Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
The present nvention relates to new dihydropyridine
amides, processe~ for their preparation, and their use in
medicament~, in particular their use as medicaments which
act on the circula~ion.
I~ ha~ already b~en disclo8ed th~t 3-aminocarbonyl-1,4-
dihydropyridine-5~carboxylic acid ester derivatives have
an antiarrhythmic and anti-hyperten~ive action ~cf. J
01075-467-~ and European Patent 288,758 A2].
Dihydropyridine amides which act on the circulation are
lQ described in German Offenlesungs chrift 2,228,377 and
also in EP-A-220,653.
It is ~lso known that the polypeptide hormone ANP (atrial
natriuretic peptide) can i.nduce diuresi~, natriure~is and
vasorelaxatio~ [cf. Nature ~38~ 78-83, 1989; Biochem. J.
~32, 313-321 (1985)]. This ef~ect i8 cau~ed by cyclic
guanosine monopho~phate (cGNP), whose excxetion via the
kidneys represen~ a ~peci~i~ marker ~or ~he aa~ivation
of the ANP ystem. ~. Europ. J. Pharmacol. ~
165-168, 1986 and Am. J. Phy~iol. ~ F1220-F122~, 1988~.
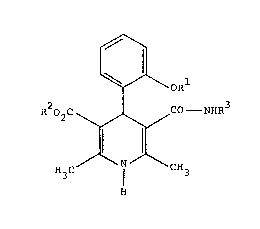
20 Th~ pre~ent in~ention now relake~ to now dlhy~r~pyridlne
amid~ o~ ~ho gonor~l ~ormula (I)
J!~aO2C~CO ~ NHP~3
: H
Le A 27 962 - 1 -
.
~b~
in which
Rl - represents straight-chain or branched alkyl which
has up to 6 carbon atoms ~nd which is optionally
substituted by phenoxy or cyclohexyl~ or
- represents hydrogen or trifluoromethyl,
R2 _ represents straight-chain or branch~d alkyl which
ha~ up to 4 carbon atom~ and which i~ optionally
substituted by cyano, hydroxyl or acetoxy,
R3 - represent~ ~traight-chain or branched alkyl
having up to 5 carbon atoms, or
. - represents cycloalkyl havin~-3 ~o 5 carbon a~oms.
- The compounds according to the invention exist in ~he
form of sterQoisomers which either behave as image ~nd
mirror image tenantiomers~ or which do not behave as
image and mirror image ~diastereomer~. The invention
relates to the antipodes as well as the racemic forms and
also the dia tereomer mixtures. The racemic ~orm~ as well
a~ the dia~t~reomer3 may be re~olved in ~ known mannor to
give the ~tereo~omerically uni~orm aom~onenk~
~Gf. E.~. ~liel, Stereo~hemi~try o~ Carbon Compound~,
~cGraw Hill, 1962).
Pro~0xred ~ompound~ o~ th~ goneral ~ormula (I~ are tho~e
in which
Le A 27 962 - 2 -
,
Rl - repre~ent~ straight-chain or branched alkyl which
has up to 4 carbon atom~ and which i3 optionally
substituted by pheno~y or cyclohe~yl, or
- represents hydrogen or trifluoromethyl,
R2 _ represents straight~chain or branched alkyl which
ha~ up to 3 carbon atoms and which i9 optionally
~ub~tituted by cyano, hydro~yl er acetoxy,
R3 ~ repre~ent~ 3traight-ch~in or branched alkyl
having up to 4 carbon atom~, or
~ lO ~ - repre ents cyclopFopyl or cyc;lopentyl.
: Particularly preferred compounds of the general formula
I) are those
in which
: :
Rl - represent3 straigh~-chain or branched alkyl which
has up to 4 carbon atoms and which is optionally
substituted by cyclohexyl or phenoxy, or
: - repr~sent~ hydrogen or trlfluorome~hyl,
R2 _ repre~n~ m~thyl, 0~hyl or propyl,
R3 - repr~o~nts methyl, ethyl, propyl~ i~opropyl,
cyclopropyl or cyclepentyl.
~ In addikion, proce~ses for the preparation of ~he com-
: pound~ ~ccording to the invention of the general formula
::
~L~L~LZ~ 3 -
~ 3~/1ç~
(I) were found, which procesaea are characteri~ed in khat
[A] aldehydes of the general formula (II)
1 (I~3
C~O
in which
S R1 is as defined above,
are reacted~either with ~-ketocarboxylic acid esters
of the general formula~(III)
R20 2C~
(III~
3 '~
in which
0 R2 i8 ~8 deined above,
and ~-ketocarboxamidee o~? the general ~rmula (IV)
ONHR~
(IV)
H3
i~ which
~ ~3 i ~ a- detined abovo,
:
?
~'' ~'' '
and ammonia, or
the compounds of the ~eneral formulae (II) and (III)
are reacted directly with enaminocarboxamides of the
general formula (V)
,¢~oNHR3
H2 H3
(V)
in which
R3 is a~ defined above,
if appropriate in the presence of an inert solvent,
or
compounds of the general formula (VI)
~OR 1
F1200t::~H
I (VI)
H3C~o
ln whiah
R1 and R2 are as defined above
are prepared first a~ intermediatas and then reacted
Le A 27 962 - 5 _
with compound~ o~ the goneral foxmula (V)
or
EB~ ylidene-~-ketocarboxamides of khe general formula
(VII)
~E:tl '
H~CoNHR3
~ H3 (VII)
.
in which
Rl and R3 are a~ defined above,
are prepared first and reacted with compounds of the
general formula (VIII),
~202C~
H3C ~ NHz ~VIII)
in which
RZ :Ls ~ de~in~d aJ~ove,
in inert ~olvents,
or
Le A 2?~9~2 - 6 --
. .,
' ~ ,~' ; .
, ~:: . . .
'. - : ' , " ' :
~C] dlhydropyridinemonocaxboxylic aaid~ o~ the gener~l
formula (IX)
R202C~OOH
H (IX)
in which
R' and R2 are ~ deflned above,
are reacted, if appropriate via a reactive acid
deri~ative, wlth amlnes o~ tho general formula (X)
~I2N-R3 (X)
in which
R3 i~ as de~ined above,
1~ ~ppropri~te in the ~re~ena~ o~ an inerk organi~
~olv~
The proce~es accordin~ to the invention can be illus~ra-
ted by way o~ exampl~ by the following aquation:
.
LI~ Z-25~ - 7 -
t A ~
H3COZC ~CO-NHCH3
OCF3 t `1 ~ ~ NH3
H 3 C--~o o~~C H 3
CHO
~CF3
H3C02C~CCO-NH-CH3
H3C NH3
tB]
[~0- ( t:H2 ) 3-(~ H3C02C
H~C O - NH <¦ --~
H ~C~NH2
o~CH3
CH~ ) 3-~
H3C02C~O-NH~
~3C N H3
lC~ ~
~ Cf~2{>
H3GOOC~OOH
~3C H3
~N 1 I NJ
L~3 A 2 7 9 6 2 - 8 -
.
,, , . , .. . , ~ ,, . : ,
.' , - ' : . ' ' ' :
,: .
.J J; .i, ~ J ; .~.
~o-CH2{~
f5N
H3COOC~O-N~5
H3C H~
H
H2N -C~H5
~o-cHz-c6Hl 1
H3COOC~O-NH-C2H5
H3C H H~
Proces~ variants A and B
Suitable ~,olvents are water or all inert organic 301vent~
which do not undergo change~ under the rea~ion condi~
tions. r~hese preferahly inalude alcohols 0uch a~
mekhanol, ethanQl, propanol, i~opropanol, ethora ~uch a0
di~th~l 0th~r, dioxano, tet.rahydro~uran, qly~r~ol mono-
m~thyl ether or glyaol dimethyl 2ther, or ,~mide~ such as
dimethyl~ormamide, dimethylacet,~mide or hex,~methylphos
phoric ~riamide, or glacial ace~ic acid, dimethyl ~ul-
phoxide, acetonitrile or pyridine.
e~A 27 962 - 9 _
.
The reaction temperature~ can be varied withln a sub~ta~-
tial range. In general, th~ proces~ is carried out
b~tween +10C and +150C, preferably between +20C and
+100C, in particular at the boiling point of the solvent
S in que~tion.
The reaction can be carried out under atmo~pheric pxe~
sure, bu~ also under increa~ed or reduced pres~ure. I~
iæ ganerally carried out under atmospheric pres6ure.
When carrying out process variant.~ A and B according to
the invention, the ~ubstance involved in the reac~ion
can be used in any dQsired ratio. In general, however,
the reactant~ are used in molar amounts. The ~ubstances
according to the invention are preferably isolated and
purified in such a manner that the solvent i8 distilled
off in vacuo and the residue, which may be obtained
in cry~talline ~orm aft~r ice-cooling, i~ recrystalli ed
from a fiuitable golvent. In some ca~es, purification of
the compound~ acaording to the in~ention may have to be
carried out by mean~ o~ chromatography.
The aldehyde~ o the general o~nula ( II ~, whleh a~e
employed a~ ~kar~ing ~ub~kanG~, are lcnown or can bo
pre~?~r~d by known method~ ~G~rman O~enlegunS~chri~t
2,165,260; 2,401,66g; T.D. ~rri~, ~;.P. }~oth, ~J. Org.
Ch~am. ~, 2004 (1979); W.J. Dale, ~.E. Henni~, J. Arn.
Chem. Soc. ~,2543(1956); Chem. ~bstr. ~, 13929 (1963)~.
The ~-ketocarboxylic acld esters of the general formula
Le A 27~ 962 - 10 -
', ~
'-
.
~ 3;~.~. 3.
(III), which are employed as s~arting sub~tancas, are
known or can be prepared by known methods ~D. Borrmann in
Houben Weyl's "Methoden der organischen Chemie" C"Methods
in Organic Chemistry"] Vol. VII/4, 230 (1968); Y. Oikawa,
R. Sugano, O. Yonemit~u, J. Org. Chem. 43, 2087 (1978)].
The ~-ketocarboxamides of the general formula (IV~, which
are employed as st~rting sub~tances, are known or can be
prepared by knewn methods tGerman Qffenlegungs chrift
1,142,859~.
The enaminocarboxamides of the general formula (V), which
are Pmployed a~ starting substances, are known or can be
prepared by known methods [German Offenlegungsschrift
2,228,377].
The enaminocarboxylic acid esters of the general formula
(VIII), which are employed as starting ~ubstances, are
known or can be prepared by known methods [F.A. Glichman,
A.C. Cope, J. Am. Chem. Soc. 67, 1017 (1945)].
The ylidene-~-ketocarboxamide~ o~ the general ~ormula
(VII), which ~re employed a~ ~tartlng ~ub~tanc~, and the
~lideno-~koto~arboxyli~ ~cld ~er~ o~ the general
~ormula (V~), which ~re ~mployed a8 ~arting substanca~,
are know~l or can be prepared by known methods ~G. Jones
"The Rnoevena~el Conden~ation" in Organic Reactions Vol.
XV, 204 (1967)].
The way in which proces~ variant C according to the
invention i~ carried out i~ ba~ed on the mekhodology
known ~rom the literature fo.r converting carboxylic acid~
into carboxamides. In this method, the carboxylic acid is
fir~t converted into an activated form such as, for
example, the acid chloride or the Lmidazolide, and these
are either isolated a~ such or reacted in a ~econd
reaction step, or amidated directly in-situ ke give the
compounds according to the invention. Example~ of
activating reagents which may be mentioned apart from the
inorganic halides such as thionyl chloride, phosphoru~-
trichloride or phosphoruR pentachloride, or
carbo~yldiimida201e, are carbodiimides such as
cyclohexylrarbodiimide or l-cyclohexyl-3-[2-(N-methyl-
morpholino)-ethyl]car~odiimide p-toluenesulphonate or N~
hydroxyphthalLmide or N-hydroxybenzo~riazole in the
presence of dicyclohe~ylcarbodiimide. Nakurally, it is
also possible fox the dihydropyridinemonocarboxylic acids
to be employed in th~ form of their salts. [The
methodology of the amidation is described, for example,
by: Fieser & Fieser, Reagents for Organic Synthesis, John
Wiley & Son~ Inc. (1967), page 231 - 236; J,C. Shihan and
G.P. Hess, J. ~m~ Chem. SocO 77, 1067 (1955); V. Goodm~n,
G.W. Xennex, Adv. ln Protein Chem. ~, 488 ~1957); W.A.
Bonner, P.I. McNamee~ ~. Org. Chem. ~, 254 (19S1); H.A.
Sta~b/ ~ngew. Chemi0 Int. Ed. 1l 351 ~1962); Fie~er
Fieser, ~ea~nt~ ~or Organlc Synthe~i~, John Wiley & Sons
Inc. ~ , 116, 114; H.C. Beyerman, U.O. van d~r Brink,
Re. Trav. ~Q, 1372 (1961); C.A~ Buehlex, D.~. Pear~on,
~ohn Wiley & Sons, Volume I (1970), page 895 o~ward~,
Volume II, (1977)].
Le A 27 962 - 12 -
Suitable solvents for proves variant C are, besides
water, all inert organic solvents which do not undergo
changes under the reaction conditions. These preferably
include ethers such as diethyl ether, dioxane, tetra-
hydrofuran, glycol dimethyl ether, or halogenated hydro-
carbons such as dichloromethane, trichloromethane or
tetrachloromethane, or amides such as dimethylformamide,
dimethylacetamide or hexamethylphosphoric triamide, or
hydrocarbons, such as benzene, toluene or xylene, or
acetonitrile, nitromethane, pyridine, dimehtyl sulphoxide
or ethyl acetate. Mixtures of the solvents mentioned can
also be used. If the activated intermediates of the
dihydrpopyridinemonocarboxylic acids are isolated, it is
also possible to ue the amines of the formula (X) on
their own as diluents.
The reaction temperatures can be varied within a wide
range. In general, the reaction is carried out in a range
from -70°C to +140°C, preferably from -20°C to +100°C.
The reaction can be carried out at atmospheric pressure,
but also under increased or reduced pressure. It is
generallyc arried out under atomospheric pressure.
When carrying out process variant C according to the
invention, the substances involved int he reaction may be
used in any desired ratio. However, the reactants are
generally employed in molar amounts, However, it has
proven advantageous to employ the amine in 5- to 10-fold
molar excess. It is particularly expedient to employ a
large excess o~ the amine directly as the solv0nt.
The dihydxopyridlnemonocarboxylic acids of the general
formula ~IX), which are employed a~ starting ~ubstance3,
are known or can be prepared by known me~hods [German
Offenlegungsschrift ~,847,236; 3,20~,671; 2,962,241],
~he amines o~ the general ~ormula (X), which are employed
a~ starting substance~, are known or can be prepared by
known methods [Houben Weyl~ Methoden der organischen-
Chemie~ Methods in Organic Chemistry") Vol. XI/l;
Paulsen, Angewande Chemie 78t 501 566 (1966~].
The compound~ according to the invention which were
selected ~ow a valuable pharmacological spectrum of
action which could no~ have been anticipated. Compared
with Xnown dihydropyridines which have a similar struc-
ture, ~uch a~, for example, representative~ de~cribed inGerman Offenlegunys~chrift 2,228,377, the compound~
according to the invantion ~how a dlfferent spectrum of
action, both quantitatively and from the point of view o~
~uality~ Apart rom acting a~ calcium antagoni~t~, thoy
increa~0 the ~NP-dependen~ na~riure~l~ when ~he ANP
~y3te~ i~ ~timulated by ~olume stre~. Thi~ addltlonal
componen~ o~ ac~ion w~ d~tect0d in r~t~ ln a model o~
acute hypexvol~mi~t the te~t sub~tance~ were
admini~terod or~lly in a tylo~e suspen~ion. ~n acute
volume ~tre~3 was triggered by in~ectin~ 15 ml/kg o~
phy~iological sodium ¢hloride 801ution into the caudal
vein, and tha urine was ~ub~equently collected over one
: ' .
I,e A ?7 ~2 - 14 -
,
hour. The compound~ according ko the invention
unexpectedly increase the excretion o~ ~odium, see ~rable
I. At the s.ame time, the excretion of cGMP, whic~ is a
specific marker for the activation of the ANP ~ystem, is
increased (for example i~ Example l9 by 100%).
Table I:
EEm~le Dosage rate in mg/~g, Inc~ in Na+
Nb. pex oral ~L~istration . e~ion in %
~ 10 80
11 10 gS
19 lO lO0
The compound~ according to the invention can therefore
be employed in medicament3 for treating pathologically
changed blood pressure and cardiac insufficiency, and
15 al50 as substances for use in coronary ~herapy.
In addition, they can be employed for treating cardiac
arrhykhmias, renal in~u~iciancy, cirrho~i~ o the li~e~,
a~clte~, oedema o~ the lun~s, oedema o~ ~he b~ain, oedema
o px~nancy, ~lauaoma o~ diabe~e~ mellitu~.
~he new aative compQunda can be aonr~r~ed in a known
manner into the cu~toma~ fonmulations, ~uch a~ tablets,
coated tablet~, pill~, granules, aexosols, ~yrup~,
emul~ions, su~pen~ion~ and 801ution8, with the use of
iner~, non-toxic, pharmaceutically acceptable excipients
L~ A 27 962 - 15 -
f)~3~
or solvent~. In ~hi~ CQn~eXt, the therapeutlcally actlve
compound should in each case be pre~ent ln the ~l~al
mixture in a ~oncentration of approximat01y 0.5 to 90% by
weight, that is to say, in amounts which su~fice to
obtain the do~age range indicated.
For example, the formulations are prepared by ex*ending
the active oompound~ with ~olvents and/or excipients, if
appropriate wikh the use of emulsifiers and/or disper-
~ants, it being po~sible ~or organic solvents to be used
as auxiliary solvents if appropriate, for example in
casQs when wster i3 used as the diluent.
Administration is carried out in the cu~tomary manner,
preferably orally or parenterally, in particularly
perlingually or intravenously.
In the ca~e of parenteral administration, solutions of
the actiYe compounds can be employed while ~uitable
liquid excipients are being used.
To obtain af~eckive result~ ha~ generally proven
advantageou~ to adminisker amounks o~ approxlm~kely 0.001
to 1 mg/kg, preerably appro~imately 0.01 ~o 0.5 mg/kg o~
body wel~ht when adminl0t~xed in~r~enou~ly, ~nd, ln the
a~e o~ orAl ~dmini~r~i.on, khe do~a~e r~ke 1~ approxl-
mat~ly 0.01 ko 20 mg/kg, pre~erably 0.1 to 10 mg/kg of
body weight.
~owever, it may be nece~a~y to deviate from the amoun~
Le A 27 g62 - 16 -
,
mentioned, n~mely a~ a function o~ the body weight or the
nature of the route of administration~ on the individual
behaviour towards the medicament, the nature o its
formulation, and the point in time or interval of admini-
stration. For ex~mple, in some sase~ it may 8uf f ice tomake do with le8s than the abovementioned minimum amount,
whila the abovementioned upper limit must be exceeded i~
other cases. I~ larger amounts are administered, i~ may
be expedient to spacQ several individual dosages over one
day.
Preparation ~xample~
!3E~L
Methyl 1,4~dihydro-2,6-dimethyl-4-[2-(2-phensxy-ethoxy)]-
phenyl-3-cyclopropyl-carbamoylpyridine-5-carboxylate
¢ ~ o-(CH2)2-0-C6H5
H3C02C ~ O-MH- a
H3C N H~
~.8 g (14.1 mmol) o~ methyl 2-(2-phenoxy-ekho~)-
benx~ ene-~cotonae~Ate ~nd 1.97 g (14.1 mmol) of
N-cyclopropyl-3-aminocarboxamide are bolled overnlght in
40 ml of i~opropnnol. ~he mixture iB concentrated and
purified over a ~ilica gel col~mn u~ing toluene/ethyl
acetate mixture~. The purified fraction3 are collected
h~ A 27 962 - 17 -
3 ~
and ~oncentrated. 'rhe product crystallises ~rom iso-
propanol. 3.0 g o~ colourless crystals o~ melting poink
151C axe obtained.
The examples listed in Table 1 were prepared analogously
S to the procedure of Example 1:
R l
H3Co2C~o-NHR3
H3C H3
Example No. Rl R3 m.p.(C)
2 -~CH2)3-0-c6HS ~ 178
3 ~C~2)2-0 C6H5 -C2H5 135
4 -CH~ ~ b 165
H ~ 1a~
6 C~3 ~ 257
Le A 27 962 - 18
~ 3li
Example No. Rl R3 m.p.(C)
7 CF3 -C3H7 251
8 CF3 -CH? 236
9 CH3 C2H5173-175
CH3 -C3H7 174
~ .
11 CH3 1 I L85-187
12 -CH~2 ~ ~ 169
13 -~2 ~ -C3H7 145
14 CH2 ~ -C2HS 150
: ' :
~S -CH3 -CH3 186
16 -CH2 ~ -CH~ llO
17 -CH3 ~ 1~2
18 -CF3 C2H5 240-242
~: 19 CF3 ~ 243-245
~@LJL~ k~ - 19 -
:: ~ . ,; ', ' ' .' ' ''
.
,
',