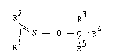Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ PP 357~1
2V~ 3 ~
NOVEL COMPOUNDS
The present invention relates to oxime ether derivatives which are
useful as herbicides, to process for their preparation and herbicidal
compositions containing them.
According to the present invention there is provided a compound of
formula (I):
where Rl is alkyl, optionally substituted aryl or optionally substituted
heterocyclyl;
R is hydrogen, optionally substituted lower alkyl, lower alkoxy,
cycloalkyl, haloalkyl, lower thioalkyl, halo or cyano; or the group
is a group of sub formula (i): R2.
where q is O or an integer of from l to 4 and groups R6 are selected
independently from hydroxy, alkyl, alkoxy, alkylcarbonyl, halogen, nitrile,
nitro, haloalkyl and haloalkoxy; X is oxygen or sulphur, p is O or 1, n is
15 0 or l and m is 1, 2 or 3 provided that when n is 0, p+ m is 2 or 3 and
when n is 1, p+ m is 1 or 2;
R3 and R4 are independently selected from hydrogen, halo, alkyl or
alkoxy or R3 and R4 together with the carbon atom to which they are
attached form a carbocyclic ring; and
R5 is CN; Co2R7 where R7 is hydrogen, a cation or an esterifying
group; a group -CYNR8R9 where Y is oxygen or sulphur and R8 and R9 are
independently selected from hydrogen, hydroxy, alkyl or alkoxy or together
with the nitrogen atom to which they are attached form a heeerocyclic ring;
or R8 together with R3 and the group -C(O)N- to which it is attached form a
heterocyclic ring, or R5 is a group of sub formula (ii)
where R10, R11, R12 and R13 are independently seleceed from hydrogen or
alkyl; or a group S()r R14 where r is 0, 1 or 2 and R14 is hydroxy, lower
alkyl or aryl.
In particular, the invention provides compounds of formula tI) where
Rl is alkyl, optionally substituted aryl or heterocyclyl;
R is hydrogen, optionally substituted lower alkyl, lower alkoxy9
cycloalkyl, haloalkyl, lower thioalkyl, halo or cyano; or the group
is a group of sub formula (i):
- 2 - 6 2 ~ ~d3 7
where q is 0 or an integet of from 1 to 4 and groups R are selecte
independently from hydroxy, alkyl, alkoxy, alkylcarbonyl, halogen9 nitrile,
nitro, haloalkyl and haloalkoxy; X is oxygen or sulphur, p is 0 or 1, n is
0 or 1 and m is 1, 2 or 3 provided that when n is 0, p+ m is 2 or 3 and
when n is 1, p+ m is 1 or 2;
R3 and R4 are independently selected from hydrogen, halo, alkyl or
alkoxy or R3 and R4 together with the carbon atom to which they are
attached form a carbocyclic ring; and
R5 is CN; C02R7 where R7 is hydrogen, a cation or an esterifying
group; or a group CoNR8R9 where R8 and R9 are independently selected from
hydrogen, alkyl or alkoxy or together with the nitrogen atom to which they
are attached form a heterocyclic ring; or R8 together with R3 and the group
-~N- to which it is attached form a heterocyclic
ring, or R5 is a group of sub formula (ii):
where R10, R11, R12 and R13`are independently selected from hydrogen or
alkyl; or a group S()r R14 where r is 0, 1 or 2 and R14 is hydroxy, lower
alkyl or aryl.
As used herein the terms "alkyl" and "alkoxy" refer to straight or
branched chain alkyl or alkoxy groups respectively, suitably having from 1
to 10 and preferably from 1 to 6 carbon atoms. The qualification "lower"
means that the number of carbon atoms present is suitably from 1 to 4.
Similarly the term "haloalkyl" refers to an alkyl group as defined
above substituted by one or more halogen atoms. Suitable halogen atoms for
haloalkyl and halo groups include fluorine, chlorine, bromine and iodine.
~5 The term "aryl" used herein includes phenyl and napthyl. The term
"heterocyclyl" includes ring systems having 5 or 6 ring atoms, up to 3 of
which are selected from oxygen, nitrogen and sulphur.
Suitably R1 is an optionally substituted aryl in pareicular phenyl or
heterocyclic group.
Examples of substituents for aryl or heterocyclic groups Rl include
one or more groups selected from hydroxy, alkyl, alkoxy, alkylcarbonyl,
halogen, nitrile, amino, nitro, haloalkyl, haloalkoxy, or a group S(o)tR15
where t is 0, 1 or 2 and R15 is alkyl.
Suitable substituents for aryl groups R1 includes one or more groups
selected from hydroxy, alkyl, alkoxy, alkylcarbonyl, halogen, nitrile,
2 ~
-- 3 --
nitro, haloalkyl and haloalkoxy.
Preferred substituents for aryl groups R1 are C(1 6) alkyl, C(1 6)
alkoxy, lower alkyl carbonyl, halo C~1 6) alkoxy or halo wherein the halo
groups are selected from fluorine, chlorine, bromine and iodine.
Particular examples of the substituents are methyl, methoxy, ethoxy,
bromo, fluoro or chloro.
Further examples include amino or S02CH3.
Preferably the substituents are in the ortho or meta position on the
phenyl ring, most preferably on the ortho position.
Examples of heterocyclic groups Rl include pyridyl, thiophenyl,
pyrrole, furyl and thiazolyl.
Suitable heterocyclic groups R1 include pyridyl, thiophenyl, pyrrole
and furyl and thiazolyl.
Suitable substituents for heterocyclic groups R1 include alkyl in
particlar methyl. The substituents may be attached to a carbon or
heteroatom where possible. In particular the substituted may be attached
to a nitrogen atom in the ring.
Suitable optional substituents for alkyl groups R include those
listed above in relation to aryl or heterocyclic groups Rl.
Examples of groups R2 are hydrogen, lower alkyl, lower alkoxy, lower
haloalkyl, C3_6 cycloalkyl, lower thio alkyl, halo or cyano.
In particular R is hydrogen, methyl, ethyl, tri~luoromethyl,
iso-propyl, methoxy, cyclopropyl or thiomethyl.
Suitably R2 is hydrogen, lower alkyl, lower alkoxy, C3 6 cycloalkyl,
lower thio alkyl, halo or cyano.
In particular R2 is hydrogen, methyl, ethyl, iso propyl, methoxy,
cyclopropyl, thio methyl or cyano.
When R1 and R2 together form a cyclic group of sub formula (i)
suitable groups are those where n is 0. Particular groups are those where
30 n is 0, p is 0 and m is 2 or 3 or n is 0, p is 1 and m is 2.
Preferably X is oxygen.
Suitable groups R6 include those substituents list above for R1.
Preferably R6 is halogen such as fluorine. Preferably q is 0 or 1, most
preferably 0.
Suitable groups R3 and R4 include hydrogen, lower alkyl, lower alkoxy
or halo.
2 ~ i~ 3 ~ ~3 L~
Examples of groups R3 and R4 include halogen, methyl, methoxy, ethyl,
ethoxy, iso-propyl or fluoro.
Par~icular examples of R3 or R4 are hydrogen, methyl, ethyl, methoxy,
ethoxy or fluorine.
Preferably R3 and R4 are other than hydrogen.
Where R3 and R4 together form a carbocyclic ring, the ring suitably
contains from 3 to 7 carbon atoms.
Suitable groups R5 are -CYNR8R9 where Y is 0 or S and R8 and R9 are
independently selected from hydrogen, lower alkyl such as methyl or ethyl
or hydroxy.
Suitable groups R5 are -CONR8R9 where R8 and R9 are independently
selected from lower alkyl such as methyl or ethyl.
When R8 and R9 together with the nitrogen atom to which they are
attached form a heterocyclic ring, suitable rings include pyrrolidine,
morpholine or azetidine rings.
When R8 together with R3 and the C(O)N group to which it is attached
form a heterocyclic ring, it suitably contains from 5 to 7 ring atoms,
preferably 5 carbon atoms.
Uhere R5 is C02R7, suitable esterifying groups R7 include alkyl,
alkenyl, alkynyl or phenyl any of which may be optionally substituted.
Suitable optional substituents for R include one or more groups
selected from halo such as fluoro, chloro, bromo or iodo; hydroxy; C1 6
alkoxy; nitro; cycloalkyl; heterocyclic optionally substituted by oxo;
nitrile; phenyl optionally substituted by nitro, halo such as chloro,
alkoxy or carboxy or salts or C1 6 alkyl esters thereof; or alkylsilyl
groups such as trimethylsilyl.
When R7 is a cation, it is suitably selected from agriculturally
acceptable cations such as sodium, potassium, calcium, anomonium or
substituted ammonium ions. +
Suitable substituted ammonium ions are NRaRbRCRd where Ra, Rb, Rc and
Rd are independently selected from hydrogen or optionally substituted
alkyl.
Preferably Y is oxygen.
Preferably R5 is a group CYN~3R9.
S it bly R10 R11 R12 and R13, in sub formula (i) are hydrogen.
`::
2~ ~37~
~ 5 --
When R5 is a group S(o)rR14, R14 is suitably lower alkyl in particular
methyl or ethyl.
Particular examples of compounds of formula (I) are se~ out in Tables 1 to
3 below.
. :. : , . :. : ~
C~
~ ~ c~ m~ C~
5: m m m m m : :: m ::C m
V ~ V ~
æ z :z z æ æ z; z z z
m ~ ~ ~ m
¦ ~ m ~ 3: m :~ m ~ ~ :~ ts
i~l
C~; ~ ~ ~ ~) C~ t~ ~ C~ ~'~ ~
~ t~
~u~ mu~ mu~ mu~ 3 mn u) ~
~ ~D ~ ~D ~D ~ ~
~; C~
æ ~ C~ ~ ~ ~ ~ ,~ GO o~ o
o
C~
- :: -- -
- 7 - ~. 3
C~
2 3~
z z; z æ æ æ
U~ o o o o o o
IY; ~
~ :~ V ~
~; ~ ~
~1 '`~; 5
3 W
~ ~
,~; . : -
;: ~i, .
.: , .
. :, . . . . . .
. .
:
- 8 - 2~3 ~37 13l.1
C~
u~ 5~
P; æ æ z æ z æ æ z
~o, o ~o, O C, O o ~o,
; 3~
~ ~lY; ~ .'
~ '
tl::~ o~
C~ ~ 3:: ~ tq q
~ ~ tq
~; ~ V~ 3
o o I~ 0~ O~ o
5~ ~ ~
..,
.. ...
2~ ~'7 ~
_ 9 _
C~
tC C~
U~
~;
Z o o ~ o~ o~ o o~
o~
~'`' m~ ~'`~
P;
c~ c~ D: ~:G~ ~ ~ :a~
E~
C~
~; ~ C~
O O u~ ~ O
æ Z ~
~ .
- : ~ . - : : ' : : .
- ' -: ' ''' '' ' ' :'
~:, I . .
,
~.
j;:
: : '
lo - 2 ~
3:~ r~ ~ f o~
U~
z z z æ ~;' z z; ~ z
~ 8
.~ ~ ~,
~; t~ ~ o o o
Hl c~ ~
~7
~;
.
~;
o o ~ ~ ~ ~r~ co a~ o _I
~ z ~ ~ ~ ~ ~ ~ ~ ~ ~
. ~ ~
- , . .
- 11 - 2~l~37~
U I V ~ ~ ~
c~ ~
z o~ o~ o~ ~) o~ o
O ~ 3 ~
C ~ ~
~1
.
~Y; ~
~Y
z ~ F ~ æ
... ..
- .
,
,
~ . .
-- 12 --
; C~
o
V~
.
~1 ~ ~ 5,
~ ~ ~ C`~
t~
C~ C~ ` ~ ~ C~
~; tq
æ c~
Z ~ ~ ~ ~ U
0 ~ u~ n ~ ~ ~ ~ u~
~)
,, ~.
13
37~
m m c~
U~
!Y; ~ ~ ~ ~ c~l
o o
U~
c ~ m
~!1 r~ ~
C~ ~ ~
~IY; ~ ) V ~ W
:- ~ ~ V~
~Z o ~ CO
- ., . . ~ .
.. . .~ , ... .
,. , ~, ..
.. ,.,. . `, , ~
. .` .
; ` ~ ,,
. . ~ , , ,~ . . ., :~
. .
.~
- 14 ~ 3~
Z z z; æ æ :z z z ~;
~K
~;
~; Zo;
~; ~
o o ~ o ~ C~ ~ ~ U~ ~ I~
P~ æ ~ r~
,
?
- 15 -
2 ~
~_ N
~ ~ O ~ ~~1
U~ t~ O
,Y: ~0 æ'' O~Z, Z~ ZO
~; ~
~J
wl ~ ~ v ~
~1
C~ ~
P~ ~ )
~C ~
o o ~ o ,~ ~ ~
~ Z r~
,.. . . ..
.,
- 16 -
20 ~37~!~
Q~ ~ ~ , ~,~
~P~
Z Z Z Z Z Z
r!~
C~ C::~ ~ ~ t~ ~ ~,~
~Y; C~
,, "
C~ V
Z ~ u~ ~D C~ cr
,
` . ,
. i
2 3~ _ 3 7 ~
C~
3:~ m~
u~ V
V
Z 0~ 0~ 0~ 0~ 0~ 0
~ o
C~ V C~
m
C~ ~ ~ ~ ~ ~ ~ C"
H ~ c~
lY~
`~
; V V C~
~1 h p~
3/
tY; I:4 Z
O O O ~ C~l ~ ~ U~ ~ r
` a:~
~4 Z ~
' ` : ~ ` , ` :. :` ' `
;
-- 18 --
2~1~3
r~ 3
v v 8 v
* c~
~; t~ ) v v
o ~p5 = = 3
m m q m v
v ~q m
~ \~ 3
.,
. ~ .
.
. ~ . . .
.
.. . ,
-- 19 --
20~37Bl.~
5~ 3
~Y; ~
~ ~ ~ C`l æ z z
o o o o o o o
m m c~ m m e: m
¦"~ =.-) =~ =o~ r =~
~ ~ ~ ~ ~7 ~ ~
m m m m m m
P; ~
~; ~ Z~ "
~: Z o O O O O o
~) ~1
,
_ 20 --
2 ~ 1 2 ~ 7
CO C~
U~ C~
~; æO ~ Z Z Z o
C.) ~ ) V ~)
`J~; ~
C~ ~ ~ ~ ~ ~ ~
~1 ~; c~
.
c~ e~
0~ C~
~ ~Z~
2 æ ~ ~ ~
2 ~
.' ' ' ' . `
,
. . . . .
:` ~'
-- 21 --
2~3~
~C ~I:
U~ C~ V
~Y; ~ ~ o o o o o
C~
17 t~
~; ~ o C~ V
o
C'~ ~ 3~ ~ ~ ~ I:q
~ ~;
~ ~ q m
~ [~ Z~ ~
, . ~
. o o
~ Z 0 ~ o
o ~ ~ ~
~ ` ~
2 2 - 2 ~ ~?~
U~ ~
C~ g
3~
~ C`J ~ C~
~IY;
C~ . ~ ~ ~ ~
H ¦ ~
~Yl
C~;
~;
"
P~ æ ~ ~ r~ o
:~: ~ C`l
C~
-- 23 --
2~31~ 9~
,~ ~ ~
~ 5:
.,~ C~
Z Z ~;
o ~, o
o o C~
~ D ~ ~
~ ~ es ~
,~ \~
P~ Z ~ ~ ~
o ,, ~ ,,
, ... . . ~ . .
_ 24 -
TABLE II 2 Q ~ ~ 7 o ~
R6 ~X)p(C~2)m 3 4 5
(GH2)n/
COMPOUND m p n X R3 R4 R5 R6
140 3 0 - H CH3 CON(CH3)2 H
141 2 0 ~ CH3 CH3 CON(CH3)2 H
142 2 0 0 - H CH3 CON~CH3)2 H
143 2 1 0 0 H CH3 CON(CH3)2 H
144 2 0 ~ CH3 CH3 C02CH3 _
~ '
- 25 -
TABLE III
3 7 ~ ~
R. (CH2)S
>---N-O--C ~1
R2 R4 \ C/ R9
COMPOUND NO Rl R2 R4 R9
145 Cl CH3 H CH3
Compounds of formula (I) can be prepared by reacting a compound of formula
(II)
wherein R1 and R2 are as defined in relation to formula (I);
with a compound of formula (III)
wherein R3, R4 and R5 are as defined in relation to formula (I) and Z is a
leaving group in the presance of a base.
Suitable bases include strong bases such as alkali metal hydrides in
particular sodium hydride. Other suitable bases are weaker bases such as
alkali metal carbonates for example potassium carbonate.
The reaction is suitably effected in organic solvent such as
dimethylformamide (DMF), dimethylsuphoxide, elevated temperatures for
examples of from 20 to 120C are suitably employed in the process.
Suitable leaving groups Z include halogen in particular chlorine and
bromine, mesylate and tosylate.
Compounds of formula ~II) can be prepared by reacting a compound of
formula (IV);
wherein R1 and R2 are as defined in relation to formula (I) with
- 26 -
hydroxylamine or a salt thereof in the presence of a base ~ ~ ~ 7p~ Lsium
carbonate. The reaction is suitably effected in an organic solvent such as
a lower alcohol in particular methanol at temperatures of from 20 to 60 C.
Compounds of formula tII) and (IV) are known compounds and can be
prepared from known compounds by conventional methods.
Compounds of formula (I) where R5 is a group Co2R7 can be converted to
compounds of formula (I) where R5 is CONR8R9 by (a) removing the group R7,
(b) converting the resultant acid ~o the acid chloride and (c) reacting the
acid chloride with an appropriate amine. Each of the steps (a) to (c)
involve standard chemical manipulations and suitable conditions and
reactions are well known in the art. Processes of this type are included
in the examples given hereinafter. Alternatively compounds of formula ~I)
where R5 is C02R7 can be converted to a group where R7 is a different
esterifying group by reacting the acid obtained in step (a) above with an
appropriate esterifying reagent such as an alcohol in the presence of an
acid. Again suitable conditions are exemplified hereinafter.
Uhen R5 is a group CSNR8R9 it may be prepared by thiolation of the
corresponding compound where R5 is CoNR8R9 as exemplified hereinafter.
Compound of formula (I) where R8 is S(o)rR14 and r is 1 or 2 can be
prepared by oxidation of corresponding compounds where r is 0. Oxidation
is suitably effected by means of conventional oxidising agent such as
potassium permanganate in a medium of glacial acetic acid under standard
conditions.
Compounds of formula (I) wherein R5 is CO3R7 can be converted directly
to compounds of formula (I) where R is CONR R by reac~ion with a compound
of formula (V)
wherein R8 and R9 are as defined in relation to formula ~I) and R16 and R17
are independently alkyl groups, for example methyl. The reaction is
suitably carried out in an inert organic solvent such as dichloromethane at
temperatures of from 25 to 50C. Suitably the reaction is effected under
an inert atmosphere for example of nitrogen.
Compounds of formula (V) can be prepared ln situ by reacting a compound
of formula (VI)
wherein R16, R17 and R18 are independently alkyl groups with an amine of
formula (VII)
- 27 - 2 ~ l~ 3 ~ ~ ~
wherein R8 and R9 are as defined in relation to formula (I) at low
temperatures for example of from 0 to 10C
A suitable compound of formula (VI) is trimethylaluminium.
This type of reaction is illustrated in Tet. Letts. 4171, 1977, S
Weinreb, Colorado State University. In some cases, the compound of formula
(I) where R5 is C02R7 can be converted to compounds where R5 is CoNR3R9 by
reaction directly with a compound of formula VII. This reaction is
particularly useful when R8 and R9 together with the nitrogen atoms to
which they are attached form a heterocyclic ring. The reaction is suitably
carried out in an inert organic solvent such as methanol at moderate
temperature of from 20 to 60C, conveniently at ambient temperature.
The compounds of the invention are capable of controlling the growth
of a wide variety of plants and in aprticular some show a useful
selectivity in crops such as soya, mai~e, rice and winter wheat. They may
be applied to the soil before the emergence of plants (pre-emergence
application) or they may be applied to the above ground parts of growing
plants (post-emergence application). In general the compounds are more
active by pre emergence application. In another aspecy~ therefore, the
invention provides a process of inhibiting the growth of unwanted plants,
~ by applying to the plants, or to the locus thereof, a compound of the
formula (I) as hereinbe~ore defined. The rate of application required to
inhibit the growth of unwanted plants will depend on, for example, the
particular compound of formula (I) chosen for use, and the particular
species of plant it is desired to control. However, as a general guide, an
~5 amount of from 0.01 to 5.0 kilograms per hactare, and preperably 0.025 to 2 kilograms per hectare is usually suitable.
The compounds of the invention are preferably applied in the form of a
composition, in which the active ingredient is mixed with a carrier
comprising a solid or liquid diluent. In another aspect, therefore, the
invention provides a herbicidal composition, comprising as an active
ingredient a compound of the formula (I) as hereinbefore defined, in
admixture with a solid or liquid diluent. Preferably the composition also
comprises a surface active agent.
The solid compositions of the invention may be for example, in the
form of dusting powders, or may take the form of granules. Suitable solid
diluents include, for example, kaolin, bentonite, kieselguhr, dolomite,
2~-~37~
- 28 -
calcium carbonate, talc, powdered magnesia and Fuller's earth. Solid
compositions also include soluble powders and granules which may comprise a
compound of the invention in admixture with a water soluble carrier.
Solid compositions may also be in the form of dispersible powders or
grains comprising in addition to the active ingredient, a wetting agent to
facilitate the dispersion of the powder or grains in liquids. Such powders
or grains may include fillers, suspending agents and the like.
Liquid compositions include solutions, dispersions and emulsions
containing the active ingredient preferably in the presence of one or more
surface active agents. Water or organic liquids may be used to prepare
solutions, dispersions, or emulsions of the active ingredient. The liquid
compositions of the invention may also contain one or more corrosion
inhibitors for example lauryl isoquinolinium bromide.
Surface active agents may be of the cationic, anionic or non-ionic
type. Suitable agents of the cationic type include for example quaternary
ammonium compounds, for example cetyltrimethylammonium bromide. Suitable
agents of the anionic type include for example soaps, salts of aliphatic
mono-esters of sulphuric acid, for example sodium lauryl sulphate; and
salts of sulphonated aromatic compounds, for example
dodecylbenzenesulphonate, sodium, calcium and ammonium lignosulphonate,
butylnaphthalene sulphonate, and a mixture of the sodium salts of
diisopropyl- and triisopropyl-naphthalenesulphonic acid. Suitable agents
of the non-ionic type include, for example, the condensa~ion products of
ethylene oxide with fatty alcohols such as oleyl alcohol and cetyl alcohol,
or with alkyl phenols such as octyl=phenol, nonylphenol, and octylcresol.
Other non-ionic agents are the partial esters derived from long chain fatty
acids and hexitol anhydrides, for example sorbitol monolaurate; the
condensation products of the said partial esters with ethylene oxide and
the lecithins; and silicone surface active agents (water soluble surface
active agents having a skeleton which comprises a siloxane chaib e.g.
Silwet L77). A suitable mixture in mineral oil is Atplus 411F.
The compositions which are to be used in the form of aqueous
solutions, dispersions or eulsions are generally supplied in the form of a
concentrate containing a high proportion of the active ingredient, the
concentrate being diluted with water before use. These concentrates are
usally required to withstand storage for prolonged periods and after such
~ 29 ~
storage to be capable of dilution with water in order to form aqueous
preparations which remain homogeneous for a sufficient time to enable them
to be applied by conventional spray equipment.
The compositions of the invention may contain, in addition to carriers
and surface active agents, various other constituents to increase their
usefulness. They may contain, for example, buffering salts to maintain the
pH of the compositions within a desired range; antiireeze agents, for
example oils and humectants; and sequrstrants, for example citric acid and
ethylenediaminetetracetic acid, which help to prevent the formation of
insoluble precipitates when the compositions are diluted with hard water.
Aqueous dispersions may contain anti-settling agents and anti-caking
agents. The compositions may in general contain a dye or pigment to impart
a characyeristic colour. Agents for increasing viscosity may be added to
reduce the formation of fine droplets during spraying, and thereby reduce
spray drift. Other additives useful for particular pruposes will be known
to those skilled in the formulation art.
In general concentrates may conveniently contain from 10 - 85% and
preferably from 25 to 60% by weight of active ingredient. Dilute
preparations ready for use may contain varying amounts of the active
ingredient, depending upon the purpose for which they are to be used;
however, dilute preparations suitable for many uses contain between 0.01%
and 10% and preferably between 0.1% and 1% by weight of the active
ingredient.
The compositions of the invention may comprise, in addition to one or
more compounds of the invention, one or more compounds not of the invention
but which possess biological activity. Accordingly in yet a still further
embodiment the invention provides a herbicidal composition comprising a
mixture of at least one herbicidal compound of formula (I) as hereinbefore
defined with at least one other herbicide.
The other herbicide may be any herbicide not having the formula (I).
It will generally be a herbicide having a complementary action in the
particular application.
Examples of useful complementary herbicides include:
A. benzo-2,1,3-thiadiazin-~-one-2,2-dioxides such
as bentazone;
, ~
2 ~ ~ 3 ~
B. hormone herbicides, particularly the phenoxy
alkanoic acids such as MCPA, MCPA-thioethyl,
dichlorprop, 2,4,5-T, MCPB, 2 9 4-D, 2,4-DB,
mecoprop, trichlopyr, clopyralid, and their
derivatives (eg. salts, esters and amides);
C. 1,3 dime~hylpyrazole derivatives such as
pyrazoxyfen, pyrazolate and benzofenap;
D. Dinitrophenols and their derivatives (eg.
acetates) such as dinoterb, dinoseb and its
ester, dinoseb acetate;
E. dinitroaniline herbicides such as
dinitramine, trifluralin, ethalflurolin,
pendimethalin, oryzalin;
- ' ~
2~37~
F. arylurea herbicides such as diuron,
flumeturon, metoxuron, neburon, isoproturon,
chlorotoluron, chloroxuron, linuron,
monolinuron, chlorobromuron, daimuron,
-5 methabenzthiazuron;
G. phenylcarbamoyloxyphenylcarbamates such as
phenmedipham and desmedipham;
H. 2-phenylpyridazin-3-ones such as
chloridazon and norflurazon;
I. uracil herbicides such as lenacil, bromacil and
terbacil;
J. triazine herbicides such as atrazine, simazine~
aziprotryne, cyanazine, prometryn,
dimethametryn, simetryne, and terbutryn;
K. phosphorothioate herbicides such as piperophos,
bensulide, and butamifos;
L. thiolcarbamate herbicides such as cycloate,
vernolate, molinate, thiobencarb, butylate ,
EPTC , tri-allate, di-allate, esprocarb,
tiocarbazil, pyridate, and dimepiperate;
M. 1,2,4-triazin-5-one herbicides such as
metamitron and metribuzin;
N. benzoic acid herbicides such as
2,3,6-TBA, dicamba and chloramben;
' ~ '': ' . , ;' . :~
- 32 -
2~3~a
0. anilide herbicides such as pretilachlor,
butachlor, alachlor, propachlor, propanil,
metazachlor, metolachlor, acetochlor, and
dimethachlor;
P. dihalobenzonitrile herbicides such as
dichlobenil, bromoxynil and ioxynil;
Q. haloalkanoic herbicides such as dalapon,
TCA and salts thereof;
R. diphenylether herbicides such as lactofen,
fluroglycofen or salts or ester thereof,
nitrofen, bifenox, aciflurofen and salts and
esters thereof, oxyfluorfen, fomesafen,
chlornitrofen and chlomethoxyfen;
S. phenoxyphenoxypropionate herbicides such as
diclofop and esters thereof such as the methyl
ester, fluazifop and esters thereof, haloxyfop
and esters thereof, quiæalofop and esters
thereof and fenoxaprop and eseers thereof such
as the ethyl ester;
T. cyclohexanedione herbicides such as
alloxydim and salts thereof, sethoxydim,
cycloxyidim, tralkoxydim, and clethodim;
,
: ' `~. ' ; .
. ~ : ' ' , ~ ;:
,
:- .
' , . :,
- 33 -
U. sulfonyl urea herbicides such as
chlorosulfuron, sulfometuron, metsulfuron and
esters thereof; benzsulfuron and es~ers thereof
such as DPX-M6313, chlorimuron and esters such
as the ethyl ester thereof
pirimisulfuron and esters such as the methyl
ester thereof, 2-[3-(4-methoxy-6-methyl-1,3,5-
triazin-zyl)-3-methylureidosulphonyl) benzoic
acid esters such as the methyl es~er thereof
(DPX-LS300) and pyrazosulfuron;
V. imidazolidinone herbicides such as imazaquin,
imazamethabenz, imazapyr and isopropylammonium
salts thereof, imazethapyr;
W. arylanilide herbicides such as flamprop and
esters thereof, benzoylprop-ethyl,
diflufenican;
X. - amino acid herbicides such as glyphosate and
glufosinate and their salts and esters,
sulphosate and bialaphos;
Y. organoarsenical herbicides such as monosodium
methanearsonate (MSMA);
Z. herbicidal amide derivative such as
napropamide, propyzamide, carbetamide,
tebutam, bromobutide, isoxaben, naproanilide
and naptalam;
- 34 -
AA. miscellaneous herbicides including
ethofumesate, cinmethylin, difen~oquat and
salts thereof such as the methyl sulphate salt,
clomazone, oxadiazon, bromofenoxim,
barban, tridiphane, flurochloridone,
quinchlorac and mefanacet;
BB. Examples of useful contact herbicides include:
bipyridylium herbicides such as those in which
the active entity is paraquat and those in
which the active entity is diquat;
* These compounds are preferably employed in
combination with a safener such as dichlormid.
The complementary herbicide is suitably present in the mixture or
composition in an amount such that it is applied at its conventional rate.
The following Examples illustrate the invention.
EXAMPLE 1
Preparation of N,N-Dimethyl-2-chloroacetophenon-eoxime-o-iso-butyramide
(Compound No 6 in Table I)
A solution of 2-chloroacetophenoneoxime (1.695g, lOmmol) in
dimethylformamide (DMF) (dry, 4ml) was added dropwise to NaH(50% oil
dispersion) (1.1 equiv; 0.264g, l~mmol) suspended in DMF (dry, 15ML) under
N2, and the resulting mixture stirred at room temperature for 1 hour.
N,N-Dimethyl-a -bromoisobutyramide (1.94g, lOmmol) in dry DMF (3ml) was
added dropwise to the mixture and the mixture heated at 100C for 36 hours.
~5 Another equivalent of N,N-dimethyl -a- bromoisobutyramide (1.94g, lOmmol)
in dry DMF (3ml) was added and the resulting mixture heated for a further
20 hours. The reaction mixture was cooled, poured into H20 and extracted
with EtOAc. The combined organic extracts were washed with brine, dried
(MgS04), filtered and concentrated under reduced pressure to yield an oil
residue. The product was purified by flash chromatography (eluted with 50%
:
~ - .
,; ,
- 35 - -
hexane~Et20) to afford a white crystalline compound (0.39g, 14~)
H NMR ~CDCl3): 1.6 [6H, s, C(CH3)2]; 2-25 (3H, s, 2 ~ ~ 3 7 Ol~
CH3-C=N); 2.~ 13H, bs, N(CH3)2], 3-1 13H,
bs, N(CH3)2], and 7.3 (4H, m, aromatic
C-H)-
m.s. M+ 283
EXAMPLE 2
Preparation of Methyl-2-chloroace_oph-n-n-eoxime
isobutyrate(Compound 32 in Table 1).
2-chloroacetophenoneoxime (4.24g, 25mmol) in DMSO (dry 5ml) was added
dropwise to NaH (50~ oil dispersion) (1.2 equiv; 0.72g, 30mmol) suspended
in DMSO (dry, 15ml) and the resulting mixture stirred at room temperature
for 1 hour. Methyl-a-bromoisobutyrate (4.525g, 25mmol) in DMSO (dry, 5ml)
was added dropwise to this green solution and the reaction mixture heated
at 90C for 4 hours, then 110C for a further 8 hours. The mixture was
cooled diluted with ice water and extracted with Et20. The combined
organic extracts were washed with brine, dried (MgS04), filtered and the
filtrate concentrated under reduced pressure to yield the product (4.73g,
70~).
1H NMR (CDCl3): 1.6 l6H, s, C(CH3)2]; 2-25 (3H, s,
CH3-C=N); 3.70 (3H, s, C02CH3); and 7-3
(4H, m, aromatic C-H).
EXAMPLE 3
Step a
Preparation of 2-chloroacetophenoneoxime-0-isobutyric acid.
Aqueous NaOH (1.2 equiv; 0.48g, 12mmol dissolved in 5ml of H20) was
added dropwise to methyl-2-chloroacetophenoneoxime-0-isobutyrate (2.695g,
lOmmol) dissolved in isopropanol (IPA) (25ml) and the resulting solution
.
..
- 36 -
heated under reflux for 6 hours. The reaction mixture was cooled, IPA
removed under reduced pressure, to leave a semi solid residue. H ~ ~ s~ 7
added and the mixture acidified (2N HCl), and extracted with Et20. The
combined organic extracts were washed with brine, dried tMgS04), filtered
and the filtrate concentrated under reduced pressure to yield the product
(2.38g, 93%).
lH NMR (CDCl3): 1.6 16H, s, C(CH3)2]; 2-3 (3H~ s,
CH3C=N); and 7.3 (4H, m, aromatic C-H).
Step b
Preparation of N?N-Dimethyl-2-chloroacetophenoneoxime-0-isobutyramide
(Compound No. 6 in Table 1)
A mixture of 2-chloroacetophenone-0-isobutyric acid (lg, 3.9mmol) and
thionylchloride (5ml) was heated at 70C for 2 hours, and the reaction
mixture was concentrated under reduced pressure. The resulting
2-chloroacetophenoneoxime-0-isobutylchloride was dissolved in a mixture of
toluene (5ml) and Et3N (4 drops) and the solution cooled to 0C (ice bath).
Dimethylamine (2 eq., 0.35g, 7.8mmol) in toluene (2ml) was added dropwise
and the mixture stirred at room temperature overnight. The reaction
mixture was concentrated under reduced pressure, the residue diluted with
water and extracted with EtOAC. The combined organic extracts were washed
with brine, dried (MgS04), filtered and the filtrate concentrated under
reduced pressure. The product was purified by preparative TLC (eluted with
50% hexane/Et20) to afford an oil which crystallised on standing (0.~5gp
77%).
lH NMR ( CDC13 ): See Example 1
E~AMPLE 4
Preparation of Azetidine-2-chloroacetophenoneoxime-0-isobutyramide
(Compound No. 27 in Table 1).
. : . ~ . : .
;.
: .
.
.;
' !
- 37 - 2~37~l~
Trimethylaluminium (2M solution in hexane, 0.27g, 2ml, 3.8mmol) was
added dropwise to azetidine (0.23g, 0.3ml, 45mmol) dissolved in CH2Cl2
(dry, 4ml) at -5C (ice salt ba~h) under N2, and the resulting mixture
stirred at room temperature for 30 minutes.
Methyl-2-chloroacetophenoneoxime-0-isobutyrate (lg, 3.7mmol) in CH2Cl2
(dry, 3ml) was added dropwise and the reaction mixture heated under reflux
for 6 hours. The reaction mixture was cooled, concentrated under reduced
pressure to yield an oily residue. A mixture of Et20/EtOAc was added and
the solid filtered off, the filtrate was concentrated under reduced
pressure, dissolved in warm EtOH, and the insoluble solids again filtered
off. The filtrate was concentrated under reduced pressure and the
resulting oil triturated with pentane to yield the corresponding product
(0.469g, 43~)
1H NMR (CDCl3): 1.5 [6H, s, C(CH3)2]; 2-2 15H, m~
CH3C=N and NCH2CH2CH2]; 4 0 l2H, t,
NCH2CH2CH2]; 4-3 [2H, t, NCH2CH2CH2l;
and 7.3 (4H, m, aromatic C-H)
ms 294 M+.
EXAMPLE 5
Preparation of 2-chloroacetophenoneoxime-0-isobutyronitrile. (Compound No.
28 in Table 1).
1. A mixture of 2-chloroacetophenoneoxime (1.61g,
lOmmol),a-bromoisobutyronitrile (1.48g, lOmmol), K2C03 (anhydrous, 1.4eq,
2.07g, 15mmol), amd DMSO (dry, 15ml) were heated at 120C for a period of
28 hours. The reaction mixture was cooled, diluted with H20 and extracted
with Et20. The combined Et20 extracts were washed with brine, dried
(MgS04), filtered, and the filtrate concentrated under reduced pressure, to
give an oily residue. The product was purified by flash chromatography
(eluted with 50% Et20/hexane) in 14% yield.
- 38 - 2~37~
1H NMR (CDC13): 1.75 16H, s, C(CH3)2], 2-25 (3H, s,
CH3C=N; and 7.35 (4H, m, aromatic C-H).
ms 236 M+
2. A mixture of 2-chloroacetophenaneoxime (1.61g, lOmmol),
a-bromoisobutyronitrile (1.48g, lOmmol), sodium hydride 10.28g, 12mmol) a
catalytic amount of potassium iodide and DMSO (dry, 14ml) were heated at
110C for a period of 8 hours. The reaction mixture was cooled, diluted
with H~O and extracted with Et20. The combined E~20 extracts were washed
with brine, dried (MgS04) filtered, and the filtrate concentrated under
reduced pressure, to give an oily residue. After purification by flash
chromatography (eluted wi~h 50% Et20/hexane) the product was obtained
(0.41g)-
EXAMPLE 6
Preparation of Ethyl-2-chloroacetophenoneoxime-0-isobutyrate. (Compound
No. 29 in Table 1).
-
A mixture of 2-chloroacetophenaneoxime-0-isobutyric_acid (0.3g,
12mmol), EtOH (dry, lOml?, and a catalytic amoun~ of p-toluenesulphonic
acid were heated under reflux for 2 hours. The reaction mixture was
cooled, concentrated under reduced pressure, and the residue dissolved in
Et20. The Et20 layer was washed with water, dried (MgS04), filtered and
the filtrate concentrated under reduced pressure. The product was purified
by preparative TLC (eluted with 50% pentane Et20) to afford an oil.
1H NMR (CDCl3): 1.25 (3H, t, OCH2CH3) 1.55 [6H, s, C(CH3)2]; 2-25 (3H, s,
CH3- C=N); 4.20 (2H, q, OCH2CH3), and 7.30 (4H, m, aromatic C-H~.
EXAMPLE 7
Preparation of Iso-propyl-2-chloroacetophenoneoxime-0-isobutyrate.
(Compound No. 30 in Table 1).
... ..
. . . : : ................................................. .
'~ :
- 39 -
This compound was prepared as described in Example 6 exce~t~ ~ ~ 7
isopropyl alcohol was employed in place of ethanol.
H NMR (CDCl3): 1.25 [6H, d, CH(CH)3~2], 1-55 (6~, s,
C(CH3~2], 2-25 (3H, s, CH3C=N~, 5.0 (lH,
s, CH(CH3~2], and 7.30 (4H, m, aromatic C-H~.
EXAMPL~ 8
The following compounds were prepared by methods analogous to those
described in Example 1, except that the appropriate a-chloroaceto, propiono
or isobutyramide derivatives was employed in place of the
N,N-dimethyl-a-bromoisobutyramide:
N,N-Dimethyl-2-fluoroacetophenone-oxime-0-propionamide (Compound No. 13 in
Table 1) (oil) structure confirmed by n.m.r.
N,N-Dimethyl-2-fluoroacetophenone-oxime-0-acetamide ~Compound No. 12 in
Table 1); oil - structure confirmed by n.m.r.
N~N-Dimethyl-2-chloroacetophenone-o-acetamide (Compound No. 20 in Table 1);
pale yellow oil; structure confirmed by n.m.r.
N,N-Dimethylacetophenoneoxime-0-acetamide (Compound No. 1 in Table 1); m.p.
65-66C.
N,N-Dimethyl-2-chloroacetophenoneoxime-0-propionamide (Compound No. 24 in
Table 1); oil, structure confirmed by n.m.r.
N,N-Dimethyl-isobutyrophenoneoxime-0-propionamide (Compound No. 19 in Table
1); oil structure confirmed by n.m.r.
N,N-Dimethyl-a-indaneoneoxime-0-propionomide (Compound No. 142 in Table 2);
m.p. 96-97C.
`. ~` ~ `
,
~ 40 - 2 ~ 1~ 3 7 ~ l~
N,N-Dimethyl-a-Tetraleneoneoxime-0-propionamide (Compound No. 140 in Table
2).
N,N-Dimethyl-2-Acetylthiopheneoxime-0-propionamide ~Compound No. 31 in
Table 1); pale yellow oil - structure confirmed by n.m.r.
N,N-Dimethyl-2-methylacetophenoneoxime-0-isobutyamide (Compound No. 11 in
Table 1); m.p. 33-88C.
N,N-Dimethyl-2-methylacetophenoneoxime-0-propionamide (Compound No. 10 in
Table 1); oil; structure confirmed by n.m.r.
N,N-dimethyl-propiophenoneoxime-0-propionamide (Compound No. 9 in Table 1)
oil, structure confirmed by n.m.r.
N,N-Dimethyl-2-ethoxyacetophenoneoxime-0-propionamide (Compound No. 7 in
Table l) pale yellov oil, structure confirmed by n.m.r.
N,N-Dimethyl-2-chloroacetophenoneoxime-0-propionamide (Compound No. 25 in
Table 1) oil, structure confirmed by n.m.r.
N,N-Diethylacetophenoneoxime-0-propionamide (Compound No. 4 in Table 1)pale yellow oil; structure confirmed by n.m.r.
N,N-Dimethyl-2-methoxyacetophenoneoxime-0-propionamide (Compound No. 5 in
Table 1) oil; structure confirmed by n.m.r.
N,N-Dimethyl-4-Chloropropiophenoneoxime-0-propionamide (Compound No. 53 in
Table l) m.p. 70-71C.
N,N-Dimethyl-l-acetonaphthoneoxime-0-propionamide (Compound No. 41 in Table
1) pale yellow viscous oil, structure confirmed by n.m.r.
Compound No. 33 in Table 1;
oil, structure confirmed by n.m.r.
:. .
,.
' ` `' ": '
-~1- 2a~3~
Compound No. 44 in Table 1; pale orange oil, structure confirmed by i.r.
EXAMPLE 9
The following compounds were prepared using methods analogous to those
described in Example 1:
N,N-Dimethyl-2-fluoroacetophenoneoxime-0-isobutyramide (Compound No. 12 in
Table 1) oil structure confirmed by n.m.r.
N,N-Dimethyl-2-fluorobenzaldoxime-0-isobutyramide (Compound No. 17 in Table
1) oil - structure confirmed by n.m.r.
N,N-Dimethylacetophenoneoxime-0-isobutyramide (Compound No. 2 in Table 1
oil structure confirmed by n.m.r.
N,N-Dimethylpropiophenononeoxime-0-isobutyramide (Compound No. 8 in Table
1) m.p. 49-50C.
Compound No. 18 in Table 1; oil, structure con~irmed by n.m.r.
Compound No. 21 in Table 1; oil, structure confirmed by n.m.r.
Compound No. 16 in Table 1;
m.pt 108 - 110C
Compound No. 23 in Table 1; oil, structure confirmed by n.m.r.
methyl-2-fluoroaceteophenoneoxime-0-methoxyacetate ~Compound No. 43 in
Table 1).
EXAMPLE 10
This example illustrates the preparation of
N-pyrrolidine-2-fluoroacetophenone oxime-0- methoxyacetamide (Compound No.
36 in Table 1).
~' ~
- 42 - 2 ~ ~ 3 ~
Compound 43 (1.4g~ prepared by methods analogous to those described in
Example 1 was dissolved in methanol (20ml) and pyrrolidine (1.17g) added.
The reaction mixture was allowed to stand at ambient temperature for 18
hours after which the methanol was removed under reduced pressure. The
residue was dissolved in hexane, washed with dilute hydrochloric acid,
dried using magnesium sulphate and the solvent evaporated under reduced
pressure. The product was purified by column chromatography on a silicon
column eluted with dichloromethane. The product was then extracted into
ethyl acetate which was then removed under reduced pressure to yield the
desired product as a colourless oil (yield 0.83g). The structure was
confirmed by n.m.r.
EXAMPLE 11
The following compounds were prepared by methods analo~ues to those
described in Example 10:
N-morpholine-2-fluoroacetophenoneoxime-0-methoxy acetoamide (Compound No.
37 in Table 1) oil structure confirmed by n.m.r.
N,N-Dimethyl-2-fluoroacetophenoneoxime-0-methoxyacetolamide (Compound No.
35 in Table I) yellow oil, structure confirmed by n.m.r.
Compound No. 42 in Table 1; oil, structure confirmed by n.m.r.
EXAMPLE 12
This example illustrates the preparation of Compound No. 34 in Table 1
Step a
Hydroxylamine hydrochloride (7.0g), cyclopropyl benzaldehyde t7.0g~, dry
pyridine (lOml) and ethanol (50ml) was heated together under reflux for 2
hours. The solvent was removed under reduced pressure and water added.
The resultant solid was filtered off, dried under suction and
recrystallised from carbon tetrachloride/60-80 petroleum ether.
. ' :
.
_ 43 - ~ ~ ~ 3~
Step b
The product from step (a) (1.89g) was dissolved in dry acetonitrile t30ml)
and N,N-dimethyl-~-bromoisobutyramide (2.0g) added together with a
catalytic amount of potassium iodide. The mixture was heated under reflux
with stirring for 16 hours, then cooled, water added and the mixture
extracted into diethyl ether. The ether layer was washed with 5% sodium
hydroxide solution (3xl5ml), dried over magnesium sulphate and evaporated
under reduced pressure. The product was obtained as an oil whlch
crystallised on standing.
After recrystallisation from hexane the desired product was obtained
as a white powder (0.43g) m.p. 53-54C
Structure confirmed by n.m.r.
EXAMPLE 13
This example illustrates the preparation of Compound 46 in Table 1.
Step (a)
Preparation of (1-chloroethyl)ethylsulphide.
To a stirred solution of diethylsulphide (31.7g) in carbon
tetrachloride (lOOml) was added chlorine gas (25.0g) in carbon
tetrachloride (300ml) dropwise over a period of 45 minutes. A temperature
of -20C was maintained throughout the addition. After the addition was
complete, the reaction was allowed to come to room temperature and then
maintained at room temperature for 2 hours during which time hydrogen
chloride gas evolved. The solvent was then removed under reduced pressure
during which time the temperature was not allowed to exceed 30C. The
resultant colourless oil was removed and distilled at a 40mm (51C).
Step (b)
Acetophenoneoxime (lg), the product from step (a) (0.92g), sodium hydride
(0,18g) and dry dimethylsulphoxide (5ml) were stirred together at room
temperature for 2 hours. The reaction mixture was then poured into water
' ~
2~'~37~
- 44 -
and extracted into hexane. The hexane layer was washed with 5% sodium
hydroxide solution, dried over magnesium sulphate and evaporated under
reduced pressure to yield a pale yellow oil. This was distilled on a high
vacum pump to give the desired product as a colourless oil (0.5g) which
distiiled at 87C/O.lnm. The structure was confined by n.m.r.
EXAMPLE 14
The following compounds were prepared by methods andogous to that
described in Example 13.
Compound 55 in Table 1; structure confined by n.m.r.
EXAMPLE 15
Preparation of Compound No. 60 in Table I.
Compound 55 (2.0g) in glacial acetic acid (20ml) cooled in a water
bath and potassium permanganate (1.84g) in water (50ml) dripped in over a
period of 15 minutes. The reaction mixture was stirred at room temperature
for 2 hours after which sulphur dioxide gas was passed through the
solution. Water (25ml) was added and the reaction mixture extracted into
trichloromethane (50ml) dried over magnesium sulphate and the solvent
removed under reduced pressure to give the desired product as a colourless
oil. (Structure confined by n.m.r.).
EXAMPLE 16
The following compounds were prepared by methods analogous to these
described in Example lS:
Compound 47 in Table l; m.pt. 63-65C
Compound 45 in Table l; m.pt. 50-51C
~5 EXAMPLE 17
The following compounds were prepared by a method analogous to that
described in Example 4 except that dimethylamine was used instead of
azelidine:
-;
,~
,
: .
2~ ~37 ~i~
N,N-Dimethyl-2-aminoacetophenone oxime-0-isobutyramide (Compound No. 69 in
Table 1~ - Pale brown solid - structure confined by n.m.r.
N,N-Dimethyl-trifluoroacetophenone-0-isobutyramide (Compound No. 70 in
Table 1) oil - structure confirmed by n.m.r.
N,N-Dimethyl-2-nitroacetophenone oxime-0-isobutyramide (Compound No. 71 in
Table 1) - oil - structure confirmed by nmr.
N,N-Dimethyl-2-chloro,6-trifluoroacetophenone oxime-0-isobutyramide
(Compound No. 75 in Table 1) oil - structure confirmed by n.m.r.
Compound No. 77 in Table 1 - oil structure confirmed by n.m.r.
N,N-Dimethyl-2,6-dichloroacetophenone oxime-0-isobutyramide ~Coimpound No.
81 in Table 1) - oil structure confirmed by n.m.r.
N,N-Dimethyl-2-methylsulphonacetophenone oxime-0-isobutyramide (Compound
No. 82 in Table 1) - off white solid - structure confirmed by n.m.r.
N,N-Dimethyl-2,6-difluoroacetophenone oxime-0-isobutyramide (Compound No.
83 in Table 1) - oil structure confirmed by n.m.r.
N,N-Dimethyl-2,5-dichloroacetophenone oxime-0-isobutyramide (Compound No.
84 in Table 1) - oil structure confirmed by n.m.r.
N,N-Dimethyl-2,3-dichloroacetophenone oxime--0-isobutyramide (Compound No.
85 in Table 1) - oil, semi-solid on standing - structure confirmed ny
n.m.r.
N,N-Dimethyl-3,5-bis-trifluoromethylacetophenone-oxime-0-isobutramide
(Compound No. 86 in Table 1) oil - structure confirmed by n.m.r.
Compound No. 87 in Table 1 - oil-structure confirmed by n.m.r.
N,N-Dimethyl-2,3-di~luoroacetophenGne oxime-0-isobutyramide (Compound No.
88 in Table 1) - oil, semi-solidified on standing - structure confirmed by
n.m.r. and m.s.
EXAMPLE 18
The following compounds were prepared substantially as described in
Example 4 except that dimethylamine was used instead of azetidine and
slightly different reaction conditions were used. In particular the
reaction mixture was stirred at room temperature ins~ead of under reflux
and work-up was effected by dilution with water, extraction into ethyl
acetate and dried over magnesium sulphate.
Compound No. 109 in Table 1 - orange oil structure confirmed by n.m.r.
Compound No. 110 in Table 1 - pale yellow oil structure confirmed by n.m.r.
Compound No. 111 in Table 1 - orange oil structure confirmed n.m.r.
- 46 ~ 3 7 ~
Com2ound No. 112 in Table 1 - pale yellow oil structure confirmed by n.m.r.
Compound No. 113 in Table 1 - pale yellow oil structure confirmed by n.m.r.
Compound No. 114 in Table 1 - orange oil - structure confirmed by n.m.r.
Compound No. 115 in Table 1 - orange oil structure confirmed by n.m.r.
Compound No. 116 in Table 1 - orange oil structure confirmed by n.m.r.
Compound No. 117 in Table 1 - orange oil structure confirmed by n.m.r.
Compound No. 118 in Table 1 - dark orange oil structure confirmed by n.m.r.
Compound No. 119 in Table 1 - oil structure confirmed by n.m.r.
Compound No. 130 in Table 1 - orange oil structure confirmed by n.m.r.
Compound No. 131 in Table 1 - oil structure confirmed by n.m.r.
Compound No. 133 in Table 1 - oil structure confirmed by n.m.r.
EXAMPLE 19
The following examples were prepared using the method of Example 3
step (b) except that where necessary dimethylamine was replaced by the
appropriate alkylamine:
N-isopropyl-2-chloroacetophenoneoxime-0-isobutyramide (Compound No. 72 in
Table 1) - oil - structure confirmed by n.m.r.
N-t-butyl-2-chloroacetophenone oxime-O-isobutyramide (Compound No. 73 in
Table 1 - oil stricture confirmed by n.m.r.
Compound No. 74 in Table 1 - oil structure confirmed by n.m.r.
Compound No. 76 in Table 1, - oil structure confirmed by n.m.r.
N-Ethyl-N-methyl-2-chloroacetophenone oxime-O-isobutyramide (Compound No.
78 in Table 1) - oil structure confirmed by n.m.r.
EXAMPLE 20
This example illustrates the preparation of Compound No. 80 in Table
1.
To a stirred mixture of hydroxylamine hydrochloride (2 equiv, 0.695g,
lOmmol) in dry methanol (lOcm3) was added potassium hydroxide (3 equiv)
(0.84g, 15mmol in methanol (3cm3)). This mixture was stirred for ~ hour at
room temperature, then a solution of methyl-2-chloroacetophenone oxime-O-
isobutyrate (1.35g, 50mmol) in methanol (lOcm3) added dropwise.
The whole mixture was stirred over the weekend. A white precipitate
was filtered off and the filtrate concentrate to give an oily residue.
The residue was di~solved in water and acidified with dilute HCL. The
aqueous mixture was extracted into ethyl acetate and the extract washed,
:
.
- 47 - 2~37~
dried and concentrated to give an oil which was purified by preparative
TLC. After trituration with pentane a white solid was obtained.
H NMR (CDCl3): 1.55[(6H,s,C(CH3)23, 2.25(3H,s,CH3-C=N), 7-35
(4H,m,aromatic C--H),
8.90(1H,s,NH or OH).
EXAMPLE 21
This example illustrates the preparation of Compound No. 79 in Table
Compound No. 80 prepared as described in Example 20, (0.249g, 9.2mmol)
was dissolved in dry DMF (5cm3) and 1,2-dibromoethane (0.52g, 27.6mmol)
added dropwise, followed by potassium carbonate ~0.26g, 18.4mmol). The
resulting mixture was stirred overnight. The reaction mixture was diluted
with water and extracted with ethyl acetate. The extract was washed, dried
and concentrated to give a viScous yellow oil. Trituration with pentane
gave the desired compound as a white solid that was filtered off and dried.
lH NMR (CDC13): 1.55(SH,s,C(CH3)2), 2.25(3H,s,(CH3-C-N),
4.20(4H,dt,2xCH2(0CH2CH20), 7.30(4H, m, aromatic C H).
EXAMPLE 22
This example illustrates the preparation of N,N-Dimethyl-2-
chloroacetophenoneoxime-O-Isobutyrothioamide (Compound No. 89 in Table 1).
To a solution of the N,N,Dimethyl-2-chloroacetophenoneoxime-0-iso-
butyramide (0.29g, 1.03mmol) in dry toluene (lOcm3) was added Lawesson's
Reagent (0.416g, 1.03mmol) and the resulting mixture stirred and refluxed
for 7 hours. The mixture was then concentrated to give a yellow oil
residue which was applied to preparative TLC plates (silica, eluant: Et20
(l)/Hexane(l)).
The relevant band (top) was isolated to give the desired product as a
yellow viscous oil.
lH NMR (CDCl3): 1.0(6H,s,c(CH3)2), 2.24(3H,s,CH3-C-N), 3.47(3H,s,CH3 of
N(CH3)2, 3.56(3H,s,CH3 of N(CH3)2, 7.3(4H,m,aromatic C-H).
2 ~
- 48 -
EXAMPLE 23
The following compounds were prepared by method analogous to those
described in Example 22:
N,N-Dimethyl-2-Fluoroacetophenone oxime-0-isobutyrothioamide. (Compound
No. 90 in Table 1) - yellow viscous oil, structure confirmed by n.m.r.
N,N-Dimethyl-2-Bromoacetophenoneoxime-0-isobutyrothioamide. (Compound No.
91 in Table 1) - yellow viscous oil structure confirmed by n.m.r.
N,N-Dimethyl-2-Bromoacetophenone oxime-0-methoxacetamide (Compound No. 132
in Table 1) - oil structure confirmed by n.m.r.
EXAMPLE 24
The following Example was prepared by methods analoguos to those set
out in Example 2 modified as necessary. In all cases the structure was
confirmed by n.m.r.
Methyl-2-3-difluoroacetophenoneoxime-0-isobutyrate (Compound No. 92 in
Table 1) oil.
Compound No. 93 in Table 1 - yellow oil.
Methyl-3,5-bis-trifluoromethylacetophenone oxime-0- isobutyrate (Compound
No. 94 in Table 1) oil, that solidified on standing.
Methyl-2,3-dichloroacetophenoneoxime-0-isobutyrate (Compound No. 95 in
Table 1). oil, which on standing and trituration with pentane gave a
crystalline solid.
Methyl-2,5-dichloroacetophenone oxime-0-isobutyrate (Compound No. 96 in
Table 1) oil.
Compound No. 97 in Table 1 - oil
Methyl-2,6-dichloroacetophenone oxime-0-isobutyrate (Compound No. 98 in
Table 1) - oil
Methyl-2-chloro-6-fluoroacetophenone oxime-0- isobutyrate (Compound No. 99
in Table 1) oil . (Except that in the method 2-chloro,6-fluoroacetophenone
oxime was used instead of the 2-chloroacetophenoneoxime).
Compound No. 101 in Table 1 (except that methyl ethyl ketone and K2C03 was
used instead of NaH/DMS0).
Compound No. 103 in Table 1 - oil (except that 2-butanone and K2C03 was
used instead of NaH/DMS0).
Methyl-2-Nitroacetophenone oxime-0-isobutyrate (Compound No. 104 in Table
1) - viscous oil.
'~ .
_ 49 _ 2 ~f~ 2
[2-Nitroacetoacetophenone oxime was used instead of 2-Chloroacetophenone
oxime].
Methyl-trifluoroacetophenone oxime-0-isobutyrate (Compound No. 105 in Table
1) - oil.
Methyl-2-chloroacetophenone oxime-0-propionate (Compound No. 106 in Table
1) - oil. Methyl-2-bromopropionate is as used instead of methyl-~-
bromoisobutyrate.
Methyl-2-chloroacetophenone oxime-0-methoxy-acetate. (Compound No. 107 in
Table 1) [except THF was used instead of DMS0].
Methyl-2-aminoacetophenone oxime-0- isobutyrate. (Compound No. 108 in
Table 1 - oil.
Compound No. 144 in Table II - (equimolar amounts of starting materials and
NaH used).
Compound No. 120 in Table 1 - (equimolar amounts of star~ing materials and
NaH used with no heating at all).
Compound No. 121 in Table 1 - (equimolar amounts of starting materials
used, with no heating).
Compound No. 122 in Table 1 - (equimolar amounts of starting materials with
no heating).
Compound No. 123 in Table 1 - (equimolar amounts of starting materials used
with no heating).
Compound No. 124 in Table 1 - (equimolar amounts of starting materials used
with no heating).
Compound No. 125 in Table 1 - (equimolar amounts of all reagents without
heat)~
Compound No. 126 in Table 1 - (equimolar amounts of all reagents heated to
60C for 6 hours).
Compound No. 127 in Table 1.
Compound No. 128 in Table 1.
Compound No. 129 in Table 1.
EXAMPLE 25
The following compounds were prepared by a method analogous to that
described in Example 3 step a.
Compound No. 100 in Table 1 - semi-solid. 1H NM~ (CDCl3):
1.65(6H,s,C(CH3)2), 7.15(3H,m,aromatic C-H), 8.45(1H,s,-CH=N-0).
_ 50 _ 2 ~ ~~. 3 ~
Compound No. 102 in Table 1 - semi-solid. 1H NMR (CDCl3): 1.65ppm
(6H,s,c(CH3~2), 7.25-7.70(4H,m,aromatic C-H), 8.55(1H,s,-CH=N-0).
Biological Data
The herbicidal properties of compounds of the invention was tested as
described below.
Each compound was formulated for test by mixing an appropriate amount
of it with 5ml of an emulsion prepared by diluting 160ml of a solution
containing 21.8 grams per litre of Span 80 and 78.2 grams per litre of
Tween 20 in methylcyclohexanone to 500ml with water. Span ~0 is a Trade
Mark for a surface-active agent cornprising sorbitan monolaurate. Tween 20
is a Trade Mark for a surface-active agent comprising a condensate of 20
molar proportions of ethylene oxide with sorbitan monolaurate. The mixture
of the compound and the emulsion was then shaken with glass beads and
diluted to 40ml with water. The spray composition so prepared was sprayed
on to young pot plants (post-emergence test) of the species named in Table
2 below, at a rate of equivalent to 1000 litres per hectare. Damage to
plants was assessed 14 days after spraying by comparison with untreated
plants, on a scale of 0 - 5 where 0 is 0 to 20% damage and 5 is complete
kill. In the table of results a dash (-) means that no test was made.
A test was also carried out to detect pre-emergence herbicidal
activity. Seeds of the test species were placed on the surface of fibre
trays of soil and were sprayed with the compositions at the rate of 1000
litres per hectate. The seeds were then covered with further soil. Three
weeks after spraying, the seedlings in the sprayed fibre trays were
compared with the seedlings in unstrayed control trays, the damage being
assessed on the same scale of 0 to 5.
The results of the tests are given in Table IV and V below, where
Table IV shows the results on the 0-5 scale and Table V shows the results
on a percentage scale.
:
', : ,
.
2~3~
C U~ ., o o
,' ~ C`~ o o
V~ ~ ~ ~ U~ o o
U~
v~ In c~In c~
~ , . . . . .
~, ~,
, ~, ~, ~,
P ~ , o
~ ~, o , o, o
~ ~ ~ ~ ~ ~o C~
~ P~, o ,, o,
U~ ,
P ~ P'
H E~
H ~ ~1 O C`l O O
C
~; ~ O ~ O O ~
O
N C~ lO O
~) O ~ ) O O
P~
C~l O
~ D o ~" o
O Z
- :, , :
2~f~37~
C~ ~o o o ~
u~ o ~
~ ~ ~ ~ ~ ~ o o C~
U~ U~ C~ U~
¢,,
~ ~ o u~ , ~ o ~n o
H , ~ ~ Ic~J I
'~:IO IOIO IO
a~ ~
V I OI O I O I O
~d 8
E~ 'C ~ ~ `~ C~ O
~ X I ~I `J O I I I
~ ~ ~ O~t r-l r-l I ~ I
~:1 '1;
CJ ~ I
:~ H O _I O C`lO O O ~--I
V~ ~ ~ ~ ~e~l O ~ _l
; O O~ O ~ O
~) OLt~ ~ O O~1 0
~ ~ C`l~ C~ ~ OO O
O O O O
~ c~ o ~ ~ o ~
O æ .
O ~ ~4 ~ O h O Ll O Ll O
~'
o æ
~ ' !
. .
- 53
2 ~ 4 3 17 ~ ~r
c~ o ~ o c~
¢ ~1 0 U) ~ O O U~
c c~ O ~ I ~ I ~ I
v ~ ~ ~ u~
~ I I I I I
o ~ o u~
bO u~ .n c~
o ~ o ~I I 11'~ 1 It'~ I
~ ~ I O
I o I o I o
Q~ 1
~0 ~
~ H O~ OO O O O O
E~ O~ c~
~ ~ O ~ I ~ ~ ~ I
X C~ O~ tN ~1 ~ ~ ~
V~
~ O C~ ~ ~ O O
D ~ ~
v
~! o , ~ a~ ~1) ~ v
~4 o
o sz
v
: ~ :
2 0 ~ 3 7 ~ ~L
C U~ ~ O ~
¢ U~ ~I O ~ ~
~ O~ O ~ ~
~ U~ ~ U~ ~ ~J
,~
¢ I I I I I I I I
~ o u~
C~ J
D ~ I O I
~1 C,) I -11 01 0 1 0
E~ ¢ ~ ~ ~ ~ ~ ~ ~ C`J
~ o o o ~ C~ C~l
U~ )~ O`J C~l
-- Z ~ ~ `;t~ ~U'l ~ I
~; .
C ~ ~ I ~~ ~ ~ ~ ~ o
o E~ ¢ ~
~ H O C`l~1 00 0 0 0
H C
V~ `J ~~ ~~ O ~ ~1
E~ n ~ ~ o u~
~ O U~ I
S ~ t~
v c~ ~~ ~o o o o
~; ~ ~~ ~U~ C~ cr~ ~
~ o
v
~ J v
o
~ ~ ~c
o ~ U~
o æ
V
. . ~
: . ~: . . ,
-- 55 --
20~370!1
C`J ~ o
C~ o ~ ~ ~ C~
V
o ~ ~ U
~ I I ~ I I I I I
U~ ~ o o U~ ~ ~ ~
U~ ~ ~ ~ ~ ~ ~ ~ '.
O ~ I O I In I u~ I ,
P~ I o I o I ~ I C~ ,
:~ , o , o I I
t~l 5
aJp~ ~ ~ I O I
U~~o ~ ~
I
o E~ O ~ ~ ~
P ~ O O O ~ ~ O O ~
ra U~ I
~ O ~ O ~ ~ U~
:3 Ul I O O If~ ~
:~: ~ ~ O O ~ C`l
v c~ o o ~ ~ ~
ty; ~ ~ c`~
v
:c o ~ O
~ o z ~
o 00 O~ o
~`
- 56 -
7 ~ ~
U~ o U~ ~ o
V~ ~ o U~ o
o
~n o n d` U~
eSII 11 11 11
~ U~ o U~
a ~ ~ ~ ~ n ~ ,
O C~ I U~ I U~ I ~ I
~P~ I o I ~ I ~ I o
a~~) I OI C~l I O I O
~ D ~
Q) ~ ~ I ~ O I
V~ ~ `J ~ ~ ~ ~
o E~~ ~ o ~ ~ ~ ~ ~ I
PH ~1 0 0 C~ OC~l O O
HC ~ ~;~. ~ ~ ~ ~
E~ ~ O u~ o ~
o o ~ o
N ~ "~
~; ~ ~ ~ ~ ~ ~ ~ ~
D ~ ~ `d' c~l ~ ~ ~ t"
O æ .
~, o ~ V
~ ~ C "
c ~ bO
~ CC~
o~ ~ ~ ~ U~
:~: Z
: . ,
2~q 3~
~ ~ o~ o ~ o
~ U~ o , o ~ o o o
U~ o o ~ o o o
d` C~ ~ O ~ O ~ O
V ~~ ~ ~ ~ o ~ ~
~ , . . . . . . .
o ~ o
d~ d~ ~ C' ~ O O O
O ~--I I~1 10 0 0
,~ , o , ~ , o , o
~,,o ,o ,o ,o
E~~ ~ ~ ~ ~ ~ ~ c~l ~
a~x o o~ e~l~ ~ o o
_ o
V~ ~ ~ C`l o
~ ~ ~ o ~ ~
t. U~~ ~ ~ ~ ~ ~ ~ ~ o
H O c~O O O O O c~l
H
.t ~O OO O O O
3 C~ 1 0 C~l~10 0
:~ ~ ~ O Oc~ O O O
~) O ~ C`~ OO O
V~ O ~1 ~ O ~ ~ ~ O
W
O ~ ~ ~ a~
~ o ~ ~
o æ
.~
. .. . . ::. :~
L ~ :
: , , ,: :
- 58 - ~ 3 7 ~ ~
~, o C`l o o~ C~ ~ o
o o o ~ o ~ o
C~ ~ o o o ~ o ~ ,
~ ~ o ~ ~ U~
U~ ~ ~ ~ o ~ ~ C~
~C,. .. .. ..
o U~ C~l ~ o
~, ~ o ~ o ~ ~ ~ o
o o, o , ~ o o ,
~¢ I OI OI OI ~
,a~ I OI O I ~ I o
E~ ~ oc~ I~ O
aa~~ o oI oI o I o
o o ~~ ~ ~ o
o
` ~~, ~~ o ~ ~ ~ o
V~
~ H O OO O~1 ~ O O
,~ O ~ ~ C~ O
~ ~; O OO O~ O ~ O
3 C`~ OO O~1 0 0 0
--I OO ~ J O
~ O O O C`~ ~ ~ O O
~ ~ O~ C~~'1 00 0
O
O O
~ O ~ L O Ll O L O Ll O
~i ~
~0 ~ 0 ,~
Z
~ ~, . `, ~; ,
"''` . `.' ~` ` ' ',`'~' . ' `
: : ,.,
2~37~
~, o o o C~l o o
¢ o ~ o o ~ o
~ o ~ o C~ ~ o
~ o ~ ~ o
v~ In ~ u~ c~l ~ ~
~, ~ U~ o U~ o
C~ ~ ~ o
o Ut ~ ~, ~,
D ~: ~ ~ I O I O
~ - ~ , o , o , o
rd D
E~ ~ ~ ~ ~ ~ ~ . c~
Q~ ~
~ P~ , o o o o o
o
~n ~ ~ ~ ~ ~ ~ c~
~ ~ V ~
C ~ ~ ~ ~ ~ , , o
V ~
:~ H O ~1
~ V~ ~ C~l
~ O C~ O C`~ O
3 c~ o C~l ~ ~ o
æ o ~ u~ ~ ~ O
~ o o
~; ~ ~1~ c~ ~ ~
~ æ .
~! O ~ h OS-l O Ll
C4 ~ ~ ~¢ ~ ~ ~ ~4 ~
O
Z; ~
Ul
.` ~ ¢
:
~ c~
æ o
.~ c~Z
`:
:`
: :
:. ~ , ,
-- 60 --
2~3~
o ~o oo U~O
V ~ I ~ ~ In O~
t~~ O I~ In O I O
~ ~ ~ ~ o~ ~
bOI O I O I n ~ O
~D ~D ~ ~
~J~ o ,~ o U~ o C~ o
~q ~ cs~ r~ ~ U~ ~
U~ o ,~ ~ U~ o ~ o
V~ ~ Cr~ ~ ~ C~ ~
~ I ~o I ~o I o
o o ~n u~ O O O
¢ o ~ a~ ~
~ Uo~ CO~ o o o
H ~ O l O l O l O
~ O I ~0 1 O I
tl~ 1 0 O U) In OIr~ U~
E~ ~ ~ r~ u~ 00 1~
a~ P~ ~ o o o o o o
~q H oO ~1 `;t . ~
O0 11'1 O O O O
t~ ~ O C~l C`~ ~) ~
E~ O O O O O O
~ O ~ ~_ c~ ~ u-~
~ ~ ~ O O U) O O O U~
P~ ~ ~1 1_
~ ~ ~ I I o r~ I ~~
~ E-l Id O O O ,n o o 1-'~
~ VI Irl u~ I~ ulLf) I`
E~ '~ I ~ o I O ~ O
~ O o I O O O O O
O
t~ O I O O O O O
~:L;o
N~--I O O O O O In
~ O O Ir~ O In o In
cqLr~ ~ ~D ~
~ O ~ UO~ U~ ~ ~ ~ U~
C~ 1 O O O O Ln O
.0 0 O O O O O O
V~ O-~ ~ ~'1
~ a~ tq a~ u~ ~ ~q
~; 0 2 ~ )-I O h O Ll O Ll O
t ~ ~ ~
~':C4' :
o æ ~ n co
.
,
:
.: -- .; ' ` ' ` '
-- 61 --
2~37
~, U l oo Uo~ CO~O
~ In U~O ~U~ 8u~
~ ~ o ~ ~ ~ o~ ~ ~
bO I o l c~ l U~ O ~
~ U~ In U~ U~ U~ O O
u~ a~ o a~ ~ o~ ~ ,~ I~
o o o n O ~ O
u~ In O ~ ~ a~ ~ ~ ~
bO I O O
¢ o, o, U~ , ~
,,~ Ul oU~ o U~ o
¢ a~ o~o ~ u~ ~ ~
~ o o o o o o
¢ ~ o~ ~ ~ U~ ~ C~l
~p p~ I OI o Oln I u~
~ ~ o
" P~ ~, o, o, o
~ ~ o oo o o U~ U~
E~ ~ u~
~ ~ OI ~, I CYl O ~
U~ ~ UO~ ~U~ ~o ~o ~o o
~o ~o U~ ~ ~o U~ o
E O1~ 0O Ifl n
~ or~ c~~1 ~ O ~J
t~ ~ ~ O O
1~ I Il l I ~ U~ ~1
Et t~ o L~lO u~u~~ u~O O
~ ~ o~ C~1~ ~:1U') ~`
E-~ ~rl In O It~ O
t4 ~ IO I I tt~
3 L~'l
r~ o oo o o ~ o I
~ O O~ O U~ ~ O O
æ o c~o o ~ In o ~D
~ OO O U~ U~
V~ O _~If~ 111O ~DO u'l
O O U~ O O
' ~":> ~ OO c~lO ~D i~ ~
~ U'~ ~ ~ U~ U~ 1`
~ OO O U~ ~
V~ o ,_~U~ U~O ~ O ~
g æ .
I E~ ~ v ~-
~ U~ Q ~ ~ ~ U~ ~ U~
IY; o ~ O s~ o ~ O ~ o
O.
,1::
~t
:~
a
o ~ ~ ~ ~
~ o ~ c~ ov oo
~) æ
-- 62 --
21~i 37
o
~, o o ~ o
o
~Y ~ o U~ C~
bO U~ o 1 ~0
V U~U~
u~ a~ o a~ ~
o o U~ o
U~ ~ Ul ~ ~
~, oI o
U~ ~ ~
U~ o o
o ~ ~ ~
, ~, o, ~o
Q~ ~
~ ~ o,o,
~ 5~ o U~ o o
E~ c~ ~
Q~ ~ O
Q~ ~ O O O ~
u~ Iy O In eo~l n
~ ~ o ~ o ~
~4o o ~ o
~ ~ o
~ o oo
~o oo
~; o o ,o, ~
:~E ~ O O
~ o
U~ o ~ o
~ U~U~
C~ O ~D O
~ U~C~ CO~I
~ oo o
V~O'~,~u~
O ~ 1~ h Oh 0
2 ~
- 63 - 2 ~ ~ 3 7 ~ ~
TABLE VI
Sb Sugar beet
Rp Rape
Ct Cotton
Sy Soya bean
Mz Maize
Ww Winter wheat
Rc Rice
Sn Senecio vulgaris
Ip Ipomoea purpurea
Am Amaranthus retroflexus
Pi olygonum aviculare
Ca Chenopodium album
Po Portulaca oleracea
Xa Xanthium spinosum
Ab Abutilon theophrastii
Cv Convolvulus arvensis
Ot/Av Oats ~cultivated in pre-emergence test and
Avena fatua (wild oats) in post-emergence
test~
Dg Digitaria sangiunalis
Pu Poa annua
Al Alopecuris myosuroides
St Setaria viridis
Ec Echinochloa crus-galli
Sh Sorghum halepense
Ag Agropyron repens
Cn Cyperus rotundus
Ga Galium aparine
Am Amaranthus retroflexus
Bd Bidens pilosa
Eh Euphorbia heterophylla
Xs Xanthium strumarium
Ce Cyperus esculentes
..
- 64 - 20~ 7~
CHEMICAL FORMULAE
(in description)
R2
R3 4
I N - O - C -R (I)
Rl R
_ ~ (X)p(CH~)m (i)
(CH2)n
R13 R12 (ii)
0~ Rll
O R10
N
~ N-O-H (II)
Rl
R3 H-NR~R9 ~VII)
Z - 1- R4 (III)
R5 AlR16R17R18 (~I)
R~
~ o (IV)
Rl
R9R8N - Al R16R17 (V)
- ~ . : . .