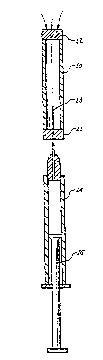Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO90/14798 PCT/US90/01918
1 2~7`~
1DEVICE ~ND METHOD FOR AVOIDING CONTAMINATION OF
2~ULTI-DOSE MEDIC~MENT VIALS
4 BACKGROUND OF THE INVENTION
The present invention relates generally to a
6 tube or reservoir which provides a source of clean air
7 for injection into a multi-dose medicament vial prior to
8 withdrawing medicament from the vial for injection into a
9 patient. The invention also relates to a method for
loading clean-filtered air into the barrel of a syringe
11 prior to use of the syringe to withdraw medicament from a
12 multi-dose viaI.
13 Liquid medication which is to be injected by needle
14 is often sold in multi-dose containers. In some cases
(e g., insulin), as many as 50 or 60 doses or shots are
16 contained in a single vial. The vials are fitted with a
17 rubber diaphragm, and when a dose is to be administered,
18 the needle of a syringe is pushed through the rubber
19 membrane and the proper amount of liquid medicament is
withdrawn for injection into the patient.
21 Since the vial is airtight, withdrawal of liquid
22 medicament creates a partial vacuum inside the vial, and,
23 after a few doses have been withdrawn, the vacuum becomes
24 enough of a factor to make it difficult to withdraw any
further doses. To compensate for this, the standard
26 practice, each time a dose is to be administered, is to
27 inject a quantity of air into the vial first, and then
28 withdraw jthe medication. As described by Sorensen et al
29 in Basic Nursing, page 949 et seq. (W. B. Saunders
Company, Philadelphia, 1979), the standard procedure
31 includes the following steps:
32 1. Cleanse the stopper of the vial with alcohol or
33 Betadine.
34 2. Draw into the syringe an amount of atmospheric
air about equal in volume to the dose to be
36 withdrawn from the vial.
WO90/14798 PCT/US90/01918
205701~ 2
l 3. Push the syringe needle through the stopper of
2 the vial, and inject air into the vial. Then
3 withdraw the amount of medication needed.
4 4. Proceed with injection of the patient.
A source of potential problems in the above standard
6 procedure is that, if the atmospheric air should be
7 contaminated, the contamination is incorporated in the
8 dose of medication and is injected through the skin
9 (normally the body's first line of defense against
infection). Pathogens in the atmospheric air are thus
ll introduced directly into the body tissues or blood, where
12 they can cause serious infections. The problem is
13 aggravated if the liquid medication (e.g., NPH insulin)
14 contains suspended solids and must be shaken before the
dose is withdrawn from the vial. In such case, the
16 shaking causes the contaminated air to be thoroughly
17 mixed with the medicament. The problem is especially
18 aggravated after 30 or 40 shots of contaminated air have
l9 been injected into the vial.
It is an object of the present invention to provide
2l a device and a method for overcoming the above-mentioned
22 problems associated with the injection of atmospheric air
23 into medicament vials.
24 It is a further object of the invention to provide a
specially designed clean air reservoir for furnishing the
26 air to be injected into medicament vials.
27 It is a still further object of the invention to
28 provide a sequence of method steps resulting in loading a
29 medical syringe with clean-filtered air and using such
air to obtain a dose of medication for parenteral
3l administration to patients.
32 Other objects and advantages will become apparent as
33 the specification proceeds.
34
SUMMARY OF THE INVENTION
36 The present invention relates to a clean air
37 reservoir comprising a container having substantially
38 rigid, air impermeable walls and at least two apertures,
WO90/14798 2 ~ ~ 7 o ~ ~ PCT/~S90/0l9l8
l one of said apertures being sealed by an air i~permeable
2 membrane capable of penetration by the needle of a
3 syringe, and the other aperture being sealed by a clean-
4 filtering material.
The invention also relates to a method of
6 administering liquid medication to a patient by injection
7 through the skin, comprising the steps of loading air
8 from a purified air reservoir into the barrel of a
9 syringe, pushing the syringe needle distally through the
septum of a medicament vial, expelling treated air from
ll the barrel of the syringe into the interior of said vial,
12 moving the syringe plunger proximally to withdraw the
13 desired dosage of medicament from the vial, and injecting
14 said dosage through the skin of the patient.
A preferred embodiment of the invention relates to a
16 method of loading clean-filtered air into the barrel of a
17 syringe, comprising the steps of pushing the syringe
18 needle distally through a first septum of a vessel
l9 containing clean-filtered air, and moving the syringe
plunger proximally to withdraw clean-filtered air from
21 the vessel into the syringe, whereby the differential in
22 pressure thus created within the vessel causes
23 atmospheric air to be drawn into the vessel through a
24 second septum fitted with a clean-filtering membrane.
26 BRIEF DESCRIPTION OF THE DRAWINGS
27 The objects, features and advantages of the
28 invention will be apparent to those skilled in the art
29 from the following detailed description, taken together
with the accompanying drawings, in which:
3l FIG. l is a longitudinal section of the clean air
32 reservoir of the present invention, together with an
33 associated syringe, prior to withdrawal of clean-filtered
34 air from the reservoir.
FIG. 2 is a longitudinal section of the reservoir
36 and the syringe, after clean-filtered air has been
37 withdrawn from the reservoir into the syringe.
, ~ ,, ,,, , . ~, - i . : -
WO90/14798 PCT/US90/01918
2~7~
l FIG. 3 is a longitudinal section of a multi-dose
2 medicament vial and a syringe, after clean-filtered air
3 has been injected from the syringe into the vial.
4 FIG. 4 is a longitudinal section of the vial and
syringe, after a dose of medicament has been withdrawn
6 from the vial into the syringe.
8 DETAILED DESCRIPTION OF THE INVENTION
9 Referring to the drawings, the device of the present
l0 invention is shown as a reservoir l0, having an aperture
ll at each end. The first aperture is fitted with an air
12 impermeable membrane or plug ll, and the second aperture
13 is fitted with a membrane or plug 12 made of a clean-
14 filtering material. The reservoir l0 thus comprises a
15 container for clean air, with a septum ll capable of
16 beinq penetrated by the needle 13 of a syringe 14, and a
17 septum 12 capable of clean-filtering atmospheric air
18 which passes into the container when air is wi~hdrawn
l9 from the container by the syringe.
The reservoir l0 may be in any suitable form or
21 shape, although its preferred form is that of a cylinder
22 or tube, with apertures at each end. The walls of the
23 reservoir are made of any suitable air impermeable
24 material, such as glass, acrylic resin, or the like. In
25 the preferred embodiment, the reservoir is an acrylic
26 tube approximately 4" in length, with an inside diameter
27 of l/2", and having a wall thickness of l/16".
28 At one end, the tube l0 is fitted with an air
29 impermeable closure ll. The preferred material for the
30 closure is the standard rubber stopper currently used on
31 multiple dose medicament vials. As in the case of the
32 medicament vials, the closure ll may comprise a rubber
33 stopper or diaphragm, covered by a soft metal cap (e.g.,
34 aluminum), which is removed prior to use. In place of
35 rubber,-any other suitable material may be used if it is
36 penetrable by the needle of a syringe and is self-sealing
37 after the syringe has been removed.
, ,, .. , . . , . . ... ,, .. , , , ., .. , ., .,, . . . . ., , , , . , ,, ,, , , . ~ ", . . . ... ..
...
W090/14798 PCT/~S90/01918
,~ a ~
l At the other end, the tube l0 is fitted with a
2 clean-filtering membrane 12. One purpose for the
3 membrane is to act as a seal between the interior of the
4 tube and the atmosphere when there is little or no
pressure differential between the two. A further purpose
6 is to allow atmospheric air to pass into the tube l0 when
7 pressure is reduced in the tube and to clean-filter such
8 air as it enters. A preferred material for the membrane
9 is a polytetrafluoroethylene/fabric laminate sold under
the trademark Gore-Tex by W. L. Gore & Associates, Inc.,
ll Newark, DE. Any suitable material comprising or
12 incorporating a porous plastic filtering membrane such as
13 polytetrafluoroethylene, also known as PTFE or Teflon,
14 may be used. The pores in the membrane should be small
enough to filter out dust particles and the
16 microorganisms or pathogens associated with them, as
17 found in the ambient air. Pores having dia~eters in the
18 range from 0.1-40 um are generally suitable for the
l9 present purpose, although membranes having pore diameters
outside this range can be useful, depending on the
21 character of the particles and the microorganisms
22 involved. Other suitable PTFE-based materials include
23 filter membranes sold under the trademark Ghia, by Ghia
24 Corporation, Pleasanton, CA; and membranes sold under the
mark Fluoropore, by Millipore Corporation, Bedford, MA.
26 The clean air tube l0 described above, when ready
27 for use, is initially filled with purified air. The
28 filling may be accomplished, at~-the manufacturing site,
29 by charging the tube with air which has been sterilized
by chemical or heat treatment. As another option, the
31 purified air may be introduced at any time by repeatedly
32 withdrawing air from the tube through a syringe until the
33 air within the tube has been completely replaced by
34 atmospheric air which has been clean-filtered by passing
through the membrane 12.
36 In the operation of the invention, the aluminum cap
37 is removed from the end of the clean air tube l0,
38 exposing the rubber diaphragm ll. The outside surface of
WO90/14798 PCT/US90/01918
205701~ 6 ~
l the rubber diaphragm is cleansed with an alcohol pledget,
2 and then, as shown in FIG. 1, the needle 13 of the
3 syringe 14 is guided distally through the rubber
4 diaphragm ll to position the tip of the needle well
within the interior of the clean air tube lO. The
6 relative positions of the tube lO and the syringe 14 will
7 then be as shown in FIG. 1, with the plunger 15 still
8 adjacent the distal end of the syringe barrel, ready to
9 be moved proximally to withdraw air from the clean air
tube.
11 As the next step, the plunger 15 is moved proximally
12 to assume the position shown in FIG. 2. Such movement
13 causes purified air to be withdrawn from the tube lO and
14 loaded into the barrel of the syringe 14. The movement
of the plunger 15 should be sufficient to withdraw a
16 volume of air substantially equal to the volume of the
17 medicament dose to be administered to the patient. As
18 purified air is drawn from the tube 10, the lowered
l9 pressure within the tube causes ambient air to be ta~en
into the tube through the filter 12, as shown by the
21 arrows in FIG. 2.
22 Next the outer surface of the rubber diaphragm 16 of
23 a medicament vial 17 is cleansed with an alcohol pledget,
24 and the needle 13 of the syringe 14 (which now contains
only purified air within its barrel) is guided distally
26 through the rubber diaphragm 16 into the interior of vial
27 17. The plunger 15 of the syringe is then moved distally
28 to expel the charge of air into the interior of vial 17,
29 thus increasing the air pressure within the vial. At
this stage, the syringe 14 and the medicament vial 17 are
31 positioned as shown in FIG. 3.
32 Finally, the plunger 15 of the syringe 14 is moved
33 proximally to the position shown in FIG. 4, and in the
34 course thereof a dose of liquid medicament is withdrawn
from the vial 17 into the barrel of the syringe. The
36 syringe is thPn removed from the vial, and the medicament
37 is administered to the patient by injection through the
38 skin.
WO90/14798 2 ~ 5 7 ~1 ~ PCT/US90/01918
l The device and method of the present invention
2 provide the following features which are significantly
3 advantageous in terms of effectiveness, safety and
4 economics:
l. The necessary step of injecting air into a
6 multiple dose medicament vial prior to
7 withdrawing the medicament can now be carried
8 out without introducing contaminated air into
9 the medicament.
2. The clean air tube with which this is
ll accomplished has a simple, uncomplicated,
12 inexpensive structure which can be mass-
- 13 produced on conventional machinery.
14 3. Since the clean-filtered air which is withdrawn
from the clean air tube is instantly
16 replenished with freshly filtered air, the tube
17 can be used again and again without
18 deterioration in the purity of the air
l9 furnished.
4. The simple, light-weight structure of the clean
2l air tube allows it to be packaged as a
22 companion item with the medicament vial itself.
23 The resulting tandem package thus furnishes not
24 only the medicament but also the means for
clean-filtering the air used for obtaining the
26 medicament dose.
27 It will be understood that use of the term
23 "purified" herein contemplates materials or conditions
29 which have been treated to remove substantial proportions
of microorganisms or other contaminants. Such treatment
3l may be by means of clean-filtering or standard
32 sterilizing techniques using heat or chemical means. The
33 spirit of the invention would not be avoided by use of
34 materials or conditions which have been substantially
improved from the standpoint of aseptic goals, even
36 though the theoretical goal of 100% asepsis may not have
37 been achieved.
WO90/14798 PCT/US90/01918
2~701~ 8 ~
l Althou~h preferred embodiments of the invention have
2 been described herein in detail, it will be understood by
3 those skilled in the art that other variations may be
4 made thereto without departing from the spirit of the
invention.
6 WHAT IS CLAIMED IS: