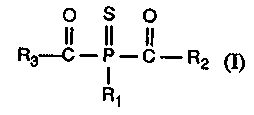Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~; a
-1-
A-18496/A
Bisacylphosphine sulfides
The present invention relates to novel bisacylphosphine sulfides, to
photocurable
compositions which contain these compounds, to the use of bisacylphosphine
sulfides as
photoinitiators for the photopolymerisation of such compounds containing
ethylenically
unsaturated double bonds, and to a process for photopolymerising such
compounds with
bisacylphosphine sulfides as photoinitiators.
Monoacylphosphine sulfides and the use thereof as photoinitiators are
disclosed in
DE-A-3 034 697.
Bisacylphosphine oxides as initiators for light-induced polymerisation
reactions are
disclosed in EP-A-184 095.
K. Issleib et al. have reported in Z. anorg. allg. Chem. 408, 266-274, (1974)
on the
synthesis of cyclic carbonyl phosphides, as well as on the reaction of 2-
phenyl-2-benzo-
phospholene-1,3-dione with sulfur to the corresponding sulfide. The synthesis
and
spectroscopic data of the cited sulfide are also found in the paper by Andrew
R. Baryon et
al., published in J. Chem. Soc., Chem. Commun. (23), 1753-4, (1987).
It has now been found that bisacylphosphine sulfides are most effective
photoinitiators for
polymerising compounds containing ethylenically unsaturated double bonds.
Accordingly, the invention relates to compounds of formula I
O S O
II II II
R3-C ~ - C - R2 (~
R~
wherein R1 is unsubstituted C1-Clgalkyl or C1-C8alkyl which is substituted by
phenyl,
-CN, C1-Ct2alkoxy or halogen, C2-Clgalkenyl, unsubstituted CS-Cgcycloalkyl or
CS-Cgcycloalkyl which is substituted by Ct-Ct2alkyl, Cl-Cl2alkoxy or halogen,
unsubstituted C6-C12ary1 or C6-CtZaryl which is substituted by halogen, Cl-
Ct2alkyl or
-2-
C1-Cl2alkoxy, or a 5- or 6-membered aromatic heterocyclic radical which
contains
oxygen, sulfur and/or nitrogen and is unsubstituted or substituted by halogen,
Cl-C4alkyl
or C1-C4alkoxy, and
R2 and R3 are each independently of the other unsubstituted Ct-Ct8alkyl or Cl-
Cgalkyl
which is substituted by phenyl, halogen or Cl-Cl2alkoxy, C2-C6alkenyl,
unsubstituted
CS-Cgcycloalkyl or CS-C8cycloalkyl which is substituted by Cl-Cl2alkyl, Cl-
Cl2alkoxy or
halogen, unsubstituted C6-C12ary1 or C6-C12ary1 which is substituted by Cl-
Cl2alkyl,
C1-Cl2alkoxy, C2-Cl2alkoxya.lkyl, Cl-C4alkylthio or halogen, or a 5- or 6-
membered
aromatic heterocyclic radical which contains oxygen, sulfur and/or nitrogen
and is
unsubstituted or substituted by halogen, Ct-C4alkyl or Cl-C4alkoxy.
R1, R2 and R3 as Cl-Cl8alkyl may be branched and unbranched alkyl, including
methyl,
ethyl, propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, pentyl,
hexyl, tert-hexyl,
heptyl, 2,4,4-trimethylpentyl, octyl, nonyl, decyl, dodecyl, tetradecyl,
heptadecyl or
octadecyl. R1, R2 and R3 may preferably be Cl-Ct2alkyl.
R1, R2 and R3 as Ct-Cgalkyl which carries one or more, conveniently one to
three and,
preferably, one or two, substituents, may be benzyl, 1-phenylethyl, 2-
phenylethyl,
a,a-dimethylbenzyl, 2-methoxyethyl, 2-ethoxyethyl, diethoxymethyl, 2-
butoxyethyl,
2-isopropoxyethyl, 2-butoxypropyl, 2-octyloxyethyl, chloromethyl, 2-
chloroethyl or
trichloromethyl, and are preferably substituted Cl-C4alkyl, more particularly
benzyl. R1
may also be cyanomethyl, cyanoethyl and the like.
R1 as C2-Clgalkenyl may be allyl, methallyl, 1,1-dimethylallyl, butenyl, 2-
hexenyl,
octenyl, undecenyl, dodecenyl or octadecenyl, and is preferably C2-Cl2alkenyl,
most
preferably C2-C6alkenyl.
R2 and R3 as C2-C6alkenyl may be vinyl, propenyl, butenyl or hexenyl.
R1, RZ and R3 as CS-Cgcycloalkyl may be cyclopentyl, cyclohexyl or cyclooctyl,
preferably cyclopentyl and cyclohexyl, preferably cyclohexyl. R1, R2 and R3 as
substituted
CS-Cgcycloalkyl, conveniently mono- to tetrasubstituted CS-Cgcycloalkyl, may
be methyl-
cyclopentyl, dimethylcyclopentyl, methylcyclohexyl, dimethylcyclohexyl,
diethylcyclo-
hexyl, methoxycyclopentyl, dimethoxycyclopentyl, ethoxycyclopentyl,
diethoxycyclo-
pentyl, methoxycyclohexyl, dimethoxycyclohexyl, ethoxycyclohexyl,
diethoxycyclohexyl,
chlorocyclohexyl, chlorocyclopentyl, dichlorocyclohexyl or
dichlorocyclopentyl. Substi-
-3-
tuted cycloalkyl is preferably Ct-C4alkyl-substituted cycloalkyl.
Rt, R2 and R3 as C6-Cl2aryl may be phenyl, a-naphthyl, (3-naphthyl or 4-
diphenylyl,
preferably phenyl. R1, R2 and R3 as substituted C6-C12ary1 preferably carry 1
to 3
substituents and may be chlorophenyl, dichlorophenyl, trichlorophenyl,
difluorophenyl,
tolyl, mesityl, ethylphenyl, tert-butylphenyl, dodecylphenyl, methoxyphenyl,
dimethoxy-
phenyl, ethoxyphenyl, hexyloxyphenyl, methylnaphthyl, isopropylnaphthyl,
chloronaph-
thyl or ethoxynaphthyl, preferably dimethoxyphenyl, chlorophenyl and mesityl,
preferably
dimethoxyphenyl. R2 and R3 as substituted aryl may also be methoxyethylphenyl,
ethoxy-
methylphenyl, methylthiophenyl, isopropylthiophenyl or tert-butylthiophenyl.
Alkyl and
alkoxy as substituents of aryl contain 1 to 4 carbon atoms and are preferably
methyl or
methoxy.
A heterocyclic radical Rt, R2 and R3 may be mononuclear or polynuclear,
preferably
mono- or binuclear, typically with a fused benzene ring, and may be furyl,
thienyl,
pyrrolyl, pyridyl, indolyl, benzoxazolyl, benzimidazolyl or benzothiazolyl.
Such a hetero-
cyclic radical preferably contains 4 to 12 carbon atoms. These heterocyclic
radicals may
carry one or more, conveniently one or two, substituents. Illustrative
examples are
dimethylpyridyl, methylquinolyl, dimethylpyrrolyl, methoxyfuryl,
dimethoxypyridyl or
difluoropyridyl.
Halogen is preferably chloro, bromo or fluoro, most preferably chloro.
A preferred embodiment of the invention relates to compounds of formula I,
wherein Rt is
unsubstituted C1-Cl2alkyl or Cl-C8alkyl which is substituted by phenyl, -CN,
Ct-C4alkoxy or halogen, CZ-Ct2alkenyl, unsubstituted CS-Cgcycloalkyl or CS-
C8cyclo-
alkyl which is substituted by Ct-Ct2alkyl, Cl-C4alkoxy or halogen,
unsubstituted
C6-Cl2aryl or C6-Cl2aryl which is substituted by halogen, Ct-Cl2alkyl or Cl-
C4alkoxy or
a 5- or 6-membered aromatic heterocyclic radical which contains oxygen, sulfur
and/or
nitrogen and is unsubstituted or substituted by halogen, C1-C4alkyl or Ct-
C4alkoxy, and
R2 and R3 are each independently of the other unsubstituted Ct-Cl2alkyl or Ct-
Cgalkyl
which is substituted by phenyl, halogen or Ct-C4alkoxy, C2-C6alkenyl,
unsubstituted
CS-Cgcycloalkyl or CS-C8cycloalkyl which is substituted by Ct-Ct2alkyl, Ct-
C4alkoxy or
halogen, unsubstituted C6-C12ary1 or C6-Ct2ary1 which is substituted by C1-
Cl2alkyl,
C1-C4alkoxy, C2-Cgalkoxyalkyl, Ct-C4alkylthio or halogen, or a 5- or 6-
membered
aromatic heterocyclic radical which contains oxygen, sulfur and/or nitrogen
and is
-4-
unsubstituted or substituted by halogen, C1-C4alkyl or C1-C4alkoxy.
Interesting compounds are also those of formula I, wherein Rt is unsubstituted
C1-Cl2alkyl or C1-C4alkyl which is substituted by phenyl, -CN, C1-C4alkoxy or
halogen,
C2-C6alkenyl, or unsubstituted CS-Cgcycloalkyl or CS-Cgcycloalkyl which is
substituted
by Cl-Cl2alkyl, C1-C4alkoxy or halogen, or unsubstituted C6C12ary1 or
C6C12ary1 which is
substituted by halogen, C1-Cl2alkyl or C1-C4allcoxy and R2 and R3 are each
independently
of the other unsubstituted C1-Cl2alkyl or Cl-C4alkyl which is substituted by
phenyl,
halogen or Cl-C4alkoxy, C2-C6alkenyl, or unsubstituted CS-Cgcycloalkyl or CS-
C8cyclo-
alkyl which is substituted by C1-Cl2alkyl, Cl-C4alkoxy or halogen, or
unsubstituted
C6-Cl2aryl or C6-Ci2aryl which is substituted by Cl-Cl2alkyl, Cl-C4alkoxy, C2-
CBalkoxy-
alkyl, C1-C4alkylthio or halogen.
Other preferred compounds of formula I are those wherein Rl is unsubstituted
C1-Cl2alkyl, phenyl-Cl-C4alkyl, unsubstituted or Ci-Cl2alkyl-substituted
cyclopentyl or
cyclohexyl, or unsubstituted phenyl or phenyl which is substituted by halogen,
C1-ClZalkyl or C1-C4alkoxy, and R2 and R3 are each independently of the other
unsubstituted Cl-Cl2alkyl, phenyl-Cl-C4alkyl, unsubstituted or Ct-Ct2alkyl-
substituted
cyclopentyl or cyclohexyl, or unsubstituted phenyl or phenyl which is
substituted by
C1-Cl2alkyl, C1-C4alkoxy or halogen.
Also preferred are those compounds of formula I wherein R1 is Ci-Cl2alkyl,
cyclohexyl or
phenyl-Cl-C4alkyl, and R2 and R3 are each independently of the other phenyl
which is
substituted by Cl-C4alkoxy, halogen or Cl-C4alkyl.
Interesting compounds of formula I are also those wherein R2 and R3 are
identical.
Compounds of formula I which also merit interest are those wherein Rt is Cl-
C8alkyl or
benzyl and R2 and R3 are Cl-C4alkyl- or C1-C4alkoxy-substituted phenyl.
The compounds of formula I may be prepared by reacting the appropriate
phosphines with
elemental sulfur:
O O O S O
R3-C-P-CI-R2 + S ~ R3-C-IP-C-R2
I I
R~ R~
-5-
This method is described in DE-A-3 034 697 for the preparation of
monoacylphosphine
sulfides. The bisacylphosphines are reacted as such or in a suitable inert
organic solvent,
as in a hydrocarbon such as toluene, cyclohexane or chlorobenzene, or in an
aliphatic or
aromatic ether, such as dibutyl ether, dioxane, diethylene glycol dimethyl
ether or
diphenyl ether, with an equimolar amount of elemental sulfur. The resultant
bisacylphosphine sulfide, or solution thereof, is freed by filtration from any
remaining
sulfur. The reaction is conveniently carried out in an inert gas atmosphere,
as in nitrogen,
argon or carbon dioxide, preferably nitrogen. The reaction temperatures are in
the range
from 20 to 200°C, preferably from 60 to 120°C, depending on the
solvent and the educts.
After removal of the solvent, the bisacylphosphine sulfide can be isolated in
pure form by
distillation or recrystallisation.
The preparation of the bisacylphosphine starting materials is known from the
literature to
those skilled in the art and is described, for example, in EP-A-184 095. Thus
they may be
prepared by reacting a suitable acid halide with a phosphine in the presence
of a base,
preferably an amine base. The phosphine used in this reaction may conveniently
be
bis(trimethylsilyl)phenylphosphine. In the reaction with the acid halide, the
trimethylsilyl
groups are then replaced by the acid radical.
The invention further relates to compositions comprising (a) at least one
ethylenically
unsaturated photopolymerisable compound and (b) at least one compound of
formula I.
In the practice of this invention, the compounds of formula I can be used as
photoinitiators
for the photopolymerisation of ethylenically unsaturated compounds or mixtures
which
contain such compounds. Component (a) may suitably be selected from
ethylenically
unsaturated monomers, oligomers and polymers which react by
photopolymerisation to
form products of high molecular weight and thereby change their solubility.
The unsaturated compounds may contain one or more olefinic double compounds.
They
may be low molecular weight compounds (monomers) or high molecular weight
compounds (oligomers).
Particularly suitable unsaturated compounds include esters of ethylenically
unsaturated
carboxylic acids and polyols or polyepoxides, and polymers containing
ethylenically
unsaturated groups in the chain or in side groups, including unsaturated
polyesters,
polyamides and polyurethanes and copolymers thereof, polybutadiene and
butadiene,
.~
-6-
polyamides and polyurethanes and copolymers thereof, polybutadiene and
butadiene,
copolymers, polyisoprene and isoprene copolymers, polymers and coplymers
containing
(meth)acrylic groups in side-chains, as well as mixtures of one or more such
polymers.
Unsaturated carboxylic acids are typically acrylic acid, methacrylic acid,
crotonic acid,
itaconic acid, cinnamic acid, unsaturated fatty acids, such as linolenic acid
or oleic acid.
Acrylic acid and methacrylic acid are preferred.
Suitable polyols are aromatic polyols and, preferably, aliphatic and
cycloaliphatic polyols.
Illustrative examples of aromatic polyols are hydroquinone, 4,4'-
dihydroxydiphenyl,
2,2-bis(4-hydroxyphenyl)propane, as well as novolaks and resols. Polyepoxides
include
those based on the cited polyols, preferably on the aromatic polyols and
epichlorohydrin.
Further suitable polyols are polymers and copolymers, which contain hydroxyl
groups in
the polymer chain or in side groups, for example polyvinyl alcohol and
copolymers
thereof or polymethacrylic hydroxyalkyl esters or copolymers thereof. Other
suitable
polyols are oligoesters containing hydroxyl end groups.
Illustrative examples of aliphatic and cycloaliphatic polyols are
alkylenediols containing
preferably 2 to 12 carbon atoms, such as ethylene glycol, 1,2- or 1,3-
propanediol, 1,2-,
1,3- or 1,4-butanediol, pentanediol, hexanediol, octanediol, dodecanediol,
diethylene
glycol, triethylene glycol, polyethylene glycols having molecular weights of
preferably
200 to 1500, 1,3-cyclopentanediol, 1,2-, 1,3- or 1,4-cyclohexanediol, 1,4-
dihydroxy-
methylcyclohexane, glycerol, tris((3-hydroxyethyl)amine, trimethylolethane,
trimethylol-
propane, pentaerythritol, dipentaerythritol and sorbitol.
The polyols may be esterified partially or completely with one or with
different
unsaturated carboxylic acids, in which case the free hydroxyl groups of the
partial esters
may be modified, for example etherified, or esterified with other carboxylic
acids.
Illustrative examples of esters are: trimethylolpropane triacrylate,
trimethylolethane
triacrylate, trimethylolpropane trimethacrylate, trimethylolethane
trimethacrylate,
tetramethylene glycol dimethacrylate, triethylene glycol dimethacrylate,
tetraethylene
glycol diacrylate, pentaerythritol diacrylate, pentaerythritol triacrylate,
pentaerythritol
tetraacrylate, dipentaerythritol diacrylate, dipentaerythritol triacrylate,
dipentaerythritol
tetraacrylate, dipentaerythritol pentacrylate, dipentaerythritol hexacrylate,
tripenta-
erythritol octacrylate, pentaerythritol dimethacrylate, pentaerythritol
trimethacrylate,
kG.
-..~
-7-
dipentaerythritol dimethacrylate, dipentaerythritol tetramethacrylate,
tripentaerythritol
octamethacrylate, pentaerythritol diitaconate, dipentaerythritol
trisitaconate, dipenta-
erythritol pentaitaconate, dipentaerythritol hexaitaconate, ethylene glycol
diacrylate,
1,3-butanediol diacrylate, 1,3-butanediol dimethacrylate, 1,4-butanediol
diitaconate,
sorbitol triacrylate, sorbitol tetraacrylate, pentaerythritol-modified
triacrylate, sorbitol
tetramethacrylate, sorbitol pentacrylate, sorbitol hexacrylate, oligoester
acrylates and
methacrylates, glycerol di- and -triacrylate, 1,4-cyclohexanediacrylate,
bisacrylates and
bismethacrylates of polyethylene glycol having molecular weights of 200 to
1500, or
mixtures thereof.
Also suitable for use as component (a) are the amides of identical or
different unsaturated
carboxylic acids of aromatic, cycloaliphatic and aliphatic polyamines
containing
preferably 2 to 6, more particularly 2 to 4, amino groups. Exemplary of such
polyamines
are ethylenediamine, 1,2- or 1,3-propylenediamine, 1,2-, 1,3- or 1,4-
butylenediamine,
1,5-pentylenediamine, 1,6-hexylenediamine, octylenediamine, dodecylenediamine,
1,4-di-
aminocyclohexane, isophoronediamine, phenylenediamine, bisphenylenediamine,
bis(~3-
aminoethyl) ether, diethylenetriamine, triethylenetetramine, bis((3-
aminoethoxy)ethane or
bis((3-aminopropoxy)ethane. Other suitable polyamines are polymers and
copolymers
which may contain additional amino groups in the side-chain and oligoamides
containing
amino end groups.
Exemplary of such unsaturated amides are: methylenebisacrylamide, 1,6-
hexamethylene-
bisacrylamide, diethylenetriaminetrismethacrylamide,
bis(methacrylamidopropoxy)-
ethane, (3-methacrylamidoethylmethacrylate, N-[((3-
hydroxyethoxy)ethyl]acrylamide.
Suitable unsaturated polyesters and polyamides are derived typically from
malefic acid and
diols or diamines. Malefic acid can be partially replaced by other
dicarboxylic acids. They
can be used together with ethylenically unsaturated comonomers, as with
styrene. The
polyesters and polyamides can also be derived from dicarboxylic acids and
ethylenically
unsaturated diols or diamines, especially from those with long chains
containing typically
from 6 to 20 carbon atoms. Polyurethanes are typically those derived from
saturated or
unsaturated diisocyanates and unsaturated and saturated diols.
Polybutadiene and polyisoprene and copolymers thereof are known. Suitable
comonomers
include olefins such as ethylene, propene, butene, hexene, (meth)acrylates,
acrylonitrile,
styrene or vinyl chloride. Polymers containing (meth)acrylate groups in the
side-chain are
_g_
also known. They may typically be reaction products of epoxy resins based on
novolak
with (meth)acrylic acid, homo- or copolymers of polyvinyl alcohol or their
hydroxyalkyl
derivatives which are esterified with (meth)acrylic acid or homo- and
copolymers of
(meth)acrylates which are esterified with hydroxyalkyl(meth)acrylates.
The photopolymerisable compounds can be used by themselves or in any desired
mixtures. It is preferred to use mixtures of polyol(meth)acrylates.
Binders may also be added to the compositions of the invention. The addition
of binders is
particularly useful if the photopolymerisable compounds are liquid or viscous
substances.
The amount of binder may be from 5-95, preferably 10 -90 and, most preferably,
50-90,
percent by weight, based on the entire composition. The choice of binder will
depend on
the field of use and the desired properties therefor, such as the ability of
the compositions
to be developed in aqueous and organic solvent systems, adhesion to substrates
and
susceptibility to oxygen.
Suitable binders are typically polymers having a molecular weight of about
5000-2 000 000, preferably 10 000-1000 000. Illustrative examples are: homo-
and
copolymers of acrylates and methacrylates, including copolymers of methyl meth-
acrylate%thyl acrylate/methacrylic acid, poly(alkylmethacrylates),
poly(alkylacrylates);
cellulose esters and ethers such as cellulose acetate, cellulose
acetobutyrate, methyl
cellulose, ethyl cellulose; polyvinyl butyral, polyvinyl formal, cyclised
rubber, polyethers
such as polyethylene oxide, polypropylene oxide, polytetrahydrofuran;
polystyrene,
polycarbonate, polyurethane, chlorinated polyolefms, polyvinyl chloride,
copolymers of
vinyl chloride/vinylidene chloride, copolymers of vinylidene chloride with
acrylonitrile,
methyl methacrylate and vinyl acetate, polyvinyl acetate,
copoly(ethylene/vinyl acetate),
polymers such as polycaprolactam and poly(hexamethylene adipamide), polyesters
such
as polyethylene glycol terephthalate) and poly(hexamethylene glycol
succinate):
The unsaturated compounds can also be used in admixture with non-
photopolymerisable
film-forming components. These components may be physically drying polymers or
solutions thereof in organic solvents, for example nitrocellulose or cellulose
acetobutyrate.
They may, however, also be chemically or thermally curable resins such as
polyiso-
cynates, polyepoxides or melamine resins. The concurrent use of thermally
curable resins
is important for the use in so-called hybrid systems which, in a first step,
are
photopolymerised and, in a second step, crosslinked by a thermal
aftertreatment.
y
-9-
The photopolymerisable compositions of this invention conveniently contain the
photoinitiator (b) in an amount of 0.05 to 15 % by weight, preferably of 0.2
to 5 % by
weight, based on the composition.
The invention also relates to compositions which comprise, in addition to the
photo-
initiator (b), at least one further photoinitiator and/or other additives.
Further different additives which may be present in the photopolymerisable
compositions
in addition to the photoinitiator are typically thermal inhibitors which,
especially during
the preparation of the compositions by mixing the components, prevent
premature poly-
merisation. Such further additives are typically hydroquinone, hydroquinone
derivatives,
p-methoxyphenol, ~i-naphthol or sterically hindered phenols, such as 2,6-
di(tert-butyl)-
p-cresol.
To enhance storage stability in the dark it is possible to add copper
compounds, including
copper naphthenate, copper stearate or copper octoate, phosphorus compounds,
including
triphenylphosphine, tributylphosphine, triethyl phosphite, triphenyl
phosphite, or tribenzyl
phosphite, quaternary ammonium compounds, such as tetramethylammonium chloride
or
trimethylbenzylammonium chloride, or hydroxylamine derivatives, such as N-
diethyl-
hydroxylamine.
The exclusion of atmospheric oxygen during the polymerisation may be effected
by
adding paraffin or similar wax-like substances which, at the onset of
polymerisation,
migrate to the surface owing to lack of solubility in the polymer and form a
transparent
film which prevents air from entering the system
Minor amounts of UV absorbers, typically those of the benzotriazole,
benzophenbne or
oxanilide type, may be added as light stabilisers. Light stabilisers of the
sterically hindered
amine type (HALS) can also be added.
In specific cases it can be advantageous to use mixtures of two or more
photoinitiators of
this invention. Further photoinitiators used in addition to the
photoinitiators of formula I
may be those selected from the following types: benzophenones, acetophenone
derivatives, such as a-hydroxyalkylphenylketones, benzoin alkyl ethers and
benzil ketals,
or acyl phosphine oxides, bisacylphosphine oxides or titanocenes.
~~ ~,
- 10-
The photopolymerisation can be accelerated, especially in pigmented
formulations, by
adding amines, such as triethanolamine, N-methyl-diethanolamine, ethyl p-
dimethyl-
aminobenzoate or Michler's ketone. The action of the amines can be intensified
by the
addition of aromatic ketones of the benzophenone type.
The photopolymerisation can further be accelerated by the addition of
photosensitisers
which shift or broaden the spectral sensitivity. These photosensitisers are
preferably
aromatic carbonyl compounds such as benzophenone, thioxanthone, anthraquinone
and
3-acylcoumarin derivatives as well as 3-(aroylmethylene)thiazolines.
Depending on the envisaged end use further customary additives are fillers,
pigments,
dyes, adhesion promoters, wetting agents or levelling agents.
The photopolymerisable compositions can be used far a variety of purposes,
including
their use in printing ink compositions, in clear coating formulations, in
white enamels, in
paints, in paints for exterior coatings, and for photographic reproduction
processes, for
image recording processes or for the production of printing plates, for making
three-
dimensional objects, as by stereolithography or mass hardening, as dental
filling
compositions, as adhesives, as coatings for optical fibres, for printed
electronic circuits or
for coating electronic components.
In coating formulations there are frequently used two-component mixtures of a
prepoly-
mer with a polyunsaturated monomer or three-component mixtures which contain
an addi-
tional mono-unsaturated monomer. The prepolymer primarily determines the
properties of
the coat and, by varying it, the skilled person can influence the properties
of the cured
film. The polyunsaturated monomer acts as crosslinker which makes the coating
film
insoluble. The mono-unsaturated monomer acts as reactive diluent with the aid
of which
the viscosity is lowered without having to use a solvent.
Two- and three-component systems based on a prepolymer are used for printing
inks as
well as for coating compositions, photoresists or other photocurable
compositions. Single
component systems based on photocurable prepolymers are also often used as
binders for
printing inks.
Unsaturated polyester resins are normally used together with a mono-
unsaturated
~a~~.j~.
-11-
monomer, preferably with styrene. Specific single component systems are often
used for
photoresists, for example the polymaleimides, polychalcones or polyimides
disclosed in
DE-OS 2 308 830.
The photocurable compositions of this invention may suitably be used as
coating
compositions for substrates of all kinds, such as wood, paper, ceramics,
synthetic resins
such as polyesters and cellulose acetate films, and metals such as copper and
aluminium,
to which it is desired to apply a protective layer or an image by
photopolymerisation.
The substrate can be coated by applying to said substrate a liquid
composition, a solution
or suspension. This is done typically by dip-coating, brushing, spraying or
reverse roller
coating. The add-on (layer thickness) and the nature of the substrate
(support) will depend
on the desired field of application. Suitable substrates for reco~ing
photographic
information are sheets of polyester, cellulose acetate or resin-coated papers.
Specially
treated aluminium is used for offset printing formes, and copper-clad
laminates for making
printed circuit boards. The layer thicknesses for photographic materials and
offset printing
formes are normally about 0.5 to about 10 Vim. If solvents are concurrently
used, these can
be removed after coating.
Photocuring is of great importance for printing inks, as the drying time of
the binder is a
decisive factor in the rate of production of graphic products and should be in
the order of
fractions of seconds. UV curable printing inks are of particular importance
for screen
printing.
The photocurable compositions of this invention are also very suitable for
making printing
plates. For this utility mixtures of soluble linear polyamides or
styrene/butadiene rubber
with photopolymerisable monomers, typically acrylamides, and a photoinitiator,
are used.
Films and plates of these systems (wet or dry) are exposed via the negative
(or positive) of
the original and the non-cured parts are subsequently eluted with a solvent.
A further field of use of photocuring is metal coating, as in the coating of
sheet metal and
tubes, cans or bottle caps, as well as the photocuring of resin coatings, for
example PVC
floor or wall coverings.
Illustrative of the photocuring of paper coatings is the colourless coating of
labels, record
sleeves or book jackets.
~a~j~~
- 12-
The use of photocurable compositions is also important for imaging techniques
and for the
optical production of information carriers. For these utilities, the layer
(wet or dry) applied
to the substrate is irradiated through a photomask with shortwave light and
the unexposed
areas of the layer are removed by treatment with a solvent (= developer). The
application
of the photocurable layer can also be effected by electrodeposition on metal.
The exposed
areas are crosslinked-polymeric and hence insoluble and remain on the
substrate. Visible
images are formed by appropriate colouration. If the substrate is a metallised
layer, then
the metal can be etched away after exposure and development at the unexposed
areas or
reinforced by galvanising. In this manner it is possible to make printed
circuit boards and
photoresists.
The invention further relates to a process for photopolymerising monomeric,
oligomeric or
polymeric compounds containing at least one ethylenically unsaturated double
bond,
which comprises adding to said compounds a compound of formula I and
irradiating with
light of wavelengths in the range from 200 to 600 nm.
Polymerisation is carried out by known methods of photopolymerisation by
irradiation
with sunlight or with light which is rich in shortwave radiation. Suitable
light sources are
typically mercury medium-pressure, high-pressure and low-pressure lamps,
superactinic
fluorescent tubes, metal halide lamps or lasers the maximum emissions of which
are in the
range from 250-450 nm. Laser light sources have the advantage that no
photomasks are
necessary, as the controlled laser beam writes direct onto the photocurable
layer. Where
combinations with photosensitisers are used, it is also possible to use light
of longer
wavelength or laser beams of up to 600 nm.
The compositions are conveniently prepared by mixing the individual
components.
The invention further relates to a cured composition which is obtained by the
above
described process.
The bisacylphosphine sulfide photoinitiators of this invention have good
solubility in
conventional, preferably apolar, resins such as silicones.
A further advantage is the insignificant yellowing of the compositions cured
with the
inventive photoinitiators.
Z0
-13-
The invention is described in more detail by the following Examples in which
and
throughout the remainder of the description and in the claims, parts and
percentages are by
weight, unless otherwise indicated.
Example 1: Bis(2,6~iimethoxybenzoyl)-(2-methylpropyl)phosphine sulfide
a) Bis(2,6-dimethoxybenzoyl)-(2-methylpropyl)phosphine
A mixture of 5 ml (0.0425 mol) of (2-methylpropyl)phosphine and 13 ml (0.0935
mol) of
triethylamine is added dropwise at 100-110 °C over 30 minutes to 18.8 g
(0.0935 mol) of
2,6-dimethoxybenzoyl chloride in 100 ml of toluene. The reaction is brought to
completion by stirring the reaction mixture at the same temperature for 6
hours,
whereupon the product, as well as triethylammonium chloride, fall out as a
yellowish
precipitate. After cooling the reaction mixture, the ammonium salt is
dissolved by
addition of water and the product is isolated by filtration and dried under
vacuum,
affording 11.1 g (62.5 % of theory) of the title compound in the form of a
white powder
with a melting point of 138-140 °C.
Elemental analysis: C calcd: 63.15 % H calcd: 6.50 %
found: 63.19 % found: 6.52 %
b) Bis(2,6-dimethoxybenzoyl)-(2-methylpropyl)phosphine sulfide
10.0 g (0.024 mol) of bis(2,6-dimethoxybenzoyl)-(2-methylpropyl)phosphine are
added to
toluene and the mixture is heated to 60°C while introducing nitrogen,
whereupon the educt
dissolves completely. Then 0.8 g (0.024 mol) of sulfur are added at
60°C.The reaction
mixture is thereafter stirred for 6 hours, cooled, and concentrated under
vacuum. The
residue is recrystallised from ethyl acetate, affording 6.9 g (63.9 % of
theory) of the title
compound in the form of a yellow powder with a melting point of 145-
147°C.
Elemental analysis: calcd: C 58.6b % found: C 58.59 %
H 6.04% H 6.01%
S 7.12% S 7.03%
Examples 2-3: The compounds of Examples 2 and 3 are prepared using the
appropriate
starting phosphines in accordance with the general procedure described in
Example 1.
29276-216
- 14-
OCH3 CH30
O S O
I_II
R~
OCH3 CH30
Table 1
ExampleRt Melting Elemental analysis
point [%]
calcd. C H S
found
CH3
2 ~ 5 8.66 6.04 7.12
H 117-118 58.48 6.04 7.30
CH2- CH3
61.98 5.20 6.62
3 ~ ~
-CH2 138-139 61.88 5.25 6.83
Example 4: Initiator activity in a white enamel formulation
A photopolymerisable composition comprising the following ingredients is
prepared:
(the parts are parts by weight)
13.5 parts of ~Ebecryl 830 (polyester acrylate, UCB, Belgium)
0.5 part of trimethylolpropane trisacrylate (Degussa)
1 part of 1,6-hexanediol diacrylate (Rohm)
5.0 parts of titanium dioxide (rutile type; ~R-TC2 Tioxide, France)
To this composition is added the amount of test photoinitiator given in Table
2. The
formulation is applied in a layer thickness of 100 p,m to aluminium sheets.
The samples
are then irradiated in a PPG exposure apparatus with mercury medium-pressure
lamps
(2 x 80 W/cm). The sample is passed under the lamps on a belt moving at a
speed of
m/min for as often as is necessary to obtain a wipe-resistant coating surface.
The fewer
~'~~~~.
-15-
the number of passes (n), the higher the reactivity of the tested compound.
The hardness of
the sample is determined by means of the pendulum hardness test using the
apparatus of
Konig (DIN 53 157). The greater the number of seconds, the harder the tested
sample. The
yellowing of the sample is determined by measuring the Yellowness Index (YI)
in accord-
ance with ASTM D 1925-70. The lower the value, the lesser the yellowing of the
sample.
The pendulum hardness and the yellowing are determined immediately after the
cure and
after an additional exposure for 15 minutes and 16 hours under 4 40 W Philips
TL 40/03
lamps.The gloss of the sample is measured in accordance with ASTM D 523 at an
angle of
incidence of 20° and 60° after an additional exposure of 15
minutes and 16 hours. The
degree of reflected light is given in %. The higher the values, the better the
gloss. The
results are summarised in Table 2.
Table 2:
Compound Reactivity Pendulum hardnessYI Gloss 20/60
of [s]
Example [n x 10 immed. 15 min immed. 15 min [in %]
m/min] 16 h 16 h
[wt. %]
1 % Ex. 6 106 157 181 1.7 0.3 -0.3 65/86
1 b
2 % Ex. 4 125 160 193 3.3 0.4 -0.2 78/90
1 b
Example 5: Initiator reactivity in a white enamel formulation
A photopolymerisable composition comprising the following ingredients is
prepared:
30 % of ~Ebecryl 608 (epoxy acrylate, ex UCB, Belgium)
15 % of trimethylolpropane trisacrylate
% of N-vinylpyrrolidone
50 % of titanium dioxide (rutile type; ~R-TC2; Tioxide, France)
Two percent by weight of the photoinitiator of Example 1b) is incorporated in
this
formulation. The formulation is applied in a layer thickness of 100 p.m to an
aluminium
sheet. The 100 um layer is cured by irradiation with a Hanovia mercury medium-
pressure
lamp (80 W/cm). The sample is passed under the lamps on a belt moving at a
speed of
m/min for as often as is necessary to obtain a wipe-resistant coating surface.
The fewer
the number of passes (n), the higher the reactivity of the tested compound.
The hardness of
the sample is determined by means of the pendulum hardness test using the
apparatus of
Konig (DIN 53 157). The greater the number of seconds, the harder the tested
sample. The
~p~ T /~
w ~~i~,~C~ .,~ Ate.
- 16-
yellowing of the.sample is determined by measuring the Yellowness Index (YI)
in accord-
ance with ASTM D 1925-70. The lower the value, the lesser the yellowing of the
sample.
The pendulum hardness and the yellowing are determined immediately after the
cure and
after an additional exposure for 15 minutes and 16 hours under 4 40 W Philips
TL 40/03
lamps.The gloss of the sample is measured in accordance with ASTM D 523 at an
angle of
incidence of 20° and 60° after an additional exposure of 15
minutes and 16 hours. The
degree of reflected light is given in %. The higher the value, the better the
gloss. The
results are summarised in Table 3.
Table 3
Reactivity endulum YI Gloss
hardness 20/60
[s] [in
%]
[n x lOm/min]immed.15 16 immed.15 16 15 min 16 h
min h min h
2 102 139 167 2.7 1.5 2.4 23/66 15/59