Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~C~ 33
~ITLE OF ~YE INVENTTON
HIG~ HYDROHEAD FIBROUS POROUS wE8
WITH I~PROVE~ RETENTIVE ABSORPTION ~ND ACQUISION RATE
3ACXGROUND OF ~HE I~VE~TION
The present invention relates to a method of treating a
high hydrohead fibrous porous web material and, for example,
to a method that increases the retentive water absorbency of
the web. The retentive water acquision rate may also be
increased. As used herein the term "absorbency" generally
refers to the ability of a material to acquire a fluid and the
acquision rate refers to the rate of such acquision. An example
of a use where higA absorbency and high acquision are desired
would be wiper type materials. In addition to having the
characteristics of high absorbency and high acquision rate,
wipers desirably also should have the characteristic of high
retentive absorbency and high retentive acquision rate. The
; 25 term retentive acquision rate is used herein to designate
; comparison of the rate of acquision of a fluid by a material
when the material is first used to acquire the fluid as
compared to the second, third and fourth times the the material
is used to acquire the fluid. Improved retentive acquisition
rate is evidenced by by smaller decreases in the rate of
acquisition with multiple uses. Likewise, the term retentive
absorbency is used to designate comparison of the amount of
fluid acquired by a material when the material is first used
to acquire the fluid as compared to the amount of fluid
acquired when the material is used a second, third, fourth time
to acquire the fluid. Improved retentive absorbency is
evidenced by smaller decreases in the amount of fluid absorbed
by the material with multiple uses. In other words, the ability
of the material to reabsorb fluid after having, in our test,
~0 been exposed to fluid, wrung out and allowed to dry.
In the copending, concurrently filed application serial no.
07/603,103 _ of Benard Cohen and Michael T. Morman entitled
2C ~3
_~w -.ydronead ~lbrous ?orous ~eb ilth _mDroved .~etentive
~ettability ~he nventors ~isclose that the application of
corona discharye treatment to low hydrohead webs whose surface
inciudes a surface active agent having a hydrophile-lipophile
balance of 6 or greater results in a significan~ increase in
the retentive wettability, as defined therein, of such webs.
This application is hereby lncorporated by reference. Low
hydrohead webs of that type would generally be unsatisfactory
for use as a wiper material due to their open pore structure
which would greatly reduce the ability of the web to acquire
fluids. Conversely, high hydrohead webs, as defined herein,
~ould generally be undesirable for use in applications where
rapid transmission of large amounts of fluid through the
material is desired. This undesirability arises from the
generally tlght, closed pore structure of high hydrohead
materials. Such pore structure would inhibit the passage of
fluids therethrough in rapid fashion.
In the past, hydrophobic wipers have been subjected to
treatment with surfactants to improve their charactersitics.
The wipers have been treated with surfactant by (1) passing the
formed wiper through a bath containing the surfactant in either
neat or solution form and drying the wiper as needed so that
a given amount of the surfactant is deposited on the wiper, or
(2) spraying a surfactant in either neat or solution form on
the fibers as they are beinq formed or on the fibrous porous
web and drying the wiper as needed so that a given amount of
the surfactant ls deposited on the wiper, or, (3) adding
surfactant to a thermoplastic resin prior to extrusion and
formation of the resin into a thermoplastic porous web
material. In the later situation, under known process
conditions, the added surfactant exudes or migrates to the
surface of the fibers of the porous web material during or
shortly after fiber formation. This phenomenon has been
referred to as ~'blooming" the surfactant. It is believed that
blooming results from the insolubility of the surfactant in the
thermoplastic polymer as the polymer cools. See U.S. Patent No.
4,535,020 to Thomas et al (hereafter Thomas et al 020) which
3~3
~emonstrateS sur;~ac~anl ~ oomlng ln a ~iaper __ner _rme~ rom
a per~orated film.
~ wiper made from a hydrophobic material, such as a
_hermoplastic polymer, will n9t readily acquire or absorb
spllled f'uids ~ecause the surface tension of the fluid is
greater than the critical sl~rface energy of he hydrophobic
material. Surface tension is the contrac~ile surface force of
a fluid where the fluid tries to assume a spherical form and
to present the least possible surface area. It is usually
measured in dynes per centimeter. Accordingly, because of its
effect on the insulting fluids, surfactant has been previously
applied to wipers. Application of a surfactant onto a wiper
material may make a nonabsorbing wiper absorbant by at least
two mechanisms: (1) Surfactants present on the wiper can
:_ dissolve into a fluid and iower the surface tension of .he
resulting solution to more eoual the critical surface energy
of the wiper material. Accordingly, when a surfactant coated
wiper is used to wipe up a fluid such as water, the surfactant
acts to lower the surface tension of the fluid and allow the
fluid to be acquired at a faster rate and for a larger amount
of fluid to be absorbed into the wiper. In this situation, a
certain amount of the surfactant on the wiper is lost with each
wiping and wringing and unacceptable acquision rate and
absorbency occurs at some following wiping due to the lack of
availability of surfactant to lower the surface tension of the
fluid. (2) The surfactant can be coated onto the fibers making
up the wiper, making the fiber surface of the wiper more
hydrophilic, i.e., lncrease the apparent ~ritical surface
energy of the fibers. In this situation the wiper would have
permanent absorbency if the surfactant did not dissolve in the
fluid the wiper was used to pick up.
As any anyone will testify, it is an aggravating event when
a disposable wiper fails in its appointed task of rapidly
acquirinq and absorbing a fluid spill.
Accordingly, it has ~een a goal of those in the art to
provide a high hydrohead porous web wiper material which has
an improved acquision rate and absorbency. This was the initial
33
oai ~ecause f _~e ma~erlai _annot acqulre and aDsor~ fluld
all, she materlal cannot runction as a wiper. ~dditionally,
it has been a goal of those in the art .o provide an high
hydrohead ~orous web wiper material which has an improved
_.etentive acquision rate and improved retentive absorbency.
That is, ~hen dried and wrung-out between wipings, the wiper
has a signlficant increase in the number of 'imes it can be
used to absorb fluid. This goal is desirable not only from the
standpoint of allowing a given disposable wiper to be used more
10times but also from an environmental standpoint in that fewer
wipers will be disposed into the environment.
Corona discharge treatment of films is old in the art and
it is known that corona discharge treatment of a polymer film
in the presence of air entails su~stantial morphological and
_5chemical ~odifications in the polymer film's surface reqion.
See Catoire et ~1, "Physico-chemical ~odifications of
superficial regions of low-density polyethylene !LDpE) film
under corona discharge," Poly~er, vol. 2S, p. 766, et. seq,
June, 1984.
20Generally speaking, corona treatment has been utilized to
either (1) improve the print fastness on the film, or (2) to
perforate the film. For example, U.S. Pdtent No. 4,283,291 to
Lowthe~ describes an apparatus for providin~ a corona
discharge, and U.S. Patent No. 3,880,966 to Zim~erman et al
2Sdiscloses a method of using a corona discharge to perforate a
crystalline elastic polymer film and thus increase its
permeability. U.S. Patent No. 3,471,597 to Schirmer also
discloses a method for perforating a film by corona discharge.
U.S. Patent No. 3,754,117 to Walter discloses an apparatus and
30method for corona discharge treatment for modifying the surface
properties of thin layers or fibers which improve the adhesion
of subsequently applied inks or paints or of subsequent
bonding.
It also is possible to treat a diaper liner material with
35a corona discharge and then immediately dip the film in a
surfactant solution. Because the corona effect on the material
generally starts to immediately decay, it is important to get
33
~.~e _orona --eated ma~erlai into ~~e _ath as ~ulc~l~ ,s
~osslble. Such a method is discussed in Japanese KOKAI 2atent
Number S~063!1g88]-211375. ~his document discloses a method for
producing a nonwoven fabric having a lonq lasting
_ hydrophilicity. rhe method lnvolves first t-eating a nonwoven
fabric o~ synthetic fiber by a corona discharge and then
coating the treated fabric with about ,-10 grams per square
meter of fa~ric of surface active agent.
Of particular lnterest is the fact that Thomas~ et al 020
L0 is directed to the utilization of corona discharge in
conjunction with surfactant treated films to effect improved
wettability, i.e. higher fluid transmission rates and therefore
decreased run-off of fluid. In this reqard Thomas et al 020
states that a perforated film which has been treated with
:5 surfactant and which is then corona discharge treated results
in a film with very low, zero or near zero fluid run-off on the
first run-off test. ~homas et al 020 reports that this effect
is accomplished because the corona discharge treatment acts on
the chemical additive, the surfactant, to provide the
perforated film with a zero or near zero percent run off.
T~5~ æ~ al 020 postulates that this effect is achieved due
to the surfactant providing a greater polarizability to the
film than the film would have without the surfactant being
added. The corona discharge treatment provides additional
polarizing effect and, in combination ~lth the surfactant,
provides improved wettability. Because homas et al 02~Q is
directed toward use of the perforated film as a diaper liner,
it does not appear to address the questions of acquision rate
and absorbency. Acquision rate, as defined herein, usually does
not apply to a film and diaper liners are generally designed
to be permeable to fluids as opposed to absorbing them. Lastly,
Thomas et al 020 does not appear to address retentive
capabilitites at all because the testing reported therein is
directed to one-time exposure to fluid.
In view of the forgoing, and the discovery by Messers.
Cohen and Morman that treating a low hydrohead porous web with
a surface active agent having a hydrophile-iipophile balance
~C~ 33
-r _OOul 5 or creater -^-llowed by c_rona a~scharge treatment
~lelded signlficantly improved reten~lve wettabillty values for
the material, we decided to determine if such treatment had
advantageous effects on the retentive water acquision rate and
retentive water absorbency of high hydrohead porous webs. If
such was the case an improved wiper would result.
SUMMARy_OF T~E INVENTION
In response to the above, we have devised a method of
treating a high hydrohead fibrous porous web material to
increase the web's retentive water acquision rate (averaged
normalized rate of water absorption in subsequent reabsorptions
as compared to the lnitial absorption rate) and retentive water
absorbency (averaged normalized amount of water absorbed in
subsequent reabsorptions as compared to the amount initially
absorbed). The method generaLly includes the steps of: (1)
providing a high hydrohead fibrous porous web having a surface
concentration of at leas~ about 0.05 percent, by weight of the
web, of a surface active agent having a hydrophile-lipophile
balance of at least about 6: and (2) applying a corona
discharge equivalent to a charge of at least about O.8 watt
minute per square foot per side of the web to the surface
active agent bearing web. The resultant web will have a percent
2S decrease in the averaged normalized water absorbed, at two
minutes, of less than about 50 weight percent in each of the
second, third and fourth times the material is tested in
accordence with absorbency test A when compared to the averaged
normalized water absorbed upon being initially tested in
accordence with absorbency test A. In some embodiments, the web
will have an average absorbency decrease, as defined above, of
less than about 25 percent.
In some embodiments the resultant web will have a percent
decrease ln the averaged normalized water absorbed, at one
minute, of less than about 50 weight percent in each of the
second, third and fourth times the material is tested in
accordence with absorbency test A when compared to the averaged
~C r ~ 333
.ormaii-ea -~aler -osorDea upon -elna ~nlt ally ~estea _n
accordence -~lth absor~ency test A. .~ddit onally, n some
emDodiments, .he resultant web will have a percent decrease in
the averaged normalized water absorbed, at one minute, of less
than about 25 weight percen~ in each of the second, third and
fourth times the material is tested in accordence with
: absorbency test A when compared to the averaged normalized
water absorbed upon being initially tested in accordence with
absorbency test A.
iOThe treated webs generally also have improved retentive
averaged normalized rates of water absorption. Thus, generally,
the resulting webs have a percent decrease in the averaged
normalized rate of water absorbed, in the first 2.4 seconds of
absorption, of less than about 50 percent in each of ~he
_5 second, third and fourth times the material is tested in
accordence with absorbency test A when compared to the averaged
normalized rate of water absorbed upon being lnitially tested
in accordence with absorbency test A. For example, the webs may
have such improved retentive averaged normalized rates of water
absorption that the averaged normalized rate of water absorbed,
in the first 2.4 seconds of absorption, decreases less than
about 25 percent in each of the second, third and fourth times
the material is tested in accordence with absorbency test A
when compared to the averaged normalized rate of water absorbed
upon being initially tested in accordence with absorbency test
A. Even more particularly, the webs may have such improved
retentive averaged normalized rates of water absorption that
the averaged normalized rate of water absorbed, in the first
2.4 seconds of absorption, decreases less than about 10 percent
in each of the second, third and fourth times the material is
tested in accordence with absorbency test A when compared to
the averaged normalized rate of water absorbed upon being
initially tested in accordence with absorbency test A.
From about 0.05% to about 3%, by weight of the web
material, of surface active agent may be adhered to the web
material. For example, from about 0.1% to about 1%, by weight
of the web material, of surface active agent may be adhered
~3
~~ ~he weD materlai. Yore ~art cuiarly, rom about ~
aboul ~.4~, ~y weight of the web material, Ot surface active
agent may be adhered to the web material- Even more
particularly, from about 0.2% to about 0.3%, ~y weight of the
web material, of surface active agent may be adhered to the web
materlal.
The equivalent of from about 0.8 to about 15 watt minute
per square foot per side of the web material of corona
discharge may be applied to the web materlal. For example, the
~0 equivalent of from about 1 to about 10 watt minute per square
foot per side of the we~ material of corcna discharge is
applied to the web material. More particularly, the equivalent
of from about 2 to about 8 watt minute per square foot per side
of the web material of corona discharge is applied to the web
material.
In one embodiment our process includes the steps of (1)
forming a melt from a thermoplastic fiber forming material;
(2) adding, to the melt, an amount of surface active agent
havinq a hydrophile-lipophile balance of at least about 6
sufficient to effect a surface concentration of the surface
active agent of at least about 0.05%, by weight of the
resulting fibrous porous web material; (~) forming the melt
into fibers and the fibers into a high hydrohead fibrous porous
web under conditions which allow at least 0.05%, by weight of
the fibrous porous web, of the surface active agent to bloom
to the surface of the fibers of the porous web; and (4)
applying a corona discharge equivalent to a charge of at least
about 0.8 watt minute per square foot of the porous web to the
surface active agent bearing web material.
Because not all of the surface active agent added to the
melt blooms, the amount of surface active agent added to the
melt is generally greater than the amount desired to be present
on the surface. Accordingly, the amount of surface active agent
; added to the melt may vary with the surface active agent used,3S the thermoplastic material used to form the web and/or the
process conditions of forming the web.
'
:
:
is is t.~e case generai~ n this emDo~lmen~ ~he equlvaienl
.r rom about 0.8 ~o abou~ 5 watt minu~e ~er square root o.
the web material of corona discharge may be applied to the web
material. For example, Ihe equivalenr of from about 1 to about
_ watt minute per square foot of ahe web material of corona
discharge is applied to the web material. ~ore particularly~
the equivalent of _rom about 2 to about ~ watt minute per
square foot of the web material of corona discharge is applied
to the web material.
In all embodiments the surface active agent may be selected
from the group includin~ one or more wetting agents, emulsions
and dispersants.
In all embodiments the hydrophile-lipophile balance of the
surface active agent will be about 6 or greater. For example
:5 the hydrophile-lipophile balance may range from 6 to about o.
More particularly, the hydrophile-lipophile balance of the
surface acti~e aqent may ranqe from 8 to about 20. Even more
particularly, the hydrophile-lipophile balance of the surface
active agent may range from 10 to about 20.
The present invention is also directed to products prepared
by or preparable by our process. That is, the invention is
generally directed to a fibrous porous web which has a high
hydrohead when tested in accordence with Test A prior to
surfactant and corona treatment in accordence with our
invention and which has improved retentive avera~ed normalized
absorbency and improved retentive averaged normalized water
acquision rates after surfactant and corona treatment.
The fibrous porous web material may include a polyolefin
or a blend of polyolefins or any other suitable material which
may be formed into a fibrous porous web. For ~xample, the
fibrous porous web may be formed from polyethylene or
polypropylene.
The fibrous porous web material may be formed by any of the
wide variety of processes which provide a high hydrohead
fibrous porous web. For example, the fibrous porous web may be
formed by meltblowing so that the fibrous porous web includes
meltblown fibers.
J33
OBJECTS OF T~E -~VENTION
Accordingly, _t is a general object of ~he presen~
invention tO provide a method whereby the retentive averaged
normalized water absorbency of high hydrohead porous webs ~s
improved.
Another general object of the present invention 1s to
provide a higA hydrohead fibrous porous web material having an
lncreased retentive averaged normalized rate of water
acquision.
still further objects and the broad scope of applicability
of the present invention will become apparent to those of skill
in the art from the details given hereinafter. However, it
should be understood that Ihe detailed description or the
presently preferred embodiments of the present invention is
~iven only by way of illustration because various chanqes and
modifications well within the spirit and scope of the invention
will become apparent to those of skill in the art in view of
the followin~ description.
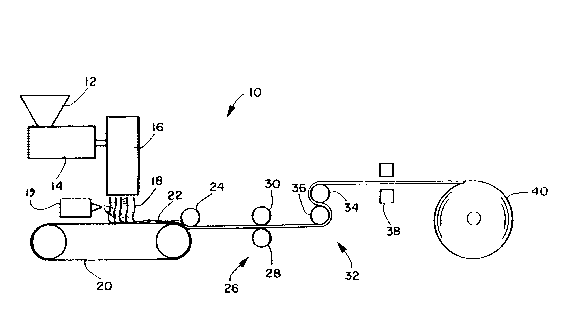
BRIEF DESCRI~IQ~_OF THE DRAWINGS
Figure l ls a schematic representation of one process for
carryin~ OUt the present invention.
Figure 2 is a schematic representatlon of a second process
for carryin~ out the present invention.
DEFINITIONS AND TESTS
As used herein the term "high hydrophile-lipophile balance"
refers to a surface active agent having a hydrophile-lipophile
balance of about six (6) or greater.
As used herein the term "surface active a~ent" refers to
any compound that reduces surface tension when dissolved in
water or water solutions or which reduces lnterfacial tension
between two liquids, or between a liquid and a solid. There are
, 10
~C~ 33
~hree aenerai c~teaorles o- sur~~ce ac~:ve aaents: leter~enls~
~ett_ng agents (l.e. surfactan~s) and emulslfiers.
~ he term 'lhydrophile-iipophile balance" (HL8) is well known
to those in ~he art. ~he HLB of a nonionlc surfactant is the
approximate weight percent or ethylene oxide n the surfactant
divlded by 5. ~he numerical scale of HLB values ranges from l
(completely ipophilic or oil-lovlng) ~o 20 (completely
hydrophilic or water-loving). Refer to W.C Griffin, J. Soc.
Cosmetic Chemlsts 317-326 (1949). In some lnstances the HLB of
a material is determined by comparing its activity to known
materials having known HLB's.
As used herein the term "high hydrohead material" refers
to a por~us web material which supports more than about 25
centimeters of water when its hydrohead is ~easured _n
:~ accordance with Method 5514 - Federal Test Methods Standard No.
l91A. In all cases the hydrohead of the porous web material ls
determined by measurement either before the web has been
treated with surface active agent and corona discharge as is
required by the present invention or, if such is not possible,
after extraction of the surface active agent from the web.
As used herein the term "water absorbency" refers to the
amount, in grams, of water that a three inch b~ eight inch
sample (folded as described in Test A, below) of high hydrohead
porous web material can vertically acquire within a given
amount of time.
As used herein the term "normalized ~ater absorbencyl~
refers to the calculated amount, in grams, of water per qram
of web that a one gram sample of high hydrohead porous ~eb
material can vertically acquire within a given amount of time.
This value is calculated by multiplying the "water absorbency
value for a given time period by (l/the weight of the sample).
As used herein the term "averaged normalized water
absor~ency" refers to the average of three "normalized water
absorbency~ replicates of the material treated in accordance
with our invention. In the example, the "averaged normalized
water absorbency" value of the non-corona treated material was
attained by averaging four replicates.
11
~C~3..3
is usea :~ereln the -erm "-ale or wa~er _bsor~eà" ~ate~
-efers .o -he rate _n arams per second ~r ~ertical ~ater
acquision of a three inch by eight inch sample (folded as
described _n Test ~ below) of high hydrohead ~orous -~e~
~aterial within a given amount of time.
~ s used herein the term "normalized rate of ~ater
absorbency~ .efers to the calculated rate in reciprocal
seconds that a one gram sample of high hydrohead porous web
material can ~ertically acquire within a glven amount of time.
This value is calculated by multiplying the "rate of water
absorbed~ (rate) value for a siven time period by (lJthe weight
of the sample in grams).
As used herein the term "averaged normalized rate of water
absorbed~ refers to the averaqe of three "normalized rate of
' 2 water absorDed" ~eplicates of the material treated in
accordance with our invention. In the example the llaveraqed
normalized rate of water absorbed" ~alue of the non-corona
treated material was attained by averaginq four replicates.
All absorbency and rate of acquision data given herein
were obtianed through the use of water Absorbency Test A
hereinafter Test A. The purpose of absorbency Test A is to
quantitatively measure the absorbency and rate of acquision
properties of a porous fibrous web such as a nonwoven web.
Test A requires the following materials/equipment: (1)
samples of materials to be tested cut ln 3 inch by 8 inch size;
(2) staples: (3) distilled water; (4) one 2SO ml.beaker; (5)
one small lab jack; (6) an Instron model 1122 with strip
recorder; (7) a Lab Wringer a #LW838 Atlas Electric Devicess
Co. of Chicago Ill was used by us: (8) one 500 gram load cell
for the Instron and (9) one standard ten gram weight.
Sample preparation for Test A is as follows: (1) 3 inch by
8 inch samples of the material to be tested are obtained; (2)
the sample is folded in on itself lengthwise one inch from one
side; (3) the sample is folded ir. on itself lengthwise one inch
from the other side to produce a three ply 1 inch by 8 inch
sample; (4) the sample is folded widthwlse in half; and (5) the
sample is stapled one-eigth of an inch from the widthwise fold.
12
23
~he -esultant sample ~s a butterfly conr guration ~lth eacn
"wing~ havlng three plies of sample mater~al.
In order to conduct Test A, the Instron must first be
prepared. ~is is done ~y installing the 500 gram load cell in
S the Instron and calibrating the machine with the lO gram
weight. The strip recorder should read O to 10 gra~s (1 inch
per ~ram~. Next the lower jaws are removed from the Instron and
replaced with a lab jack. The beaker which is filled with
distilled water is placed on the lab jack. The side of the
beaker is marked to record the height of the water in the
beaker. It is important that this level be maintained at as
constant a ievel as possible.
Placement of a sample in the Instron should be consistent
and is accomplished as follows: (1) a start-up sample is placed
'5 in the upper jaws of the Instron with the stapled end down: (2)
the lab jack is used to raise the beaker so that the level of
the water will be one-eighth inch above the staple (the folded
edge of the sample will be one-fourth inch below the surface
of the water); and (3) the height of the jack is recorded. It
is important that the beaker be raised to the same height for
each test.
Sample testing is accomplished as follows: (1) a sample to
be tested is placed in the jaws of the Instron as stated above;
(2) the strip recorder of the Instron is started and allowed
to run for ten seconds to obtain a reading of the sample
weight; (3) the level of fluid in the beaker is checked to
ensure that it is at the mark that has been placed on the
beaker; (4) the lab jack is used to raise the sample to the
same height as was recorded with the start-up sample [this step
should be done quickly and smoothly to minimize irre~ularities
in the climbing portion of the curve~; (5) the test is allowed
to proceed for three minutes: a chart speed of S inches per
minute was used in all cases; (6) once the three minutes has
elapsed, the recorder is turned off, the lab jack is used to
lower the beaker and the sample is removed from the jaws of the
Instron; (7) the staple is carefully removed from the sample
but the sample is maintained in its six-ply configuration; (8)
. i
13
~3
lab wrlnqer :s used t~ remove excess water rom the sample:
30 pounds added to the wrlnger arm is adequate~ (9) after the
sample is put through the wringer, it is unfolded and allowed
to dry ~5 hours is ample for a 2 ounce per square yard
~eltblown sample3.
The data obtained in test A are as follows: (1) total
sample weight is the value read from the baseline of the
Instron recorder plot. [The scale of the paper in these tests
was 1 inch per gram with a zero to ten gram range.]; (2~ actual
10sample weight is the value calculated to ~e the total sample
weight minus the weight of the staple used to hold the sample
~old intact; (3) the water absorbed value is read as the gram
weight absor~ed amount recorded at 1.2 seconds, 2.4 seconds,
1 minute and 2 minutes of elapsed time. Early points are used
15to calculate acqulsion rate: later points are used to compare
overall absorption capacity. Total water absorbed is calculated
to ~e the difference between the baseline total sample weight
and the weight read from the curve for a given time. [Note: If
the weight on the curve is less than the baseline weight, the
20amount of water absorbed is recorded as zero. This occurs as
the result of a buoyant effect as the acquision rate
decreases.] (4) the rate is the value of the slope of the
climbing portion of the curve and is calculated by linear
regression using water absorbed readings for early points, i.e.
25"the points (0 sec., 0 grams), (1.2 sec., ~. grams) and ~2.4
sec., Y2 grams). Note that ~1= water weight at 1.2 seconds
(weight absorbed at 1.2 seconds minus total sample weight1 and
Y2 = water weight at 2.4 seconds (weight absorbed at 2.4
seconds minus total sample weight).
30All data have also been normalized and given in terms of
grams of water absorbed per gram of tested material. Actual
sample weight were used in these calculations.
As used herein the term "decrease in averaged normalized
rate~' refers to the percentage decrease in the rate of water
35absorption of a given sample in its second, third, and fourth
times of testing, in accordence with Test A, as compared to the
rate of water absorption calculated in its first time of
14
~C~J33
~est n~ when ~one n acccrdance wlth test ~. ~ny _ncrease n
.he rate is reported as a zero decrease.
As used herein the term "decrease in averaged normalized
water absor~ed~ refers to the percentage decrease in the amount
of water absor~ed by a given sample in its second, third, and
fourth times of testing, in accordence with Test A, as
compared to the amount of water absorption calculated in its
first time of testing when done in accordance with test A. For
consistency, the point in time of measurement of the amount of
water absorbed must be the same. Thus, this data can ~e
reported at, for example, one minute, two minutes or any other
convenient time. The values are reported at 1 and 2 minutes
herein.
DETAILE~ DESCRI~TlON OF ~HE PREFERRED EMBODIMENT
Referring now to the drawings where like reference numerals
represent like structure or like process steps and, in
particular, to Figure l which schematically illustrates
apparatus 10 for forming and treating a high hydrohead fibrous
porous web material to improve the retentive water absorbency
and retentive water acquision rate of the material. The process
may be initiated by supplying pellets (not shown) of a fiber-
forming thermoplastic material which may be, for example a
polyolefin or a blend of polyolefins such as polypropylene or
polyethylene into the hopper 12 of an extruder 14.
While any thermoplastic fiber forming material may be
useful, one desirable material is a polypropylene which may be
obtained from the Shell Chemical Company under the trade
designation 5A09. The Shell 5AO9 polypropylene has a melt flow
rate of about 40 decigrams per minute when measured in
accordance with ASTM D 1238 at 230 degrees Centigrade.
Many other thermoplastic polymers are suitable for use as
the fiber forming polymer. Specific, non-limiting examples of
such polymers include: polyolefins such as low density
polyethylene, linear low density polyethylene and high density
polyethylene. The materials may be plasticized with suitable
3~3
-iastlcizerS~ ~nd other additives ~nown in the art may be aaded
.o acnieve the deslreà physical char~cteristlcs.
Elastomeric polymers may be used to form the fibrous porous
web. Such polymers include: polyester elastomeric material5,
polyurethane elastomeriC materials, polyetherester elastomeric
materials, polyamide elastomeric materials, and the various
elastomeric A-B-A' block copolymer materials disclosed in U.S.
Patent No. 4,663,220 to Wis~ç~ki et al, which is hereby
inCorporated by reference.
Neat or a solution of a surface active agent is sprayed
onto the fibers as they are formed or on the formed web 22 from
a spraying apparatus which may be a spray boom 19. The surface
active agent may be, for example, an emulsion, a wetting agent
or a detergent having a hydrophile-lipophile balance of at
least about 6 or ~reater. The surface active agent may be
nonionic, cationic or anionic. If the surface active agent is
` nonionic, it is desirable that it have at least 3 ethylene
oxide groups. One desirable surface active agent is a
surfactant is Na-di(2-ethlyheXyl) sulphosuccinate which may
be obtained from American Cyanamid under the trade designation
Aerosol OT. Aerosol OT has an equivalent hydrophile-lipophile
balance of greater than about 13. It has been reported that the
hydrophile-lipophile balance of Aerosol 0~ is about 13.5. See,
U.S. patent number 4,013,863 to van Osenbruggen. et. al. at
Table I, therein, and U.S. patent number 3,904,728 to Davls,
et. al. Another surface active agent which may be used may be
obtained from the Rohm & Haas Company under the trade
desi~nation Triton X-102. Rohm & Haas literature states that
the X-102 is a nonionic octylphenol liquid surfactant having
from 12-13 ethylene oxide units. The material is about 73%, by
weight, ethylene oxide, has a Brookfield viscosity at 25
degrees C. (12 rpm) of 330, and has a calculated hydrophile-
lipophile balance of about 14.6. Other Triton brand name
materials may be utilized in the present invention. Exemplary
of which are Triton X-35 which is a nonionic octylphenol series
material having three ethylene oxide units and a calculated
hydrophile-lipophile balance of 7.8; Triton RW 50 which is a
X~ 3
-at onlc .materlai ~ .H(CX.CH~G!cH)~ -avlnq an average ^-
fi~e ethyiene oxlde unlts anà a measured hydrophlle-~ipophile
balance of '2-14; ~.iton RW 100 which is a cationic material~
(t-c.214~H(cH~cH2o)~o;i)~ having an average of 10 ethylene oxide
_ unlts and a measurea hydrophile-iipophile balance of 16; Triton
DF 12 which is a nonionic modified polyethoxylated alcohol that
has a calculated hydrophile-lipophile balance of 10.6 and
~riton DF 18 which is a nonionic biodegradable modified alcohol
that has a calculated hydrophile-lipophile balance of 11.3.
It is desirable for the surface concentration of the
surface active agent on the surface of the fibers of the web
to be at least about 0.05 weight percent of the web. For
example, from about o.OS percent, by weight, to about 3
percent, by weight of the web. More particularly, from about
__ 3.10 percent, by welght, _o about 1.0 percent, by weight of the
web. ror example, ~rom about 0.1 percent, by ~ei~ht, 'o about
0.4 percent, ~y weight, of the web. Even more particularly~
from about 0.20 percent, by weight, to about 0.30 percent by
weight of the web. ~n one embodiment the surface concentration
is about 0.~0 percent by weight of the web 22.
Because not all of the sprayed surface active agent remains
on the fibers, the amount of surface active agent applyed to
the fibers is generally greater than the amount desired to be
present on the surface. Accordingly, the amount of surface
~5 active agent sprayed on the fibers may vary with the surface
active agent used, he thermoplastic material used to form the
web and/or the process conditions of forming the web.
The temperature of the blend is elevated within the
extruder 14 by a conventional heating arrangement (not shown)
to melt the polymer and pressure is applied to the polymer by
the pressure-applying action of a turning screw (not shown),
located within the extruder, to form the polymer into an
extrudable composition. Preferably the polymer is heated to
a temperature of at least about 175 degrees Centigrade if
~5 polypropylene is utilized as the fiber forming polymer. ~he
polymer is then forwarded by the pressure applying action of
the turning screw to a fiber forming arrangement 16 which may,
17
~C5~ 3
_r -xampie _e a conven~lonai ~eitblowlng -? e arrancemen~.
~eltblowing iie arrangemen~S are descr bea _n ~.S. ?atent
numbers ~,978,185 to ~untin e~ al and 3,849,241 to Buntin et
al. 30th of these patents are hereby incorporated by reference
_ The eievated temperature of the polymer is maintained in the
fiber forming arrangement 16 by a conventional heating
arrangement (not shown). The fiber-forming arrangement
generally extends a distance in the cross-machine direction
which may be about equal to the width of the fibrous porous
nonwoven web which is to be formed by the process. The fiber-
forming arrangement 16 extrudes and attenuates the fibers 18
and directs them onto a moving forming screen 20. Upon
impacting the forming screen 20, the fibers 18 may, depending
upon known process conditions, adhere to each other to form the
fibrous porous web 22. L C not, a nip roller 24, in com~ination
with the forming screen 20 can act to make the web 22 self
supporting. Lf desired, the web 22 may be passed through a
thermal point bonding arrangement 26 including rollers 28 and
30 to consolidate the web 22 even further. The combination of
elevated temperature and elevated pressure conditions which
effect extrusion of the polymer will vary over wide ran~es.
For example, at higher elevated temperatures, lower elevated
pressures will result in satisfactory extrusion rates and, at
- higher elevated pressures of extrusion, lower elevated
temperatures will effect satisfactory extrusion rates.
During or shortly after formation of the fibrous porous web
22, the high hydrophile-lipophile surface active agent is
sprayed onto the surface of the fibers forming the web 22. In
many instances the heat of the molten fibers 18 cooling after
extrusion will be sufficient to effect drying of the high
hydrophile-lipophile balance surface active agent. However, in
so~e instances, the web 22 will have to be passed through a
heating arran~ement ~2 which can include heating cans 34 and
36 to effect drying. The heating can drying temperature will
vary with the surface active agent and polymer utilized. In any
event the drying conditions are to be adjusted so that at least
about 0.05, ~eight percent of the resultant ~eb 22, of surface
18
~C ~3
-c~_-Je agent wll _e on -he sur.~ce o. ~he weD 22. .-or examDie
from aDout 0. 05 percen~, _v ~elght, -o aDoul percent~ by
weight of the web 22 or surface acti~e agent will be on the
surrace of _he web 22. More particularly, .rom about 3.10
percent, by weight, ~o aDOUt 1.0 percent, by weight of the web
22, of surface active agent will be on the surface of the web
22. ~or example, Crom about 0.1 percent, by weight, ~o about
0.4 percent, by weight, of the web 22, of surface active agent
will be on the surface of the web 22. rven more particularlyl
0 from about 0.20 percent, by weight, to about 0-30 percent by
weight of the web 22, of surface active agent will be on the
surface of the web 22.
~etermination of the weight percentage of the surface
active agent on the surface of the web at this point in the
:5 process can be determined by: (l) weighing the initial sample
of material; (2) quantitatively extracting the surface active
agent from the surface of the web 22 using an appropriate
solvent; (3) determining the amount of surface active agent ln
the extraction solvent by such means as ultraviolet
spectroscopy, infra-red spectroscopy, gravimetric analysis etc.
(This may require making up a series of concentration standards
of the surface active agent in the extracting fluid to
calibrate the analytical equipment/method/technique.
Manufactures of surface active agent often will supply methods
~5 for determining surface active agent quantitatively ~nd
qualitatively.); and (4) dividing the amount of surface active
agent by the lnitial web 22 sample weight and multiplyin~ by
100 .
once the high hydrophile-lipophile balance surface active
agent has been applied to the surface of high hydrohead the web
22, the web 22 is passed through the gaps of two conventional
corona discharge units 38. The two corona units are arranged
so one treats one side of the web 22 and the other corona unit
treats the other side of the web 22. one desirable corona
discharge unit can be obtained from Enercon lnd. Corporation
under trade designation Model SS 1223. The gaps of the corona
discharge treatment apparatus may be maintained at about 0.065
i9
33
..c..es. ~tanaarà ...elai -oiis are useà a s t~.e around eiectroàeO
~he ~ase ~etal ground elec~rode roll may De ~uffered with 1
wrap of 0.5 mil polyes~er to substantially prevent arcing of
_he corona unit and pinholing in the higA hydrohead fibrous
- ?orous web ~2. Such buffering reduces the erfectiveness of the
corona discharge unit by approximately 20% for each wrap of 0.s
mil film used. ~he 'ine speed of the high hydrohead ~eb
material 22 and the voltage and amperage of the corona
discharge unit 38 are adjusted so that the equivalent of at
least about 0.8 watt minute per square foot per side of corona
discharge is applied to the web material 22. For example, the
equivalent o~ from about 0~8 to about 15 watt minute per square
foot per side of the web material 22 of corona discharge may
be applied to the web material 22. Accordingly, the equivalent
I~ of rom about l to about I0 watt minute per square foot per
side of the we~ material 22 of corona discharge may be applied
to the web material 22. .~ore particularly, the equivalent of
from about 2 to about 8 watt minute per square foot per side
of the web material 22 of corona discharge may be applied to
the web material 22.
Once the corona discharge unit 38 has applied the
appropriate amount of charge to the web material 22, the web
material 22, may be wound up on a storage roll 40. The corona
treated web material 22 may later be used in a wide variety of
2S applications which require or desire utilization of a material
having acceptable retentive water absor~ency and retentive
water acquision rates. This method of treating a high hydrohead
fibrous porous web material 22 has been found to increase the
web's retentive acquision rate (averaged normalized rate of
water absorption in subsequent reabsorptions as compared to the
initial absorption rate) and retentive absorbency (averaged
normalized amount of water absorbed in subsequent reabsorptions
as compared to the amount initially absorbed).
Another embodiment is schematically illustrated in Figure
2. In this situation the surface active agent may be applied
in neat form or from solution by any of a number of
conventional application methods. Exemplary of which is dip-
23
--nà-squee2e. ~~.e ~lD-anQ-soueeze -~elnoc , _llustr~eà _n
.-igure ~ ~ith ~he dip-and-sqUeeZe apparatus ~2 _ncluàing a
ipplng bath 44 and a palr of squeezln~ rollers 46 and 48. ln
.his process at _east about 0.05%, 3y wei~ht, ~f .he web
material 22 or high hydrophile-iipoPhile balance surface active
agent ,s applied to the web material 22. ror example, from
about 0.05% to about q%, by weight of the web material, of high
hydrophile-lipophile ~alance surface actlve agent may be
applied to the web material 22. Even more particularly, from
about 0.1~ to about 1%, by weight of the web material 22, of
high hydrophile-lipophile balance surface active a~ent may be
applied to the material 22. More particularly, from about 0.1%
to about 0.4~, by weight of the web material 22, of high
hydrophile-7ipophile balance surface active agent may be
:5 applied to t~e web material 22. Even more particularly, from
; about 0.2% to about 0~3%, by weight of the web material 22, of
high hydrophile-lipophile surface active agent may be applied
to the web material 22. The remainder of the process is the
same as the process described with respect to Figure 1.
Of course, other conventional methods can be used for the
production of the nonwoven web 22.
EXAMPLE
In order to demonstrate the improved retentive ~ater
~5 absor~ency and _mproved retentive ~ater acquision ~ate of
corona discharge treated high hydrophile-lipophile balance web
materials of our invention, samples of commercially available
wet wipers available from the Kimberly-Clark Corporation under
the trademark Kimtex were treated in accordence with the
teachings of the present invention. ~he wiper material was an
approximate 2 ounce per square yard meltblown polypropylene
material which had already been treated with a sufficient
amount of Aerosol OT, Na-di(2-ethlyhexyl) sulphosuccinate, to
have a surface concentration of Aerosol OT of about 0.30 weight
percent or about 0.006 ounce per square yard. This web material
was subjected to corona dischar~e treatment .n accordence with
our invention. ~he amount of corona dischar~e applied to the
33
,ampie was ;ar1eà ~ ar.~ e ne spee~ c- _he weD ~ater1al
as _- -.oved ~hrougn -he aps vr each ~- ~he -wo -orona
discharge e~ectrodes. ~ach of the two electrodes were three
feet in iength and had their gap set at 3.065 _nch and the
_ power suppiy was set al 1.25 Kilowatts .or each of the .wo
electrodes. ~he ground r~ll of each electrode was buffered with
one wrap of 0.5 mil polyester to prevent arcing and pinholing.
As has been previously stated this buffering reduces the
effectiveness of the corona discharge by about 20 percent.
S~mples were made with the llne speed (ls) of the web set at
25, 50, 100, ~00, 400 and 600 feet per minute. The
correspondlng watt-min per square foot per side of corona
- discharge values are 13.3, 6.6, 3.3, 1.1, 0.83 and 0.55,
respectively. For example, the the corona charge placed on each
:, side of the ~00 eet per ~inute sample can be calculated as
follows: 1250 watts per side times 0.80 efflciency divided by
three feet electrode length divided by 400 feet per minute
equals 0.83 watt ~in. per square foot per side.
Testing of these materials and samples of non-corona
treated material was conducted in accordence with Test A. The
results of this testing is reported below in the Table, below.
A .~
--e ~J _
2n'
v r
~ e re ~ _
~ 5 ~ c
'J -- ~ Z ~ --o ~ 2 ~ ~n -- e
~ E O
~, <
3 ,~ ~o o _ ~o ,' ~
~ ~J O N --
v 2
~c z ~ e 0 o~ ~ ~ ~ ~ .
~ O O 1~ ~I N
V ~
'A ~1 ~
v ~ -- eo, ~ 0 2
C~ C o
-- Z
~ e e~ 0 c o o ~ ~ c o o ~ ~ o c
z
~ ~ E ~ o 8 ~ ~ ~o o o~ o X eo ~ u- O 0~ 0 ~ O O O
2 0 I N ~ ~ ~ ~ N N N N O _ _ _ O O ~ ~ ~ ~t ~ ~ ~ rl ~
E V C
o " _ ._ ~ 0 o~ ,_ 0 V- O 0 8 ~. ~o ' 8 ~o e~ -- o~ e~ ,~ o ~ o _ o ~ ~ ~ ~ ~ ~ ~
5 V~-- N N N N _ e~J N _ O _ _ -- O O -- -- ~ ~i ~ i ~ N N N N e~J ~ ~ 10 ~0 N N N e~J N e~J ~ e~J
-- 0 ~ 0 ~o ~ ~ ~ e~l 8 o~ 8 o~ . o ~ v~ ~ o 0 U~ N N t~ O 0 0 ~ ~ O -- -- ~ N `O ~
_ ~ ~ ~ ~ ~ ~ e~ e~l e~ O _ _ _ o O _ _ ~ ~ N e~
< e71
V N ~ 0 o ~ 8 ~ _ o~ 2 0 ~ 0 ~ 0N V 2 u~ o
~ ~ e~J e~J N ~ _ O _ _ _ O O _ _ ~1 ~ ~ 1~ N N N N oJ N N N ~ '1 N ~ N ~ N N N I~J
V
_ _ 0 ~ O _ _ ~ I~ Vl ~ O 1~ 0 -- O O O O `O O~ O U~ _ O ~ o O ~O ~ -- ~ ~ N ~ '~ ~ N 0 0 -- ~ ~'
o `n
u û _ u~ m o. o~ 0 e~ o eo 8 e~J g o o o 0 0 ~~ o ~ 3 0 t-- ~ ~ r~ O ~~ ~ eo -- 0 ~ o r~ u~ o
5 V~ ~ N N O O O -- O O O O
OOOooooc~oooooooo oOOOOO~oOOOOO OOOOOOOOOOOO
U~,
o o _ o 1~ ~ 0a 1~ o N O .'~1 0 0 0 0 -- O N V~ ~ o o o 0 o _ o 0 -- ~ -- e~ N O ~ O~ O~
N -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- --
U
'-- 0 ~ N O g g O g g g O O ~ ~O 0 O~ O $ ~O u~ O 0 ~0 ~ O O ~O ~ ~
iOOOOOoOoOOOOoOo OOOoOoOOOOOO OOOOO~OOOOOOO
_
_ e~ _
J ~ -- O -- -- O C ~ 1~ ~-- O 0 O~ O Yl N ~O O~ O~ 1~ N 0 ~ C O O O O O O O O O g
U5V_ ----------------------------~~ '
eg _
~ O~ ~ O _ _ ~- O ~ _ _ ~ O _ O~ ~ 00~ æ 0~ O~ oN~ ~0 ~ o~ o o, o 0 r~ ~ ~ O _ 0 0 0 v~ ~
O~Oen ________________ OOOOOOOOOOOO ____________
v . ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ eo eo 0 ~ ~, U U U ~ ~ ~ ~ 5 eo n 0 ~ U u u ~ ~--
-- ~ ~ ~ _ N ~ ~ ~ ~ _ e~ N r _ e~J ~ _ ~ ~t _ ~J ~ -- N ~ -- ~- ~ -- ~J ~ _
e~
~3
--. ~
~ C .
_ :n
t U '`~
"~ 0 ~ ~ .
- ~ a ~ ~
;~ ~ _ C~ ~ Z -- ~ Z -- ~ -- Z
~ _ ~ 3 _
_: Z
a ~ a
_ ~ ~ tn
~ o ~ _ ~ , O
.. = ~, ~._ a ~ O~ ~'
-- ~ ~ N~I ~ N
~ tJn li~
" ~ E tlt ~Z ` `O A~
t~ _a ~ -- ~ o a ~ a
tl0t`tt~ r ~ , tO ,~
~ ~ ~` -- O AA O ~~ ~ ~ '~
~~E --`otV~o~o`r`~NNorJ ~tot~ o~too~o~~ tO~O~oOtto~O"U~oo~N~
gL~t~ ~ ~N~ ~NNNN_NNNN
,_r~
C
O~E ~o~ooN~o~N ,,,,,,,.~, ONN~t~t~ONo`
~~ ~N~NNNNNN ~N~NNNNNNN ~ N NNN-NNN_
E tooo~ao~No~NO o~tao~~o~o~_t~ o~ot~-~oo~o~o~
~NNNN~N _~t~N_Nt~NN
<ttn
~ - N~o_~Oo~a~$ O~O~Not~oN~ ~N~N~O--
~ ~t~NNNNNNN ~N~NNt~NNNN ~NNNN_~NNN
-~t~ aN~~`t~ `~`~~d`~ ta~tOtO~o~o~_
~tc~n ~N~o~N~ta~ta ~`O~OOOO~N~O o~-~N~to~Ntao
o tn
t~ OOOotatd~to~o~ ~0~0~-0~ _Otd~O~ONo__~ON
~o~t~o~a~ ~to~laN~-~N~o ~NOO~O_~O~_
OoOooooooooo oOoOOOOOOOOO OOOOOOOOOOOO
n 0~NO_OONOON~ -taNoo~0oooNN ~N~o~N~o~o~_
OO~NtO_~O~_t~O_ ~OO_~oOooo_ ~ota~_o~o~otoo~
~N -~~~~-~~ ~~~~~~~~ ~~~
Dn
,_J ~0~~~~~ tO~otoota$$~oo~ ~-t~No~Ng~
~~ OOOoOoooot~OO OOOOOOOOOOoO OOOOOOOOOOOo
_ to ~
~^n_~ ~ot~ot~oo~o`~oNo~o~ ~o~o~o~o~o~o~a~oo~o~ ~t~C~t~CC~CC~t~t~ot~
t~ O--------OO__O_o oOOOoOoooooo ____________
--' NO~O~ON o ~ O tO t~ - - to ~o t~ o o o ta o ta ~ tO tO tO tO ~0 0 tO tO o~o~
~ ~ ~ O _ o t~ o o o _ o o o o O O O~ O O~ O 0. O, t~ O, O~ C~ ~ O
,_ ~ ~t
____________ ____________ ____________
~- -N~_N~_N~ t~-N~_N~-N~ -N~-~-~_~
o$$o__o$o oo$oo$ooooo $$$$$
XC~ 33
~, ~,
.
.s ~ `?
>_ 7, , _ _
_ _ - 3 N
Z 3
' 6
,, 0 ~
:J ~ ~ ._ C
0
-- 6 3 --
<
n _ 'A 1~
Z Ll = N O ~ O
~ _ ~ N
C
;~) 0
U ~ Z-- ~ ~
C O
J ~ ~
> 6 0~`~0 ~ ~ - J ''`
O O ~ O
3~ 8. E o o. 0 ~ o~ o~ Oo` N ~ d
o ~ N ~ ~1 ~ i 10 ~ O _ _ O _ N
~ E o ~ ~ 0 d d o 0 ~-- oO ~
-- ~ ~ ~ N 1~i ~1 0 _ _ O O ~J
O t` ' 0 /~1 -- ~ 0 r'-- O. N ~ 0
n ~ N ~1 1~ ~ ~ 0 N O _ _ O _ N
~n
C N U~ d 0 0 ~, 8 ~r 0 $ æ --
-- --~ ~ ~ N N N O _ _ _ O N
N 0 0 O C~ o o o C`
o ~ 0 0 0 C; C; C; O O O O C; O O
-
O ~ U~ N ,~ ~ O N o o o O
0 0 0 0 0 0 0 0 0 0 Ci O O
0 U
' O C0 8 CO 0` -- $ $ ~
o N --
U
U , ~ d ~ N V O O O O 0
-- N O O O O O O O O O O O O
_ ~ ~
-- ~ -- n ~ ` ` ` ` ` `
u0 _ __oooo_ooooo
~ ~.
o~ ._ 0 , , , , , , O 0, 0 0
<~ _ _
~ _ _ _ _ _ _ _ ~ ~ ~ _
~ ~ ~ 3 3 ~1 U ~ U ~1 ~ `~7
:.1 . -- N ~ _ N ~ -- N 1~ _ N ~'1
ooooooooooo
V~ `O d d `O d d d
33
~he data cr ~able _ ~av be nterreted âS follows: (1) UT
-epresen~s t~e son-corona .-ealed sampies ~hereas the ~5,
-o, oo, ~o, oo, ~oo -epresents ~he fee~ per minute of
- ~ _he web as it passed through the corona discharge gap; (2)
Ihree replicate samples of each treated material were taken
-~ith each of these being represented by the number i or 2
or 3; (3) each of the samples was subjected to testing in
accordence with Test A four times with the first test being
iesignated by the letter (a) the second represented by the
letter (b) the third being represented by the letter (c)
and the fourth being represented by the letter (d). Thus
600-3(d) stands for the results of the fourth time the
third replicate sample of material treated at 600 feet per
minute was tested in accordence with Test A.
From the above results reported in Table I, it is
~lear tha~ ~a~erials -reated in accordance ~ith our
invention line speeds of about 400 feet per minute or less
(about 0.8 watt-min. per square foot per side or greater)
have significantly smaller decreases in average normalized
rate of water àcquision and in averaged normalized water
absorbed at one minute and t~o minutes. Such materials can
be repeatedly reused as wiper materials helping both the
environment because of less wipers being used and the user
because less materials may be purchased.
While the invention has been described in detail with
respect to specific embodiments thereof, it ~ill be
appreciated that those skilled in the art, upon attaining
an understanding of the foregoing, may readily conceive of
alterations to and variations of these embodiments. Such
alterations and variations are believed to fall within the
, scope and spirit of the invention and the appended claims.
::`