thiobacillus
ferrooxidans, oxidize sulphide through the reaction:
S2 + 202 ----~ S042
22658/29/1-2-1-14/900
2o39gs3
-2-
These bacteria are most suitable for the oxidation of
hydrogen sulphide in an industrial process.
Thiobacillus thiooxidans is especially suitable being
biologically active at an extremely low pH (e. g. <1.0),
resulting from the fact that sulphuric acid is the
ultimate end-product of hydrogen sulphide oxidation.
Thiobacillus thiooxidans was discovered and named by
Waksmann & Joffe, Science LIII: 216, 1921. Bacterium
from the group Thiobacillus thiooxidans require reduced
sulphur compounds such as elemental sulphur or sulphide
as their sole source of energy.
Several microbial processes for the oxidation of
hydrogen sulphide have been described in the patent
literature. U.S. Patent No. 4,879,240 discloses a
process for controlling hydrogen sulphide production
using the bacterium Thiobacillus denitrificans under
aerobic conditions to oxidize hydrogen sulphide to
sulphate compounds. U.S. Patent No. 4,760,027
discloses the use of the bacterium Thiobacillus
denitrificans to desulphurize a gas stream by
converting hydrogen sulphide to sulphate compounds.
U.S. Patent No. 4,869,824 discloses an apparatus and
process for the biological purification of outgoing air
and waste water. U.S. Patent No. 4,723,968 discloses a
method for the purification of waste air containing
biologically decomposable impurities. U.S. Patent No.
4,179,374 discloses an apparatus for the treatment of
waste water by denitrification using faculative
organisms.
The aforementioned prior art methods are deficient
in that these methods do nat operate at a low pH. This
is a particularly desirable trait since sulphuric acid
is the end-product of hydrogen sulphide oxidation.
Thus, the need exists for a method operable at a low pH
which will convert hydrogen sulphide to non-toxic,
manageable products. Another benefit of a low PH
environment is the fact that most bacteria will not
22658/29/1-2-1-14/900
_ 3 _ 2Q39863
tolerate this condition, hence contamination from
undesirable bacterial species is virtually eliminated.
SUMMARY OF THE INVENTION
According to an embodiment of the present
invention, there is provided a microbiological process
for desulphurizing a gas containing hydrogen sulphide
comprising contacting said gas with a culture of the
bacterium Thiobacillus thiooxidans. This reaction
takes place in an acidic environment with the pH < 3
and oxidizes the sulphides to sulphur or sulphates.
Preferably, this reaction takes place in a
countercurrent flow by injecting gas in the bottom of
the chamber and injecting liquid bacterial medium in
the top.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is an illustration of the laboratory scale
flow scheme.
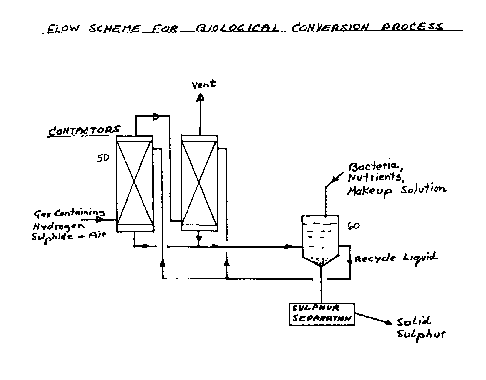
Fig. 2 is a flow diagram of the preferred
embodiment of the invention.
Fig. 3 is a plot showing improvement in bacterial
performance with successive exposures to hydrogen
sulphide.
Fig. 4 is a graph illustrating the difference in
rates of hydrogen sulphide removal and sulphate
formation.
Fig. 5 is a graph showing the effect of gas flow
rate on hydrogen sulphide removal.
Fig. 6 is a plot showing improvement in hydrogen
sulphide removal with time.
DETAILED DESCRIPTION OF THE INVENTION
The present invention utilizes a chemoautotrophic
bacterium to convert sulphides to sulphates. Although
several chemolithotrophic bacteria are capable of
oxidizing sulphides and using sulphur compounds as
22658/29/1-2-1-14/900
2039863
-4-
their source of energy, the bacterium Thiobacillus
thiooxidans has been discovered to be uniquely suitable
for achieving the objects of the present invention.
This chemolithotrophic bacterium is also
acidophilic, surviving only at a pH < 3. In addition,
Thiobacillus thiooxidans is capable of oxidizing
hydrogen sulphide gas in an oxygen-containing
environment and at room temperature. These unique
conditions and the use of the bacterium Thiobacillus
thiooxidans sets the present invention apart from the
prior art.
The method of culturing used in the present
invention is well known in the art. Any of the known
methods of culturing bacteria may be used. An example
of a suitable method would be to culture the bacteria
in a countercurrent contacter containing inert support
medium.
The culturing temperature is generally any
temperature that enables the bacteria to be cultured.
Generally, the temperature is in the range of about
10°C to about 50°C; preferably in the range of about
20°C to about 40°C.
The process of the present invention may be
conducted generally at any pressure that enables the
bacteria to be cultured and allows the bacteria to
desulphurize gas. Generally, the pressure is in the
range of from about 0 psig to about 100 psig;
preferably in the range of from about 0 psig to about
10 psig.
The support medium used in the present invention
is also well known in the art. Any inert material may
be used, e.g. glass beads, silica beads, ceramic chips,
etc., or any of the commercial media developed for the
application of biofilm growth.
The amount of bacteria used in the present
invention is from about 106 cells/ml to about 1010
22658/29/1-2-1-14/900
2039863
-5-
cells/ml; preferably, about 10$ cells/ml to about lOlo
cells/ml bacteria are used.
The pH of the aqueous solution is from about 0.8
to 3Ø Preferably, the pH is from about 2.0 to 2.5.
Fig. 1 is an illustration of the present
invention. Hydrogen sulphide is removed from the feed
gas stream 10 by any established techniques, such as
extraction or absorption, providing a purified gas
stream for use or sale. The concentrated hydrogen
sulphide stream is oxygenated and then fed to a
contacter 20 wherein the bacterium Thiobacillus
thiooxidans oxidize the hydrogen sulphide to either
sulphur or sulphate. Preferably, countercurrent
contact is used arid is accomplished by injecting the
gas stream into the contacter bottom 30 while the
aqueous bacterial medium is injected at the contacter
top 40, flows to the bottom and is recycled. Part of
the gas stream may be recycled around the contacter for
increased efficiency.
For commercial scale operation, 2 or more
contacters 50 could be connected in series for the gas
flow, as shown in Fig. 2. Sulphur could be recovered
continuously from the aqueous stream recycled out of
container 60 by a separation method, such as gravity
settling, filtration or centrifugation.
The following examples are given for illustrative
purposes only and are not meant to limit the scope of
the present invention.
EXAMPLES
Example 1
The experiment consisted of passing hydrogen
sulphide by way of a recirculating gas mixture through
a column packed with glass beads. The bacterial strain
SM-7 (Thiobacillus thiooxidans) was constantly
recirculated in the presence of a medium containing
O.lg KH2P04, 0.4g (NH4)2S04, 0.4g MgS04 ~ 7 H20 per
liter, adjusted to pH 2.3 with H2S04.
22658/29/1-2-1-14/900
X039863
-6-
Microbiologically-induced rates of hydrogen sulphide
removal were compared with spontaneous chemical removal
rates (control). Two columns with identical
air/hydrogen sulphide mixtures were run and hydrogen
sulphide levels were measured over time. Sulphide,
sulphate and pH were determined at the start and end of
the experiment. Bacterial viability was confirmed by
the growth of a sulphur flask inoculated with effluent
from the bacterial column. The results are summarized
in Table 1.
Table 1 Conversion of hydrogen sulphide to
sulphate by bacteria and control columns
Bacteria Control
H2S (mmoles) start 2.26 2.26
5042-(mmoles) start 1.84 0.84
end 3.33 0.78
pH start 1.67 3.00
end 1.44 7.70
The bacterial strain SM-7 oxidized hydrogen
sulphide to sulphate whereas the control column did
not. The decrease in pH seen in the bacterial column
further indicates that oxidation of sulphide is taking
place, producing sulphuric acid. This was not seen in
the control column. In addition, sulfur deposition was
observed in the bacterial column but not in the
control.
Example 2
A constant stream of hydrogen sulphide in air was
passed through a column packed with glass beads and
onto a scrubber. The bacterial strain SM-7
(Thiobacillus thiooxidans) was constantly recirculated
countercurrent to the gas flow. Microbiologically-
induced rates of hydrogen sulphide removal were
compared with spontaneous chemical removal rates
(control). Two columns with identical air/hydrogen
22658/29/1-2-1-14/900
aossss3
_7_
sulphide mixtures were run. Inflow and outflow
hydrogen sulphide levels were monitored at various
times. The results are summarized in Tables 2 and 3.
Table 2 Summary of initial trials with gas
flowthrouqh system using bacteria
Duration Gas Flow Hydrogen sulphide (ppm) Conversion
(hours) (mL/miny In Out lmmol,/hour~
0 15
16 15 3500 60 0.13
23 15 3500 55 0.13
Medium changed
88 20 3250 45 0.16
Sulphur deposit observed
94 50 3250 900 0.29
112 50 3250 150 0.38
Medium changed
117 50 3250 150 0.38
Volume of liquid was 70 mL, recirculated at a rate of
63 mL/min.
NM = not measured
Table 3 Control experiments showing chemical hydrogen
sulphide conversion rates using a gas
flowthrough system
Run Gas Flow Hydrogen sulphide (ppm) Conversion
~_m~~,~min)~ In out (mmol,/hour~,
1 23 8600 8200 0
2 18 8200 7800 0
3 18 8000 7800 0
Volume of liquid was 70 mL, recirculated at a rate of
63 mL/min.
Good hydrogen sulphide conversion rates were
observed (see Table 2) which were higher than those
seen in the gas recycle experiments. After only a few
days, sulfur could be seen accumulating at the bottom
of the column bed.
22658/29/1-2-1-14/900
X039863
_8_
Example 3
An experiment was run as per Example 1 but only
with the column containing the bacterial strain SM-7.
The column was run until all the hydrogen sulphide was
removed from the recirculating gas mixture, then hooked
up to a second gas reservoir and run as before without
changing the medium or reinoculating with bacteria. At
the end of the experiment bacterial activity was
confirmed by the growth of a sulfur flask inoculated
with effluent from the bacterial column. Consumption
of hydrogen sulphide took place in both runs. The
second run gave a faster hydrogen sulphide removal rate
(Fig. 3). As shown in Table 4, the bacterial oxidation
rate almost doubled from about 0.06 mmoles/hour to 0.10
mmoles/hour. This experiment involved recycling of
air/hydrogen sulphide gas mixtures using two 10 L gas
reservoirs sequentially. This is a clear indication of
adaptation by the bacterial strain SM-7 to hydrogen
sulphide, enabling the organism to oxidize it faster.
Table 4 Effect of subsequent exposure of bacteria
to hydrogen sulphide
Run 1 Run 2
Hydrogen sulphide (mmoles) start 2.46 2.50
end 0.01 0.01
Time required for H2S
removal (hours) 42 27
Bacterial oxidation rate 0.06 0.10
(mmoles H2S/hour)
A repetition of this experiment under identical
conditions gave similar results. As before, the
bacterial hydrogen sulphide oxidation rate doubled from
0.06 to 0.11 mmoles/hour (see Fig. 3). As in other
experiments, elemental sulphur could be seen in the
liquid medium.
22658/29/1-2-1-14/900
2U39863
_g_
Example 4
An experiment was run as per Example 1 but only
with the column containing the bacterial strain SM-7.
The column was run well past the point when all the
hydrogen sulphide was removed from the recirculating
gas mixture. This allowed all of the elemental sulphur
to be oxidized all the way to sulphate. Sulphate was
determined at the start of the experiment, once all the
hydrogen sulphide was gone, and at time intervals after
that. The bacterial hydrogen sulphide removal rate was
0.07 mmoles/hour and is comparable to past observed
rates. The formation of sulphate, however, was much
slower at 0.02 moles/hour. Thus, the rate of hydrogen
sulphide removal was 4 times that of sulphate formation
(see Fig. 4). This immediately suggests the
accumulation of an intermediate, namely elemental
sulfur which was visible in the column liquid.
A repetition of this experiment using twice the
usual volume of air/hydrogen sulphide gas mixture gave
similar results. the rate of hydrogen sulphide
oxidation was 0.20 mmoles/hour while the rate of
sulphate formation was only 0.05 mmoles/hour.
Examgl,e 5
An experiment was run per example 2 but with both
columns containing the bacterial strain SM-7. One
column contained glass beads that were 3 mm in diameter
while the other contained glass beads that were 5 mm in
diameter. Both columns were run in parallel, using the
same source of air/hydrogen sulphide gas mixture, the
same volume of liquid, and the same liquid flow rates.
Various gas flow rates were used and the corresponding
bacterial hydrogen sulphide removal rates were
determined. All the measurements were carried out
within 9 hours to minimize any effect of performance
improvement over time as seen in past experiments. The
results obtained are shown in Table 5 and Fig. 5.
22658/29/1-2-1-14/900
2039163
-10-
Table 5 Influence of gas flow rate on hydrogen
sulphide conversion rates
Column 1 Column 2
Gas rate Conversion Gas rate Conversion
(mL,/min) (mmoljhour) (mLlmin) (mmol,/hour)
12 0.08
18 0.16 18
23 0.19 23 0.10
34 0.28
38 0.28 38 0.14
38 0.12
100 0.26
Columns 1 and 2 contained 3 mm and 5 mm glass
beads, respectively. Each time the flow rate was
changed the system was allowed to equilibrate for at
least 30 minutes prior to taking a gas sample. The
entire experiment was carried out within 9 hours.
At similar gas flow rates the difference between
conversion rates correlated with the differences in
total reaction surface area for each column. With both
columns having the same bed volume, the difference in
glass bead size accounts for a difference in reaction
surface area by a factor of about two. Column 1 which
had almost twice the surface area as column 2 (3mm beds
versus 5 mm beads) also had about double the conversion
rate.
The rate of hydrogen sulphide removal increased
with gas flow rate. This is to be expected, up to the
point where mass transfer from the gas to liquid phases
no longer represents the rate-limiting step.
Examgle 6
An experiment was run as per Example 5 but
maintaining a constant gas flow rate through both
columns for several days in order to investigate the
variation in H2S conversion with time. The results are
shown in Table 6 and Figure 6.
22658/29/1-2-1-14/900
-11- ~~9863
Table 6 Performance of two columns with different
size cLlass beads
Column 1 Column 2
Duration Gas rate Conversion Gas rate Conversion
,Lhours) (mL/min~ (mmol,/hour) ~(mL~/minJi ~(mmol~hour)
0
1.5 12 0.09
34 0.25
16 12 0.09
10 21 34 0.26
23 12 0.10
24 34 0.25
45 34 0.29
46 12 0.11
15 63 34 0.30
64.5 12 0.11
Columns 1 and 2 contained 3 mm and 5 mm glass
beads, respectively.
For each column, conversion rates were observed to
increase with time. The bacterial activity (in this
case hydrogen sulphide conversion rate) is related to
their concentration and can be used to represent
population size. Bacteria reproduce by binary fission,
hence their populations increase exponentially. In
such cases the best way to describe the growth rate of
a bacterial population is its generation time: the
time required for the population to double in size. In
this case, the generation time would be the time
required for the hydrogen sulphide conversion rate to
double.
The rate increases with time were extremely slow
for both columns (see Fig. 6). Doubling times were
estimated at about 200 hours. These generation times
are extremely low; when grown in sulfur flasks,
Thiobacillus thiooxidans shows typical generation times
of 6 to 10 hours. Sulfur, however, is a much more
desirable substrate than hydrogen sulphide which must
dissolve in water in order to be accessible to the
bacteria. The main significance of this is that the
22658/29/1-2-1-14/900
2039863
-12-
majority of the active microorganisms in the columns
came from the original inoculum and not from growth.
This is a great advantage from a process standpoint
since the rate of biomass increase will be manageable.
Example 7
The experiment consisted of passing hydrogen
sulphide by way of a constant stream through two
columns arranged in series and onto a scrubber. Each
column had the bacterial strain SM-7 (Thiobacillus
thiooxidans) constantly recirculating in the present of
medium as per Example 1. Hydrogen sulphide was
supplied as a mixture with air. Column 1 containing 3
mm beads was first on line followed by column 2 packed
with 5 mm beads. Inflow and outflow hydrogen sulphide
levels were monitored upstream of column 1 (inflow) and
downstream of column 2 (outflow). The results are
summarized on Table 7.
Table 7 Total removal of hydrogen sulphide by columns
in series.
2 0 Column 1 Column 2 Total
H2S Gas Flow HZS Con- H S Con- Con-
Duration In Rate out version ~ut version version
Jhours) (vumZ (mL/minl fpm) (mmoJhr Lj,ppm) (mmol/hr) (mmol/hr)
0 3000 23 17 0.17 0 0.001 0.17
2 5 24.5 2100 58 330 0.25 0 0.05 0.30
95.5 2100 58 550 0.22 0 0.08 0.30
T a co umna were arranged n ser es.
Putting the two columns in series was successful
30 in removing all of the hydrogen sulphide from the gas
stream. Most of the hydrogen sulphide was removed in
column 1, but this is to be expected since the amount
of hydrogen sulphide entering column 2 was extremely
low to begin with. In a separate experiment the
35 positions of the columns were reversed to that the gas
stream passed through column 2 followed by column 1.
The results were analogous with those shown on Table 7
22658/29/1-2-1-14/900
209863
-13-
in that most of the hydrogen sulphide was removed in
the first column (column No. 2 in this case).
22658/29/1-2-1-14/900