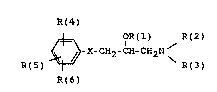Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
f 2 9 ~ 6
HOECHS~ ARTIENGESELLSCHAFT HOE 90/F 164 Dr.v.F./St
Description
Use of substltuted ~-hydrosyethyl~ ~nes ~B potent
S inhibitors of the esoen~yme- of fungi
The invention relates to the u~e of ~ubstituted
p-hydroxyethyl~mines and of pharmaceutlcal composltiQns
containing these compounds a~ pharmaceuticals, in
particular a8 growth inhibitors of the pAthogenic phase
of dimorphic yeast cells, a8 antimycotics and plant
protection agents, for ex~mple fungicides and growth
regulators, it being possible for the compounds to be
used both in prophylaxis and in therapy.
Of the few ~-hydroxyethyl~mines which are used a8 beta
blockers, it is known, however, that they have a slight
effect on the inhibition of the liposomal phospholipase
Al of mammals. (cf. R.Y. Hostetler et al., Biochemical
Pharmacology 34, 521-524 (1985)). The potency and tolera-
bility of these compounds, however, are not satisfactory.
(Examples sre propranolol or metoprolol).
OH OH
O- CH2 NH2 CH3- - CH2- C~2 ~ ~ C
t~H
propranolol metoprolol
It has now surprisingly been found that substituted
~-hydroxyethylamines, depending on the structure of the
respective ~ubstituents, are potent inhibitors of the
exoenzymes (pathogenicity factors) of fungi, have fungi-
cidal and antimycotic activity and in particular are
outstanding growth inhibitors of the pathogenic phase of
dimorphic yeasts.
- 2 -
The inventLon therefore relates to the use of ~ub~tituted
~-hydroxyethylamines of the formula III
olR(l) R(2)
A - X - CH - CH2 - N ~
~(3) (III)
and of their salts with pharmaceutically acceptable acids
and also of pharmaceut~cal preparations containing these
compounds III for the production of ~ medicament for the
prevention of and for the treatment of fungal disea~es,
the radicals in formula III having the following meaning:
R(l) is H, (Cl-C1O)-alkyl (straight-chain or branched),
(C2-Cl~)-alkenyl (straight-chain or branched, mono-
or polyunsaturated~, benzyl, tun~ubstitu~ed or mono-
or polysub~tituted by F, Cl, Br, CF3, (Cl-C4)-alkyl
(straight-chain or branched), OCH3, O-phenyl or
phenyl], C(O)-(C1-CB)-alkyl (straight-chain or
branched) or C(O)-phenyl,
R(2) is H, (C1-C18)-alkyl (straight-chain or branched),
(Cl-C6)-alkyloxy-(Cl-C6)-alkyl (straight-chain or
branched), (C1-C6)-alkyloxyphenyl (straight-chain or
branched), (C2-C16)-alkenyl (straigh~-cha~n or
branched, mono- or polyunsaturated), (C3-C~)-cyclo-
alkyl (mono-, bi- or multicyclic, such a~ norbornyl,
adamantyl or decahydronaphthalenyl), phenyl-(cl-c6)-
alkyl (straight-chain or branched, the alkyl chain
being unsubs ituted or mono- or disubstitut~d by
OH), phenyl-(C2-C6)-alkenyl (straight-chain or
branched, mono- or polyunsaturated), diphenyl-
(Cl-C6)-alkyl (straight-chain or branched) or
phenyl~ the phenyl systems being unsubstituted or
mono- or polysubstituted by substitu~nts from the
group compri~ing F, Cl, Br, ~C1-C1O)-alkyl (straight-
chain or branched), (C3-C1~)-cycloalkyl, OH, SH,
(C1-ClO)-alkoxy ~straight-chain or brAnched),
r~
~ 3 ~
(Cl-C~)-alkylenedioxy (straight-chain or branched),
dimethylaminoethoxy, (Cl-C4)-alkoxycsrbonylmethoxy
(straight-chain or branched), phenoxy, phenyl,
benzyl, phenethyl, thiophenyl and C~F~*l where n i8
s equal to 1-6,
or
R~2) is an indol-3-yl-(Cl-C~)-alkyl radical (strai~ht-
chain or branched), thie~yl, thienylmethyl, the
thienyl radical being unsubstituted or substituted
by F, Cl, (Cl-C4)-alkyl or O(C~-C4)-alkyl t~traight-
chain or branched)~
R(3) is defined as R(2), R(2) nd R(3) each having the
~ame or a different meaning, or
R(2) forms a -(CH2)m-chain with R(3) where m i5 equal to
4-6, in which a CH2 group can be replaced by oxygen,
sulfur or nitrogen, the additional nitrogen carrying
a hydrogen atom, a CH3, phenyl, benzyl or a phenethyl
group as a further bonding component, and
A i6 (Cl-C18)-alkyl (straight-chain or branched),
(C2-Cl8)-alkenyl (straight-chain or branched, mono-
or polyunsaturated) or a group of the formula II
R(4)
,~,
R(5) ~ II
R(6)
in which the radical~ R(4), R(S) ~nd R(6) have the
follow$ng meaning:
5 R(4) is H, (Cl-Cl8)-alkyl (~traight-chain or branched)~
(C2-Cl8)-alkenyl (~traight-chain or branched, mono-
or polyunsaturated), (C3-C20)-cycloalkyl, tmono-, bi-
or multicyclic, unsubstituted or mono- or disub-
stituted by (Cl-C4)-alkyl (~trsight-chain or
2 ~ 2 1 6
branehed), ~C~-C~)-alkoxy (~traight-ehaln or
br~nched)~ C~F~*~ where n is equ~l to 1-4, F, Cl, Br
or OH], Y-(C~-C20)-~lkyl (str~ight-ehain or
branched)~ Y-(C2-C~ lkenyl (-traight-ehaln or
br~nehed, mono- or polyun~aturated), Y-(Ca-C~O)-
eycloalkyl (mono-, bi- or multieyclle, unsubstituted
or substituted aB lndicated above)~ phenyl,
Y-phenyl, phenyl-(C~-C~ lkyl (~traight-ehain or
branched), phenyl-(C1-C~)-alko y (stra$ght-chain or
branched)~ biphenylyl, F, Cl, Br, CDF~ (where n iB
equal to 1-8), CC13, YH, naphthyl, CN or NO2, the
phenyl systems being unsubstituted or mono- or
disub~tituted by F, Cl, CF3, (C~-C~)-alkyl (straight-
ehain or branehed) or (C1-C~)-alkoxy (straight-ehain
lS or branehed),
where Y is equal to oxygen or sulfur, SO or SO2,
R(5) is defined as R(4), R(4) and R(S) being identieal or
different, or
R(4) together with R(5) form6 a (CH2)p ehain where p is
equal to 3 or 4 in the ease in whieh the 6ub~titu-
ent6 are bonded to ad~acent positions on the phenyl
ring, and
R(6) is H, (C1-C15)-alkyl (6traight-ehain or branehed),
(C2-C~s)-alkenyl (straight-chain or branched, mono-
or polyunsaturated), Y-(C~-C~5)-alkyl (straight-ehain
or branched), Y-(C2-C~5)-alkenyl (straight-chain or
branehed, mono- or polyunsaturated), phenyl,
Y-phenyl, benzyl, biphenylyl, F, Cl, Br, I, CnF~*~
(where n iB egual to 1-8), CCl9, naphthyl or YH, and
30 X iB oxygen or sulfur.
A preferred compound of eompounds of the formula III iB
one of tho6e in which the radical6 have the following
meaning:
2 ~ ?~
- 5 -
R(l) is H, (Cl-C~-alkyl (stralght-ehaln or br~nched),
~C3-C~)-alkenyl (~traight-ehaln or branehed, mono- or
diunsaturated)~ benzyl ~unsub~tituted or mono- or
disubstituted by F, Cl, CF~, (C,-C~)-alkyl (~tralqht-
S ehain or branehed) or OCHal or C(O)-~Cl-C~)-alkY
(straight-ehain or branehed),
R(2) is H, (Cl-Cl6)-alkyl (-traight-ehain or branched),
(Cl-C~)-alkylo y-(Cl-C~)-alkyl (~traight-ohaln or
branehed), (Cl-C0)-alkyloxyphenyl (straight-ehain or
branehed), (C2-C10)-alkenyl (str~ight-ehain or
branched, mono- or polyunsatur~ted), (CJ-C1O)-CYC1O-
alkyl, (mono-, bi- or multieyelie), phenyl-(C1-C6)-
alkyl (straight-ehain or branched) or diphenyl-
(C1-C6)-alkyl (straight-ehain or branehed) or phenyl,
the phenyl sy~tems being unsubstituted or mono- or
disubs~ituted by ~ubstituent~ from the group eom-
prising F, Cl, (C,-C~)-alkyl (straight-chain or
branched), (C3-C~)-eyeloalkyl~ (Cl-C8)-alkoxy
(~traight-chain or branched), (C1-C2)-alkylenedioxy,
(Cl-C~)-alkoxycarbonylmetho y, phenyl, benzyl,
phenethyl and thiophenyl,
or
R(2) iB ~n indol-3-yl-(Cl-C~)-alkyl radieal (straight-
chain or branched) or thienylmethyl, the thienyl
radical being unsubstituted or substituted by Cl,
(Cl-C~)-alkyl (straight-chain or br~nehed) or
O(Cl-C~)-alk~l (straight-chain or branched)~
R(3) i~ defined ~8 R(2), R(2) and R(3) each having the
same or a different meaninq, or
,~
R(2) form~ a -(CH2)~-chain with R(3) where m i~ equ~l to
4-6, in whieh a CH2 group ean be replaced by oxygen,
~ulfur or nitroqen, the additional nitrogen earrying
; a hydrogen atom, a CH3, phenyl, benzyl or a phenethyl
group as a further bonding eomponent, and
- 6 -
i8 (Cl-C~0)-alkyl ~tralght-chaln or branched),
(C9-C~ alkenyl (straight-chaln or branched, mono-
or polyunsaturated) or a group of the formula II,
in which the radicals R(4), R(5) and ~(6) have the
S following meanings
R(4) 1s H, (C1-Cl6)-alkyl (~tr~ight-chain or branched),
(C2-C1e)-alkenyl (straight-ch~in or branched, mono-
or polyun~aturated)~ (c3-c~2)-cycloalkyl tmono-, bi-
or multicyclic, unsub~tltuted or monosubstituted by
(C1-C~)-alkyl (6tralght-chain or branched) or (C1-C~)-
alkoxy (straiqht-chain or branched)], Y-(C1-C16)alkyl
(straight-chain or branched), Y-(C3-C16)-alkenYl
(strsight-chain or branched, mono- or polyun-
saturated)~ Y-(C3-Cl2)-cycloalkyl (mono-, bi- or
multicyclic, unsubstituted or sub~tituted as
indicated above), phenyl, Y-phenyl, phenyl-(C,-C2)-
alkyl, phenyl-(Cl-C~)-alkoxy (straight-chain or
branched), biphenylyl, F, Cl, C~F2~l (where n iB
equal to 1-4), CC13, naphthyl or CN, the phenyl
systems being unsub~tituted or mono- or disubsti-
tuted by F, Cl, CF3, (Cl-C~)-alkyl or (Cl-C~)-alkoxy,
where Y is equal to oxygen or ~ulfur,
R(S) is defined as R(4), R(4) and R(5) being identical or
different, or
R(4) together with R(5) forms a (CX2)p chain where p is
equal to 3 or 4 in the case in which the substitu-
ents ~re bonded to nd~acent positions on the phenyl
ring, and
R(6) is H, (Cl-C~)-alkyl (straight-chain or branched),
(C3-C~)-alkenyl (straight-chain or branched, mono-
or polyunsaturated), Y-(C1-Cl2)-alkyl (straight-chain
or branched), Y-(C9-Cl2)-alkenyl (~traight-chain or
branched, mono- or polyunsaturated), phenyl,
Y-phenyl, benzyl, biphenylyl, F, Cl, Br, C~F~
r
-- 7 ~
(where n iB equal to 1-4 ), CC1J or naphthyl, and
X is oxygen or sulfur.
A particularly preferred compound of oompounds of the
formula III is one in which the radical~ ln formula III
have the following meaning-
R(l) is H, C(O)-(Cl-C~)-alkyl (straight-chain or branched)
or benzyl,
R(2) is H, (Cl-Cl2)-alkyl (stra$ght-chain or branched),
(C1-C~)-alkyloxy-(Cl-C~)-alkyl, (C3-C12)-alkenyl
(straight-chain or branched, mono- or polyunsatura-
ted), (C3-C10)-cycloalkyl (mono-, bi- or multicyclic)
phenyl-(C~-C6)-alkyl (qtraight-chain or branched),
diphenyl-(C1-C6)-alkyl (~traight-chain or branched)
or phenyl, the phenyl systems being un~ubstituted or
mono- or disub~tituted by substituents from the
group comprising CF3, F, Cl, (C1-C~)-alkyl ~straight-
chain or branched), (C1-C8)-alkoxy (straight-chain or
branched), (C1-C2)-alkylenedioxy, phenyl, benzyl and
phenethyl,
':
or
R(2) i8 an indol-3-yl-(C1-C4)-alkyl radical tstraight-
chain or branched) or thienylmethyl, the thienyl
radical being unsubstituted or sub~tituted by Cl,
(C1-C~)-alkyl or O(C1-C~)-alkyl,
R(3) is H,
R(2) formQ a -(CH2)~- chain with R(3) where m is equal to
4-6, in which a CH2 group can be replaced by osygen,
sulfur or nitrogen, nitrogen carrying a hydrogen
atom, a CH3, phenyl, benzyl or a phenethyl group as
a further bonding component, and
2 ~ f ~ t, ,~ 53
- 8 -
- A is (Cl-C~)-~lkyl ~-tr~lght-¢haln or br~nehod),
(C3-C~ lkenyl (~traight-ehain or br~nehed, mono-
or polyun~aturated) or ~ qroup of the formula II,
in whieh the radieal~ R(~), R(5) ~nd R(6) have tho
S following meaning-
R(4) is H, (Cl-Cl~)-alkyl (~tralqht-ehaln or branehed),
(C2-Cl2)-alkenyl (~traiqht-ehain or branehed, no-
or polyun~aturated), (C~-C~)-oyeloalkyl ( no-, bl-
or multieyelle and un~ubstituted), Y-(cl-c~)
(straiqht-ehain or branehed), Y-(c3-cl2)-~lk~nyl
(straight-ehain or branehed, ~ono- or polyun~atura-
ted)~ Y-(C3-Cl2)-eycloalkyl (mono-, bi- or ~ulti-
eyelie and unsubstituted), phenyl, Y-phenyl, phenyl-
(Cl-C2)-alkyl, phenyl-(Cl-C~ lkoxy (~tralght-ehain
or branehed), biphenylyl, F, Cl or naphthyl, the
phenyl systems being unsub~tituted or mono- or
disubstituted by F, Cl, CF3, (Cl-C~)-alkyl (straight-
ehain or branched) or (Cl-C~)-alkoxy (straight-ehain
or branched),
20 where Y i8 equal to oxyqen or sulfur and
R(S) is defined as R(4), R(4) and R(S) being identieal or
different, and
R(6) is H, (C1-C10)-alkyl (straight-chain or branched),
(C3-C10)-alkenyl (~traight-chain or branched, mono-
or polyunsaturated), Y-(Cl-Clo)-~lkyl (straight-ehain
or branehed, mono- or polyunsaturated), phenyl,
Y-phenyl, benzyl, F, Cl or CF3, and
X iB oxygen.
Very particulsrly preferred is the use of compounds of
the formula III in which the radieals in formula III have
the following meanings
R(l) is H, C(O)-CH3 or benzyl,
- 9 - 2~,? ~
R(2) is H, (C1-C10)-alkyl ~str~ight-ch~in or branched),
( Cl-C3 ) -alkylo~ty- ( Cl-C3 ) -~lkyl, ( C3-C10 ) -alkenyl
(straight-chain or branched, mono- or polyunsatur~-
ted), (C1-Clo)-cyclo~lkyl, (mono-, bl- or multl-
cycllc)~ phenyl-(Cl-C~ lkyl (-tralght-chaln or
branched), diphenyl-(Cl-C~)-alkyl (~tralght-chaln or
branched) or phenyl, the phenyl ~ystems being unsub-
stltuted or mono- or disubstituted by ub~tituent~
from the group comprising F, Cl, (C1-C~)-alkyl
(straight-ch~$n or branched~, (C1-C6)-alkoxy
(straight-chain or branched), (C1-C2)-alkylenedioxy,
phenyl, benzyl and phenethyl,
~ or
: R(2) is an indol-3-yl-(C1-C~)-alkyl radical (straight-
lS chain or branched) or thienylmethyl, the thienyl
radical being unsubstituted or substituted by Cl,
(C1-C~)-alkyl or O(C1-C~)-alkyl,
: R(3) i8 H
and
A is tCl-Cl2)-alkyl (straight-chain or branched)~
(C3-C10)-alkenyl (6traight-chain or branched, mono-
or polyun6aturated) or a group of the formula II,
in which the radicsls R(4), R(5) and R(6) have the
~ following meanings
; 25 R(4) i8 H, (C1-Cl2)-alkyl (straight-chain or branched)~
(C2-C~)-alkenyl (straight-chain or branched, mono-
or polyunsaturated), (C3-C10)-cycloalkyl (mono-, bi-
or multicyclic and unsubstituted), Y-(C1-C~)-alkyl
:~ (straight-chain or branched), Y-(C3-Cl2)-~lkenyl
(straight-chain or branched, mono- or polyun~atura-
~: ted), Y_(CJ_C10)-CYC10a1kY1 (mono-, bi- or multi-
: cyclic and unsubstituted)~ phenyl, Y-phenyl, benzyl,
benzyloxy, biphenylyl, F, Cl or CF9, the phenyl
systems being unsubstituted or mono- or di~ubsti-
tuted by F, Cl, CF9, (C1-C~)-alkyl (str~ight-chain or
br~nched) or (C1-C~)-alkoxy (str~ight-chain or
br~nched),
S Y i8 equal to oxygen,
R(5) i~ H, tC~-C~)-alkyl (straight-chain or branched),
~C2-C~2)-alkenyl (~traight-chain or branched, mono-
or diuns~turated), (CJ-C~)-cycloalkyl, Y-~C~-C~)-
alkyl (straight-cha$n or branched), Y_(CJ_C~)_CYC1O_
alkyl, phenyl, Y-phenyl, benzyl, F, Cl or CFJ,
R(6) is equal to H, and
X i8 equal to oxygen.
It has now surprisingly been found that substituted
~-hydroxyethylamines, depending on the structure of the
respective sub~tituent6, are potent inhibitors of the
exoenzymes (pathogenicity factors) of fungi, have fungi-
cidal and antimycotic activity and in particular are
outstanding growth inhibitor~ of the pathogenic phase of
dimorphic yeasts.
The invention therefore relates to the use of substituted
~-hydroxyethylamines and pharmaceutical compositions
containing these compounds, of the formula I
R(4)
1 OR(1) R(2)
R(5) ~ X-CH2-CH-cH2N ~ I,
R(6)
for the prevention ~nd treatment of fungal di~ea~es; in
the compoundss
R(l) is H, (C1-C~0)-alkyl (straight-chain or branched)~
(c2-c~o)-alkenyl (straight-chain or branched, mono-
or polyunsaturated) or benzyl (unsubstituted or
mono-or poly~ub~tituted by F, Cl, Br, CF3,
2.7' l~
(C~-C~)-alkyl (stralght-chain or branched), OCH3,
O-phenyl or phenyl),
R(2) is H, (C1-C10)-alkyl (straight-chain or branched),
(C2-C10)-alkenyl (straight-chain or branched, mono-
or polyunsaturated), (C3-C10)-cycloalkyl, phenyl-
(Cl-C~)-alkyl (straight-cha$n or branched), phenyl-
(C2-C~)-alkenyl (straight-chain or branched, mono- or
polyunsaturated)~ diphenyl-(C1-C~)-alkyl (straight-
chain or branched) or phenyl, the phenyl system
being unsubstituted or mono- or polysubstituted by
substituents from the group comprising F, Cl, Br,
(Cl-C4)-alkyl (~traight-chain or branched), (C3-C8)-
cycloalkyl, OH, SH, (Cl-Cl0)-alkoxy (straight-chain
or branched), phenyl, benzyl, phenethyl, thiophenyl
and CnF~l where n = 1-6,
R(3) is defined as R(2), R(2) and R(3) each having the
same or a different meaning,
or
R(2) forms a -(CH2)~- chain with R(3) where m is equal to
4-6, in which a CH2 group can be replaced by oxygen,
sulfur or nitrogen, nitrogen carrying a hydrogen
atom, a CH3, phenyl, benzyl or a phenethyl group a~
a further bonding component,
and
R(4) is H, (cl-cl5)-alkyl (~traight-chain or branched)~
(C2-Cl5)-alkenyl (straight-chain or branched, mono-
or polyunsaturated), (C3-C20)-cycloalkyl tmono-, bi-
or multicy~lic, unsubstituted or mono- or disubsti-
tuted by (C1-Cl~)-alkyl (straight-chain or branched)~
(C1-C4)-alkoxy (straight-chain or branched), CDF~
(where n i8 egual to 1-4), F, Cl, Br or OH, ~uch as,
for example, norbornyl, adamantyl or decahydro-
naphthalenyl]; Y-( Cl-C15 ) -alkyl (straight-chain or
- 12 - 2~ ~?)sr~
br~nched)~ Y-~C2-C1~)-alkenyl ~-traight-chain or
branched, mono- or polyun~aturated), ~-(C3-C20)-
cycloalkyl ~mono-, bi- or multicyclic, un~ub~tituted
or ~ub~titutod a8 lndicated above), phenyl,
Y-phenyl, phenyl-(C1-C~)-alkyl, blph nylyl, F, Cl,
Br, I, CnF~*l (whore n i~ qu~l to 1-8), CCl~, YH,
naphthyl, CN or NO2 where Y i~ ~qual to oxygen or
sulfur
and
`:
R~5) is defined a8 R~4), R~4) and R(5) being identical or
different,
and
R(4) together with R(5) can form a (CH2)~-chain where p iB
equal to 3 or 4 in the case in which the substituents are
bonded to ad~acent positions on the phenyl ring,
.
and
R(6) i8 H, (C1-Cl5)-alkyl (straight-chain or branched),
(C2-C10)-alkenyl (~tr~ight-chain or branched, mono-
or polyunsaturated), Y-~C1-C1~)-alkyl ~straight-chain
or branched), Y-~C2-C~5)-alkenyl ~traight-chain or
; branched, mono- or polyunsaturated), phenyl,
: Y-phenyl, benzyl, biphenylyl, F, Cl, Br, I, CDF
~where n is equal to 1-8), CCl3, naphthyl or YH,
Y - oxygen or ~ulfur, and
X - oxygen, sulfur, SO or SO2,
and their salts with pharmaceutically acceptable acids.
: Compounds of the type I are preferred in which the
substituents have the following meanings
R(l) is H, ~Cl-C3)-alkyl (straight-chain or branched)~
- 13 - 2~
(C9-Cl~)-alkenyl (~tralght-chaln or branched, mono-
or polyunsaturated) or benzyl (unsubstltuted or
monosubstituted or disubstituted by F, Cl, CF3,
~Cl-C~)-alkyl (straight-chain or branched) or OCH3),
R(2) is H, (Cl-C~)-alkyl (straight-chaln or branched),
(C3-C10)-alkenyl (straight-chain or branched, mono-
or polyunsaturated), phenyl-(Cl-C~)-alkyl (straight-
chain or branched), diphenyl-(Cl-C~)-alkyl (straight-
chain or branched) or phenyl, the phenyl system
being unsubstituted or mono- or dioubstituted by
substituents from the group comprlsing OH, F, Cl,
Br, (Cl-C~)-alkyl (straight-chain or branched), (Cl-
C4)-alkoxy (straight-chain or branched), phenyl,
benzyl and phenethyl,
lS R(3) is defined as R(2), R(2) and R(3) each having the
same or a different meaning,
or
R(2) form~ a -(CH2)~- chain with R(3) where m i8 equal to
: 4-6, in which a CH2 group can be replaced by an
oxygen or nitrogen atom, nitrogen carrying a
hydrogen atom, a CH3, phenyl, benzyl or a phenethyl
group a~ a further bonding component,
and
R(4) i~ H, (Cl-Cl~)-alkyl (straight-chain or branched),
(C2-Cl5)-alkenyl (straight-chain or branched, mono-
or polyun~aturated), (C3-Cl5)-cycloalkyl tmono-, b~-
or multicyclic, unsubstituted or moncsubstituted by
(Cl-C~)-alkyl (straight-chain or branched) or (C1-C~)-
alkoxy (~traight-chAin or branched), such as, for
example norbornyl~ adamantyl or decahydronaphtha-
lenyl], Y-(Cl-Cl0)-alkyl (straight-chain or
branched), Y-(C2-C1O)-alkenyl (straight-chain or
branched, mono- or polyunsaturated~,
- 14 -
2~ ~32 ~ ~
Y-(C3-C~ cycloalkyl ~mono-, bl- or multlcycllc,
un~ubstltuted or ~ubstituted ~ lndic~ted ~bove),
phenyl, Y-phenyl, phenyl-(C1-C2)-~lkyl, biphenylyl,
F, Cl, CnF~1 (where n is equal to 1-4), CCl3,
S naphthyl or CN,
where Y 1~ equal to o~ygen or sulfur,
and
R(5) is defined as R~4), Rt4) and R~5) being identical or
different,
and
R(4) together with R(S) can form a -tCH2)p- chain where p
i8 equal to 3 or 4 in the ca~e in which the substituents
are bonded to ad~acent positions on the phenyl ring,
and
R(6) i8 H, (C1-C10)-alkyl (straight-chain or branched)~
Y-(C~-C10)-alkyl (straight-chain or branched),
Y-(C2-C10)-alkenyl ~straight-chain or branched, mono-
or diunsaturated), phenyl, benzyl, biphenylyl, F,
Cl, Br, CnF~1 (where n is equal to 1-4), CCl3 or
naphthyl,
Y z oxygen or sulfur, and
X = oxygen or sulfur.
Particularly preferred compounds I are those in which the
substituents have the following meanings
R(l) is H, (C1-C~)-alkyl (straight-chain or branched) or
benzyl (unsubstituted or mono- or disubstituted by
F, Cl, CF3, (Cl-C4)-alkyl (straight-chain or
branched) or OCH3),
R(2) is H, (Cl-Ca)-alkyl (straight-chain or branched),
(C3-C10)-alkenyl (~traight-chain or branched,
1S- 2~ 2~
mono- or diun~aturated), phenyl-(cl-c~)-alkyl
(~tra~ght-chain or branched), diphenyl-~C~-C~)-alkyl
(straight-chain or branched) or phenyl, the phenyl
system being unsubstituted or mono- or di~ubstltut-d
by ubstltuents fro~ the group comprlsing OH, F, Cl,
Br, (C~-C~)-alkyl (straight-ch~ln or branched), (Cl-
C~ lkoxy (~tralght-chaln or br~nched), phenyl and
benzyl,
R(3) is H
or
R(2) forms a -(CH2).- chain together with R(3) where m i~
equal to 4-5, in which a CH2 group can be replaced
by an o y gen or nitrogen atom, the nltroqen carrying
a hydrogen atom, a CH3, phenyl, benzyl or a phenethyl
qroup a8 a further bonding component,
and
R~4) i8 H, ~Cl-C10)-alkyl ~straight-ch~in or brsnched),
tC2-C10)-alkenyl (str~ight-ch~in or branched, mono-
or polyunsaturated), (C3-Cl5)-cycloalkyl tmono-, bi-
or multicyclic, (such a8, for ex~mple, norbornyl,
~dam~ntyl or decahydronaphthalenyl], Y-(C1-C10)-alkyl
(straight-ch~in or branched), Y-(C2-Cl0)-alkenyl
(straight-chaln or branched, mono- or polyunsatura-
ted), Y-(C9-Cl5)-cycloalkyl (mono-, bi- or multi-
cyclic), phenyl, ~-phenyl, ben~yl, blphenylyl, F,
Cl, CnF~*l (where n is equ~l to 1-4), n~phthyl or CN,
where Y iB equal to oxygen or ~ulfur,
and
:
R(5) is defined a~ R(4), R(4) ~nd R(5) beinq identic~l or
different,
and
~'
2~ 1~2~
- 16 -
R(6) is H, (Cl-C~)-alkyl (straight-ehain or branehed),
Y-(C~-C~)-alkyl (straight-ehain or branehed), F, Cl,
or CF3,
Y ~ oxygen or sulfur, and
X - oxygen.
Very particularly preferred eompounds I are tho~e in
whieh the substituents have the following meanings
R(l) is H or benzyl,
R(2) is H, (Cl-C~)-alkyl (straight-chain or branehed) or
phenyl-(Cl-C~)-alkyl (~traight-ehain or branehed),
the phenyl system being unsub~tituted or monosubsti-
tuted by F, Cl, (Cl-C~)-alkyl (straight-ehain or
branched) or (Cl-C~)-alkoxy (straight-ehain or
branehed),
R(3) i8 H
R(4) is H, (C1-C1O)-alkyl (straight-ehain or branehed),
(c2-clo)-alxenyl (straight-ehain or branehed, mono-
or polyunsaturated), (C3-Cl5)-eycloalkyl [mono-, bi-
or multieyclic, (sueh as, for example, norbornyl,
adamantyl or decahydronaphthalenyl)], Y-(Cl-C10)-
alkyl (straight-ehain or branched), Y-(C3-Cl5)-
eyeloalkyl (mono-, bi- or multicyclic), phenyl,
Y-phenyl, benzyl, biphenylyl, F, Cl or C~F~l (where
n is egual to 1-4),
where Y is equal to oxygen
and
R(5) is H, (C~-C6)-alkyl (straight-chain or branched),
(C2-C6)-alkenyl (straight-chain or branehed, mono-
or polyunsaturated), (C3-C6)-cycloalkyl, Y-(Cl-C6)-
alkyl (~traight-chain or branched), Y-(C9-C~)-cyclo-
alkyl, phenyl, Y-phenyl, benzyl, F, Cl or CF3
2 i~ ~ 3 2 ~
where Y 1B equal to oxygen,
R(6) is H, and
X is equal to oxygen.
Alkyl or alkenyl are in all ca~es either the straight-
chain or the branched groups.
The compounds I are prepared, for ex~mple, according to
US 4,191,765 (~OE 76/F llS). The compound~ used a~
starting material~ are either commercially available or
are obtained by known processe~.
The invention relates to the use of the co~pound~ I in
the form of the free base or of an acid addition salt a~
potent inhibitors of the exoenzymes of fungi.
Examples of pharmaceutically acceptable salt-forming
acids are inorganic acids such as hydrochloric acid,
hydrobromic acid, hydriodic acid, sulfuric acid, pho~-
phoric acid or nitric acid or organic acids such as
malonic acid, oxalic acid, gluconic acid, c~mphorsulfonic
acid, benzenesulfonic acid, acetic acid, propionic acid,
p-toluenesulfonic acid or salicylic acid.
The compounds I have at least one asymmetric c~rbon atom
~nd can therefore occur as enantiomers and diastereomers.
The invention includes both the use of the pure isomers
and of their mixtures. ~ixtures of diastereomers can be
separated into the components by customary method~, for
example by selective crystallization from suitable
solvent6 or by chromatography oa silica gel or alumina.
Racemates can be separated into the enantiomers by
customary methods, for example by salt formation with an
optically active acid, separation of the diastereomeric
salts and liberation of the pure enantiomer~ by means of
a ba~e. Moreover, the enantiomers can also be separated
by means of chromatography on chiral phases or by
en2ymatic routes.
- 18 -
The compound~ of the formula I, their aeld additlon alts
and their physlologlcally hydrolyzable derlv~tlvo~ are
useful pharmaeeutles. They partleularly have antimlcro-
bial aetivlty and are ultable for preventlon of and
treatment of fungal infectlon~ in human- and ln variou~
m~mmalian species.
The eompounds are vory highly aetive $n vitro agalnst
kin fungi uch as, for oxample, Trichophyton mentagro-
phytes, Mierosporum eanis, Epidor~ophyton flocco~um;
against mold fungi, ~uch as, for example, Asperg$11us
niger or against yeasts, ~uch as, for example, Candida
albieans, C. tropiealis, Torulopsis glabrata and Trieho-
sporon cutaneum or against protozoa such as Trichomonas
vaginalis or T. fetus, or alternatively against gram-
lS positive and gram-negative bacteria.
In vivo a~ well, for example in experimental renal
Candidiasis of the mouse, the co~pounds have a very good
6ystemic effect after oral or parenteral administration
against, for example, Candida albicans. In thi~ case, the
exoenzyme system of the yeast Candida alb$cans is in
particular affected in such a way that the pathogenicity
of the paShogens is distinctly reduced. There is likewise
a very good effect against various pathogens of skin
mycoses (for ex~mple Trichophyton mentagrophytes) in the
guinea pig after oral, parenteral or local admini~tra-
tion.
Examples of indication areas in human medicine which may
be mentioned ares
Dermatomycoses and sy~temic mycoses caused by Tricho-
phyton mentagrophytes and other Trichophyton species,Microspora species, Epidermophyton floccosum, and bi-
phasic fungi and mold fungi. In particular, deep-seated
mycoses, which are caused by Candida albicans, are
favorably affected, a6 in this case penetration of tha
fungi into the host cell is prevented or made difficult.
2 t~ J .~ ..t. ~
Ex~mple~ of lndlcation ~r ~ ln ~ terln~ry mediolne whioh
may be mentloned are~
All dermatomycoses and ~y temic myco~es, in particular
those which are cau~ed by the abovementioned p~thogen-.
The present invention lncludes phar aceutloal prepara-
tions whlch, in addition to non-tosic, inert pharmac-uti-
cally uit~ble escipient~ contain one or more active
compounds or which comprl-e one or re active compounds
used according to the lnvention and processes for the
production of these preparations.
Non-toxic, inert pharmaceutically suitable e~cipients are
understood as meaning solid, semisolid or liquid
diluents, fillers and formulatlon auxiliaries of any
type.
An inhibitor for the different phospholipnses of Candida
albic~ns must be pre~ent in suffi¢ient concentrations
everywhere in the patient where the fungus can colonize-
the parenchyma. Thi~ f~ct assume~ that the corresponding
substances have to be administered in a concentration
which has previously proved effective in anim~l exper-
iments.
In the case of the severe symptoms of deep-seated candi-
diasis, the patients are usually in a very poor general
condition. High fever and other disorders are frequently
to be found. In the dosage instructions, a different-
iation must be made between the prophylactic dose and the
therapy in the case of established infection. In the case
of prophylaxi~, it is possible to start from a better
general condition of the patients which enables oral
admini~tration. In this case, tablets, solutions, gels or
inspissated ~uice can be used. In form6 having esta-
blished deep-seated candldiasis, treatment mus~ often be
started from the fact that controlled oral absorption of
the active compounds is not always ensured. Parenteral
~ 3
- 20 -
administration forms are then ~uitable for thie purpose.
In exceptional cases, ~ubcutaneous administration can
al80 be considered.
Suitable candidates for prophyl~xi~ ar- primarlly immuno-
compromi~ed patients, who ~re in thi~ ~ltuation owinq toappropriate medicinal ~tre~ or owing to immune problem~
specific to the body. Tbe~e are in particular transplant
patients, diabetics and/or ~dipose patients, AIDS
patients, patients under chemotherapy, those receiving
long-term ventilation etc.
The compounds show inh$bitions of the phospholipase of
Candida albicans which are f~r below the minimum inhibi-
tory concentr~tions of the active compound~ against
Candida albicans found in vitro. The dosage can therefore
as a rule be below that which would be necessary for a
pure antimycotic therapy.
The action in the patient is based on the fact that the
active compounds induce two different effects in the
Candida cells which are in the vicinity of the paren-
chyma. On the one hand, the adhesion of the yeast cellsto the body cells iB prevented and on the other hand
Candida albicans is as a result prevented from penetrat-
ing the body cells with germ tubes.
As a result of this dual concept of action, the yeast
cannot expre~s its pathogenicity completely. However, it
must be mentioned that, apart from the phospholipases,
Candida albicans additionally has other pathomechanisms
~uch as, for example, protease. The attachment to the
body's own cells is, however, the primary step for
penetrntion. As this attachment is prevented by phospho-
lipase inhibitors, the other pathomechanisms are unable
to act completely.
Possible administration forms are, for example, t~blets,
coated tablets, capsules, pills, aqueous solutions,
29 il~3~ ~
- 21 -
suspension~ and emulslon~, optionally ~t-rlle in~ectable
solutions, non-aqueous emul~ions, ~u~pen~ion~ and ~olu-
tions, ointments, ereams, pastes, lotion~, ~prays ete.
The prophylaetieally and therapeutleally w tlve eompound~
should preferably be pre--nt in the ~bovementioned
pharmaeeutieal preparations ln a eoneentratlon of ~bout
0.1 to 99.5, preferably of ~b~ut 0.5 to 95 ~ by weight of
the total mi~ture.
The abovementioned ph~-rm~ceutical preparation~ can al~o
contain other pharmaceutical active compounds in addition
to the active compounds used according to the invention.
The abovementioned pharmaceuticsl preparation6 are
prepared in a eustomary manner by known methods, for
example by mixing the active eompound or the aetive
lS eompounds with the excipient or the exeipients.
The present invention inelude~ the u~e of the active
eompounds seeording to the invention and of pharmaeeuti-
~ eal preparations which eontain one or more active eom-
; pounds aecording to the invention in human and veterinary
medicine for the prevention, amelioration and/or cure of
the abovementioned diseases.
The active compounds or the pharmaceutical preparations
can be administered locally, orally, parenterally,
intraperitoneally and/or rectally.
In qeneral, it has proved advantageous both in human and
in veterinary medicine to administer the aetive
compound(~) used aeeording to the invention in total
amount6 of at least about 0.05, preferably 0.1, in
partieular 0.5 mg/kg of bodyweight up to at mo~t about
200, preferably up to 100, in partieular up to 10 mg/kg
of bodyweight every 24 hour~, if appropriate in the form
of several individual do~es to aehieve the desired
result~. The total amount is administered ~n 1 to 8,
- 22 ~ 2 ~S
proferably ln 1 to 3, lndlvldual dose~, but ln deep-
seated myeoses over cubstantlally longer perlod~ of t~me
(up to 6 weeks).
However, it may be neee~ary to depart from the do~age~
S mentloned, ln partleular dopendlng on th poelo~ and the
bodyweight of the ~ub~-et to be tr ated, the nature ~nd
the sever~ty of the d~ease, the manner of preparatlon
~nd administration of the pharmaeeutle~l and the perlod
or lnterv~l within whleh adminlstration takes pl~ee.
Thus, in ~ome e~es it may be ~ufflelent to m~nage with
les~ than the abovementioned amount of aetive eompound,
while in other ease~ the ~bovementioned amount of aetive
eompound must be exeeeded. The optimum dosage and manner
of administration of the aetive eompounds neees~ary in
lS each case c~n easily be determined by ~ny person ~kllled
in the art on the basis of his expert knowledge.
The compounds of the formula I are also aetive as
bioeide~. They are distinguished in particular by their
funqicidal activity in phytopathogenie fungi. ~ven fungal
disease pathogens whieh have already penetrated into the
plant tis~ue ean be eontrolled successfully. This 18
particularly important and advantageous in those fungal
diseases which can no longer effectively be eontrolled
using the otherwise eustomary fungieides after infeetion
has set in. The spectrum of action of the compounds ~
ineludes a large number of different phytopathogenie
fungi, such as, for example, Botrytis cinerea, powdery
mildew fungi, Drechslera teres, Venturia inaequalis, Cerospora
beticola, Phytophthora infestans, Plasmopara viticola, various
rust species, but especially Plasmopara viticola, in fruit,
vegetable, cereal and decorative plant cultivation.
The compounds ean be applied in the customary prepara-
tions as wettable powder~, emulsifiable concentrates,
~ sprayable 601utions, du~ting agents, seed treatment
; 35 agents, di~persions, granules or microgranules.
- 23 - 2 ~ ~J~J.
Wettable powders are under~tood a8 meaning preparations
which can be unlformly di~persed ln water, which apart
from the active compound ln addit$on, if appropriate, to
a diluent or inert ~ubstance, addltlonally contain
wetting agents, for ex~mple polyoxy thylated alkyl-
phenols, polyoxyethylated fatty alcohol~, alkyl- or
alkylphenylsulfonates and di-per-ing agent~, for example
sodium ligninsulfonates, ~odium 2,2~-dinaphthylmethane-
6,6'-di~ulfonate, sodium dibutylnaphthalenesulfonate or,
alternatively, sodium oleoylmethyltaurlde. They are
prepared in a customary manner, for ex~mple by grinding
and mixing the components.
Emulsifiable concentrates can be prepared, for ex~mple,
by dissolving the active compound in an inert organic
~olvent, for esample butanol, cyclohexanone, dimethyl-
formamide, sylene or, alternatively, higher-boiling
aromntics or hydrocarbons with the addition of one or
more emul~ifiers. In the case of liquid active compounds,
the solvent component can al~o be entirely or partially
omitted. Examples of emulsifiers which can be used are: -
calcium alkylarylsulfonate~, such a8
C8 dodecylbenzenesulfonate, or nonionic emulsifiers such
as fatty acid polyglycol esters, alkylaryl polyglycol
ether~, fatty alcohol polyqlycol ethers, propylene oxide/
ethylene oxide condensation products, fatty alcohol/
propylene oxide/ethylene oxide condensation products,
alkyl polyglycol ethers, sorbitan fatty acid ester~,
polyoxyethylene sorbitan fatty acid esters or polyoxy-
ethylene sorb$tol esters.
Dusting agents are obtained by grinding the active
compound with finely divided solid substances, for
example talc, or n~tural clays such a8 kaolin, bentonite,
pyrophillite or diatomaceous earth.
Granule6 can be prepared either by spraying the active
compound onto ad~orptive granulated inert material or by
applying active compound concentrates to the ~urface of
~ ~ ?
-- 24 --
carrier ub~t~nee~ ~ueh a8 ~nd, k~olinlte or of gr~nu-
lated inert materl~l by means of bindors, for ox~mple
polyvinyl aleohol, sodium polyaerylato or, alternatively,
mineral oils. Suitablo ~etivo eompounds e~n al-o be
granulated in the cu-tomary mann r ~or the preparatlon of
fertilizer granules - if de~irod mix d with fertilizers.
In wettable powders, the ~etive eompound eoneentration
is, for ex~mple, about 10-90% by weight, the remainder to
100% by weight eomprises eustomary formulatlon eom-
ponents. In the ease of emulsifiablo eoneentrates, theaetive compound eoncentration may be ~bout 10-80% by
weight of aetive eompound, in sprayable ~olutions about
1-20% by weight. In the ~ase of gr~nules, the ~etive
eompound eontent partly depends on whether the active
compound i8 present as a liquid or solid and which
granulating auxiliaries, fillers ete. are used.
In addition, said aetive compound formulations optionally
contain the adhesives, wetting agents, dispersants,
emulsifers, penetrants, solvents, fillers or carrier
sub~tances customary in each case.
For applieation, the concentrate~ present in eommereial
form are diluted, if appropriate, in a eustomary manner,
for example by means of water in the ease of wettable
powders, emulsifiable concentrates, dispersion~ and in
some eases also in the case of mierogranule~. Dust-like
and granulated preparations ~nd al80 sprayable solutions
are eustomarily not diluted further with other inert
substanee~ before applieation.
Nixtures or mixed formulations with other aetive com-
pounds, sueh as, for example, inseetieides, acarieides,herbieides, fertilizers, growth regulators or other
fungicides may also be po~sible, under eert~in eireum-
stances it also being possible to ~ehieve synergistie
increa~e~ in aetion.
2 ~ ~ r 1 ~3 ~ ~j
~ 25 ~
Some formulatlon ex~mple~ may be montloned in the follow-
ing~
A dustinq agent iB obtained by mixing 10 parts by weight
of aetive ¢ompound and 90 part~ by waight of talc a- an
S inert substanee and eomminuting ln a hammer mlll.
A wettable powder whieh i~ oasily di-per~ible in water i-
obtained by mixing 25 part~ by weight of aetive eompound,
65 parts by woight of kaolin-eontaining quartz a- an
inert substanee, 10 part~ by weight of potassium lignin-
sulfonato and 1 part by weight of odlum oleoylmPthyl-
tauride as a wetting agent ~nd disporsant and grinding in
a pinned-disk mill.
A dispersion eoncentrate which is easily dispersible in
water is prepared by mixing 20 parts by weight of aetive
compound with 6 parts by weight of alkylphenol polyglycol
ether (Triton X 207), 3 parts by weight of isotridecanol
polyglyeol ether (8 E0) and 71 parts by weight of paraf-
finic mineral oil (boiling range for example, nbout 255
to over 377-C) and grinding in a friction ball mill to a
fineness of less than 5 mierons.
An emulsifiable concentrate can be prepared from 15 parts
by weight of aetive eompound, 75 parts by weight of
eyelohexanone as a solvent and 10 parts by weiqht of
oxyethylated nonylphenol (10 E0) as an emulsifier.
As an example of the inhibition of the pathogenie phase
of dimorphie yea~t eell~, results of ~n in vitro enzyme
test are shown in which the percentage inhibition of
exoenzymes relea~ed, in particular of lysophospholipase
(phospholipase B) released, is determined as a measure of
the activ~ty.
To determine the enzyme inhibition, a suspension of
Candida albicans blastoconidia (strain 200J175), in which
the baeterial density was ad~usted photometrieally to an
- 26 ~ 92~3
absorption of 0.5 ~500 nm)~ was mixed w~th preparation
solution or, for the control, with solvent solut$on, to
be preclse
a) 100 ~1 of preparation solution (su~pension)
+ 900 ~1 of bActerial ~uspenslon
b) 100 ~-1 of solvent
+ 900 pl of bacterisl suspension
5 ~1 each of the bll~toconidia ~u~pension which had been
incubated at 21-C for 30 min were added dropwise to an
agar plate (S_bouraud agar with the ~ddition of 8~ egg
yolk, 1 M NaCl, 5 mM CaCl2).
The plate thus inoculated was incubated at 37C for 3
days.
Evaluation wa~ carried out by
1. deten~ining the diameter (mm) of the Candida
albicans colony (treated and untreated) and
2. detem~ining the total diame~er of colony and tur-
bidity halo which was caused by exoenzymes (treated
and untreated).
A value which wa~ to be regarded a8 a measure of the
enzyme activity re~ulted from the determination of the
~uotient of preparation and control group.
As can be ~een from Table 1, the compounds used according
to the invention inhibit the exoenzyme~ released sub~t_n-
tially more strongly than propranolol.
Propranolol i~ de~cribed in the literature (Pappu A.S. et
al. in Biochem. Pharmacol. 34, 521-24, 1985) a8 the most
active substance which showed inhibitory action ~gainst
phospholipase from liver cells in an ~ppropriate in vitro
30 test.
2 ~ C,,~
- 27 -
The pr$or art ~8 surpri~lngly clearly excelled by the
compounds I used according to the invention.
- 28 -
T~ble 1 2 ~
Percent~ge inhibition of the ~o~nzyme~ rele~sed from
C~ndid~ ~lbic~nJ in vltro
Prep~r~tionConcentr~tlon % inhibltlon of
l~m~lJL-- ~ho~pholip~e
, 1 . 1 ,~
~ W
2-CH2-NH S 62,5
~ ~ HO-CH-CH2-O
.~ 1.2
CH3 OH
CH3-C-NH-CH2-CH 3 5 50
HCl ~
CH3
1.3
CH3 ~ OH
J N CH2 C~H ~ 10 42,8
~ 1.4
: HO- ~ -CH2 - C- NH -CH2 ~ 50 100
CH3 HO-~H-CH2-O
COOH-COOH
.._
- 29 - 2Q l~3 ~ 1 ~
Co~t~nu~tlon of ~ble 1
Prop~r~tlonConcentr~tion ~ lnhibltlon of
(~m/ml) pho-phollp~-e
1.5
CH3 ~ -CH2-C-NH-CH2_CH SO SO
8Cl 3 ~ ~
~ a
1.6
~ OCH3- ~ _CH2 _ C -NH -C~2 10 87.5
: CH3 HO-CH-CH2-
~ . HCl
:
~ 1.7
; .
CH3
; CH-NH-CH2 ~ lO 50
HCl ~
.
1.8
CH3~ . 50 ~5
CH-N~-CH2 ~ .
CH3 HO-CH-CH2-0 ~ No2 10 25
HCl
:L9
CH-NH-~CH2 ~ lO . 75
CH3 HO-CH-CH2- ~ Cl
HCl ~
- 30 -
Continuation of Table 1 . 2
Preparation Concentration % inhibition of
(um/ml)~hospholl~e
-
1 .1,0
CH3~
CH-CH2 ~ 10 75
CH3 ~ CH-NH-CH2
CH3 HO~H-~H2-0
~Cl
. .
1.11
CH-NH-CH2 3 ~ 40
CH3 HO-CH-CH2.0- ~ C~3
HCl ~
1.12 Cl
~ o-cH2-CH-CH2-NH-cH2 ~ Cl 100 1OO
Cl ~
.13 50 100
CH3 10 30
CH3-0- ~ -CH2 - C - NH - CH2
~Y I ~0- ~-CH2-0
CH3
.. _ _ . .....
1.14
100
CH2-CH2 N~-CH2 f~ 10 33
HO- CH- CH2- O- ~_ C~2 ~=7
,
- 31 -
Contin~tion of Table 1
Preparation Concentration ~ inhibition of
(~m/ml) phosphollp~e
l.lS 50 ~6
; ~ -CH2-CH2-NH CH2 10- . 29
CH O CH CX O ~ ~
~ '' 100 ' ioo
1.16 ~ ~IH 50 100
C~3 CH3 . ~Cl
CH ~CH3 o~ . HCl 100 100
1.17 C ~ 2 CH ~ 50 100
~q -
CH3 50 91
H~ ~ NH ~ CH . 10 .14,3
Ho-t!-CI-oH ~
C113 .
-
- 32 - 2~
Continuation of Tabl~ 1
Preparation Concentration % lnhibition of
~m~ml) pho~phollDa~e
Cl ~ 10O 100
C-CH2-o~2-NH-lCH2)3-cH3 50 87,5
Cl ~
CH3
Cl ~ ~CH CH3 100 100
Cl ~ CH~ CH
1.21 100 100
Cl ~ ~ 25
~ .
1.22
CH3~ , CH3 100 100
CH 50 100
; ~CH2
C~2 oH CH3
CH2~o~2-N~ClH3
. HCl
_ _.
1.23 oH CH3
O~ ~-N~ 100 100
~CH2)4 ~ CH3 S0 100
CH3 ~ . HCl
1.24
CH CH3 100 100
0-CH2-c~-c~2-N*~c~3 50 1OO
CH3-(CH2)4 ~ c~3
. HCl
Propranolol 100 30
33 - ~ 3?J'~
Continu~tlon of ~ble 1
Prepar~tion Concentr~tion % inhibition of
/ml) ~hoJ~holi~e
t,~ 25 OH
o~ so ~00
t.26 OH
~,r ~ ~ ~ ~O ~00
s æ
t.27
~r ~ ~, , lSoO ,oo
t.28 o
O ~ 100
1 .29
~NH~O~ 10
t . 30 OH
100
N O~ 00
- 34 -
2 ~ A 9 ~
Continu~tion of ~ble 1
Prep~r~tion Concentr~tion ~ inhibit~on of
(~m/ml~ pho~holl~e
o~ul~t ~~ l ~o
-3~ ~ 1OO 100
- 10 100
'33 ~ 100 1OO
1.34 . ~ ~ ~ 100 loo
~_~ 50 100
~ ~ ( lo loo
lOC
'-3~f~ ~3 100
- 35
Continu~tion of T~ble 1
Prep~r~tion Concentr~t$on ~ inhibition of
(~m/ml) pho-pholip~e
1.36
,~,~O~
100 100
l~,c~ so 100
100
H~ a- 5 8
OH
1-37 ~ r~ ~ ~ ~ C~ lOo lOo
~ ~ 50 loo
~,~ ~
~3
0~0 "
1.3B ~ a~ loo loo
~0
H~C
1 .39
CH
H~IC ~o~NH~CH3
H3~a ''ff~, 100 loo
H~o~
~'
2~'~32 ~ ~
- 36 -
Contlnu~tlon of T~ble 1
Prep~r~t$on Concentr~tion~ inhibition of
~m/m~ Lpho~phollp~
.40
~ 50 100
~ ~:~ 1 10 100
~ 5 17
1.41 C 100 100
~o~ ~ SO 100
!iO
1 .42
100 100
~ 0~1 C"H~ 50 100
1 .43
100
O ,C"H" 5 100
.44
~ ~ 100 100
\~ OH ~ S0 100
~N ~ HN~J----I~J 10 4~i
1 .45
~ ~ 100 ~00
HO ~ ~_1 S0 ~`
HN _~NHJ o ~I
- 37 ~ 2~ $
Contlnuation of Table 1
Preparation Concentration % inhibition of
(um/ml) pho~pholi~ase
1, 46 HO 10 100
~NH~O~ ,~ S 100
1. 47 HO l00 100
O~O~NI-J O~¢~
.48
~--~ HO 10 100
$~NHJ O~~ 5 ~
~00 100
'~ 49 \ --~NHJ~O~ 50 100
1. 50 OH l00 56
~,~J~,~13~ 23
OH 100 100
o~OH OH 10 107~
100 100
.51 OH
~V~~,~ 50 100
- 38 - 2 ~ ~ 3 2 l ~
Contlnu~tlon of Table I
Preparation Concentration ~ inhibition of
( um/ml ) phosphollDa~e
.52
- o
)=\ OH 100 100
/ ~N~ ~o~ so loo
.s3
_O OH
~J
1.54 OH
~ 100 loo
1.55
~ ~ 50 lOD
1.56
100 loo
- 39 -
Continu~tion of T~ble 1
Prepar~t$on Concentr~tion % inhibltion of
(p4lml) ~ho~phollp~e
1.57 r o
F ~ ~ ~ 100
1.58
~~ 100 100
f~ ~ 50 lOO
1.59 a loo loo
~: ~~ 50 lOO
1.60 ~
0~ 100 100
lO0
l,61 ~f ~ o ~ ~ lOO lO0
~a~ 50 60
.,
- 40 ~ ?~ ~J ',~
Cont$nu~tion of T~ble 1
Prep~rntlon Concentr~tion ~ lnhibition of
(um/ml) pho~phollp~e
1.6~ ~ ~
1.63 ~ a~ ,a loo loo
~ ~~ W 50 100
~ 10 100
~ 5 34
1. 64 OH 100 100
~0~ 10 30
1.65
100
~ O S ~
: t.66
So 100
~0~
1.67
100
~ OH ~; 100
I~,HN ,~O~
_ 41 ~ 3 ~ i t~
Continuation of Table 1
PreparationConcentration % inhibition of
(um/ml) phospholi~ase
. 1.68
~o ~oo ~oo
OH ~ S0 ~
l~ ~oJ~
HO 100 100
~NHJ ~k 100
~ Q i~ l?
- 42 -
Sy~temic Candlda albican~ infection ln the mou~e i8 used
as an ex~mple of the ~y-temlc in vivo actlvity of the
compounds.
It i8 the aim of thl~ method to det rmlne the effect of
S preparations on a ~y~temie Candlda albicans lnfeetion.
The infeetion leads to the death of the anim~l~ wlthin
2-4 d~ys.
Deseription of the methods Albino miee (NNRI, male,
15-20 g bodyweight) ~re lnfeeted intravenously with
; 10 Candida ~lbicans yeast eells (str~in 200/175) whieh h~ve
been reeently precultured on m~lt ~gar ~nd suspended in
physiological saline solution. The infeetious dose which
leads to mortality within the timescale indicated was set
photometrically and contained a mlllion yeast cells per
mouse.
The compounds to be tested are administered either on
their own or in combination with a standard antimycotic.
In the te6t carried out, a combination preparation was
used, fluconazole (Pfizer) being used as the standard
antimycotic. Administration routes differ depend$ng on
the substance group, oral administration being given
preferenee. The mice are observed for 4 weeks, the
mortality i~ recorded daily, and the survival time6 of
the individusl groups are averaged and compared with each
other. Group size is on average 10 ~nimals.
The mean values are compared using the students T test.
As Table 2 shows, the survival time of mice infected with
Candida i8 increased by 65% (the ~ur~ival time of the
animals treated by means of flueonazole monotherapy was
defined as 100, whereupon 165 resulted for the eombina-
tion therapy) on admini~tration of, for example, compound
1.16 (14 x 50 mg/kg p.o. 1 x daily).
'~Qfl ~3~
-- ~.3 -
Table 2 Survival tLmes of miee infeeted with Candida on
eombination therapy with flueonazole
Compound Survlval tLme (b~-ed on
flueon~ole - 100%)
Flueonazole 100%
Flueonazole + eompd. 1.7 127
Flueonazole ~ " 1.9 1319
Flueonazole + " 1.10 149%
Fluconazole + " 1.16 165%
Dosages s
Flueonazole in each ca~e 8 x p.o. 50 mg/kg/day
p-Hydroxyethylamine in each ca~e 14 x p.o. 50/mg/kg/day
The fungicidal, antimycotic activity of the compound~ WaB
15 verified in some examples (~ee Table 3) by means of the
percentage destruction of Trichophyton mentagrophytes in
vitro (standard preparation rilopirox a~ a comparison
substance).
.
Table 3
Compound ~g/ml
2.5
1.1 100 100 99.8 99.498.7 80.8
1.2 100 100 100 100 98.7 92.3
1.3 100 100 99.8 98.974.9 52.7
1.17 100 100 100 99.887.1 73.4
1.18 100 100 100 100 95.7 79.3
Rilopirox100 100 100 100 96.2 94.1
(Value~ in 96 deEtruction of Trichophyton mentagrophytes
30 after 14 h in distd. water.)
2~ ~3~
- 44 -
ExumpleJ for th- fung~cldal ~etlvlty
V~ne ~eedllng-, Abo~t 6 ~eok~ old, w-~- unlformly ~-ttod
wit~ An aqueou- su~pon~on 0f tbo clal~od compound-
~ftor the ~pray eoatlng ~d dried on, th- pl-nt- wo~-
lnoculatcd wlth an ~gueou~ ~po~- u~pen~ion of ~ mopara
v~ticol~ and ~ncub-ted In ~ cllmat~c ch~mber for a ~hort
timo at 23 C and 80-90 X rel-tlve atmo~p~erlc humldlty
After ~ tlme of 7 ~ay~, ~h- pl~nt~ (f~ng~) sporul-t~d ~n
the above m-ntloned ch~mber The degr-e of ~nfe~tatlon o
tho vlne leaves W-8 ~s~e~cd as a,porcen~ag~ of th-
infested le~ves ln ¢ompari-o~ ~o the untr-~ted, lnfected
control plant- ~nd c~n b- ~oen from ta~le 4
Table 4
Compound~ according Degr-e of infestatlo~ of the ~lne
to example 1 leave~ wlth Plasmop~ra vltlcol~
in % at ppm act~ve lng~edient
(mg ~etlv- ~gent for liter
agueou6 ~u~pon~on)
S00 250 125
_
1 32 0 0 0
1 37 0 0 0
untre-t-d, inf-eted 100
control pl~nts
.
It 18 obvlou6 that tbe plant~ tre~ted wlth the compounds
ccordlng to the lnventlon are free of Infest~tlon