Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2()4~536
Method for treatment and recycling of pulp mill bleach
plant effluents
The present invention relates to a method for treatment
and recycling of pulp mill bleach plant effluents. In
particular, the present invention relates to a method
for separation of chlorine-containing chemicals from the
fluid obtained from the first bleach stage performed
with these chlorine-containing bleach chemicals on
washed and partly dewatered pulp.
Thus the purpose of the present invention is to achieve
a method for treatment and recycling of the effluents
caused in pulp bleaching so that the effluent is
separated into separate fractions, of which one fraction
can be recycled to the mill's chemical recovery system
and another fed to equipment with which contaminating
substances can be separated from the fluid, which then
can be re-utilized in the process and the contaminants
transformed into a state harmless to the environment.
In production of fully bleached pulp according to the
sulphate or sulphite method there is no other choice
than to use chlorine-based oxidating bleach chemicals.
These bleach chemicals are mostly molecular chlorine,
chlorine dioxide and hypochlorite. In the bleach process
the chlorine, as a result of chemical reactions, is
mainly transformed into chloride ion, but about 10 ~ of
the chlorine is bound to organic material. The bleaching
of chemical pulp is divided into stages with different
chemicals added. Partly the chlorine compounds are used
in the first bleach stage as a reactant to degrade
lignin contained in the unbleached pulp and partly in
the last stages to eliminate chromophor groups from the
pulp and thus give it its final brightness. All
oxidative degraded lignin is not water soluble in the
acid environment of the first bleach stage. In the
second bleach stage, degraded lignin is therefore
extracted from the pulp using sodium hydroxide, at the
same time as a further oxidative lignin degradation is
carried out with oxygen and possibly hydrogen peroxide.
2041536
In the first bleach stages principally the residual
lignin in the pulp is extracted, which thus is contained
in the waste liquors from these bleach stages. For
example in production of fully bleached softwood kraft
pulp, the extracted organic substance is 3-5 % of the
pulp production. This organic substance has a high
biologic oxygen demand (BOD) and a very dark colour and
it contains the major part of the chlorinated organic
material, measured for example as adsorbable organic
halogen (AO~). Partly the chlorinated organic material
is high-molecular with a more or less unknown
composition, and partly low-molecular. The low-molecular
part contains substances that have turned out to be
toxic, mutagenic and having a tendency to bioaccumulate
in the ecological system.
The bieach plant effluents are so far discharged to a
water recipient, mostly though via a process-external
effluent treatment in an aerated lagoon or activated
sludge plant. The effluent treatment efficiently reduces
the BOD concentration but not the biologically inactive
organic material. Even the most efficient biologic
effluent treatment plants are capable of reducing the
total concentration of organic material and also the
concentration of organically bound chlorine with only
50 %. Thus the bleach plant effluents constitute a
considerable environmental dilemma to the pulp mills.
Various methods have been explored to solve this
problem. What mainly has been examined is the
possibilities to recycle the bleach plant effluents
within the mills' chemical recovery systems for
destruction of the organic substance for example in the
recovery boiler of the kraft pulp mill. Well-known is
the mill scale trial carried out at Great Lakes Paper
Co's pulp mill in Thunder Bay, Canada ten years ago. The
difficulty in recycling bleach plant effluents is that
the chlorides in the water are concentrated in the
process thus causing process failures and accelerated
corrosion in the equipment. To solve this problem,
evaporation of white liquor and a partial
crystallization of sodium chloride was introduced in the
above-mentioned pulp mill. At this stage, however, the
3 20~1536
chloride-containing black liquor had already passed
through the liquor evaporation plant and been combusted
in the recovery boiler. Great difficulties forced the
mill to return to the common praxis of discharging the
bleach plant effluents to an external water recipient.
Well-known i5 also the ultrafiltration technique for
treatment of effluents containing high-molecular
substance. In this case the effluents flow over
microporous membranes, which let through low-molecular
material but retain high-molecular material. This
technique is used for example at Taio Paper Co in Japan
for separation of the high-molecular substance from the
effluents of the second (alkaline) bleach stage. The
major part of the organic material as well as the
chloride ions in the effluents from the bleach stage
where chlorine-containing bleaching agents are used do
pass through the commercially relevant membranes,
though. Therefore this technique is not suitable for
mill scale treatment of chlorine-containing bleach plant
effluents.
With presently existing technology it is thus impossible
to separate the contaminants in the bleach plant
effluents with process-internal measures so that the
water could be recycled into the process and the
contaminants destructed in a way not harmful to the
environment. This can be seen from the fact that all the
world's pulp mills today discharge their bleach plant
effluents to an external water recipient.
The present invention relates to a new method for
enabling partial or total recycling of bleach plant
effluents through process-internal interconnecting of
individually known devices and with simultaneous
transforming of both chlorinated and non-chlorinated
organic substance extracted in the bleach plant into a
state harmless to the environment.
The primary characteristics of the invention appear from
claim 1 of the patent claims presented below.
204153~
According to a presented type of the invention, the pulp
is dewatered by pressing out the liquid from it, with
for example a screw press, preferably to a dryness of
25-35 w.p.
The fluid discharged from the bleach stage can be
evaporated in several parallel or consecutive stages
preferably at a relatively low temperature below 70C.
The condensate achieved through recompression of the
evaporated vapour is preferably completely or partially
recirculated from the evaporation to some subsequent
bleach stage for use as wash liquor. The evaporation is
preferably performed up to a dryness high enough to let
the evaporation residual be dried and combusted to
inorganic material.
Below the present invention is described more in detail
with reference to enclosed drawings, in which figure 1
shows a connection diagram of a typical modern pulp
bleaching plant in a simplified manner, whereas figure 2
shows a connection diagram for application of the method
according to the present invention.
To an expert it is clear that the description and
drawings only constitute an example of the application
of the invention and thus not include all the
alterrlatives covered by the patent claims.
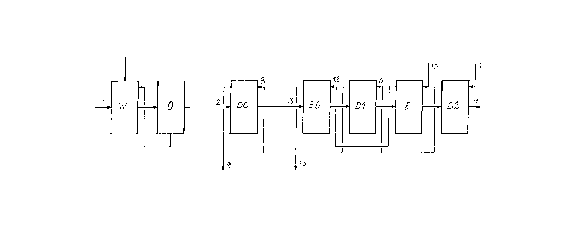
The typical connections of a modern pulp bleaching plant
are shown in figure 1 in a simplified manner. Here W
indicates the wash room, in which the waste liquor from
the digesters is separated from the unbleached stock.
Generally the wash room consists of several counter-
current wash filters or combinated devices where
counter-current washing can be achieved. The washed
brown stock is taken to a process stage with oxygen
delignification, in figure 1 indicated with 0. This
stage is omitted in many ~pplications and is not
essential to this invention. After a possible oxygen
delignification with a subsequent washing stage, the
brown stock is taken to the bleach plant. The bleaching
is performed in four or five consecutive bleach stages.
2(3 4153~
Each one of these consists of devices for dosing of
bleachiny chemicals into the pulp suspension, a bleach
reactor to achieve enough retention time for the
reaction, and a device for washing of the stock. In
figure 1 the first bleach stage is indicated with DC.
Chlorine gas and chlorine dioxide are fed to this stage
in various proportions. After washing, which normally
leads to a pulp concentration of 10-12 % after the
washing stage, the stock is fed on to the alkaline
bleach stage, in figure 1 indicated with E0. Sodium
hydroxide and often also oxygen gas are fed to this
stage. After the chemical treatment, the stock is also
washed in the E0 stage resulting in a pulp concentration
of 10-12 ~ after the washing stage. Now the stock is
taken to final bleaching in the bleach stages indicated
with D1, E and D2. Normal chlorine dioxide is fed to Dl
and D2 and sodium hydroxide to E. In some cases the E
stage is omitted, which is not essential to this
invention.
In figure 1 some main flows between the separate devices
are indicated. The flows indicated with 1-4 show the
pulp suspension flowing through the plant, flow 1 is the
brown stock suspension from the digester house and flow
4 the bleached pulp suspension. Each bleach stage
contains a pulp washing stage and figure 1 shows the
main flows of wash liquor, which transport dissolved
solids in the bleach plant. 5 and 10 indicate clean
washing water - often white water from the bleached
stock cleaning - coming to the bleach plant. 6 is wash
liquor discharged from the D2 stage and containing
chlorine substances and relatively small quantities of
extracted organic material, a liquor which is fed as
incoming wash liquor to the D1 stage, the outgoing wash
liquor of which is indicated with 8 and often used as
wash liquor in the DC stage. Wash liquor 9 from this
stage is heavily contaminated by both organic material
and chlorine substances, and in conventional bleach
plants it is taken to an acid waste water canal.
In conventional bleach plants fresh water is used for
washing water 10 to the E stage. 12 is wash liquor
discharged from this stage, a liquor which is fed as
2041536
incoming wash liquor to the E0 stage, the outgoing wash
liquor of which is indicated with 13 and heavily
contaminated and, in conventional bleach plants, taken
to an alkaline waste water canal. The waters in the acid
and alkaline waste water canals are combined and taken
via process-external effluent treatment to an external
recipient.
This description of the wash liquor distribution in the
bleach plant does not include the flows of wash liquor
leading from one washing stage to the preceding stage,
because this wash liquor only displaces the water
contained in the pulp and does not essentially mix with
the wash liquor discharged from this stage. Furthermore
there is an internal fluid circulation within the bleach
stages for transport and formation of the filter cake in
some washing devices. This circulation has not been
indicated in figure 1 and is unessential to this
invention.
The method according to this invention is described with
figure 2. This method differs from the known process as
described above primarily with respect to three new
devices that have been introduced in the bleach plant,
in figure 2 indicated with P1, P2 and EV. Pl and P2 are
dewatering devices, with which the consistency of the
pulp suspension to and from the DC stage is raised above
the normal, for example from 10-12 % to 25-35 %. The
purpose of these devices is to reduce the volume of
water brought with the pulp to and taken out from the DC
stage. The pressed out liquid is returned to the
preceding stage and used there as dilution liquor. Since
the carryover of dissolved solids in the fluid is
proportional to the volume of transferred fluid, the
raise of the pulp consistency in P2 will reduce the
carryover of chlorine-containing material to the E0
stage, especially as a suitable quantity of the
discharge water from the E0 stage is used to displace
the major part of the chlorine-containing discharge to
the normal washing device of the DC stage. When the
carryover of chlorine-containing material to the E0
stage has thus been considerably eliminated, wash liquor
flow 13 from this stage can be recycled to the mill's
Z041536
chemical recovery system. According to the example in
figure 2 this would be implemented by combining the flow
in question with discharge flow 14 from the oxygen
delignification to flow 15 to be used in a suitable
manner together with the normal washing water flow 16 to
tha wash room W.
To balance the water flows in the three last stages of
the bleach plant, a certain overflow 7 of wash liquor
from the Dl stage can occur. This is then led to the
waste water canal. The concentration of organic material
in the discharge water from the ~inal bleach stages is,
however, considerably lower than from the first two
bleach stages, and therefore such an overflow - if it
occurs - does not reduce the value of the invention as
method for cleaning the bleach plant effluents.
The function of the dewatering device Pl is to reduce
the incoming water to the DC stage. If the water volume
in flow 2 to the DC stage is bigger than the water
volume in flow 3 out from the stage, the difference will
be discharged in discharge wa-ter flow 9. To considerably
reduce this water volume, which would load device EV,
the dewatering device Pl is needed.
The devices Pl and P2 can be separate devices, for
example well-known screw presses, or their function can
be integrated with the washing device as in well-known
pressure washers.
The device EV is an evaporation unit. In this unit added
discharge water is evaporated. Heat is taken from
condensing vapour to evaporate the water in the
discharge and the vapour thus formed is compressed to a
higher pressure and used as a source of heat for
evaporation of more water. The condensing vapour and the
boiling discharge are separated by a non-permeable heat-
conducting membrane. The condensate formed when the
vapour is condensated contains no non-volatile compounds
and is thus free from salts and also all organic
material with cooking temperatures above the temperature
at which the evaporation takes place in the device.
.
2041~36
The device EV is thus a well-known, e.g. from patent
application FI 79948, evaporation device. EV can consist
of such a device, but in general several devices of this
type are needed, connected in parallel or in series.
These types of connecting are known. For this invention
it is, however, suitable that the evaporation unit
functions through thermal recompression, thus having no
continuous vapour demand. Further it is preferable but
not necessary that it operates at a low temperature
level, typical evaporation temperatures being below
70C, to avoid unnecessary heating of the discharge
water and to prevent evaporation of organic material and
stripping of hydrochloric acid. This means that the
device in that case operates at a pressure below
atmospheric pressure and that the pressure differences
over thP non-permeable heat-conducting membranes in the
device are small. The membranes can therefore be thin
and made for example from plastic material.
With the device EV the heavily contaminated and
chlorine-containing discharge water flow 9 can thus be
divided into an essentially clean water flow and a
concentrate flow. The concentration of dissolved solids
in this flow is typically above 10 ~. The purified water
fraction is clean enough to be used as process water in
the mill, according to figure 2 for example as wash
liquor flow 10 to the E stage of the bleach plant (or
the E0 stage if there is no E stage). In some cases
stripping of hydrochloric acid gas can take place
together with the evaporation of the water, when the
concentration of dissolved solids in the evaporation
becomes higher. To avoid any disturbing return of
chlorine to the bleach plant, the device EV can consist
of evaporation units connected in series in the water
section, all operating with recompression of stripped,
condensated water vapour but with different
concentration levels of the solution to be evaporated.
The condensate coming from the devices operating at a
high concentration level and being contaminated with
chloride ions but not with organic material, is
discharged to the waste water canal, and only the non-
contaminated condensate is returned to the bleach plant.
204~5~6
The concentrate contains the chlorine compounds, mainly
chloride ions, and the non-volatile organic material. It
is taken out as flow 17 from the device EV. This
concentrate is passed to the incinerator IN, where it is
dried and combusted using the air stream 18. When the
acid chloride-containing concentrate is dried, a
stripping of hydrochloric acid gas takes place. This
hydrochloric acid can be absorbed into an added flow 21
of water or liquor solution. Stripped hydrochloric acid
is then contained in the outgoing absorption water 22.
The organic material is completely combusted and it
passes out as carbon dioxide and water vapour with the
flue gas stream 19 from the incinerator. All organically
bound chlorine is transformed into inorganic state and
tanken out as ash stream 20 from the incinerator. The
techni~ue of incinerating wet material is well-known and
there are many variants for its implementation, e.g. the
one described in U.S. Patent 4 159 682. Which one of
these that would be used is not essential to this
invention.