Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
OXYGEN-PERMEABLE POLYMERIC MEMB~AN~S
BACKGROUND OF T~E INVE.NTION
This invention ~elates to oxygen-permeable polymeric
membranes to be used in processes for producing oxygen- or
nitrogen-enriched air for industrial, medical, and other
applications. More particularly, the invention concerns
polymeric membranes which contain, as dispersed therein, a
metal complex capable of adsorbing and desorbing oxygen rapidly
and reversibly.
Oxygen is one of the chemicals most widely used on indu~-
trial scales, specifically in the manufacture of iron, steel,
and other metals and glass, in chemical oxidatlon and combus-
tion, and in wastewater disposal~ It has also very extensive
usage in the field of medical care, lncluding the therapy for
lung disease patients by means of oxygen inhalation. Nitrogen,
on the other hand, is a chemical conveniently and extensively
used to maintain a nitrogen atmosphere, for example, for the
preservation of foods, in fermentation processes, and in
electronic circuit fabrication. For these reasons the develop-
ment of processes for concentrating oxygen and nitrogen out of
air is an important problem with far-reaching effects on
various sectors of industry. While low-temperature and adsorp-
tion techniques are in use as industrial processes for atmo-
spheric oxygen and nitrogen concentration, membrane separation
is considerecl promisirlg from the energy-saving viewpoint.
Success of membrane separation depends primarily on the
discovery of a membrane material that ~ould permit selective
and efficient oxygen permeation relative to nitrogen from air.
Currently available membranes capable of perrneating and
concentrating atmospheric oxygerl (known as oxygen-permeable
membranes) are those of silicone, silicone polycarbonate, and
the like. Some of them are in practical service. They do not
have high oxygen-permeation selectivity (02/N2) value (oxygen-
permeability coefficient/nitrogen-permeability coefficient),
the value being approximately 2, and yet exhibit high
permeability coefficient (10-3 [cm3 (S~P) cm/cm2 sec cmHg]).
With this feature the membranes are incorporated in modules,
multistage processes, and other systems to obtain oxygen-
enriched air, with oxygen concentrations of about 30%. In
order to obtain highly oxygen-rich air useful for industrial
and medical applications by a single, continuous permeable-
membrane pass, it is essential that the membrane have an
(02/N2) value of at least 5.
The first requisite for an enhanced selectivity (O2/N2) is
to make oxygen more soluble than nitrogen with respect to the
membrane.
We have hitherto continued the synthesis of metal complex-
es capable of rapid, reversible adsorption and desorption of
oxygen molecules. We clarified essential requirements of the
-- 2 --
metal com~)lexes that ~an adsorb and desorb oxygen molecules
selectlvely, rapidly, and reversii)ly, even in a solid-phase
membrane polymer. We successfully synthesized the novel
complexes and taught their use for o~ygen-permeable membranes
(Patent Application ~'ublic Disclosure No. 171730/19~7).
~ ighly o~ygen-rlch air is useful ~or industrial and
medical applications, and large quantities of highly nitrogen-
rich air are used as inert gas in many sectors of industry. If
they are to be obtained continuously by a single pass through
an economical membrane, it is essential that the mernbrane have
a selectivity (O2/N2) value of 5 or upwards.
~ ;e have hitherto continued the synthesis of metal complex~
es capable of rapid, reversible adsorption and desorption of
oxygen molecules. As a result, we successfully synthesized
novel ~etal complexes that can adsorb and desorb oxygen mole-
cules selectively, rapidly, and reversibly, even in a solid
phase. we further found that the metal complexes carried in
polyr~eric solid-phase membranes are kept from irreversible
oxidation and permit stable, selective permeation of oxygen.
r.owever, polymeric membranes incorporating such complexes,
when used in air permeation, did not always achieve the object
satisfactorily in the region where the feed oxygen pressure was
high (20 mmHg or above), although the (02/N2) value exceeded
the ta-get value of 5. Thus, a further improvement in the
(O2/N~) value was sought.
- 3 -
s`~
SUMMARY OIF TI~E INVF,N ~ION
In view of the above, we have made further intensive
research for the improvem2nt in performance o the complex that
can adsorb and desorb oxygen. ~e have now successfully synthe-
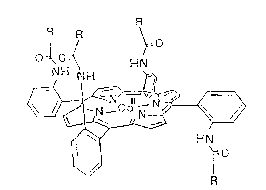
si~ed a novel porphyrinato transition metal (II) complex, i.e.,
a meso-tris(~ -o-substituted-amidophenyl~-mono-(~-o-substi-
tuted amidophenyl)porphyrinato cobalt (II), represented by the
formula (I):
~Nro
R
in which M stands for a transition metal (II). The complex,
when combined with a polymeric ligand, gives a membrane with
desired oxygen permeation performance. In the complex of the
formula (I), the transition metal (II) is preferably cobalt
(II) and substituents R's are preferably acetyl, acryl, meth-
acryl, or pival. Since one of the four substituents on the
porphyrin of the complex faces downward, oxygen adsorption and
desorption take place very rapidly through the steric inter-
stices. In a solid membrane combining this complex with a co-
poly~er of an alkyl acrylate or alkyl methacrylate and a vinyl
aromatic amine, the life of the complex of the formula (I) for
oxygen adsorption and desorption is extended sufficiently for
practical use, and the concept has led to the present invention
as oxygen-permeable polymeric membranes.
The invention thus resides in the following oxygen-
permeable polymeric membranes:
1. An oxygen-permeable polymeric membrane characterized by
a complex comprising (a) a transition metal ~II) ion, (b) a
ligand comprising a meso-tris(~ o-substituted-amidophenyl)-
mono-(~-o-substituted amidophenyl)porphyrinato, said metal ion
and porphyrin being of the formula (I)
R
I / ,~0
O ~OH~ N
R
in which M stands for a transition metal (II), R's are a
s` ~
substituent each, belng acetyl, acryl, methacryl, or pival, and
(c) an aromatic amine polymer.
2. The membrane of 1 above in which said transition metal
(II) comprises cobalt (II).
3. The membrane of 1 above in which said aromatic amine
polymer comprises copolymers of a vinyl aromatic amine and
either (a) an alkyl acrylate or (b) an alkyl methacrylate.
4. The membrane of 3 above in which said vinyl aromatic
amine is either (1) vinylimidazole or (2) vinylpyridine.
5. The membrane of 3 above in which said alkyl group of
the alkyl acrylate or alkyl methacrylate contains from 1 to 15
carbon atoms.
6. The membrane of 5 above in which said transition metal
(II) ion comprises cobalt (II).
7. The membrane of 1 above in which said transition metal
(II) and said ligand comprise from about 1 to 30 % by weight of
the polymeric membrane.
8. The membrane of 6 above in which said transition metal
(II) and said ligand comprise from about 1 to 30 % by weight of
the polymeric membrane.
DETAILED DESCRIPTION OF THE INVENTION
Stable, reddish brown membranes were successfully made by
uniformly dispersing the newly synthesized porphyrinato
transition metal complexes, especially cobalt complexes, in
monomeric bases under specific conditions. The (O2/N2) values
- 6 -
. ~J
of the memhranes exce~ded , even at ~l ~eed pressure of 15~
mmHg. They could collect oxyc3en-rich air corlcelltrated to 55%
or upwards by single-step permeatio~ o~ atmospheric air. At an
oxygen feed pressure of lO mm~g the (02/N2) value was more than
10. In order that the complex permeate oxygen as efficiently
in the region where the feed pressure i5 high, it is important
that the rates of combination and dissociation of the complex
and oxygen be great (Tsuchida: J. Japan Chem. Soc., 19~8, pp.
845-852 (1988)). The reaction for combination of oxygen with
the porphyrinato complex is inrluenced by the structure of the
complex and is governed by the effect of the steric substi-
tuents of the porphyrin surface. I'he rate constant increases
with the relaxation of the stereostructure over the rings of
the porphyrin, with the result that the efficiency of oxygen
permeation is improved and an increase in the (02/N2) value is
made possible.
~ hus, the above findings have now led to the present in-
vention. It provides novel oxygen-enriching polymeric mem-
branes characterized in that a complex of a specific porphyrin
structure is uniformly dispersed in a polymeric ligand.
~ he transition metal (II) ion, especially cobalt (II),
forms a complex which has reversible interactions with 2
The aromatic amine functions as the axial base in the
complex, "activating" the complex for reversible interactions
with 0~.
~ or use in the present invention the porphyrinato
transition metal comple~ is represented by the general. formula
(I) in which the transi.tion metal is de,ired -to be cohalt, and
the substituents P~'s are desired to be acetyl, acryl, meth-
acryl, or pival. If the Rs are larger than these, the
molecular weight of the compl.ex increases, reducing the amount
of oxygen adsorbed or desorbed per unit weight of the complex
and also lowering the rate of increase in the separability of
the membrane itself. The core metal of the porphyrinato
complex other than cobalt is, for example, iron, but the latter
involves difficulties in preparing a membrane that retains
activity. Desirable as the polymeric ligand is a copolymer
(with a molecular weight of 100,000 to 300,000) of a vinyl
aromatic amine and an alkyl acrylate or alkyl methacrylate in
which the alkyl group contains from 1 to 15 carbon atoms,
typified by poly(octyl methacrylate-co-N-vinylimidazole) or
poly(octyl.- methacrylate-N-vinylpyridine). If the alkyl group
contains more than 16 carbon atoms, the resulting membrane will
be brittle or hard to form. Also, if a ligand of a lower
molecular weight is used, the membrane life for oxygen adsorp-
tion and desorption will be shortened.
The cobalt ion of the porphyrinato cobalt and the ligand
residue (aromatic amine residue) that constitute a compl.ex are
in a molar ratio appropriately in the range from 1:1 to 1:30.
A porphyrinato cobalt and a polymeric ligand are separate-
ly d ssolved uniformly in an OrcJcilli( ;olvent such aschlo~o~orm, thoroughly deoxidized, and l~ixed up. In this case
the ?orphyrinato compiex content is desirably chosen from the
range of about 1 to about 30% by weight If the content is
less than 1% the selectivity (O2/N2) value will be too low to
obtain sufficiently oxygen-enriched air, but a content of 31%
or mo~e wil:l embri~tle the resulting membrane or hardly form a
membrane. The oxygen-permeable polymeric membrane of the
invention is formed by so-called solvent casting, or a process
in ~-hich the mixed solution is cast over a Teflon sheet or the
like in an oxygen-free atmosphere and the solvent is allowed to
evaporate slowly. For the manufacture of the membrane, thor-
ough oxygen removal from the solution in advance is advisable.
The thickness of the oxygen-permeable membrane according
to t;-_ invention is not specially limited but is usually chosen
fror .he range of about 1 to about lOO ~m. The membrane of the
inve..tion permits oxygen permeation with a high selectivity, at
the (C,/N2) value of 10 or upwards. For example, air at an
oxyg_-. concentration of 70% or more can be obtained by single-
stag_ concentration. The measurements of gas permeation
thrcu~h the oxygen-permeable membranes may be made using an
ordin2-y gas permeability measuring instrument conforming to
eithe- the low vacuum method or the isotactic method.
E X A M P L E S
~he invention will be more fully described below in
_ g _
connection with examples thereo~ which, 0~` course, are in 110
way limitative.
Also it is to b~ unclerstood that al~hough specifically
dense membranes are deal with in the examples, the membranes of
the invention are applicable as well to porous membranes
without departing from the spirit and scope o~ the invention.
Examplel
Meso tris(~,(x,~--o-met~lacrylamidophenyl)-mono-(~-o-meth-
acryl amidophenyl)porphyrinato cobalt (II) was synthesized in
the following manner.
Meso-tris(~ o-aminophenyl)-mono-(~-o-aminophenyl-
)porphyrin, an isomer of meso-tetra(o-aminophenyl)porphyrin (5
g), was separated and purified (Rf = 0.18, 2.5 g) using a
silica gel column and chloroform/ether (4/1) solvent. 2.5 g of
the separated meso-tris(~ -o-aminophenyl)-mono-(~-o-amino-
phenyl)porphyrin was dissolved in 100 ml of a chloroform solu-
tion. While the reactant solution was being kept at 0C or
below, 12 ml triethylamine and 17 ml methacrylic acid chloride
were added. After the reaction, the product was refined (Rf =
0.41) using a silica gel column and a developing solvent
chloroform/ether (8/1) to yield 3.1 g of meso-tris(~ -o-
methacrylamidophenyl)-mono-(~-o-methacrylamidophenyl)porphyrin,
lH NMR~ (ppm): -2.7 (s, 2H internal H), 1.1-1.2 (s, 12H, -
C(CH2)=CH3), 4.3-4.5 (s, 8H, -C(CH2)=CH3), 7.1-7.9 (m, 16H,
phenyl-H), 8.7 (s, 4H, amide-H), 8.8 (s, 8H, pyrol, ~-H).
-- 10 --
Cobalt ace~a-e ~Ind meso-tris((x,~ o-methacrylamido-
phenyl)-mono-(~-o-methacrylamiclophenyl)porphyrin were dissolved
in a mixed chloroform/methanol soLution. After 15 hours of
boiling-point reflux, the resultant was column-refined to
obtain 1.7 g of meso-tris(~ o-methacrylamidophenyl)-mono-
(~-o-methacrylamidophenyl)porphyrinato cobalt (II).
A polymeric membrane was made in the following way.
Nitrogen gas was introduced for 0.5 hour separately into 20 ml
of a chloroform solution containing 10 mg meso-tris(~ -o-
methacrylamidophenyl)-mono-(~-o-methacrylamidophenyl)porphy-
rinato cobalt (II) (hereinafter called "~3~-CoMP" for brevity)
and lO0 ml of a chloroform solution containing 0.5 g poly-
(octylmethacrylate-co-N-vinylimidazole) (TFMlm). Using three-
way tubes the two ~olutions were simultaneously deaerated under
vacuum.
Following thorough deaeration, the solutions were mixed,
and the solvent was subjected to pressure reduction under
vacuum until the total amount of the mixed solution decreased
to about 30 ml. ~lext, the solution under vacuum was trans-
ferred into a dry box, the box was swept out several times with
nitrogen, and the solution under vacuum was cast over a tetra-
fluoroethylene sheet 7 cm by 7 cm in size in an open nitrogen
atmosphere. The chloroform solution was gradually reduced in
pressure inside the dry box, down to 60, 50, 30, and 10 cmHg
over '0 hours. Finally, a polymeric membrane containing 12% by
weic~ht (~3p-coMP, !)O to 6~) ILIII ~hi.(k, red aTld c:lear, with
adequate mechanical strenc3th, was obtained.
Reversible oxyqen adsorption and desorption of the por-
ph~yrinato complex in the membrane could be confirmed from
changes in the visible spectrum (oxygen-combined type: 545 nm;
deoxygenation type: 52~ nm).
The polymeric membrane thus prepared was tested for air
permeability in conformity with the low vacuum method. The
membrane had a permeability coefficient of 4.0xlO-9 cm3 (STP) -
cm/cm2 sec cmHg and 2/N2 = 10, achieving efficient permeation
of oxygen.
Reference values of a membrane using a complex meso-tetra-
~ -o-pivalamidophenyl)porphyrinato cobalt (II), tested
under the same conditions as above, were 1.7xlO9 (cm3 (STP) -
cm/cm2 sec cmHg and O2/N2) = 4.3. The reference value of a
polymeric membrane free of the complex was (O2/N2) = 3.2,
clearly indicating the superior performance of the membrane
according to the present inventi.on.
Example 2
A polymeric membrane, 50 to 60 ~m thick, was made in the
same manner as described in Example 1 with the exception that
the ~3~-CoMP was replaced by meso-tris(~ -o-acrylamido-
phenyl)-mono-(~-o-acrylamidophenyl)porphyrinato cobalt (II).
Meso-tris(~ -o-acrylamidophenyl)-mono-(~-o-acrylamido-
phenyl)porphyrinato cobalt (II) was synthesized in the
- 12 -
follo-~ing way.
In the same manner as in Example 1, meso-tris(~ o-
aminophenyl)-mono-(~-o-aminophenyl)porphyrin was purified (2.5
g). 2.5 g of the separated meso-tris(a,~,a-o-aminophenyl)-
mono-(~-o-aminophenyl)porphyrin was dissolved in 100 ml of a
chloroforrn solution. While the reactant solution was being
kept a~ 0C or below, 12 ml triethylamine and 16 ml acrylic
acid chloride were added. After the reaction, the resultant
was column-refined to yield 2.6 g of meso-tris(~ -o-acryl-
amidophenyl)-mono-(~-o-acrylamidophenyl ! porphyrin, lH NMR~
(ppm): -2.7 (s, 2H internal H), 5.0~.1 (s, 8H, -CH-CH2), 5.8,
5.9, 6.0 (s, 4H, -CH=CH2~, 7.1-7.9 (m, 16H, phenyl-H), 8.6 (s,
4H, a~ide-H), 8.9 (s, 8H, pyrol, ~-H).
Cobalt acetate and meso-tris(~ -o-acrylamidophenyl)-
mono-(~-o-acrylamidophenyl)porphyrin were dissolved in a mixed
chloro'orm/methanol solution. After 15 hours of boiling-point
reflux, the resultant was column-refined to obtain 0.82 g of
meso-tris(~ -o-acrylamidophenyl)-mono-(~-o-acrylamido-
phenyl)porphyrinato cobalt (II) (hereinafter called "~3~-
CoArP").
'~ complex membrane containing 13~ by weight ~3~-CoArP was
made in the same way as in Example l. Reversible oxygen
adsor?tion and desorption of the porphyrinato complex in the
membrane could be confirmed from visible spectrum chanses
(oxysen-combined type: 545 nm; deoxygenation type: 528 nm).
- 13 -
'l'he polymer.ic rnembrc~ne thus prepared was tested for air
permeability in conformity wi-th the low vacuurn method. The
membrane had a permeabLlity coefficient of 5.5xlO 9 cm3 (STP) -
cmlcm2 sec cm~g and o~/N2 = 14, achieving efficient permeation
of oxygen.
Reference values o:f a membrane usiny a complex meso-tetra-
~ -o-piva.lamidophenyl)porphyrinato cobalt (II), tested
under the same conditions as above, were: permeability coeffi-
cient 1.7x10-9 cm3 (srrp) cm/cm2 sec cmHg and (02/N2) = 4.3. The
reference value of a polymeric membrane free of the complex was
(02/N2) = 3.2, clearly tes~ifying to the superior performance
of the membrane of the present invention.
Example 3
The procedure of Example l was repeated excepting the use
as the complex of meso-tris(~,~,a-o-acetamidophenyl)-mono-~p-o-
acetamidophenyl)porphyrinato cobalt (II), and permeation
measurements were made in the same manner as in Example 1.
The meso-tris(~,~,a-o-acetamidophenyl)-mono-(p-o-acet-
amidophenyl)porphyrinato cobalt (II) was synthesized as below.
In the same manner as in Example 1, meso-tris(~ -o-
aminophenyl)-mono-(~-o-aminophenyl)porphyrin was purified (2.5
g). 2.~ g of the separated meso-tris(a,~,a-o-aminophenyl)-
mono-(~-o-aminophenyl)porphyrin was dissolved in lO0 ml of a
chloroform solution. While the reactant solution was being
kept at 0C or below, 12 ml. triethylamine and 14 ml acetyl
chloride were added. After the reaction, 'he resultant was
column-refined to yield 2.1 y of rneso-tris(~ -o-acetyl-
amidophenyl)-mono-(~-o-acetylami.dophenyl)porphyrin, lH NMR~
(ppm): -2.5 (s, 2H internal H), 1.~0-1.90(s, 12H, CH3), 7.1-8.0
(m, 1.6H, pheny].-H), ~.7 (s, ~H, amide-H), 808 (s, 8H, pyrol, ~-
Cobalt acetate and meso-tris(~ -o-acrylamidophenyl)-
mono-(~-o-acrylamidophenyl)porphyrin were dissolved in a mixed
chloroform/methanol solution. After 10 hours of boiling-point
reflux, the resultant was column-refined to obtain 0.45 g of
meso-tris(~ -o-acetylamidophenyl)-mono-(~-o-acetylamido-
phenyl)porphyrinato cobalt (II) (hereinafter called "~3~-
CoAtP").
A complex membrane containing 12% by weight ~3~-CoAtP was
made in the same way as in Example 1. Reversible oxygen
adsorp~ion and desorption of the porphyrinato complex in the
membrane could be confirmed from visible spectrum changes
(oxygen-combined type: 545 nm; deoxygenation type: 528 nm).
The polymeric membrane thus prepared was tested for air
permea ility by the low vacuum method. The membrane had a
permeability coefficient of 4.9xlO-9 cm3 (STP) cm/cm2 sec cmHg
and 2/~2 = 12, achieving efficient oxygen permeation.
Reference values of a membrane using a complex meso-tetra-
~ -o-pivalamidophenyl)porphyrinato cobalt (II), tested
under .ne same conditions as above, were: permeability coeffi-
- 15 -
cient 1.7x10-9 cm3 ((~rrp) cm/crn2 sec cmHg and (O2/N~ 4.3. The
reference value of a polymeric membrane free of the complex was
(O2/N2) = 3.2, clearly proving the superior performance of the
membrane of the present invention.
Example
In Example 1 the complex used was replaced by meso-tris~
~ ,a-o-pivalamidophenyl)-mono~ -o-pivalamidophenyl)porphy-
rinato cobalt (II), but otherwise in the same manner as in
Example 1 permeation measurements were made.
The meso-tris(~,~,a-o-pivalamidophenyl)-mono-(~-o-pival-
amidophenyl)porphyrinato cobalt (II) was synthesized in the
following way.
As in Example l, meso-tris(~ -o-aminophenyl)-mono-(~-o-
aminophenyl)porphyrin was purified (2.5 g). 2.5 g of the sepa-
rated meso-tris(a,~,a-o-aminophenyl)-mono-(~-o-aminophenyl)-
porphyrin was dissolved in 100 ml of a chloroform solution.
While the reactant solution was being kept at 0C or below, 12
ml triethylamine and 18 ml acetyl chloride were added. After
the reaction, the resultant was column-refined to yield 3.7 g
of meso-tris(~ -o-pivalamidophenyl)-mono-(~-o-pivalamido-
phenyl~porphyrin, 1~ NMRX (ppm): -2.5 (s, 2H internal H), 0.10,
0.16, 0.23 (s, 27H, tert CH3), 7.1-7.9 (m, 16H, phenyl-H), 8.7
(s, 4H, amide-H), 8.8 (s, 8H, pyrol, ~-H).
Cobalt acetate and meso-tris(~ -o-pivalamidophenyl)-
mono-(~-o-pivalamidophenyl)porphyrin were dissolved in a mixed
- 16 -
chloroform/methanol ;olution. After 20 hours of hoiling-point
reflux, the resultant WclS column-refirled to obtain 3.1 g of
meso-tris(~ -o--pivaLamiclophenyl)-mono-(~-o-pivalamido-
phenyl)porphyrinato cohalt (II) (hereinafter called "~3~-
CoPP" )
A complex memhrane containing 12~ by weight ~3~-CoPP was
made in the same way as in Example 1. Reversible oxygen
adsorption and desorption of the porphyrinato complex in the
membrane could be confirmed from visible spectrum changes
(oxygen-combined type: 545 nm; deoxygenation type: 528 nm).
The polymeric memhrane thus prepared was tested for air
permeability by the low vacuum method. The membrane had a
permeability coefficient of ~.5xlO 9 cm3 (STP) cm/cm2 sec cmHg
and 02/N2 = 8.8, achieving efficient oxygen permeation.
Reference values of a membrane using a complex meso-tetra-
(~,c,c,~-o-pivalamidophenyl)porphyrinato cobalt (II), tested
under identical conditions, were: permeability coefficient
1.7x10-9 cm3 (STP) cm/cm2 sec cmHg and (02/N2) = 4.3. The
reference value of a polymeric membrane free of the complex was
(2/`~2) = 3.2, clearly proving the superior performance of the
membrane of the present invention. Also, the oxygen permeabil-
ity OI the membrane according to the invention was generally as
stable as the conventional membranes.
- 17 -