Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~:;
~'
~ 1 2 ~ L~ 8 4 ~ 9
UNITARY IN~RAVA~CULAR DEFIBRILLATIN~ CAT~ETER
WITH ~EP~RATE BIPOLAR SENSINC
BACKGROUND OF THE INVENTION
This invention relates to body implantable medical
devices, and more particularly to defibrillating catheter~
employing bipolar sensing.
- Heart disease is a major cause of deaths in the United
States and in other industrial nations. Tachyarrythmias (rapid
disturbances in cardiac electrical activity), in particular the
conditions of ventricular tachycardia, ventricular flutter and
ventricular fibrillation, are~ widely believed to be the primary
cause of sudden deaths associated with heart disease. Atrial
tachyarrythmic conditions, on the other hand, are not considered
life threatening unless they lead to rapid ventricular
disturbance.
Recent experience confirms that tachyarrythmic conditlons
frequently can be corrected by applying relatively high enerqy
electrical shocks to the heart, a technique often referred to aB
cardioversion. Cardioversion devices include implantable
electronic stand-by defibrillators which, in response to the
detection of an abnormally rapid cardiac rhythm, discharge
sufficient energy through electrodes connected to the heart to
de-polarize and restore the heart to normal cardiac rhythm.
Cardioverting or defibrillation devices typically include
means for monitoring heart activity as well as delivery of
cardioversion energy. For example, U. S. Patent No. 3,942,536
(Mirowski et al) discloses an intravascular catheter with a cap
electrode at the distal tip, a distal electrode including a
plurality of rings near the tip, and a proximal electrode also
consisting of a plurality of rings. The tip and distal
'
2~84~
electrodes are used to provide pacing pulses, while
defibrillation pulses are provided using the distal and proximal
electrodes. A probe is provided to sense pressure in the right
ventricle, to initiate cardioversion upon sensing a pressure that
5 does not exceed a predetermined threshold.
U. S. Patent No. 4,355,646 (Kallok et al) is directed to
a transvenous defibrillating lead with one tip electrode and
three additional, annular electrodes. The tip electrode and the
most distal of the annular electrodes are placed in the right
10 ventricle and used to measure impedance changes in the ventricle.
Defibrillating pulses are delivered across all four of the
electrodes.
A key factor in successful defibrillation by implantable
t devices is the timely and accurate detection of the R-waves, the
15 relatively weak electrical signals produced by ventricular
contraction. In particular, the sensing means (one or more
electrodes) of the defibrillating device must be capable of
quickly detecting abnormally high cardiac rhythm in order to
trigger the defibrillation pulse. Perhaps more importantly, the
20 sensing means preferably is able to confirm a successful
defibrillation, i.e. a return to normal cardiac rhythm, as soon
as possible after each defibrillation pulse. Otherwise, there is
the danger of the device delivering an unnecessary and possibly
i harmful defibrillation pulse.
The advantage of preventing unnecessary or undue
defibrillation pulses is recognized in U. S. Patent No. 4,614,192
(Imran et al). Imran teaches an implantable cardiac
defibrillator employing bipolar sensing, in particular a bipolar
sensing electrode assembly including a distal tip electrode and a
nearby ring electrode, along with two sensing and high voltage
-2-
D
~ O '~ 9
delivery electrodes, one in the superior vena cava and another in
the form of a patch over the myocardium, near the apex of the
heart. This system contemplates three separately implanted
electrodes or groups of electrodes. A unitary intravascular
multiple electrode catheter is disclosed in U. S. Patent No.
4,603,70~ tSpeicher et al). The catheter includes three
electrodes- a distal tip electrode, an intermediate spring
electrode and a proximal spring electrode. The tip and
intermediate electrodes are used in pacing and sensing, while the
intermediate and proximal spring electrodes are used to deliver
defibrillation pulses.
Use of a common lead for sensing and delivering
defibrillation pulses, however, interferes with the timely
sensing of R-waves. In particular, tissue proximate the
cardioversion discharge electrodes temporarily loses much of its
ability to conduct electrical impulses immediately after
discharge, resulting in an effective suppression of the R-wave
immediately following a defibrillation pulse. Thus, post-shoc~
sensing abnormalities prevent an immediate sensing that the heart
has returned to normal sinus rhythm in response to the
defibrillation pulse, presenting the risk that another, unneeded
defibrillation pulse will be delivered.
Therefore, it is an object of the'present invention to
provide a unitary intravascular implantable device in which
post-defibrillation pulse sensing abnormalities are substantially
reduced or eliminated.
Another object is to provide a unitary defibrillation
catheter with sensing circuitry independent of the defibrillation
circuitry and with increased spacing of sensing electrodes from
~34~
the nearest defibrillation electrode, for more discrete and
localized electrograms.
Another object of the invention is to provide an
implantable defibrillation device with a defibrillation pulse
_ .~. 5 delivery sy6tem with electrodes and conductqrs suited for
relatively high energy defibrillation, along with independent
sensing circuitry including electrodes and conductors suited to
sensing.
Yet another object is to provide a unitary defibrillation
catheter which simultaneously affords optimum spacing between
bipolar sensing electrodes, between a pair of defibrillation
electrodes, and between the most adjacent sensing and
defibrillation electrodes.
SUMMARY OF THE INVENTION
To achieve these and other objects, there is provided a
-- unitary intravascular cardioversion device. The device includes
an elongate, flexible and dielectric catheter body having a
proximal end region, a distal end region and a lumen means formed
in the body from the proximal end region to the distal end
region. The device has a cardioversion circuit including a
cardioversion electrode means mounted on the catheter body
proximally of the distal region, and a flexible conductor means
connected to the cardioversion electrode means, for conducting
electrical pulses between the cardioversion electrode means and
the proximal end region, and a cardioversion connector means near
the proximal end region for electrically coupling the conductor
means with a cardioversion pulse generating means, thereby to
deliver cardioversion pulses to the cardioversion electrode
means. The device further includes a cardiac sensing circuit
including a first sensing electrode mounted on the catheter body
~ 20'~4~
at the distal end region, a first sensing conductor means
connected to the first sensing electrode for detecting electrical
pulses between the first sensing electrode and the proximal end
region, a second sensing electrode mounted on the catheter body
at the distal end region proximally of the first sensing
electrode and spaced apart from the first sensing electrode by a
predetermined first distance, a second flexible sensing conductor
means connected to the second sensing electrode for detecting
electrical pulses between the second sensing electrode and the
proximal end region, and a sensing connector means near the
proximal end region for electrically coupling the first and
second sensing conductor means with a pulse sensing means,
thereby to utilize the first and second sensing electrodes as a
bipolar pulse sensing pair independent of the cardioversion
circuit.
Preferably, the first sensing electrode i8 a distal tip
electrode at the distal end of the catheter body, and the second
sensing electrode is a ring electrode surrounding the catheter
body and spaced apart from the tip electrode a distance in the
- 20 range of from one to twenty millimeters, preferably ten
millimeters.
The cardioversion means advantageously includes distal
and proximal cardioversion electrodes in the form of flexible,
electrically conductive coils. In this event, the conductor
means includes a first cardioversion conductor coupled to the
distal conversion electrode and a second cardioversion conductor
coupled to the proximal electrode. Both cardioversion conductors
are flexible and contained in the lumen means, with the
cardioversion connector means then coupling both cardioversion
conductors to the pulse generating means. Each of the proximal
2~'18449
and distal cardioversion coils can have a length in the range of
from 1 to 7.5 centimeters.
The preferred spacing between the proximal eensing
electrode or ring electrode, and the distal defibrillating
electrode, is at least one centimeter. This ensures that heart
tissue proximate and between the sensing electrodes is
effectively isolated from the tissue subject to the
; defibrillation pulse. As a result the device affords accurate
R-wave sensing immediately after applying a defibrillation pulse,
substantially eliminating the possibility of charging for and
delivering unnecessary defibrillation pulses after the heart has
returned to normal sinus rhythm.
A further advantage of the present invention is that it
permits selection of the distance between the defibrillating
electrodes for a preferred positioning of the distal
defibrillating electrode, e.g. in the right ventricle near the
apex, and of the proximal defibrillating electrode, e.g. high in
the right atrium or within the superior vena cava. Total
electrical independence of the sensing system from the
defibrillation circuit permits simultaneous optimum separation of
the tip and ring electrodes, the ring electrode and distal
defibrillating electrode, and the two defibrillating electrodes,
an advantage not attainable when a single'electrode is utilized
for defibrillation pulsing and sensing. A further advantage of
the present invention resides in the ability to tailor electrodes
and conductors specifically for the sensing system, and to tailor
other electrodes and conductors specifically for the
defibrillation circuit. The relatively high currents and
voltages involved in the defibrillation circuit require
relatively large surface area electrodes to reduce impedance, and
20'184~9
eonduetors formed of drawn brazed strand (DBS) wires or other
highly conductive material. The sensing system does not impose
these requirements. A unitary catheter with independent sensing
and cardioversion systems, in accordance with the present
invention, permits a better impedance matching of the two sensing
electrodes. Such catheter further allows seleetion of materials
and component sizes customized to either sensing or
eardioversion, for example multi-conduetor tube (MCT)
eonstruetion involving eoaxial windings for defibrillation
eonduetors, in eombination with sensing eonduetors eontained
within a eentral lumen of the eatheter.
Another aspect of the present invention is a
eardioversion and sensing system in whieh sensing eleetrodes are
mounted on a sensing eatheter for use in eonjunetion with a pair
of eardioversion electrodes. The cardioversion eleetrodes may be
provided as eoils on a separate cardioversion eatheter, as two
separate patch electrodes, or as a single defibrillation coil in
eombination with a patch electrode. The eleetrodes are plaeed in
the region of the heart, encompassing ventrieular and atrial
endoeardial plaeement, intraparacardial or extraparaeardial
plaeement, vascular positioning, and in general within the
thoraeie cavity. The use of patch electrodes for cardioversion,
alone or with a coil electrode, affords a high degree of
flexibility in eleetrode positioning.~
Thus, in accordance with the present invention, a
catheter system provides sensing electrodes in eomplete isolation
; from a defibrillation pulse delivery system, for substantially
immediate R-wave sensing following the application of each
defibrillation pulse.
'~ -
~ 2~'18~49
IN THE DRAWINGS
--~ For a further understanding of the above and other
features and advantages, reference is made to the following
detailed description and the drawings, in which:
Figure 1 is a plan view of a unitary intravascular
defibrillating catheter constructed in accordance with the
present invention;
Figure 2 is a sectional view of a portion of the catheter
of Figure l;
Figure 3 is a sectional view illustrating the positioning
of the catheter of Figure 1 within the heart;
Figure 4 is a sectional view of a portion of an
alternative embodiment catheter constructed in accordance with
the present invention;
Figure 5 is a plan view of another alternative embodiment
of the invention comprising two leads separately implanted in the
heart; and
Figure 6 is a schematic vlew of yet another alternative
embodiment using patch electrodes for defibrillation.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
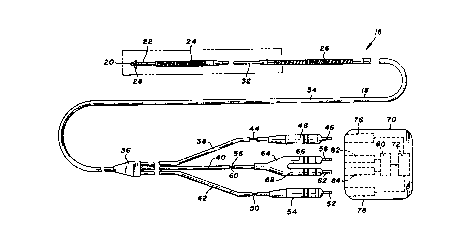
Turning now to the drawings, there is shown in Figure 1 a
unitary intravascular defibrillation catheter 16 including an
elongate and flexible catheter body 18 co~structed of a
dielectric material, for example silastic or polyurethane. Four
electrodes are mounted to the catheter body, including a distal
tip electrode 20 at the distal end of the body, a bipolar ring
electrode 22, a distal spring electrode 24 and a proximal spring
electrode 26. A plurality of tines 28 near the distal end of the
catheter, formed of the dielectric material comprising the body,
20~49
assist in the positioning and securing of the catheter during
implant.
Catheter body 18 further includes a reduced diameter
"- distal tubing portion 30 which supports the tip and ring
,,-~'' 5 electrodes, a proximal reduced diameter tubing portion 32 between
spring electrodes 24 and 26, and a sheath portion 34 encompassing
the majority of the catheter length.
A reinforcing member 36 provides a junction for sheath 34
and three lengths of electrically insulative tubing 38, 40 and
42. Tubing 38 contains a conductor 44 provided for transmitting
electrical signals from distal spring electrode 24 to a pin 46.
An electrically insulative boot 48 surrounds pin 46 and tubing
44. A conductor 50, contained within insulative tubing 42 and
sheath 34, electrically couples proximal spring electrode 26 and
a pin 52, with pin 52 and tubing 42 being surrounded by an
electrically insulative boot 54.
,Similarly, a conductor 56 electrically couples ring
, . , , .. _ ~. . . ~,
electrode 22 with a pin 58, and a conductor 60 similarly couples
tip electrode 20 with a pin 62. Pins 58 and 62 and conductors 56
and 60 are surrounded by an insulative plug 64 with boot portions
66 and 68.
In use, catheter 16, particularly at plug 64 and boots 48
and 54, is electrically and mechanically coupled to a
defibrillation control unit 70 including defibrillation pulse
generating circuitry 72, represented schematically in Figure 1.
Unit 70 includes a pair of receptacles 76 and 78 for receiving
pin 46 and boot 48, and pin 52 and boot 54, respectively, thus to
electrically couple spring electrodes 24 and 26 with
~- '' defibrillation pulse generating circuitry 72. Boots 48 and 54
40~~184~9
fit tightly within their respective receptacles to provide a
positive fluid seal.
Defibrillation unit 70 further includes pulse or heart
rate sensing circuitry represented schematically at 80. A pair
of sensing receptacles 82 and 84 receive plug 64, to electrically
couple distal tip electrode 20 and ring electrode 22 with the
sensing circuitry, Witll the boot portions of the plug member
again providing a fluid seal. Further details of defibrillation
control unit 70 are not discussed herein as they are known in the
art and not particularly germane to the present invention. In
short, the connection of pins 46, 52, 58 and 62 as described
creates two independent electrical circuits: a sensing circuit
including tip electrode 20 and ring electrode 22, and a
defibrillation circuit including spring electrodes 24 and 26.
The sensing circuit monitors heart electrical activity, in
particular to sense tachyarrythmias. In response to such
sensing, the pulse generating circuit delivers a defibrillating
pulse to the heart across spring electrodes 24 and 26.
As seen in Figure 2, tip electrode 20 is constructed of
one or more filaments, preferably a thin wire 86 of platinum or a
platinum iridium alloy. The wire is stretched, then crumpled and
packed against the distal end of catheter body 18. A screen 88,
also of platinum or a platinum alloy, is fastened to the
periphery of the catheter body distal end and maintains the
crumpled wire in place. For further information regarding this
type of electrode, reference is made to U.S. Patent No. 4,156,429
(Amundson). So constructed, electrode 20 is highly porous, for
example consisting of approximately twenty percent platinum alloy
by volume, the remaining eighty percent being open to permit
passage of bodily fluids through the tip electrode and to admit
,
--10--
~ ~ 4 ~ 4 4 g
ingrowth of tissue, which assists in anchoring the tip electrode after implant. Tip
electrode performance may be further enhanced by surface treatment to micro texturize
the tip, as disclosed in U.S. Patent 5,074,313, issued 24 December 1991, and assigned to
the assignee of this application. This treatment subst~nti~lly increases the reactive surface
area of the tip.
Conductor 60 includes a single wound coil 90 formed of a nickel alloy or
other electrically conductive material p~ g flexure. The exposed distal end of coil
90 is electrically and mechanically coupled to distal tip electrode 20. The remainder of
the coil is surrounded by a flexible, dielectric sheath 92. The rem~ining conductors are
similarly constructed. Conductor 56 includes a single wound coil 94 surrounded by a
sheath 96 and with its exposed distal end coupled to ring electrode 22. The ring electrode
is constructed of pl~tim-m, a pl~tinum iridium alloy or other appropliate electrically
conductive and body compatible material. The outer surface area of the ring electrode
exposed to bodily tissue and fluids is in the range of from ten to fifty square millimeters,
and more preferably is about the same in effective surface area as the tip. If desired, ring
electrode 22 can be subject to ~u~eling or other surface treatment to impart
microporosity. For accurate R-wave sensing, ring electrode 22 must be spaced apart
from tip electrode 20 in the range of from one to twenty millimeters, with a particularly
ple~lled spacing between these electrodes being about ten millimeters.
Proximally of ring electrode 22 is a fitting 98 which surrounds distal tubing
portion 30. Fitting 98 is joined to the distal end of spring electrode 24, and cooperates
with a fitting 100 at the proximal end of spring electrode 24 to support the
-11-
j:.
.~,"
~ ;
2~'~84~9
electrode. Distal spring electrode 24 can have a length of from
1 to 7.5 centimeters, and up to 15 centimeters if especially
smooth. Preferably electrode 24 is 6 centimeters long, to
provide a relatively large exposed surface area necessary for
effective delivery of defibrillation pulses. Spring electrode 24
is spaced apart from ring electrode 22 a distance in the range of
five to twenty millimeters, although generally a spacing of at
least one centimeter i5 recommended to ensure that heart tissue
used in sensing pulse rate, particularly tissue near ring
electrode 22, is sufficiently distant from tissue affected by the
defibrillation pulse to ensure a localized, isolated and
.. . ~ . .. .
therefore more accurate R-wave sensing.
Proximally of spring electrode 24, a pair of fittings,
one of which is shown at 102, support proximal spring electrode
26. Like spring electrode 24, spring electrode 26 is constructed
of an electrically conductive and bodily compatible material such
as titanium or platinum. Proximal spring electrode 26 can have a
length in the range of 1 to 7.5 centimeters, and is preferably
3.8 centimeters long. The spacing between proximal and distal
spring electrodes 24 and 26 preferably is about eleven
- centimeters, although a spacing of from six to fourteen
centimeters has been found satisfactory.
--- Tubing sections 30 and 32, spring electrodes 24 and 26
and sheath 34 cooperate to define a central lumen 104 running the
length of the catheter from the distal tip to reinforcing member
36. Conductors 44, 50, 56 and 60 all are contained within lumen
104. Proximally of reinforcing member 36, each of the conductors
is contained within its corresponding one of tubing sections 38,
40 and 42. Thus, the proximal tubing sections sheath, spring
electrodes, and distal tubing sections form a lumen means in
-12-
~8~9
which the conductors are contained and thus isolated from bodily
fluids.
Catheter 16 is inserted intravenously, for example into
the subclavian vein or the cephalic vein, and progressively moved
toward the heart until the distal end reaches a selected cardiac
chamber. As illustrated in Figure 3, catheter 16 preferably is
inserted to position distal tip electrode 20 and ring electrode
22 in a right ventricle 106 of the heart 108, near the apex 110.
Within the ranges for spacing and lengths discus6ed above, spring
electrode 24 preferably is within the right ventricle when tip
electrode 20 is positioned as described, with proximal spring
electrode 26 located high in the right atrium 112 or in the
superior vena cava 114.
With the distal tip positioned as shown, the lead
proximal end, still outside the body, is maneuvered to implant
the distal tip into the endocardium. Once implanted, distal tip
electrode 20, ring electrode 22, conductors 56 and 60 and sensing
circuitry 80, cooperate to monitor electrical activity in the
heart, in particular R-wave activity.
Figure 4 shows an alternative design catheter 120 with a
solid platinum or titanium tip electrode 122 and an annular
electrode 124 near the tip electrode for bipolar R-wave sensing.
A central lumen 126 of catheter 120 contai'ns a pair of conductors
128 and 130 connected to tip electrode 122 and annular electrode
25 124, respectively. Conductor 128 includes a conductive single
~- coil winding 132 surrounded by an insulative sheath 134 and
exposed at its distal end for connection to the tip electrode.
Similarly, conductor 130 includes a coil winding 136 surrounded
by an insulative sheath 138 and exposed for its connection to the
30 annular electrode. Electrodes 122 and 124 are mounted on a
20'~84~9
i dielectric and flexible dlstal tubing section 140 of catheter
120.
Defibrillation pulses are applied through a pair of
spring electrodes, a distal spring electrode 142 and a proximal
spring electrode 144. The distal spring electrode s supported
between a pair of fittings 146 and 148 at its opposite ends.
Spring electrode 144 is similarly supported between a pair of
fittings, one of which is shown at 150.
For transmission of cardioversion pulses between spring
electrodes 142 and 144, multi-filament conductors 152 and 154 are
connected to electrodes 122 and 124, respectively, and also are
electrically coupled to a pulse generator, not shown. Each of
conductors 152 and 154 includes a plurality of individual
electrically conductive filaments arranged in parallel, helical
paths about the center of catheter 120. More particularly,
conductor 152 includes filaments 152a, 152b and 152c, embedded in
a length of insulative tubing 156 and thus electrically isolated
from one another. At their distal ends, however, filaments
152a-c are exposed for electrical coupling to distal spring
electrode 142.
Similarly, conductor 154 includes filaments 154a, 154b
and 154c. Through the majority of the length of conductor 154,
the filaments are embedded in tubing 156 and thus are
electrically isolated. The distal ends of the filaments are
exposed near electrically conductive fitting 150, for electrical
coupling to this fitting, illustrated as an alternative to a
coupling of these filaments to spring electrode 144. Conductors
152 and 154 are laterally offset from one another over the entire
length of tubing 156 and thus are electrically isolated from one
another. The multi-filament construction of these conductors
-14-
2~8~49
affords the desired flexibility in catheter 120 and the increased
cross-sectional conductive area desired for handling high energy
cardioversion pulses, while permitting the catheter diameter to
remain relatively small. For a further explanation of the
helically woùnd and isolated filament technique, reference is
made to U. S. Patent No. 4,559,951 (Dahl et al).
Figure 5 discloses yet another approacll to separate
sensing and defibrillating, employing a sensing catheter 160 and
a defibrillation catheter 162, separately implantable within the
right ventricle 164 of the heart lG6. Sensing catheter 160
includes a tip electrode 168 and a ring electrode 170 near the
distal tip but separated from the tube electrode by one to ten
millimeters as previously explained. A pair of conductors,
contained within insulative tubing 172, connect tip and ring
electrodes 168 and 170 with pulse sensing circuitry near the
proximal end of sensing catheter 160. Defibrillation catheter
162 includes a distal tip with tines 174 to assist in positioning
the catheter upon implant. Proximal and distal spring electrodes
'r 176 and 178 are mounted to catheter tubing 180 as explained in
connection with Figures 2 and 4. A pair of conductors, one
associated with each of spring electrodes 176 and 178, transmit
defibrillation pulses to the spring electrodes. The conductors
may be contained in a central lumen of thè catheter, or
alternatively helically wound as explained in connection with
Figure 4. The sensing and defibrillating conductors are coupled
to pulse generating and heart rate sensing circuitry by plugs 184
and 182, respectively. If desired, a patch electrode 186, at
I least equal to spring electrodes 176 and 178 in surface area, is
secured to myocardial tissue and used in combination with the
spring electrodes or in lieu of one of the spring electrodes. As
-15-
2~8~49
compared to the embodiments in Figures 2 and 4, the two-catheter
system in Figure 5 of course requires a greater degree of skill
and effort for implantation. On the other hand, it affords the
added advantage of lateral or transverse orientation of the
sensing electrodes from the defibrillation spring electrodes, to
assure localized R-wave sensing remote from tissue subject to
--- defibrillation, and further to permit optimum positioning of the
sensing system and the defibrillation system, each fully
independently of the other.
Figure 6 schematically illustrates a system employing a
sensing catheter 190 having a tip electrode 192 and a ring
electrode 194 spaced apart from the tip electrode by one to ten
millimeters. A pair of conductors in the catheter are connected
at their distal ends to electrodes 192 and 194, respectively, and
at their proximal ends to pins 196 and 198. The pins are plugged
into a defibrillation control unit 200 similar to unit 70
described in connection with Figure 1, to electrically couple the
sensing electrodes to sensing circuitry in the control unit.
The system further includes a pair of defibrillation
electrodes in the form of patch electrodes 202 and 204, each of
which is subcutaneously implanted in the thoracic region, e.g.
secured to myocardial tissue. A conductor electrically couples
patch electrode 202 with a proximal pin 2~6, and another
conductor likewise couples patch electrode 204 to a proximal
z5 terminal pin 208. Pins 206 and 208 are plugged into control unit
200 to electrically couple the patch electrodes with a pulse
generating circuit contained in the control unit.
In this system, catheter 190 is provided solely for
sensing and defibrillation is accomplished solely through the
patch electrodes. Accordingly, this system is particularly
-16-
2aLl34~s
useful in applications calling for maximum flexibility in the
positioning of defibrlllation electrodes, and in which a single
catheter is preferred.
Figures 7 and 8 illustrate another alternative, namely
a bifurcated catheter 190 having a proximal spring cardioversion
electrode 192 and a distal spring cardioversion electrode 194.
Separate conductors are connected to spring electrodes 192 and
194 respectively, for transmitting cardioversion pulses between
these electrodes. Near the distal end of catheter 190, an
~ 10 insulative boot forms a junction 196. A first extension 198,
distally of the junction, supports a helical coil 200 used in a
known manner to secure extension 198, and thus the remainder of
the lead, to endocardial tissue.
A second extension 202 of the catheter is directed
generally proximally of junction 196 but inclined relative to the
remainder of the catheter. Two sensing electrodes including a
tip electrode 204 and a ring electrode 206, are supported on
extension 202 and constructed as previously described. Separate
conductors are connected to tip electrode 204 and ring electrode
206 respectively, each for transmitting electrical pulses between
its associated sensing electrode and the proximal end region of
catheter 190.
., . ~ . , . ~
As seen in Figure 8, catheter 190 preferably is
inserted to position the distal tip of extension 198 in the right
ventricle 208 of the heart 210, at the apex 212. Coil 200 is
secured to endocardial tissue at the apex and thus maintains
catheter 190 in the desired position. As noted previously in
connection with other embodiments, distal spring electrode 194
preferably is within the right ventricle and proximal spring
-17-
~ 20~84~9
.~
electrode 192 is in either the right atrium 214 or the superior
vena cava 216.
Extension 202 of the catheter is inclined away from the
remainder of catheter 190 toward the septum 218, preferably to
position tip electrode 204 and ring electrode 206 against the
septum along the outflow tract, again resulting in sensing
remotely of the area subject to cardioversion pulses. In view of
the reverse bend in the conductors from the sensing electrodes at
junction 196, it is recommended that these conductors be coils,
with a known reverse winding technique used to negotiate the
relatively sharp bend. In other respects, the electrodes and
conductors can be constructed as previously described.
Thus, in accordance with the present invention the R-
wave sensing system is configured in complete electrical
isolation from the cardioversion system, with a bipolar sensing
electrode means interacting with endocardial tissue remote from
tissue subject to the immediate electrical affects of
cardioversion. Consequently post-shock sensing abnormalities
encountered in connection with previous devices, particularly
unitary catheters, are substantially eliminated. A more timely
and accurate R-wave sensing is achieved, to substantially reduce
the risk of generating unnecessary and possibly harmful
cardioversion pulses after a return to normal slnus rhythm.
What is claimed is:
-18-