Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02040913 1999-07-27
v
BACKGROUND OF THE INVENTION
Competitive isotopic and non-isotopic immunoassays
or binding assays with solid phase or second antibody
separations for measuring analytes in biological fluids, have
been described in the literature for over two decades.
Numerous United States and foreign patents have been issued
dealing with one or more aspects of these basic techniques.
Competitive enzyme immunoassays for various analytes
were disclosed in U. S. patent No. 3,654,090 to Wilhelmus et
al and 3,850,752 i~o Wilhelmus et al.
In enzyrne immunoassays the enzyme label is prepared
by one of several methods in which the analyte is covalently
attached to the enzyme and the free unreacted analyte is
separated from the: enzyme labeled analyte either by dialysis
and/or chromatography.
In such methods the free unconjugated enzyme is, in
most cases, not separated from the conjugated enzyme for two
practical reasons:
1. It requires tedious affinity purification and if
accomplished produces a very unstable conjugated enzyme-
analyte label since the latter constitutes a very low ratio of
the unconjugated enzyme.
2. The analyte specific binder bound enzyme, and not
the free unbound enzyme, is the fraction that is quantitated
after exhaustive washing of the free unbound enzyme.
1
68299-100
CA 02040913 1999-07-27
t r
It follows, therefore, that in the aforementioned
type assays the zero dose concentration has the highest signal
since there is no analyte to compete with the labeled analyte
for binding sites on the analyte's specific binder.
In competitive enzyme immunoassays the absence of a given
analyte in a sample produces the highest color while the
presence of a given analyte in a sample will produce
progressively less color as compared to the zero dose
depending on the concentration of said analyte in a given
sample. For qualitative, "on site" type assays whereby a
"yes" "no" answer is needed for the detection of a given
analyte in biological fluids, the type of competitive enzyme
immunoassays described in U.S. Patent No. 3,654,090 and
3,850,752 are unsuitable for obvious reasons: (1) the decrease
in color from a reference zero dose is difficult to detect by
the naked eye, and (2) washing is required to separate the
free unbound enzyme from the bound enzyme.
It is the object of the present invention to reverse
such a trend since' it is more logical to observe the presence
of color in samples containing a given analyte while negative
samples, samples devoid of a given analyte, produce no color.
U. S. Patent No. 3,817,837 to Rubinstein et al and
U. S. Patent No. 3,852,157 to Rubinstein et al and other
follow-up patents disclose homogenous type enzyme
amplification immunoassay for haptens where the separation of
2
68299-100
CA 02040913 1999-07-27
_.. i ,
antibody bound enzyme from free unbound enzyme is not
required. In said enzyme amplification assay system the
antibody-hapten enzyme labeled complex inhibits the enzyme
from reacting with its substrate since the active site on the
enzyme molecule is sterically hindered by the antibody to the
hapten. By contacting the antibody's hapten-enzyme complex
and the enzyme complex and the enzyme substrate with a sample
containing a given hapten to the antibody it competes with the
hapten-enzyme label for antibody sites thus allowing the
enzyme to react with its specific substrate. This technique
is limited to few enzymes specifically glucose-6-dehydrogenase
(U. S. Patent No. 3,875,011) malate dehydrogenase (U. S.
Patent No. 4,191,613) and U. S. Patent Nos. 4,203,802 and
4,067,774.
U. S. Patent No. 4,434,236 to Freytag discloses a
method for the rapid determination of analytes in biological
specimens by usinc3 an analyte-analogue immobilized on a solid
phase wherein a displaceable labeled antibody to the analyte
is found. In thi:a disclosed method the antibody has a greater
affinity for the analyte than for the analyte-analogue. The
presence of an analyte in a sample specific for said antibody
will easily displace the labeled antibody. Consequently, the
amount of displaced labeled antibody is related to the amount
of analyte present: in the sample.
3
68299-100
CA 02040913 1999-07-27
Although this method of U. S. Patent No. 4,434,236
is an improvement over the previously cited patents it relies
on two important factors; namely, (i) the use of an analyte-
analogue that has a lower affinity to the analyte's labeled
antibody, and (ii) the immobilization of analyte-analogues,
especially small compounds (such as haptens), on a solid
support is not easily achieved and requires specific
functional groups on the analyte-analogue in order to affect
immobilization. Furthermore, since the affinity of the
analyte-analogue to the analyte's antibody is purposely low,
the changes of labeled antibody leaking off the solid phase is
quite probable.
U. S. Patent No. 4,446,232 to Liotta, is similar in
context to that disclosed in U. S. Patent No. 4,434,236,
wherein a given antigen is impregnated in a given matrix in
the first zone of the disclosed device. In said matrix
containing a given antigen, an enzyme-linked antibody is
reacted to said antigen. In the presence of antigen in a
biological specimen the antibody is displaced into a second
zone which contains materials capable of reacting with the
enzyme linked antibodies to produce a color. Determination of
antibodies in biological fluids is also disclosed by Liotta
wherein the antibody is impregnated in first zone and reacted
with enzyme-linked, antigen. Although this approach is a
simple modification of Freytag's approach, it suffers several
4
68299-100
CA 02040913 1999-07-27
v
technical drawbacks; (1) the impregnation of antigens or
antibodies in the first zone of Liotta's device will be prone
to antigen or antibody "leakage" in the absence of patient
antigens or antibc>dies, (ii) the affinity of enzyme-linked
antibody or antigen is not defined. The competition between
sample antigen and impregnated antigen in the first zone to
the enzyme-linked antibody is by no means instantaneous
because of steric hindrance and the ability of the sample
antigen, like hCG in Example 1 of said patent, to dislodge the
enzyme-linked antibody from the impregnated antigen is highly
improbable because: of steric hindrance and equilibrium
considerations.
It is well established in the art that large
molecules (greater than 20 kilo daltons), require longer
incubations with their specific binders to reach equilibrium.
When the specific antibody (molecular mass 150 kilo daltons)
is linked to an enzyme (molecular mass greater than 50 kilo
daltons), i.e., the effective molecular mass of the enzyme-
linked antibody is approximately 200 kilo daltons, and said
enzyme-linked or antigen is not defined. The competition
between sample antigen and impregnated antigen in the first
zone to the enzyme-linked antibody is by no means
instantaneous because of steric hindrance and the ability of
the sample antigen, like hCG in Example 1 of said patent, to
dislodge the enzyme-linked antibody from the impregnated
5
68299-100
CA 02040913 1999-07-27
S T
antigen is highly improbable because of steric hindrance and
equilibrium considerations.
It is well established in the art that large
molecules (greater than 20 kilo daltons), require longer
incubations with their specific bonders to reach equilibrium.
When the specific antibody (molecular mass 150 kilo daltons)
is linked to an enzyme (molecular mass greater than 50 kilo
daltons), i.e., the effective molecular mass of the enzyme-
linked antibody is approximately 200 kilo daltons, and said
enzyme-linked antibody is pre-reacted with an antigen (now
molecular mass is approximately 220 kD) the patient antigen
(20 kD) will require time to compete with the pre-reacted
antigen bound to t:he enzyme-linked antibody and displace it.
This is quite obvious from the following equilibria.
kl
E-Ab + Agl ~ E-Ab=Agl
k2
and
k3
E-Ab + Agl + Agl ~--. E-Ab~~Agl + Agl
k4
where kl = k2; k3 - k4 and kl » k3 because of steric
hindrance and dish>lacement of Agl from E-Ab Agl by Agl is not
6
68299-100
CA 02040913 1999-07-27
easily achieved as disclosed in U. S. Patent No. 4,446,232.
Furthermore, k3 > k4 only if the concentration [Agl] » [Agl]
that displacement will occur. This means that the amount of
Agl measured will only be at high concentrations; therefore,
low sensitivity assay.
This faces is exemplified in European Patent
Application No. 0 279 097 in which Fuerstenberg shows that
Liotta's disclosure in U. S. Patent No. 4,446,232 is quite
insensitive as shown in Example 1 of EPA 0 279 097 for
theophylline were the reflectance difference between
theophylline levels of 10.4 ug/ml (therapeutic threshold) and
19.4 ug/ml (toxic threshold) is 0.55 - 0.43 or 0.12 units.
Similarly, in Example 2 of EPA 0 279 097, 200 mIU hCG was
required to produce a change in color and displace the enzyme-
linked hCG antibody. By all analytical standards the Liotta
disclosure and the examples cited in EPA 0 279 097 using
Liotta's method show that said method as disclosed in U. S.
Patent No. 4,446,.?32 is not sensitive enough to be a reliable
analytical tool.
Other United States and foreign patent
specifications and applications dealing with elements for the
determination of biological fluids are cited here for
completion.
7
68299-100
CA 02040913 1999-07-27
r r
U. S. Patent Nos. 4,144,306; 4,366,241; 4,740,468;
4,774,192; 4,632,901; 4,774,174; 4,769,333; 4,769,216;
3,811,840 and 4,042,335.
European Patent Specification Nos. 0 042 755; 0 070
300 and European Patent Applications 0 281 201; 0 284 232 and
International Applications W084/029193 and W088/06723
8
68299-100
CA 02040913 1999-03-24
SD~ARY OF THE INVENTION
Briefly, the present invention comprises a method
for measuring analytes in biological fluids wherein a specific
binder (Ab) to a given analyte (Agl) is covalently immobilized
on a solid phase support to which a labeled analyte (Agl*) is
prereacted to saturate almost all binding sites on said
specific binder to form an immobilized specific binder-analyte
labeled complex, ~ - Ab ~ Agl*, which method comprises
contacting a sample of biological fluid to be analyzed with
said immObiliZed complex wherein an analyte (Agl), if present
in said sample, competes with the labeled analyte (Agl*) bound
to the immobilized binder for binding sites on said binder
thus displacing a given amount of labeled analyte (Agl*) which
is directly proportional to the amount of analyte (Agl)
present in the sample.
More specifically, the present invention provides a
method for measuring analytes in biological fluids which
comprisess
(1) covalently immobilizing a specific antibody binder
to a given analyte on a solid phase supportf
(2) saturating the binding sites on said specific
antibody binfi3er With a labeled analyte to the extent steric
hinderance permits to form an immobilized specific antibody
binder-analyte labeled complex
(3) saturating remaining unoccupied binding sites on the
immobilized specific antibody binder-analyte labeled complex
with unlabeled analyte prior to contacting said complex with a
sample of biological fluid
- g -
68299-100
CA 02040913 1999-03-24
(4) contacting a sample of biological fluid to be
analyzed for the presence of the given analyte With said
immobilized complex, said sample of biological fluid as
contacted with said immobilized complex being in an untreated
form as obtained from the donors and
(5) allowing an analyte, if present in said sample, to
compete with the labeled analyte bound to the immobilized
binder for binding sites on said binder thus displacing a
given amount of labeled analyte Which is directly proportional
to the amount of analyte present in the sample,
wherein the affinity of the analyte to the analyte~s
specific binder is at least about 10~ 1/mol and is higher than
the affinity of the labeled analyte to the same binder and
Wherein the analyte has a molecular Weight greater than 20 kD.
The present invention also provides a method for
measuring analytes in biological fluids which comprisess
(1) covalently immobilizing a specific lectin binder to
a given analyte on a solid phase support
(2) saturating the binding sites on said specific lectin
binder with a labeled analyte to the extant steric hinderance
permits to form an immobilized specific lectin binder-analyte
labeled complex
(3) saturating remaining unoccupied binding sites on the
immobilized specific lectin binder-analyte labeled complex
Wlth unlabeled analyte prior to contacting said complex with a
sample of biological fluid
(4) contacting a sample of biological fluid to be
analyzed for the presence of the given analyte with said
_ 9a _
68299-100
CA 02040913 1999-03-24
immobilized complex, said sample of biological fluid as
contacted with said immobilized complex being in an untreated
form as obtained from the donors and
(5) allowing an analyte, if present in said sample, to
compete with the labeled analyte bound to the immobilized
binder for binding sites on said binder thus displacing a
given amount of labeled analyte which is directly proportional
to the amount of analyte present in the sample,
wherein the affinity of the analyte to the analyte~s
specific binder is at least about 10~ 1/mol and is higher than
the affinity of the labeled analyte to the same binder, and
wherein the analyte has a molecular weight greater than 20 kD.
The present invention also provides a diagnostic
device for measuring analytes in samples of biological fluids
which comprises:
(1) a column-type assembly defining a fluid pathway
having an open end adapted to receive a sample of biological
fluid to be analyzed, said fluid pathway being bridged by a
first solid phase support, and an effluent discharge point on
the lower and of said column-type assembly, opposite said open
end f
(2) a sleeve-type container having an open end and a
closed end, said column type assembly being received in said
open and of said sleeve-type containers
(3) a specific antibody binder covalently immobilized to
a given analyte on said first solid phase support, the binding
sites on said specific antibody binder being saturated with a
labeled analyte to the extent steric hinderance permits to
- 9b -
68299-100
CA 02040913 1999-03-24
form an immobilized specific antibody binder-analyte labeled
complex, remaining unoccupied binding sites on the immobilized
specific antibody binder-analyte labeled complex being
saturated with unlabeled analyte prior to contacting said
complex With a sample of biological fluid, said solid phase
support being adapted to have displaced therefrom a given
amount of labeled analyte which is directly proportional to
the amount of analyte present in the sampler and
(4) a second solid support, spaced apart from said first
solid phase support, housed at the closed end of said sleeve-
type container and in proximity to said effluent discharge
point, said second solid support, when contacted by the
displaced labeled analyte, being adapted to produce a visible
color either directly or after the addition of a substance
capable of reacting with the labeled analyte to produce a
visible color.
The present invention also provides a diagnostic
device for measuring analytes in samples of biological fluids
which comprises:
(1) a column-type assembly defining a fluid pathway
having an open end adapted to receive a sample of biological
fluid to be analyzed, said fluid pathway being bridged by a
first solid phase support, and an effluent discharge point on
the lower end of said column-type assembly, opposite said open
end,
(2) a sleeve-type container having an open and and a
closed end, said column type assembly being received in said
open and of said sleeve-type container,
- 9c -
68299-100
CA 02040913 1999-03-24
(3) a specific lectin binder covalently immobilized to a
given analyte on said first solid phase support, the binding
sites on said specific lectin binder being saturated with a
labeled analyte to the extent steric hinderance permits to
form an immobilized specific lectin binder-analyte labeled
complex, remaining unoccupied binding sites on the immobilized
specific lectin binder-analyte labeled complex being saturated
with unlabeled analyte prior to contacting said complex with a
sample of biological fluid, said solid phase support being
adapted to have displaced therefrom a given amount of labeled
analyte which is directly proportional to the amount of
analyte present in the samplef and
(4) a second solid support, spaced apart from said first
solid phase support, housed at the closed and of said sleeve-
type container and in proximity to said effluent discharge
point, said second solid support, when contacted by the
displaced labeled analyte, being adapted to produce a visible
color either directly or after the addition of a substance
capable of reacting with the labeled analyte to produce a
visible color.
This invention also comprises a diagnostic device
for measuring analytes in samples of biological fluids which
comprisess
a column-type assembly defining a fluid pathway having an
open end adapted to receive a sample of biological fluid to be
analyzed, said fluid pathway being bridged by a first
- 9d -
68299-100
CA 02040913 1999-07-27
solid phase support, and an effluent discharge point on the
side of said support opposite said open end,
a sleeve-type' container having an open end and a closed
end, said assembly being received in said open end of said
sleeve-type container,
a specific binder (Ab) covalently immobilized on said
solid phase support to which an analyte label (Agl*) is pre-
reacted to saturate almost all binding sites on said binder to
form a first solid phase specific binder-analyte label
complex, ~Ab~Agl*, said solid phase complex when contacted
with a biological fluid sample containing a specific analyte
(Agl), being adapted to have displaced therefrom labeled
analyte (Agl*) in an amount directly proportional to the
concentration of Agl,
a second solid support, spaced apart from first solid
phase support, housed at the closed end of said sleeve-type
container and in proximity to said effluent discharge point,
said second solid support, when contacted by the displaced
labeled analyte (Agl*) form the effluent discharge point said
first solid phase complex, being adapted to produce a visible
color on said second solid support either directly or after
the addition to said second solid support a substance capable
of reacting with the analyte label to produce a visible color.
68299-100
CA 02040913 1999-07-27
In accordance with the present invention a method
and diagnostic device are disclosed for the determination of
analytes in biological fluids wherein a specific binder for a
specific analyte is covalently immobilized onto a solid
support, preferably microparticles but not restricted to same,
to which a labeled. specific analyte is pre-reacted whereby all
binding sites on the specific binder are completely occupied
with the labeled a.nalyte and in certain instances the binding
sites are saturated with a combination of the labeled analyte
and unlabeled analyte. Determination of a given analyte
proceeds according to the following, preferred but not
restricted to, analytical steps: (a) admixing a biological
sample, suspected of containing a given analyte, with a
diluent in a separate tube; (b) pouring the analyte/diluent
mixture onto a diagnostic device, as illustrated in the
drawings, whereby the analyte/diluent is contacted with the
immobilized binder-analyte label complex; (c) the resultant
reaction mixture flowing through the solid phase microparticle
bed is contacted with a substrate specific for the displaced
label and a colored product is developed in which is directly
proportional to th.e amount of analyte present in the sample.
The affinity of a specific analyte to its specific
binder is higher than the affinity of the specific analyte-
label to the given binder.
11
68299-100
CA 02040913 1999-07-27
1
DETAILED DESCRIPTION OF THE INVENTION
Turning to the drawings,
Figure T_ is a side plan view of the column assembly
present in the device of this invention.
Figure 2 is a sectional view of the sleeve-type
container used in the device of this invention.
Figure .3 is a sectional view of the device of this
invention showing the elements of Figures 1 and 2 as fully
assembled and ready to receive a sample of biological fluid at
the top or open end.
Figure ~6 is a top plan view of the device of Figure
3.
Turning to the drawings in more detail, the method
of the present invention is conducted in a novel self-
contained diagnostic device in which a specific binder is
covalently immobi7.ized on microparticles and reacted with a
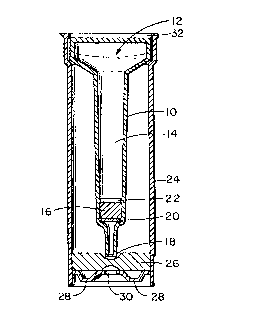
specific labeled analyte and placed in a column assembly 10
which includes a chromatographic-type compartment. The
assembly 10 has an open end 12 to receive the sample of
biological fluid t:o be analyzed. The fluid pathway 14 runs
longitudinally of the assembly 10. The solid phase support 16
bridges the assembly 10. An effluent discharge point 18 is at
12
68299-100
CA 02040913 1999-07-27
the lower end. The solid phase support 16 separated by two O-
ring type frits 20 and 22 made of a porous substance. The
assembly of Figure 1 has a funnel-shaped cross-section and is
inserted in an opaque outer-sleeve type container 24 shown in
Figure 2 that houses a lcm porex*-type material 26 inserted at
the bottom of the outer-sleeve 24. The porex*-type material
26 is used to absc>rb excess sample/diluent volume. The base
28 of the outer-sleeve 24 is also opaque but contains in the
center of it a thin (approximately 10 mm) clear white
absorbent element 30 wherein the displaced labeled analyte is
concentrated either through simple absorption and/or through a
secondary immunological reaction involving label-antilabel or
analyte-ligand anti-ligand. The colored product of the label
is thus formed on the surface of clear absorbent 30 either
through direct reading of the label, in the case where the
label is a colored! dye or a colored latex particle, or through
the reaction of the label with its substrate, in the case
where the label is~ an enzyme, or through the reaction of the
label with its enzyme, in the case where the label is a
substrate.
It is to be understood that the open end 32 of the
outer sleeve 24 ma.y be provided with a sealing cap or closure
held by a force or interference fit.
*Trade-mark
13
68299-100
CA 02040913 1999-07-27
Certain conditions have to be met in order to
achieve maximum sensitivity for a given analyte when measured
by the method of the instant invention.
The following conditions are set forth to fulfill
the requirements of the present invention: Reactions (1) and
(2) below describe the equilibria involve in such a method
kl
~Ab + Agl* ~ ~Ab Agl* (1)
k 92
and
k3
~Ab + Agl * + Agl ~- ~Ab Agl * + Agl ( 2 )
k4
where ~-Ab is the specific analyte binder covalently
immobilized on solid support
Agl* is the labeled analyte
Agl is the unlabeled analyte to be measured.
The affinity constant for reaction (1) above is
K1; K1 = k2 where
k
1
14
68299-100
CA 02040913 1999-07-27
k2 > kl, i.e. the labeled analyte should have a fairly high
affinity to the specific analyte binder and the reaction~is
always favored to the direction of the binder-analyte label
complex where
K1 = k2
kl
The affinity constant for reaction (2) above is
K1; K1 = k4 where
k3
k3 > k4, i.e. the analyte should have a higher affinity to the
specific binder than does the labeled analyte, thus k3 > kl.
In other words, the affinity of the unlabeled analyte to the
analyte's specific: binder is at least about 10~ 1/mol and
should be greater than the label analyte has to the same
binder.
For large molecular mass analytes (>20 kD) k3 could
be made greater than k4 by two different analytical
manipulations, either separately or combined:
1) Increas'i.ng the concentration of the enzyme labeled
analyte [Agl*] that is bound to immobilized specific binder in
68299-100
CA 02040913 1999-07-27
reaction (1), supra, to a point of maximum saturation wherein
there exists no binding sites unoccupied on the specific
binder. Therefore, shifting the equilibrium to the right in
reaction (1), supra, where kl > k2. This should be
accomplished withc>ut creating a steric hindrance situation on
the solid phase. This is possible by diluting the solid
phase, e.g. sepharose, with sepharose that does not contain
the specific binder thus spacing the immobilized specific
binder entities within said solid phase matrix far apart to
avoid steric hindrance.
The addition of a minimal concentration of Agl
should easily displace the label analyte Agl* from the
immobilized specific binder; therefore, k3 > k4 and a signal
is produced which is directly proportional to the
concentration of added analyte [Agl].
2) Binding sites unoccupied on the immobilized specific
binder ~Ab by the labeled analyte Agl* could cause low
sensitivity assays because the addition of analyte, Agl, from
a biological specimen will first bind to these unoccupied
sites on the specific binder and not displace the labeled
analyte. By saturating these sites with "cold" analyte prior
to the assay, the addition of analyte from a biological
16
68299-100
CA 02040913 1999-07-27
specimen will dish>lace enzyme labels analyte at low
concentrations, thus a high sensitivity assay especially~since
kl > k2 as explained, supra. A combination of 1 and 2, supra,
could even yield sensitivities in the sub-nanogram range as
will be shown in t:he instant examples of the invention.
Multiple analytes could be screened using the method
and diagnostic device of the present invention by admixing
several immobilized specific binders to various analytes in
their respective appropriate dilutions; thus, the solid phase
support will contain multiple specific binders to which a
specific analyte-labeled has been pre-reacted and stabilized
~Abl ", Agl*
~~2 ~ Ag2
~Ab3 ,~, Ag3
~Abn ~" Agn*
where ~Abl to ~P,bn are various immobilized specific binders
to which a specific analyte-labeled Agl* to Agn* have been
pre-reacted and stabilized. The mixture containing the
various immobilized pre-reacted specific binders-analyte
labels could now serve as one reagent for screening several
analytes in one given specimen without any loss of
sensitivity. This is particulary useful for drug screening
17
68299-100
CA 02040913 1999-07-27
programs where multiple drugs could be screened on a given
specimen. A positive result using said multiple approach
could then be confirmed using the single analyte approach.
Screening for other types of analytes using the
disclosed invention should be obvious to those skilled in the
art.
In the following examples the conjugation and
labeling methods used are well-known in the art and are
presented here for' illustrative purposes only and should not
be restrictive to the practice of the instant invention.
Other conjugation and labeling procedures could easily be used
by those skilled in the art. Furthermore, the types of solid
phase used to immobilize the analyte's specific binder and the
type of enzyme or other label used are not restrictive and are
obvious to those skilled in the art.
The analyte's specific binders used in the following
examples were antibodies, polyclonal or monoclonal, raised
against various analytes. The analyte's specific IgG from
these various antibodies was routinely purified by Protein-A
chromatography using immobilized recombinant Protein-A
(Repligen, Cambridge, MA 02139) following well-known
established procedures.
18
68299-100
CA 02040913 1999-07-27
The Protein-A purified specific IgG was covalently
bound to cyanogen bromide activated sepharose* 4B by the'
method of March, C'.S. et al., 1974. Anal. Biochem. 60:149-152.
Macromolecular antigens (molecular mass greater than
20 K.D.) were enzyme (horseradish peroxidase) labeled using
the periodate method of Boorsma, D.M., et al (1979). J.
Immunol. Meth. 30:245-255.
Haptens were coupled to horseradish peroxidase
enzyme using the carbodiimide method of Staros, J.V. (1986).
Anal. Biochem. 156:220-222.
The device can be built as an integral unit or
alternatively elements 10 and 24 can be assembled at the time
of use.
The following Examples are illustrative of the
invention, and are not intended to be limiting in any way.
EXAMPLE 1
MORPHINE ASSAY IN URINE
Morphine-3-glucuronide was conjugated to horseradish
peroxidase enzyme using a modification of the method of Staros
et al, 1986, supra, as follows:
(1) 2.95 mg of morphine-3-glucuronide (6.4 x 10-3) mmol)
were dissolved in 0.5 ml of normal saline to which 20 mg of
horseradish peroxidase (RZ > 3) dissolved in 3.0 ml of normal
saline were added.
*Trade-mark
19
68299-100
CA 02040913 1999-07-27
(2) 15 mg oi= N-hydroxysuccinimide was added to the
mixture in (1) and stirred until it was dissolved to which 40
mg of EDC (N-ethyl-N-(3-dimethylaminopropyl) carbodiimide)
dissolved in 0.5 ml normal saline was added dropwise over 30
minutes.
(3) The reacaion mixture in (2) was stirred for an extra
60 minutes at ambient temperature and a further 10 mg EDC
added as powder. The reaction mixture was stirred overnight.
(4) The enzyme conjugate was then dialyzed for 48 hours
against 2 x 5 liters of phosphate-buffered saline and finally
charcoal absorbed twice using 20 mg activated charcoal in an
ice bath and filtered through 0.22 micron filter. 40 mls of
morphine antibody (polyclonal) was purified on Protein-A
column as described, supra, to yield 378 mg IgG which was then
conjugated to cyanogen bromide activated sepharose 4B as
indicated to yield 3.6 mg IgG/ml of gel, and diluted at an
appropriate dilution in unreacted sepharose 4B in the ratio of
1:100 (1 part morphine antibody coated gel to 100 parts
unreacted sepharo~;e gel). The working gel could be stored in
phosphate-buffered saline solution containing 0.025 (w/v)
sodium azide at 4°C for extended time periods without any loss
or "leakage" of Ig~G.
68299-100
CA 02040913 1999-07-27
Binding of morphine-horseradish peroxidase conjugate
to the solid phase morphine antibody was accomplished as~
follows:
The diluted morphine antibody (IgG)-sepharose gel
was washed with a diluent prepared in 0.05 M phosphate buffer
pH 7.0 containing 0.01 (w/v) thimerosal, 0.2~ (w/v) alkali
treated casein, O,.l~s (w/v) charcoal absorbed human serum
albumin and 20 ug/ml gentamicin sulfate, and was reconstituted
such that 2 ml of gel suspension in said buffer contains 1 ml
of settled gel volume. The morphine-enzyme conjugate was then
added to the morphine-antibody gel suspension to give a final
concentration of 7.:1000 and the mixture was incubated for 60
minutes at ambient: temperature on a rotary mixer. This
process causes the' binding of morphine-enzyme label to the
immobilized morphine specific antibody on the sepharose.
Unbound or free enzyme conjugate is washed off the solid phase
with the 0.05 M phosphate buffer, pH 7.0 diluent. The washed
gel containing they immobilized morphine antibody-morphine
horseradish peroxi.dase complex is transferred to the
diagnostic device (Figure 1) as described, supra, and each
device now contains 250 ul of settle gel (3, Figure 1). Few
millilitres of diluent are passed through the gel to ensure
21
68299-100
CA 02040913 1999-07-27
that no more enzyme elutes from the gel. The gel containing
the immobilized antibody-analyte label complex could be stored
at 4°C either lyopholized or suspended in the 0.05 M
phosphate, pH 7.0, diluent in the diagnostic device until
used.
The affinity constant of morphine to the morphine
antibody was calculated by the method of J. D. Teale
("Radioimmunoassa;r". In David Williams, Ronald Nunn, Vincent
Marks (eds), Scientific Foundations of Clinical Biochemistry,
Vol. 1, 1978:299-.322, Pub. William Heinemann, London) and
found to be 4.a x 1011 1/mol. The affinity constant of
morphine-3-glucuronide to the same antibody was also
calculated by the same method and found to be 8 x 1010 1/mol.
Description of the Tnlorkiag Model
The diac3nostic device once assembled will contain
250 ul of suspended gel (16) containing the immobilized
antibody-analyte label complex in the column assembly 10 part
of the device, Figure 1, and protected on each side by two
porous frits 20 and 22. The inner part of device, the column
assembly 10, is housed in the outer-sleeve 24 shown in Figure
2, as described, supra. 126 samples were analyzed to
determine the presence or absence of morphine and/or opiates
using the described diagnostic device and reagents as follows:
22
68299-100
CA 02040913 1999-07-27
1. 2 drops of urine were added to 900 ul of sample
diluent (0.05 mg/rnl of tetramethylbenzidine in phosphate
buffered saline, pH 7.4) to give an approximate 1:10 (v/v)
dilution of sample'.
2. The contents of sample plus diluent were poured onto
the top 12 of the diagnostic assembly 10.
3. The: device was inverted and one drop of
peroxide at a concentration of 0.02% in citrate/phosphate
buffer, pH 5.0, was added to the clear white absorbent element
(7). After 5 minutes color was observed and recorded: white =
negative; blue = positive.
The results of the above assays showed that out of
the 126 samples analyzed 71 samples were negative and 55 were
positive. When compared against a sensitive RIA assay for
morphine (Coat-a-C'ount* Morphine kit Diagnostic Products
Corporation, Los P.ngeles, CA 90045) 100% agreement was
achieved. The positive samples had morphine RIA values
ranging from 162 n.g/ml to 116,200 ng/ml. All 71 negative
samples were from known volunteers not taking any drugs.
The Coat-a-Count* RIA assay had a cut-off of 25
ng/ml for free morphine. The morphine assay of'the present
invention is set at a cut-off of 300 ng/ml for morphine-3-
*Trade-mark
23
68299-100
CA 02040913 1999-07-27
glucuronide and 50 ng/ml cut-off for free morphine. Levels
below 300 ng/ml of morphine-3-glucuronide or 50 ng/ml free
morphine will not produce any visible color.
The fol7.owing compounds did not produce any visible
color when assayed in the morphine method of the present
invention at concentrations of 10,000 ng/ml:
oxazepam
cotinine
caffeine
acetaminophen
PCP
acetylsalicylic acid
secobarbital
amphetamine
cocaine
fentanyl
LSD
bupreneorphine
lidocaine
ibuprofen
fenfluramine
D-propoxyphene
methaqualone
24
68299-100
CA 02040913 1999-07-27
benzoylecogonine
methadone
EXAMPLE 2
PHENCYCLIDINE (PCP) ASSAY IN URINE
Phencyclidine (PCP) derivative, 1-[1-(phenyl-3-0-
car-boxymethyl eth.er)-cyclohexyl] piperidine, synthesized
according to the methods of Kalir, A., et al, 1969, J.Med.
Chem. 12:473 and R.ao, P.N. et al, 1980, J. Steroid Biochem,
13:1291, was conjugated to horseradish peroxidase by the
method of Staros, et al 1986, supra, as outlined in Example 1,
supra, except that the PCP derivative was dissolved in
dimethylformamide instead of normal saline.
mls of PCP antibody (polyclonal) were purified on
Protein-A column as described, supra, to yield 254 mg IgG
which was then conjugated to cyanogen bromide activated
sepharose 4B as indicated to yield l.3 mg IgG/ml of gel and
diluted at an appropriate dilution in unreacted sepharose 4B
in the ratio of 1:5 (1 part PCP antibody coated gel to 5 parts
unreacted sepharose gel). The diluted gel is stored as
20 indicated in Example 1, supra. PCP-horseradish peroxidase
conjugate was bound to the PCP antibody diluted gel after a
dilution of 1:1000 in the phosphate buffer, pH 7.0, diluent as
described under example l, supra. The gel containing the
68299-100
CA 02040913 1999-07-27
immobilized PCP antibody-PCP horseradish peroxidase label was
first washed with 0.02 M citrate/acetate buffer, pH 5.O,~then
rewashed with phosphate buffer, pH 7.0, diluent to remove
unbound or free enzyme conjugate. The washed pre-reacted gel
was transferred to the diagnostic devices, as in Example 1,
supra.
The affinity constants of PCP and horseradish
peroxidase PCP to the PCP antibody were calculated by the
method of Teale, 1978, supra, and were determined to be 1.4 x
1012 1/mol for PCP and 3.0 x 1010 1/mol for horseradish
peroxidase-PCP conjugate.
Seventy-one urine specimens were analyzed for PCP
using the described reagents and diagnostic device of the
present invention, as indicated for morphine in Example 1,
supra, and compared to a sensitive RIA PCP method (Coat-a-
Count PCP, Diagnostic Products Corporation, Los Angeles, CA
90045) .
50 samples were from known PCP addicts and were RIA
positive at a cut-off of 25 ng/ml. All 50 samples were also
positive by the method of the instant invention at a cut-off
of 50 ng/ml. All 21 negative samples were correctly
identified.
26
68299-100
CA 02040913 1999-07-27
The positive samples had PCP RIA values ranging from
151 ng/ml to 2672 ng/ml.
The following drugs gave negative results, no
visible color, when assayed in the PCP method of the present
invention at concentrations of 10,000 ng/ml
Ethyl morphine
morphine
methaqualone
cotinine
secobarbital
lidocaine
normorphine
diazepan
D-propoxyphene
phenobarbital
acetominophen
acetylsalicylic acid
amphetamine
benzoylecgonine
bupreneorpine
caffeine
ecgonine
codeine.
27
68299-100
CA 02040913 1999-07-27
The folJ.owing drugs gave positive results
(equivalent to 100 ng/ml PCP) at the concentrations indicated:
1- [1- (2-Thienyl) -c:yclohexyl] piperidine 100 ng/ml
1-(1-Phenylcylcohexyl)-4-hydroxypiperidine 1000 ng/ml
N-Ethyl Phencyclidine 10,000 ng/ml
EXAMPLE 3
URINARY HUMAN ALBUMIN ASSAY
Human albumin was conjugated to horseradish
peroxidase by the periodate method of Boorsma et al, 1979,
supra.
Monoclonal antibody raised against human albumin was
prepared according to the method of Galfre, G. and Milstein,
C. (Preparation of Monoclonal Antibodies: Strategies and
procedures. In Methods of Enzymology, Immunochemical
Techniques, vol. .'3, Langone, J. and Van Vunakis, H., eds.
Academic Press (1981) pp. 3-46).
The asci.tes fluid was purified on Protein-A column
as described, supra, and the affinity of the monoclonal
antibody to human albumin was 2.3 x 107 1/mol as determined by
the method of Adri.on, R. F. 1982, Clin. Chem. (lett); 28, p.
717. The monoclonal specific IgG was coupled to Sepharose 4B
by the cyanogen bromide activation procedure of March et al,
1974, supra, to yield 0.763 mg IgG/ml of gel. The IgG coupled
28
68299-100
CA 02040913 1999-07-27
gel was diluted with casein-coupled Sepharose 4B (4.92 mg
casein/ml of gel), using the same coupling procedure as that
for IgG, in various ratios described below. The diluted gel
containing the human albumin monoclonal antibody was then
reacted with human albumin-horseradish peroxidase conjugate
for 1 hour at ambient temperature and washed with the
phosphate buffer, pH 7.0, diluent as described in Example 1,
supra. The washed gel now containing immobilized human
albumin monoclonal. antibody-human albumin-horseradish
peroxidase ( ~Ab~~HA - E) was then reacted with 300 ug/ml
human albumin equivalent (30 ug albumin per 250 ul of gel) to
saturate all binding sites on the albumin monoclonal antibody.
The gel is rewashe:d with the phosphate buffer, pH 7.0, diluent
to remove any unreacted "cold" albumin and transferred to the
diagnostic device of the present invention. To check the
effective minimum detection level for measuring albumin in
urine using the above-described method, urinary albumin
calibrators containing 10, 20, 30 and 40 ug/ml of albumin were
diluted 1:10 in the sample diluent (0.05 mg/ml of
tetramethylbenzidi.ne in phosphate buffered saline, ph 7.4) as
described under Example 1, supra, and applied to diagnostic
devices containing the following ratios of reagents as shown
in Table 1.
29
68299-100
CA 02040913 1999-07-27
TABLE 1
~IgG ~ CASEIN Albumin- "Cold" Minimum
gel gel HRPO Albumin Detection
Dilution ug per Limit
250 ul ug/ml
gel
DEVICE 1 1 part: 25 parts 1:100 30 20
2 1 part: 50 parts 1:25 30 40
3 1 part: 50 parts 1:50 30 20
4 1 part: 25 parts 1:200 30 20
1 part: 50 parts 1:100 30 20
6 1 part: 50 parts 1:50 0 320
7 1 part: 25 parts 1:25 0 340
Thus, saturating the unoccupied binding sites by
manipulating the albumin-enzyme conjugate and the addition of
"cold" albumin enable the system to detect 20 ug/ml of urinary
albumin. Without the added "cold" albumin the detection limit
is approximately 33 ug/ml. Published studies (Mogensen, 1984,
supra) based on highly sensitive RIA for albumin have
established the upper limit of normal for adults as
68299-100
CA 02040913 1999-07-27
approximately 15 ug/minute or approximately 17 ug/ml (based on
1600 mls of urine is excreted in 24 hour period) and a range
extending from 20-~30 ug/ml to about 150 ug/ml as an
operational definition of microalbuminuria. The disclosed
methods of the present invention allows the rapid detection of
albumin in urine at levels only previously achieved with
highly sensitive immunoassays.
EXAMPLE 4
URINARY HUMAN CHORIONIC GONADOTROPIN (hCG)
hCG was conjugated to horseradish peroxidase by the
periodate method of Boorsma et al., 1979, supra.
Monoclonal antibody raised against hCG was prepared
according to the method of Galfre et al, 1981, supra.
The asci.tes fluid was purified on Protein-A column
as described, supra, and the affinity of the monoclonal
specific antibody to hCG was 1.18 x 109 1/mol as determined by
the method of Adri.on, R. F. 1982, supra. The monoclonal
specific IgG was coupled to Sepharose 4B by the cyanogen
bromide activation procedure of March et al, 1974, supra, to
yield 0.95 mg IgGfml. The IgG coupled gel was diluted with
unreacted Sepharose 4B in ratio of 1:25 (1 part IgG gel to 25
parts unreacted Sepharose 4B). The diluted gel containing hCG
31
68299-100
CA 02040913 1999-07-27
monoclonal antibody was then reacted with hCG-horseradish
peroxidase conjugate dilute 1:25 in phosphate buffer, pH~7.0,
diluent for 4 hours at ambient temperature and washed with
phosphate buffer, pH 7.0, diluent as described in examples 1
and 3, supra.
The washed gel now containing immobilized hCG
monoclonal antibody-hCG-horseradish peroxidase ( ~AbhCG'~hCG -
E) was then reacted with various amount of "cold" hCG to
saturate all binding sites to the hCG monoclonal antibody as
shown in Table 2 below. The gel was re-washed with phosphate
buffer, pH 7.0, di:luent to remove any unreacted "cold" hCG and
transferred to the diagnostic device of the present invention.
The minimum detection level for measuring hCG in
urine using the above-described method, was checked by using
urinary hCG calibrators containing 20, 30, 40, 50 and 60
mIU/ml of hCG diluted 1:10 in the sample diluent as described
under Examples 1 - 3, and applied to diagnostic devices
containing the following reagent ratios as shown in Table 2.
32
68299-100
CA 02040913 1999-07-27
TABLE 2
--IgG ge.l ~--CASEIN hCG- "Cold"hCG Minimum
gel HRPO hCG mIU Detection
Dilution per Limit
250 ul gel mIU/ml
DEVICE 1 1 part: 25 parts 1:25 0 60
2 1 part: 25 parts 1:25 1 50
3 1 part: 25 parts 1:25 2 40
4 1 part: 25 parts 1:25 3 30
1 part: 25 parts 1:25 4 20
The effective minimum detectable limit for the
urinary hCG assay is approximately 20 mIU/ml. Without the
added "cold" hCG the detection limit is approximately 60
mIU/ml. Meticulous titering of "cold" hCG in the system could
yield sensitivities even lower than 20 mIU/ml.
Having fully described the invention it is intended
that it be limited solely by the lawful scope of the appended
claims.
33
68299-100