Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
90B145/MW 2~6~2~
DISSOLVING A GAS IN A LIQUID
This invention relates to dissolving a gas in a liquid a part of a process
in which flexible containers such as thin walled cans, typically formed of
aluminium or steel, and plastics bottles, are charged with the liquid.
In the canning and bottling industries there has been a trend in recentyears to substitute containers having flexible walls for the traditional
rigid steel can and rigid glass bottle when canning or bottling an
artificially carbonated beverage. The canning or bottling process
includes saturating the beverage with carbon dioxide under pressure, and
then discharging the beverage under pressure into open containers to be
filled. The containers are then closed and sealed. Carbon dioxide comes
out of solution in the sealed container and thus creates a pressure in the
head space of each sealed can sufficient to resist deformation of the of
its walls during normal handling and stacking. Typically, more than 2.5
volumes of carbon dioxide are dissolved in each volume of liquid (when
measured at 15C) in order to create the necessary super-atmospheric
pressure in the head space of each can. In some beverages, particularly
soft drinks, such a level of carbonation is deemed not to have an adverse
effect on the quality of the beverage, and is frequently believed to be
beneficial. There are, however, a large number of other liquid food
products, including other beverages, in which such levels of carbonation
are unacceptable. Indeed, many liquid food products are required to be
free of dissolved carbon dioxide. Accordingly, modern flexible thin
walled containers cannot be used for their packaging unless another means
of providing an internal pressure above atmospheric is provided.
Most attempts to provide such other means have been based on the use ofthe so-called liquid nitrogen droplet dispenser. This is a device which
delivers a small metered quantity of liquid nitrogen to each filled
container before the latter is sealed. The liquid nitrogen vaporises
almost instantaneously and is thereby able to create a super-atmospheric
pressure in the head space of the vessel, bearing in mind that 1 volume of
liquid nitrogen will produce in the order of 600 volumes of nitrogen gas.
Since modern canning lines can operate at speeds up to and over 2,000 cans
per minute, there have been considerable problems in designing such a
droplet dispenser so as to be able to deliver up to 2,000 equal unit
,, ~"
90B145/MU 20~692~
-- 2 --
quantities of liquid nitrogen per minute. To date, these problems have
not been fully solved and there tends to be noticeable variation beyond
what is acceptable in the head space pressure of the sealed cans even at
canning line speeds well below 2,000 cans per minute. Another difficulty
often associated with the operation of liquid nitrogen droplet dispensers
is that whereas it is conventional in canning to blow a non-oxidising gas
such as nitrogen or carbon dioxide over the mouth of the of the open
container once it has been filled and up to the time when it is sealed,
the rates of flow of non-oxidising gas that are optimum for this purpose
tend to draw nitrogen vapour out of the head space of the can and give
rise to undue variations in the internal pressure of the sealed can.
Accordingly, lower than optimum flows of non-oxidising gas across the
mouth of the open container tend to be used with the result that the
oxygen content of the head space of each sealed container is higher than
is ideal. Thus, an alternative to the liquid nitrogen droplet dispenser
is needed.
There are indeed a number of alternative proposals in the art. For
example, US patent specification 4 347 695 discloses a beverage bottling
or canning method for non-carbonated beverages. An inert gas, other than
carbon dioxide, such as nitrogen is injected into a non-carbonated
beverage. The resulting beverage containing dissolved nitrogen is then
introduced into a cooler through which it passes on the way to a filler
which is employed to charge the cans or bottles with the nitrogenated
beverage. Inert gas is permitted to escape from the beverage in the
filled container before sealing the container. The amount of gas released
is sufficient to strip dissolved oxygen from the beverage and then purge
air from the head space of the container. Sufficient gas is retained in
the beverage to exert a super-atmospheric pressure after the container is
sealed. The reduction in oxygen content of the head space is stated to be
superior to that achieved when passing a stream of nitrogen purging gas
into the head space. It is further disclosed that in order to avoid or
minimise the formation of excessive foam, the gas is preferably metered
and injected into a flowing stream of the beverage. The minimising of the
formation of excessive foam is defined as meaning that when containers are
filled to a conventional volume, liquid is not carried beyond the closure
of the container.
.: . : ., : : :
. ... : ~ ... . .
i: :.,... :
-: :: , :: :: ::- ;
90B145/MW
- 3 - 2 ~ 3 6 9 ~ ~
GB-A-2 134 496 nominally relates to filling thin walled cans with
non-fizxy or substantially non-carbonated drinks. In the process
described therein it is, however, required to use carbon dioxide in
addition to nitrogen to create an internal can pressure. Such use of
carbon dioxide is unacceptable in many liquid food products.
GB-A-2 203 417 relates to charging a flexible container such as a can or
plastics bottle with non-carbonated liquid. Argon is dissolved in the
liquid. The liquid is then passed to a filler bowl and the containers are
filled with the liquid therefrom. The containers are then sealed. In
view of the greater solubility of argon than nitrogen, relatively higher
head space pressures can be created thereby. Unfortunately, argon is not
an approved food additive in the United Kingdom and other countries, and
this drawback has lead to a delay in the commercial exploitation of the
method described in GB-A-2 203 417.
GB-A-2 089 191 discloses creating a super-atmospheric pressure in a sealed
container by pre-dissolving an inert gas in a liquid food before the
container is filled ~ith the food. The gas is dissolved in the liquid
food in a gasifying device on its way to the canning filling station.
FR-A-2 636 918 discloses a method in which in order to fill a thin walled
container with a fruit juice, nitrogen is dissolved in refrigerated water
and the resulting nitrogenated water is mixed with deoxygenated
concentrated fruit juice in line on its way to the filling station. The
containers are then filled and sealed and the nitrogen comes out of
solution to create the necessary super-atmospheric pressure therein. This
method has the disadvantage that mixing the chilled water with the
deoxygenated fruit juice will reduce the level of dissolved nitrogen in
the water and hence limit the maximum pressure that can be created in the
container.
Only one of the above discussed prior patent specifications (namely US-A-
4 347 695) addresses the problem of foaming of the liquid, suggesting that
such foaming (sometimes known as "fobbing") can be minimised. We have
discovered that this is not a realistic appreciation of the situation that
arises in commercial practice. Many liquid food products contain
surfactants or other substances which promote foaming. Moreover, nitrogen
.. . .
.~. , ~ : : i i
, ' ~
. -.
90B145/MW 2~92~
tends to interact with such substances to create relatively stable foams.
Accordingly, with many liquid food products, foaming is for practical
purposes impossible to avoid when dissolving nitrogen.
It is an aim of the present invention to provide a method of charging
containers having flexible walls with a liquid food product in which
nitrogen is dissolved in the liquid so as to enable a suitable head space
pressure to be crea~ed in each sealed container and whose operation is not
adversely affected by the creation of foam when filling the containers.
According to the present invention there is provided a method of charging
containers having flexible walls with a non-carbonated liquid food
product, comprising the steps of dissolving nitrogen in the liquid food
product, holding the liquid food product containing dissolved nitrogen for
a period of at least 10 minutes under a pressure of nitrogen, said period
being of sufficient duration for foam formed during the dissolution of the
nitrogen to subside, and then introducing the liquid into the containers
and thereafter closing the containers gas-tight, the concentration of
dissolved nitrogen in the liquid that is introduced into the containers
being such that after their closure dissolved nitrogen is able to come out
of solution in each container to create therein a super-atmospheric
internal pressure that resists deformation of its walls during normal
handling.
If it is desired to deliver continuously a volume of liquid in excess of
that of a vessel in which the liquid food product is held under the said
nitrogen pressure, then a plurality of holding vessels may be used,
whereby one or more are used to deliver nitrogenated liquid to a filling
station, while the other or others are being charged with or holding the
nitrogenated liquid food product. Alternatively, a single holding vessel
may be employed, the vessel having an outlet of liquid located
sufficiently-below the inlet for liquid that liquid free of foam may be
continuously withdrawn from the outlet.
Preferably, the liquid food product has nitrogen dissolved in it under a
pressure of nitrogen in excess of the holding pressure. Typically, the
dissolving pressure is in the range of 3 to 6.5 atmospheres absolute and
the holding pressure is in the range of 2 to 3.5 atmospheres absolute.
-. . .:
, -, ,- .;; . - . :
- . ; . ,. ., -;
: ~. ... , ,,. ~ .
90B145/MW ~S~2~
Preferably, sufficient nitrogen is brought into contact with the liquidfood product to saturate it with nitrogen at the dissolving pressure,
although it is to be appreciated that not all this nitrogen may be
dissolved, and indeed it is generally not essential to saturate the liquid
food product at the dissolving pressure.
Any conventional apparatus for dissolving nitrogen in a liquid may be
used. Preferably, the nitrogen is dissolved in a turbulent, pressurised
stream of the liquid food product. Moreover, the technique of introducing
the nitrogen into the liquid food product is preferably one which
facilitates the formation of small bubbles of nitrogen. For example, the
nitrogen may be introduced into the stream through a venturi. The shape
of the venturi naturally creates turbulence in the stream which helps to
dissolve the nitrogen. An alternative technique is to use a sparger,
which is a pipe having a plurality of orifices of small size (for example
0.012 mm each) typically located within the pressurised stream so as to
create turbulence by its presence.
Dissolution of nitrogen is considerably facilitated if a turbulent stream
of the beer containing undissolved nitrogen bubbles is passed through a
heat exchanger of the plate or plate-fin type whereby the plates provide
an enhanced surface area of the transfer of nitrogen from the gas phase to
liquid phase. Thus, an appreciable quantity of nitrogen is dissolved as
the liquid nitrogen food product passes through the chiller. The chiller
also preferably adjusts the temperature of a liquid food product such that
it leaves the chiller at a temperature a~ or close to 0C. The resulting
stream leaving the chiller is typically in the form of a foam.
The nitrogenated liquid food product may be held for a substantial period
of time with a concentration of nitrogen such that, at the end of the
holding period, there is sufficient dissolved nitrogen to provide adequate
internal container pressure on completion of the method according to the
invention.
In order to maintain the holding pressure at a desired value, nitrogen is
desirably passed into the head space of the holding vessel without its
passing through the liquid food product.
.
,
90B145/MW
- 6 - 2~36~2~
The step of introducing the liquid food product into the containers
typically includes transferring the nitrogenated liquid food product from
the holding vessel to a filli~g vessel. The filling vessel may for
example be a conventional filler bowl. While it is resident in the
filling vessel, the nitrogenated liquid food product is preferably held
under a pressure of nitrogen at least equal to the pressure of nitrogen
under which the beer is held in the holding vessel. Accordingly, a
positive displacement pump is preferably used to transfer the liquid food
product from the holding vessel to the filling vessel. If desired, a
buffer vessel may be employed intermediate the holding vessel and the
filling vessel. By avoiding the use of a pressure transfer system relying
on a pressure difference between a higher pressure in the holding vessel
and a lower pressure in the filling vessel to effect transfer of the
liquid food product, it becomes possible to avoid any substantial
reformation of foam as the liquid food product flows from the holding
vessel to the filler vessel. A pressurised nitrogen atmosphere is
preferably provided in the head space of any buffer vessel. When filling
flexible walled containers, (e.g. thin walled cans or plastics bottles) by
the method according to the invention, it is preferred to pass nitrogen at
a low pressure (e.g. 0.4 psig) over the mouth of each can as it is filled
and up to the time a lid is sealed to the can (the sealing steps sometimes
being referred to in the art as "seaming"). Such nitrogen flow helps to
minimise the amount of air that enters into the head space of the cans
after filling and before closure.
During the short period of time between discharging a volume of liquid
food product from the filler and closing the container which is charged
with the liquid food product, there is tendency for nitrogen to come out ;~
of solution because in this period liquid food product is subjected to the
ambient atmosphere which is at a lower pressure than that maintained in
the filler. Accordingly, this period is typically kept to a very short
duration by operating the canning line at or near to its maximum speed.
As well as helping to reduce oxygen levels in closed cans, the blowing of
nitrogen over the surface of the liquid food product therein immediately
prior to closure of the cans helps, we believe, to enhance the
equilibrated can pressure. It is to be appreciated that shortly after
.
, , . . ~ ,
,: ~ . - i
90B145/MW 2~36~2~
closure, not all of the gas, which may subsequently come out of solution
in the liquid held in the can, would typically have done so.
Equilibration can be brought about by shaking or otherwise disturbing the
can so as to ensure good contact between the gas phase and liquid phase
therein such that an equilibrium can be established between the dissolved
gas in the liquid and the gas in the head space of the cans.
If the liquid food product requires deoxygenation, then this step is
preferably performed upstream of said step of dissolving the nitrogen
therein. If desired, the deoxygenation may be performed by passing
nitrogen bubbles through the liquid so as to drive dissolved oxygen out of
solution. Although such a deoxygenation step will help to provide some
dissolved nitrogen in the liquid food product, this level will be well
below that necessary for creation of a pressure in each filled container
sufficient to resist deformation during normal handling.
If the liquid food product is formed by mixing two or more liquids, thestep of mixing is preferably performed upstream of said step of dissolving
nitrogen in the liquid food product.
The flexible containers may be charged with any one of a wide range of
different liquid food products that have been nitrogenated in accordance
with the invention. The liquid food product may for example be a fruit
juice, milk, a soft drink, wine, an edible oil, or a vegetable juice.
The method according to the invention will now be described by way of
example with reference to the accompanying drawings, in which:
Pigure 1 is a schematic flow diagram of a first apparatus for filling
cans with a liquid food product; and
Figure 2 is a schematic flow diagram of an alternative apparatus for
filling cans with a liquid food product.
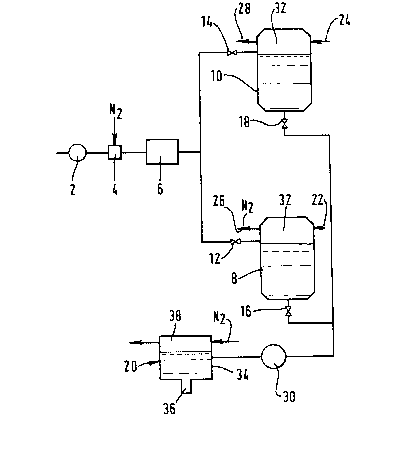
Referring to Figure 1 of the drawings, a stream of deoxygenated liquid
food product is pumped at a pressure of 5 atmospheres absolute by a pump 2
through a gas dissolution device 4 of a kind in which the gas to be
dissolved is introduced into a turbulent stream of the liquid food product
.
~. :
-, ~ . ~. - ... .
.: : .:
~. .
90B145/MW
- 8 - 20~92~
through a sparge pipe (not shown~ having a multiplicity of small orifices
for the gas to be dissolved. A stream of nitrogen, typically at a
pressure of 6 atmospheres absolute, is introduced into the turbulent
stream of liquid food product flowing through the gas dissolving device 4.
The nitrogen enters the turbulent stream of liquid food product in the
form of bubbles. The ratio of the volumetric rate of flow of nitrogen
into the gas dissolution device 4 to that of the liquid stream is
typically 1 to 3. The dissolution device 4 is typically operated at a
temperature between O and 4C. The resulting turbulent stream of liquid
food product containing dissolved nitrogen and undissolved bubbles of
nitrogen then flows to a chiller 6 which is of a plate-fin kind. While
some nitrogen bubbles dissolve upstream of the chiller 6, further
dissolution of the nitrogen takes place in the chiller 6 as a result of
the enhanced liquid-gas contact that the chiller 6 provides. As a result
of the intimate contact between the nitrogen and the liquid, the stream of
liquid food product leaves the chiller 6 in the form of a foam.
Holding tanks 8 and 10 are provided for the nitrogenated liquid food
product. Manually or automatically operable stop valves 12 and 14
associated with the tanks 8 and 10 respectively are operable to place
either of the tanks 8 and 10 in communication with the outlet of the
chiller 6. The tanks 8 and 10 are employed first to receive the
nitrogenated liquid food product and hold it under nitrogen pressure, the
holding period being of sufficient duration to allow the foam to subside,
and second to deliver the liquid to a filler 20. The valves 12 and 14 are
operated so that while the tank 8 receives and holds nitrogenated liquid
food product, the tank 10 delivers to the filler 20 liquid food product
that has been held for a sufficient period of time to enable foam to
subside. Once the liquid from the tank 10 has been delivered to the
filler 20, the roles of the two tanks 8 and 10 are reversed, and the tank
8 is used to deliver liquid food product and the tank 10 to receive and
hold such product. Each of the tanks 8 and 10 has an outlet at its bottom
through which liquid is delivered to the filler ?0. The tank 8 has a
manually or automatically operable on-off valve 16 located in its outlet,
and the tank 10 a similar manually or automatically operable stop valve 18
located in its outlet. Accordingly, by appropriately opening and closing
the respective valves 12, 14, 16 and 18, continuous delivery of the
nitrogenated liquid food product to the filler 20 is made possible. This
:. . .
.
... .
90B145/MW
_ 9 _ 20~ 6 9 2 ~
enables continuous production of canned liquid food product to be
effected.
Prior to commencement of the delivery of the liquid foot product to a
selected tank 8 or 10, both tanks 8 and 10 are purged, and then filled,
with nitrogen under pressure. This pressure is, for example, 3
atmospheres absolute, which pressure is maintained in the ullage space of
each tank by having a continuous flow of nitrogen into and out of the
ullage space 32 thereof via respective inlet pipes 22 and 24 and outlet
pipes 26 and 28. Typically, the holding period of the liquid food product
in a chosen tank is typically at least one hour. At the end of this
period the foam has almost completely subsided. This enables the exact
quantities of liquid food product to be dispensed from the filler 20 to
each can.
Transfer of the liquid food product from a chosen tank 8 or 10 to the
filler 20 is effected by a positive displacement pump 30. The filler 20
comprises a filler bowl 34 having a dispensing nozzle 36 at its bottom. A
super-atmospheric pressure of nitrogen, say 3 atmospheres absolute, is
maintained in the ullage space 38 of the filler bowl 30 by controlled
passage of nitrogen therethrough.
Cans (not shown~ are advanced one at a time under the filler 20 and filled
with a chosen volume of nitrogenated liquid food product. Nitrogen is
passed or blown across the open mouth of each can while it is being
filled and until its closure. The cans are then closed by means of a lid
which is immediately sealed thereto by a seaming machine. The contents of
the cans may then be pasteurised.
Typically, from 50 to 100 parts per million (by volume) of nitrogen may be
dissolved in the liquid food product. After equilibration, the filled and
sealed cans typically have an equilibriated internal can pressure of from
20 to 30 psig ~at 20C).
Referring to Figure 2 of the drawings, the apparatus depicted therein is
similar to that shown in Figure 1 and like parts in the two figures are
identified by the same reference numerals. The essential difference
between the apparatus of Figure 2 and that of Figure 1 is that whereas in
: :., ;. ; :
:... . . .- .:
90B145/MU
lo 2Q~92~
Figure 1, two holding tanks 8 and 10 with associated valves 12, 14, 16 and
18 are employed, in the apparatus shown in Figure 2, just one holding tank
50, relatively large in size, is used. The holding tank 50 is operated
with a super-atmospheric head space nitrogen pressure of, say, 3
atmospheres absolute. This pressure is maintained by passage of nitrogen
into and out of the head space 52, and an inlet 54 and an outlet 56 are
provided for this purpose. The capacity of the tank 50 is large enough to
enable continuous withdrawal of liquid free of foam from a bottom outlet
58 to be performed while a stream of foam is being received through an
inlet 60. Thus, the average residence time of the liquid in the holding
tank 50 is sufficient to allow the foam to subside. In other respects,
operation of the apparatus shown in Figure 2 is analogous to that shown in
Figure 1.
The filler 20 may typically form part of a conventional apparatus for
charging cans with a carbonated beverage. The nitrogen dissolving
apparatus and the vessels and associated equipment for holding the
nitrogenated liquid food product can then be retro-fitted to the filler
20.
"' '' '