Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
.... , I
20~257~
Title of the Invention
Electrophtographic Toner
Backqround of the Invention
The present invention relates to an electropho-
tographic toner, and more particularly to an electro-
photographic toner to be used for an image forming
apparatus such as an electrostatic copying apparatus,
a laser printer or the like.
In the image forminy apparatus above-mentioned,
an electrostatic latent image formed on the surface of
a photoreceptor by exposure to light is let come in
contact with an electroph~tographic developer by a
developping device. Toner in the electrophotographic
developer is electrostatically sticked to the electro-
static latent image. This causes the electrostatic
latent image to be turned into a toner image. Then,
the toner image is transferred to paper from the sur-
face of the photoreceptor and fixed on the paper, thus
achieving image forming.
As an electrophotographic toner, there may be
generally used toner particles containing a binder
resin, a coloring agent such as carbon black or the
like, an electric charge controlling agent, a release
agent, a flowability imparting agent as necessary and
--2--
Z05~57~
the like. As the electric charge controlling agent,
there is generally used an azo-type metal complex salt
dye (azo-type chromium dye or the like).
To improve the flowability of the toner parti-
cles, silica fine powder, particularly hydrophobicsilica fine powder, is generally mixed with and dis-
persed in the toner particles.
However, such a conventional electrophotographic
toner presents the problems that the electric charge
characteristics are not stabilized to provoke fog,
decrease in image density, toner scattering or a so-
called letter dispersion, i.e., spots as formed by the
toner scattering around reproduced letters, so that
stable images cannot be obtained. In particular, when
a black toner containing carbon black as a coloring
agent is used, the problems above-mentioned are re-
markable.
Summary of the Invention
It is an object of the present invention to pro-
vide an electrophotographic toner with which there can
be obtained stable images free from fog, decrease in
image density, letter dispersion, toner scattering and
the like.
To achieve the object above-mentioned, the in-
"'''''``' ~' ' "" ' ` ' -: - ......
. ~ .
--3--
2~S25~
ventors have studied hard and paid their attention to
the pH value of an azo-type metal complex salt dye
used as the electric charge controlling agent. The
inventors have found the novel fact that the electric
charge characteristics and humidity resistance of a
toner and dispersibility of the electric charge con-
trolling agent in the resin vary with this pH value to
cause a variety of problems such as defective image
(insufficient image density, fog and the like), toner
scattering and the like.
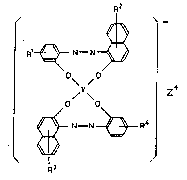
The electrophotographic toner in accordance with
the present invention contains, as the electric charge
controlling agent, a compound represented by the fol-
lowing general formula (1) and presenting a pN value
in a range from 3 to 5:
~ _
~ ~ R ~ N= N $~
O~ ~0 ~ (
1)
/Y\ Z
~ ~R~'
--4--
20S~571
[wherein Rl, R2, R3 and R4 may be the same as or dif-
ferent from one another, and each is a hydrogen atom,
a halogen atom or the following group:
- S O 2 ~ ~
R 6
(wherein R5 and R6 may be the same as or different
from one another, and each is an alkyl or aryl group),
and R , R2, R3 and R4 should not be simultaneously a
hydrogen atom; Y is a Cr, Fe, Co, Zn or Ti atom; Z is
a cation selected from the group consisting of an
ammonium ion, a hydrogen ion, a potassium ion and a~
sodium ion.]
For a toner containing carbon black as the co-
loring agent, it is preferable to use carbon black of
which pH value is in a range from 6 to 11, in addition
to the use of the electric charge controlling agent
having a pH in the range mentioned earlier.
When hydrophobic silica fine powder is mixed
with and dispersed in toner particles containing, as
the electric charge controlling agent, the compound
(1) having a pH value in a range from 3 to 5, the pH
value of the hydrophobic silica is preferably in a
.
.
- '
20~;~57~
range from 3.5 to 4.5.
The pH value above-mentioned may be measured in
accordance with the method set forth in JIS K 6221.
More specifically, 10 g of a sample is added to 100 ml
S of distilled water. The sample-water mixture is then
boiled for 15 minutes and cooled to a room tempera-
ture, after which pH value is measured.
Detailed nescription of the Present Invention
Since the compound of the general formula (1)
presents a pH value in a range from 3 to 5, it can be
uniformly dispersed, as the electric charge control-
ling agent, in the binder resin of the toner. Accord-
ingly, the electrophotographic toner in accordance
with the present invention can be stabilized in elec-
tric charge characteristics.
If the pH value of the compound (1) is less than
3, the toner is lowered in humidity resistance. If the
pH value is greater than 5, the dispersibility of the
compound (1) in the binder resin is defective. In both
cases above-mentioned, there are caused the problems
such as decrease in image density, letter disperslon,
toner scattering and the like.
The pH value of the compound of the general for-
mula (11 is greatly influenced by the polar group con-
.. . . ........... . . . . .
~'.
20S;~5~
nected to this compound. When the polar group is anelectron attractive group (e.g., a halogen atom), the
pH value is liable to decrease. It is therefore re-
quired to select the respective groups such that the
pH value is located in the range above-mentioned.
Table 1 shows the relationship between the combination
of the substituting groups and the pH value. It is
however noted that the pH value varies with a trace
amount of a by-product included in the course of pro-
duction of the compound (1) or with the presence ofunreacted substances, and is therefore not a definite
value.
Table 1
Rl R2 R3 R pH
Cl H H Cl Cr H _3.1 - 4.9
H H *1 Cl Fe H 3.6 - 4.9
H *2 H Cl Co Na 4.1 - 4.9
*3 H H Cl Ni R _ 4.2 - 4.9
*1 -SO2N(CH3)2
*2 -SO2N(C2H5]2
*3 -SO2N~C5Hll)2
-:
2 C)5;~57~
When there is used, as the coloring agent, car-
bon black of which pH value is less than 6, the humi-
dity resistance of the toner is not sufficient. When
there is used, as the coloring agent, carhon black of
which pH value is greater than 11, the dispersibility
of the compound (1) and the carbon black in the binder
resin is lowered. In both cases above-mentioned, there
are caused the problems of fog, decrease in image den-
sity, letter dispersion, toner scatterin~ and the
like.
When hydrophobic silica finé powder is to be
mixed with and dispersed in toner particles contain-
ing, as the electric charge controlling agent, the
compound tl) of which pH value is in a range from 3 to
lS 5, it is preferable to use hydrophobic silica fine
powder of which pH value is in a range from 3.5 to
4.5. In this case, the electric charge characteristics
are stabilized to produce stable images. More speci-
fically, if the pH values of the compound of the gen-
eral formula (1) and the hydrophobic silica fine pow-
der are below the ranges above-mentioned, the amount
of negative electric charge becomes great, causing the
toner to be separated from the carrier with difficul-
ty. This provokes the problem of decrease in image
.: ~
-
--8--
205;~57~
density. If both pH values exceed the ranges above-
mentioned, the amount of negative electric charge be-
comes small, causing the toner to be insufficiently
sticked to the carrier. This provokes the problem of
toner scattering, fog or the like.
Examples of the halogen atom include a fluorine
atom, a chloride atom, a bromine atom and an iodine
atom.
Examples of the alkyl group include methyl, eth-
yl, propyl, isopropyl, butyl, t-butyl, pentyl and
hexyl groups, each having 1 to 6 carbon atoms.
Examples of the aryl group include phenyl, tol-
yl, xylyl, biphenyl, naphthyl, antolyl and phenantolyl
groups.
lS As the electric charge controlling agent, the
compound (1) is used in an amount from 0.5 to 8 parts
by weight, preferably from 1 to 3 parts by weight, for
100 parts by weight of binder resin. If the blending
ratio of the compound (1) is smaller than the range
above-mentioned, the electric charge characteristics
become unstable. If the blending ratio of the compound
(1) is greater than the range above-mentioned, the
carrier is sticked to the toner, thereby to provoke
toner scattering, fog and the like.
The toner is produced by a method of mixing a
-
- 9 -
2~5~57~
binder resin, a coloring agent, the compound tl) as an
electric charge controlling aqent, a release agent (an
off-set preventive agent) and an additive such as a
flowability imparting agent or the like to be used as
necessary, and pulverizing the mixture into particles
having a predetermined particle size. More specific-
ally, the toner is produced by previously mixing and
kneading the components above-mentioned uniformly with
the use of a dry blender, a Henschel mixer, a ball
mill or the like, uniformly melting and kneading the
resultant mixture with the use of a kneading device
such as a Banbury mixer, a roll, a single- or double-
shaft extruding kneader or the like, cooling and
grinding the resultant kneaded body, and classifying
the resultant ground pieces as necessary. The toner
may also be produced by suspension polymerization or
the like.
Examples of the binder resin include styrene
resins (monopolymers and copolymers containing styrene
or a styrene substituent) such as polystyrene, chloro-
polystyrene, poly-~-methylstyrene, a styrene-chloro-
styrene copolymer, a styrene-propylene copolymer, a
styrene-butadiene copolymer, a styrene-vinyl chloride
copolymer, a styrene-vinyl acetate copolymer, a sty-
rene-maleic acid copolymer, a styrene-acrylate copoly-
--10--
2C)5;~57~
mer (a styrene-methyl acrylate copolymer, a styrene-
ethyl acrylate copolymer, a styrene-butyl acrylate
copolymer, a styrene-octyl acrylate copolymer, a sty-
rene-phenyl acrylate copolymer or the like), a sty-
S rene-methacrylate copolymer (a styrene-methyl meth-
acrylate copolymer, a styrene-ethyl methacrylate co-
polymer, a styrene-butyl methacrylate copolymer, a
styrene-phenyl methacrylate copolymer or the like), a
styrene-~-methyl chloroacrylate copolymer, a styrene-
acrylonitrile-acrylate copolymer and the like. Exam-
ples of the binder resin further include polyvinyl
chloride, low-molecular-weight polyethylene, low-mole-
cular-weight polypropylene, an ethylene-ethyl acrylate
copolymer, polyvinyl butyral, an ethylene-vinyl ace~-
tate copolymer, rosin modified maleic acid resin,phenyl resin, epoxy resin, polyester resin, ionomer
resin, polyurethane resin, silicone resin, ketone res-
in, xylene resin, polyamid resin and the like. The
examples above-mentioned may be used alone or in com-
bination of plural types. In the examples above-men-
tioned, there may be preferably used styrene resin,
particularly a styrene-(meth)acxylate copolymer and
more particularly a styrene-methyl methacrylate-butyl-
acrylate copolymer. In particular, there may be pre-
ferably used a styrene-methyl methacrylate-butylacryl-
~ ' ` ~ ' ' .
-
: ''
2~)5~57~
ate copolymer containing 75 to 85 % by weight of sty-
rene, 0.5 to S % by weight of methylmethacrylate and
10 to 20 ~ by weight of butylacrylate.
Examples of the coloring agent include: a black
coloring agent such as carbon black (furnace black,
channel black, thermal, gas black, oil black, acetyl-
ene black), lamp black, aniline black or the likei a
brown coloring agent as obtained by mixing red, yellow
and black coloring agents. Of these, the black color-
ing agent may be particularly suitably used. Thecoloring agent may be used in an amount of 1 to 20
parts by weight and preferably 3 to lS parts by weight
for 100 parts by weights of the binder resin.
Examples of the release agent (off-set prevent-
ing agent) include aliphatic hydrocarbon, aliphaticmetal salts, higher fatty acids, fatty esters, its
partially saponified substances, silicone oil, a vari-
ety of waxes and the like. Of these, there is pre-
ferably used a low-molecular-weight aliphatic hydro-
carbon of which weight average molecular weight isfrom about 1,000 to about 10,000. More specifically,
there is suitably used one or a combination of plural
types of a low-molecular-weight polypropylene, low-
molecular-weight polyethylene, paraffin wax, a
low-molecular-weight olefin polymer composed of an
-12-
~:~5~571
olefin unit having 4 or more carbon atoms and the
like. The release agent may be used in an amount of
0.1 to 10 parts by weight and preferably from 1 to 5
parts by weight for 100 parts by weight of the binder
S resin.
As conventionally done, the toner particles may
have sizes in a range from 3 to 35 ~m and preferably
from 5 to 25 ~m, but it is preferable that the distri-
bution of toner particle sizes satisfies the following
formula:
N < -172.7C + 1.45
[wherein N is the percentage by the number of toner
particles of which sizes as measured with a coulter
counter exceed 16 ~m, and C is surface dye density
(g/g) of the toner particles]
When the distribution of toner particle sizes is
in the range above-mentioned, it is possible, in view
of the relationship with the surface dye density, to
further eliminate variations in electric charging
characteristics of the toner.
To obtain toner particles presenting a distri-
bution of particle sizes which satisfies the formula
above-mentioned, the ground toner particles may be
classified to remove particles having sizes greater
than 16 ~m, or toner particles may be ground such that
2~15~57~
the peak of the toner particle-size distribution is
shifted to a smaller-size zone to reduce the content
of particles having sizes greater than 16 ~m.
According to the present invention, as the hy-
drophobic silica fine powder to be mixed with and dis-
persed in the toner particles, there may be used sili-
ca fine powder of which surface is treated with, for
example, a (poly)alkyl group, a (poly)alkylsilil
group, a (poly)alkylsilane or silicone oil. Prefer-
ably, there may be used silica fine powder of whichsurface has been treated with a compound having a pol-
ymethylsilil group such that the powder becomes hydro-
phobic. Such powder i8 higher in hydrophobic nature
than conventional silica fine powder treated with a
compound having a low-molecular-weight alkyl group.
As a commercially available product of such si-
lica fine powder, there may be mentioned "Cabosil
TS720" manufactured by Cabot Co., Ltd. This product is
hydrophobic fumed silica fine powder, which is obtain-
ed by treating high-purity fumed silica fine powder
(99.8~ SiO2) with an organic silicone compound, and on
the surface of which a polymethylsilil group is pre-
sent to increase the hydrophobic nature of the surface
of the silica fine powder.
The particle sizes of the silica fine powder so
.... .. . . . :
-14-
2C~5~5~
treated as to be hydrophobic are suitably in a range
from 0.01 to 0.04 ~m.
The pH value of the hydrophobic silica fine pow-
der varies with a variety of factors wl~ich are not
always clarified. However, it is known that the sur-
face functional group is influenced by reaction by-
products.
The hydrophobic silica fine powder may be added
in an amount of 0.01 to S % by weight and preferably
from 0.05 to 1 % by weight for the total amount of
toner. If the amount of the hydrophobic silica fine
powder is greater than the range above-mentioned, the
amount of electric charge is excessive. If this amount
is smaller than the range above-mentioned, the effect
of improving the toner flowability cannot be expected.
Examples
The following description will discuss in more
detail the electrophotographic toner in accordance
with the present invention with reference to Examples
and Comparative Examples.
Examples 1 to 9 and Comparative Examples 1 to 8
,
2~5~S71
(Component) (% by Weight)
Styrene-acrylic copolymer 86
Carbon black 10
Off-set preventive agent 2
(Low-molecular-weight polypropylene~
Charge controlling agent (Compound (1)) 1.5
Hydrophobic silica 0.5
The component_ above-mentioned were mixed. The
mixture was molten and kneaded with a double-shaft
kneader, and then cooled, ground and classified to
prepare toner particles having the average particle
size of 10 ~m. Table 2 shows the substituting groups
contained in the compounds (1) used, as the electric
charge controlling agent, in Examples 1 to 9 and Com-
parative Examples 1 to 8. Table 3 shows the pH values
of carbon black and the compounds (1) used. Each pH
value was measured in the manner that 10 g of a sample
was added to 100 ml of distilled water and the sam-
ple-water mixture was then boiled for 15 minutes on a
hot plate and cooled to a room temperature, after
which pH value was measured with a glass electrode pH
meter. The pH value of the hydrophobic silica fine
powder used was 4.1.
The moisture contents of the resultant electro-
photographic toners thus obtained were measured under
, . . . . , .. . ... . . . . . . -
-16-
205~57~
the cGndition of ambient temperature/ambient humidity
(temperature : 20C, humidity : 65%, hereinafter
referred to as N/N) and under the condition of high
temperature/high humidity ~temperature : 35C, humidi-
ty : 85~, hereinafter referred to as H/H), respective-
ly, according to the Karl Fischer method. Table 3
shows the results.
The following evaluation tests were conducted on
the electrophotographic toners obtained in Example 1
to 9 and Comparative Examples 1 to 8. Table 3 shows
the test results.
(1) Test of Dispersibility
The circumference of each of the electrophoto-
graphic toners was covered with and solidified by epo-
xy resin. Each of the toners as cut with a microtome
was observed with a transmission-type electro micro-
scope. The toner dispersibility was evaluated accord-
ing to the following cr teria:
O : Extremely finely dispersed
O : Substantially finely dispersed
~ : Some large particles observed
X : Many large particles observed
(2) Test of Image Density
Each of the electrophotographic toners was mixed
Z~5~57~
with a carrier to prepare a developer having a toner
density of 3%. With an electrophotographic copying
apparatus ~DC-7085 manufactured by Mita Industrial
Co~, Ltd.) using (i) each developer above-mentioned as
a start developer and (ii) the same toner as that con-
tained in the start developer as a resupply toner, a
solid-black document was continuously copied for
150,000 pieces under the condition of ambient tempera-
ture/ambient humidity (N/N), i.e., temperature of 20C
and humidity of 65~, except that intermediate 8000
copied pieces from 16001st piece to 24000th piece were
taken at temperature of 35C and humidity of 85%
(H/H). Every thousandth copied pieces were extracted,
as samples, from 150,000 copied pieces for each of the
developers and were measured as to the density values
thereof with a reflection densitometer (TC-6D manufac-
tured by Tokyo Denshoku Co., Ltd.). The averages were
calculated for these samples for all the developers.
(3) Test of Fog Density
With the use of the reflection densitometer
above-mentioned, the density of the blank spaces of
each sample obtained in Test of Image Density was
measured to measure fo~ density. The averages were
calculated for the samples for all the developers.
-18-
20~;~5~
(4) Test of Letter Dispersion
All the samples obtained in Test of Image Densi-
ty were visually checked for a so-called letter dis-
persion of toner spots.
(5) Test of Toner Scattering
For each developer, there were checked (i) the
blank spaces of the reproduced image of the 150,000th
piece, and (ii) the inside of the copying apparatus
after 150,000 copies had been taken. The toner dis-
persibility was evaluated according to the following
criteria:
~pparatus Reproduced
Inside Image
O : No toner scattering No toner scattering
observed observed
O : Some toner scattering "
observed
: Toner scattering Sporadic ~oner scat-
observed tering observed
X : Many toner scattering Continuous toner scat-
observed tering observed
--19--
2QS257~
l __ t ____~ _ _ 1- .. _
:1 1 Z X X Z Z Z :Z: ~ X Z
__ O _._ _ _ _ _ _ _
~- ~ ~ ~ Z ~ ~) ~ ~ (~ ~) ~
P: :; ~ :~ :~ ~ :r: :~ :~ ~: :~ \ /
o~ . ~n~ .
_ ____ _ _ __ _
.~ ~ ~ Z ~ P: :~: ~ :C :~ :: P: ~ X
O .~ .
_ _ _ . __ Q,
_ ol I I q ol o~ o, o( o~ IQ Ip , C,~
~ ~ ~Z ~ ~ V ~ L~ ~ Z ~
~- .' O :~
_ ~O _ _ _ _ _ X
~ ~` CCI a~ _l N ~ ~r tl~ ~D r c~ .
a) a) ~ ~ w ~ ~x ~x ~ ~x ~ ~x o~
X: X X X _ ~ E U o E U o
1~ -
~C~5~57~
~r o r~
In 1~ U~ ~ ~ ~r ~D
r~ ~ ~ ~ ~ r~ ~ r~
. ,, ,, ,, ,, ,,,
a~
a
Q) ~ O
~ Z ~ ~ ~ ~ ~ ~ ~ O ~
~ Z ~
H ~1 ~1
I ~
I Ul-r1
Vl ~ ~i
.,1 ~-~1 O O O O O O C~ O O
a
a) u~
~ :Cu~ x ~c~
_ ~ ~ ~ ~ _I_I
o 5:
o O O O O O o o O
~ _
h dP
--
~ Z ~ o u
t~ rl~ ~1 N_~_I ~ r 1
~0 Z o o o o o o o o o'
E~
C
O ~ ,4 ~ ~u~ r o o
R
~D ~D O Ou~
-
~ u m
:~
Or~ ~ ~o~ ~~1 ,~~n
D.. . . . . . ~ .
U
a~
~i
X X X X X X X ~ X
-
205~57
I~
. ::
~ ~ ~ ~ ~ ,~ o~
~1 :r:
~ ,, ,, ~ o o . ,, o
O U~
Z ~ N ~D X ~ o N -1
~ Z ~ ~ ~ cn o ~ o
H ~ ~ 1 0
I U~
U~
a ~'R <I ~ X ~ O ~ <I O
~J ~ ~OD
~ ~ N
~ ~ ~ ~t~ D
O ~ . . . . . . . .
U oc~ o ~1 0 0 0 0 a
_ ~tP ~
X
U~ Z
X o z N ~ ~~1 , Q~
~3 ~ O O O O O O O O ~
E~ .~
~.~C O ~ Q.
~ ~ m u~ ~
~ ~ 0
0~ ~ O U~ o ~ ~D N ~D e
e co ~ :
~x ~ x ~ x o
e e e e e o Q :
t~ ~ O U
- 7~Z -
205~7~
Table 3 ( 3J3 )
__
Fog Density Letter Toner Total
Disper- Scatter- Evalua-
N/N H/H sion inq tion
.
Example 1 0.002 0.004 None O
-
Example 2 0.003 0.004 None 0
Example 3 0.001 0.003 None ~ 0
Example 4 0.001 0.002 None O 0
Example 5 0.003 0.004 Little O O
-
Example 6 0.002 0.004 Little O O
_
Example 7 0.001 0.003 None O
Example 8 _0.003 0.004 Little O O
Example 9 0.002 0.003 Little O _ O
Comp. Ex.l 0.011 0.019 Sporadic X X
Comp. Ex.2 0.012 0.017 Sporadic ~ X
Comp. Ex.3 0.014 0.022 Contiuous X_ X
Comp. Ex.4 0.016 0.026 Continuous X X
Comp. Ex.5 0.013 0.023 Sporadic ~ X
Comp. Ex.6 0.012 0.022 Sporadic X X_
Comp. Ex.7 0.014 0.021 Sporadic ~ X
Comp. Ex.8 0.011 0.023 Sporadic ~ X
"Comp. Ex." means "Comparative Example".
21~S;~57~
It is understood from Table 3 that each of the
electrophotographic toners of Examples 1 to 9 contain-
ing, as the electric charge controlling agent, the
compound (1) presenting a pH value in a range from 3
S to 5, is superior in dispersibility to the electropho-
tographic toners of Comparative Examples 1 to 8 con-
taining a compound presenting a pH value which devi-
ates from the range above-mentioned. Further, each of
the toners of Examples l to 9 presents less variations
of moisture content under both conditions of ambient
temperature/ambient humidity (N/N) and high tempera-
ture/high humidity (H/H), and is therefore excellent
in humidity resistance.
It is also understood that the reproduced images
obtained with the use of the electrophotographic
toners of Examples 1 to 9 are superior in any of image
density, fog density, letter dispersion and toner
scattering to the reproduced images obtained with the
use of the electrophotographic toners of Comparative
Examples 1 to 8.
It is also understood that variations between
reproduced images obtained under the ambient tempera-
ture/ambient humidity (N/N) condition and reproduced
images obtained under the high temperature/high humi-
dity (H/H) condition both with the use of each of the
,' ' '
.
.
-24-
57~
electrophotographic toners of Examples 1 to 9, are
less than variations between reproduced images ob-
tained under the ambient temperature/ambient humidity
(N/N) condition and reproduced i~ages obtained under
the high temperature/high humidity (H/H) condition
both with the use of each of the electrophotographic
toners of Comparative Examples 1 to 8. Thus, stable
reproduced images can be obtained with the toners of
Examples 1 to 9.
It is also understood that, out of the electro-
photographic toners of Examples 1 to 9, the toners of
Examples 1 to 4 and 7 using carbon black presenting a
pH value in a range from 6 to 11 are particularly
excellent.
Examples 10
(Component)(% by Weight)
Styrene-acrylic copolymer 85
Carbon black 10
Off-set preventive agent 3
(Low-molecular-weight polypropylene)
Chromium-containing azo dye (pH 4.9) 2
The components above-mentioned were molten and
kneaded with a double-shaft kneader, and then prepared
as toner particles having the averag~ toner particle
20S;;~57~
size of 10 ~m with a jetmil. Table 4 shows the groups
contained in the chromium-containing azo dye used as
the electric charge controlling agent. The pH value of
the carbon black was 8.5.
Silica fine powder so treated as to be hydropho-
bic (particle size of 0.02 ~m, pH of 3.7, Cabosil TS-
720 manufactured by Cabot Co., Ltd.) was mixed with
and dispersed in the toner particles thus prepared in
an amount of 0.5 % by weight for the total amount of
the toner particles, thus preparing a toner.
Examples 11 to 13 and Comparative Examples 9 to 16
Toners were prepared in the same manner as in
Example 10 except that there were used (i) metal-con-
taining azo dyes which respectively contained groups
shown in Table 4 and of which pH values are shown in
Table 6 and (ii) hydrophobic silica fine powders of
which pH values are shown in Table 6.
-26-
2~5~57~
Table 4
5~ Groups of Compound ~1)
Rl R2 R3 R4 Y Z+
Examples 10 Cl H H Cl Cr H
Examples 11 Cl H H Cl Cr H
Examples 12 Cl H H Cl Cr H+
Examples 13 Cl H H Cl Cr H
Comparative
Examples 9 Cl H H Cl Cr NH4
Comparative +
Examples 10 Cl Cl Cl H Fe Na
Comparative +
Examples 11 H Cl Cl Cl Co
Comparative +
Examples 12 H H Br H Zn NH4
Comparative +
Examples 13 H Cl Br H Co H
Comparative +
Examples 14 Cl H Cl Cl Zn K
Comparative
Examples 15 Cl H H Cl Cr
Comparative +
Examples 16 Cl H H Cl Cr H
.. . . , . f
2~s~s~
Evaluation Tests
Ferrite carrier having the average particle size
of 80 ~m was blended with each of the toners of ~xam-
ples 10 to 13 and Comparative Examples 9 to 16. Each
mixture was uniformly mixed and agitated to prepare a
two-component developer presenting toner density of
4~. With the use of an electrophotographic copying
apparatus (DC-3255 manufactured by Mita Industrial
Co., Ltd.) using each of the developers thus prepared,
an original document was copied ~otally 80,000 pieces
under different operating conditions under which a
predetermined number of copied pieces were respective-
ly taken. All the copied pieces were checked for image
density, fog density, amount of electric charge and
toner scattering for each of the operating conditions.
More specifically, the copying operation was carried
out with the operating condition changed in the order
shown in Table 5 for a predetermined number of pieces,
and the reproduced images were checked for the items
above-mentioned. It is however noted that the measured
values of the images reproduced under the N/N condi-
tion were those obtained after 80,000 pieces were
copied.
-28-
21D5;~571
Table S
.
Copying Mark OperatingNumber of
Order ConditionCopied Pieces
1 N/N Ambient Temp. & 8,000
Ambient Humidity
~20C & 65%~
2 L/L Low Temp. & 8,000
Low Humidity
(10C & 45%)
3 H/H High Temp. &8,000
High Humidity
(35C & 85%)
_ _
4 N/N Ambient Temp. & 56,000
Ambient Humidity
(20C & 65%)
The respective tests were conducted in the fol-
lowing manners.
(1) Measurement of Image Density (I.D.)
Each image density was measured with the use of ~;'
a reflection densitometer (TC-6D manufactured by Tokyo
Denshoku Co., Ltd.)
~2) Measurement of Fog Density (F.D.)
With the use of the reflection densitometer
above-mentioned, the density of blank portions of each
reproduced image was measured and defined as fog den-
sity.
(3) Amount of Electric Charge
The amount of electric charge was measured with
.~
.. . . . . .. . ... . ..... . . . . ........ . . . . .
,
-29-
2~S;~:57~.
a blow-off electric charge measuring instrument manu-
factured by Toshiba Chemical Co., Ltd.
(4) Toner Scattering
The inside of the copying apparatus and the sur-
face of each reproduced image were visually checked
for toner scattering, and evaluated according to the
following criteria:
Apparatus Reproduced
Inside Image
O : Substantially no toner No toner scattering
scattering observed observed
O : Slight toner scattering "
observed
: Some toner scattering "
observed
: Toner scattering Toner scattering
observed observed
X : Many toner scattering Toner blanking
observed observed
The test results are shown in Table 6.
-- 30 --
205~57
~ ~ o o ~ * ~ X X ~
C ~: ~ r~ u~
0~ z o ~ ~ o o ~ ~ ~ ~
o ~, ~ o o o o o ~ ~ ~ ~
E~n Z,,~
~ ~ ~ OD ~D O 0~ U~ _~ ~r _~
~ ~ ~ ~ ~ ~ ~ r~ ~7 _1 ~
~'
O ~ ~ erLr) r~ _l,~ ,1 _~
o . oC~ o o o o o ,, o
,,o~0~ o o o o o o o o o
o ~ ~ o o o o o o o o o
~O X 3 ~
~1 O ~ ~ ~ ~ ~ CD C~ _~ C~ ('~) O
.4 ~ U~ Q a ~ ~ ~ ~ ~ _, ,, ~ ~
~ U o . . . . . . . . .
E~ '¢ Ql ~H _I~1 _~ _~ ~ _~_1 _~ _I
.,1
~ a~ ~ _l ~o ~ ~D u~ U~ C~
0 0 ~ ~7 ~ ~ ~ ~ ~ U~
3 t~ 1~ ~D ~ ~ ~ ~1 CO O O
~r ~ ~ o .~, u~ ~
_~ ~ ~ X X X X X
,~ ~ a~ a) ~ ~ w ~ w
~ ~ ~ ~ Oe o o~ ~o ~Q
El~ W IY C) t ~ ~ C ~ ~
.
- 31 -
205;~57~
.
~ ~ a x * o ~
's~ ~ e) o o
h ~ Z 3
~o t~ æ o o o
oo~
~ ~~)U-) N 1~l _I O O O
~il '. '
_ ~
~ o . oo o a~
O~ ~ O O O
~D ~ ' ~ O O O C
X ~ . u~ ~ ~D ~
~ ~ O C~ ~ ~ ~ O
1~ ~ O O . . . .
E~ ~ O H _1~1 _I O
,~ h ~ _
~ ~p ~ ~ a~ ~ o
~ . . ~ o a~
. ~r ~ ~ :~ .C
~1 ~-1
~ _~~ ~r ~r ~ ~ .
.,~ ~ o . o u~
ta ~ n ~D ~ ,~ a ~ ~ o
X X X _ 0~_10
O O O _I N ') ~
U ~_)
': ' '' ' ' ' ' '' ' ' ' :'
~ ' .
:
' - ' ' '
Z~5~i7~
It is apparent from Table 6 that, by adjusting
the pH values of the hydrophobic silica fine powder
and the electric charge controlling agent within re-
spective predetermined ranges, the electric charge
characteristics can be stabilized to remarkably im-
prove the image density and toner scattering.
.